Abstract
Background
Although there is widespread acceptance of core needle biopsy (CNB) for diagnosing solid tumors, there is reluctance by some clinicians to use CNB for aneurysmal bone cysts (ABCs) as a result of concerns of safety (bleeding, nerve injury, fracture, readmission, or infection) and reliability, particularly to rule out malignant diagnoses like telangiectatic osteosarcoma. This is especially true when CNB tissue is sent from an outside hospital, where the technique used to obtain the tissue may be spurious.
Questions/purposes
(1) Is CNB effective (provided adequate information to indicate appropriate surgical treatment without further open biopsy) as an initial diagnostic test for ABC? (2) Is CNB accurate (pathology consistent with the subsequent definitive surgical pathologic diagnosis) in differentiating between benign lesions such as primary or secondary ABCs and malignant radiolucent lesions such as telangiectatic osteosarcoma? (3) What are the complications of CNB? (4) Is there any difference in the effectiveness or accuracy of CNB performed at outside institutions when compared with a referral center?
Methods
A retrospective study of our musculoskeletal tumor board pathology database (1990-2016) was performed using search criteria “aneurysmal bone cyst” or “telangiectatic osteosarcoma.” Only patients undergoing a CNB who proceeded to definitive surgical resection with final pathology were included. Excluding outside CNBs, CNB was performed after presentation at a musculoskeletal tumor board as a result of atypical features on imaging or history concerning for malignancy. Outside CNB tissue was reviewed by our pathologists. If there was sufficient tissue for diagnosis, the patient proceeded to definitive surgery. If not, the patient underwent open biopsy. CNB diagnosis, open biopsy results, and open surgical resection pathology were reviewed. Complications, including bleeding, infection, nerve injury, readmission, or fracture, between the CNB and definitive open surgical procedure (mean 1.6 months) were documented. CNBs were considered “effective” if they yielded pathology considered sufficient to proceed with appropriate definitive surgery without additional open biopsy. CNBs were considered “accurate” if they were effective and yielded a pathologic diagnosis that matched the subsequent definitive surgical pathology. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of obtaining a malignant diagnosis using CNB were also calculated.
Results
A total of 81% (59 of 73) of CNBs were effective. Ninety-three percent (55 of 59) of CNBs were classified as accurate. Diagnostic CNBs had a sensitivity and specificity of 89% (eight of nine) and 100% (51 of 51), respectively. The PPV was 1.00 and the NPV was 0.82. There were no complications. With the numbers available, there was no difference in efficacy (90% [37 of 41 versus 14 of 15]; odds ratio, 0.97 [95% confidence interval {CI}, 0.41-2.27], p = 0.94) or accuracy (92% [34 of 37 versus 13 of 14]; odds ratio, 0.87 [95% CI, 0.08-9.16], p = 0.91) between CNBs performed in house and those referred from outside.
Conclusions
These data suggest that CNBs are useful as an initial diagnostic test for ABC and telangiectatic osteosarcoma. Tissue from outside CNBs can be read reliably without repeat biopsy. If confirmed by other institutions, CNB may be considered a reasonable approach to the diagnosis of aggressive, radiolucent lesions of bone.
Level of Evidence
Level III, diagnostic study.
Introduction
Aneurysmal bone cysts (ABCs) are benign, radiolucent lesions that enlarge the affected bone characterized by differing densities of blood contents [19]. The use of the term “cyst” is a misnomer because these lesions lack endothelial walls and instead are lined by proliferative fibroblasts, giant cells, and trabecular bone [3, 7]. ABCs are benign, solitary lesions without metastatic potential. Approximately 70% of ABCs are considered primary and have a gain-of-function mutation of TRE17/USP6 [25]. Secondary ABCs are considered to be a subset representing approximately 30% of all ABCs that do not possess this translocation and accompany another adjacent benign bone tumor.
The accurate diagnosis of ABC is critical given that their presentation and imaging may overlap with malignant bone neoplasms such as telangiectatic osteosarcoma and giant cell-rich osteosarcomas that have different treatments and prognoses [19]. Telangiectatic osteosarcoma, a malignant osteogenic tumor, can cause nonspecific musculoskeletal pain and swelling with imaging findings that at times are similar to ABCs: radiolucent bone lesion that enlarges the bone on radiography and cyst-like fluid collections with fluid-fluid levels on MRI [15, 21]. Telangiectatic osteosarcoma is treated with neoadjuvant chemotherapy followed by radical resection, a vastly different approach than intralesional curettage or, more recently advocated, percutaneous or medical therapies recommended for ABCs [19]. Given the importance of accurately differentiating between these two lesions, some surgeons and centers prefer open biopsy to establish the diagnosis [20]. However, interest in less invasive methods has led to the use of core needle biopsy (CNB) and fine-needle aspiration biopsy (FNAB) in ABC diagnosis. Additionally, because less invasive treatment modalities have been advocated for ABC (such as doxycycline and denosomab), obtaining diagnostic tissue without having to perform an open biopsy may obviate the need for any surgical procedure and its associated risks. Unfortunately, there is a limited number of studies examining these biopsy methods, and the existing studies looking at fine-needle aspirates report low diagnostic accuracy [4, 13]. In contrast to open biopsy for ABC, CNB is an accepted method of biopsy for solid tumors. Given the uncertain diagnostic accuracy of CNB and FNAB for “cystic” lesions, there has been reluctance by some treating surgeons to utilize CNB for the diagnosis of ABC. However, there remain many possible advantages of using CNB over open biopsy such as improved cost-effectiveness, decreased pain, less intraoperative risk of adjacent body compartment violation, and ability to target specific areas within heterogeneous tumors through image guidance [2, 5, 6, 10, 12, 14, 22, 23]. Despite these advantages, the question can be raised whether CNB tissue can be reliably procured outside of a large referral center, where these procedures are done on a regular basis. Cystic lesions are inherently difficult to biopsy and CNB can be performed using a variety of techniques; there is concern that tissue procured from an outside hospital may be less definitive in differentiating between benign and malignant cystic bone tumors.
We aimed to study CNB as an initial diagnostic test for aggressive radiolucent lesions of bone by asking the following four questions: (1) Is CNB effective (provided adequate information to indicate appropriate surgical treatment without further open biopsy) as an initial diagnostic test for ABC? (2) Is CNB accurate (pathology consistent with the subsequent definitive surgical pathologic diagnosis) in differentiating between benign lesions such as primary or secondary ABCs and malignant radiolucent lesions such as telangiectatic osteosarcoma? (3) What are the complications of CNB? (4) Is there any difference in the effectiveness or accuracy of CNB performed at outside institutions when compared with a referral center?
Patients and Methods
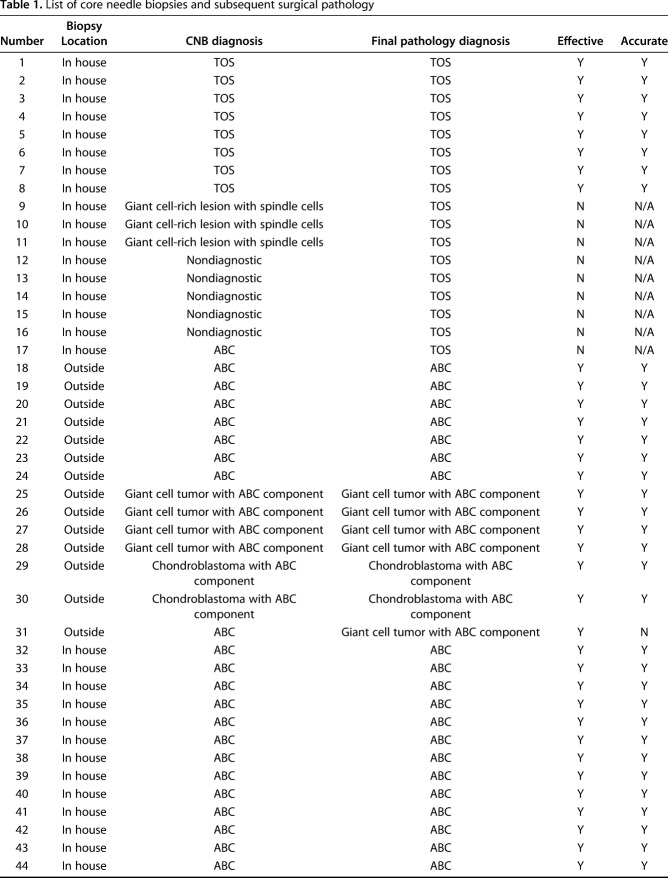
A retrospective case series was compiled by review of the musculoskeletal tumor board pathology database at a major academic sarcoma referral center. All patients from 1996 to 2016 who had CNB tissue and either “aneurysmal bone cyst” or “telangiectatic osteosarcoma” in a biopsy or final pathology diagnosis were reviewed. CNBs performed at outside institutions were included if the workup and final surgical pathology were performed at the home institution. All outside CNB tissue was reviewed at the home institution by a fellowship-trained musculoskeletal pathologist (SDN). Those outside CNBs that were read at our institution but where the patients had definitive surgery elsewhere were excluded from the study because the true pathologic diagnosis could not be confirmed. Thus, only patients who underwent definitive open surgical intervention were included in this study. Ninety-two CNBs included the designated search terms, 73 of which met the previously described criteria (Table 1). The other 19 were excluded as a result of absence of final surgical pathology. Fifteen (21%) CNBs were performed at outside institutions. The 73 patients included in the study had a mean age of 26 years (range, 2-80 years) at the time of biopsy. Fifty-three percent (n = 39) of patients were male and 47% (n = 34) were female.
Table 1.
List of core needle biopsies and subsequent surgical pathology
The patients in this study who underwent CNB all had some atypical feature on imaging or history as determined by the orthopaedic oncologist at the initial patient clinic visit. This included (1) a soft tissue component; (2) a history of pain out of proportion to the lesion; (3) aggressive cortical response; or (4) a significant family history of malignancy. Except for the 15 patients who had a CNB elsewhere, patients with atypical imaging or history proceeded to be presented for discussion at a musculoskeletal tumor board with imaging and history alone. The tumor board is a multidisciplinary conference consisting of fellowship-trained musculoskeletal radiologists; musculoskeletal pathologists; and medical, radiation, and orthopaedic oncologists. If all parties reached consensus that the lesion had no concerning features, CNB was not performed. If any member of the tumor board had concerns of diagnosis, CNB was obtained. Concurrent open biopsy and definitive surgical curettage were used in patients with classic ABC features in an anatomic area in which open biopsy with frozen section would carry little morbidity if a later diagnosis changed. These patients were not included in this study.
Home Institution Biopsy Technique
Fellowship-trained musculoskeletal radiologists performed all CNBs at the home institution. All lesions were biopsied under CT guidance. For lesions with a bony cortex that was interrupted or destroyed, a coaxial system with an 11-gauge outer cannula and a 14-gauge biopsy gun was used (Quick-Core Biopsy Needle Set; Cook Medical, Bloomington, IN, USA). Lesions where the cortex was intact were accessed with a drill set using a 10-gauge access needle and a 12-gauge core needle (OnControl; Teleflex, Wayne, PA, USA, or Bonopty; AprioMed, Londonderry, NH, USA). Multiple core samples were typically obtained from areas with the greatest amount of solid tissue with the final number depending primarily on the appearance of each acquired core. On average, six cores were taken if possible. Four of these cores were placed in formalin for primary histology, immunochemistry, and fluorescence in situ hybridization and two in saline for tissue culture or electron microscopy if deemed necessary (Fig. 1). A cytopathologist was present for all CNBs to confirm adequate tissue was obtained.
Fig. 1A-D.
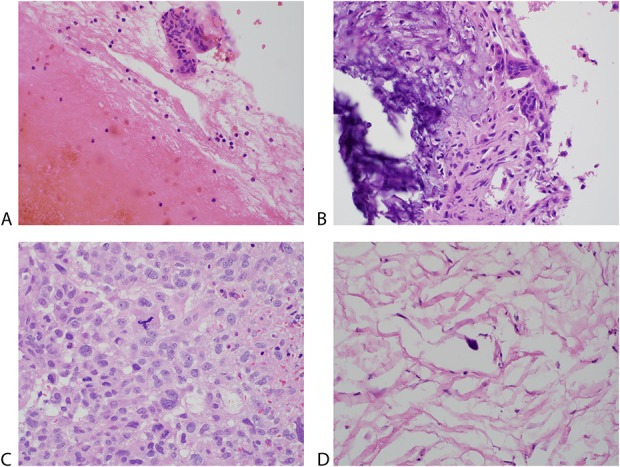
Histologic sections show both ABC and telangiectatic osteosarcoma from selected core needle biopsy and final surgical pathology. (A) Section from the core biopsy of an ABC showing abundant blood and fragments of cyst wall containing benign-appearing cells and multinucleated giant cells (Stain, hematoxylin and eosin; original magnification, x 400). (B) Section from the excision of the ABC showing the cyst wall composed of bland spindle cells, multinucleated giant cells, and calcification (Stain, hematoxylin and eosin; original magnification, x 400). (C) Section from the core biopsy of a telangiectatic osteosarcoma showing obviously anaplastic tumor cells with pleomorphic, enlarged nuclei with prominent nucleoli, atypical mitotic figures, and necrosis (Stain, hematoxylin and eosin; original magnification, x 400). (D) Section shows the excision of the treated, virtually completely necrotic telangiectatic osteosarcoma. Scattered anaplastic cells were present among hyalinized or necrotic tumor (Stain, hematoxylin and eosin; original magnification, x 400).
Per the established protocol at the home institution, all cases were again reviewed at a weekly musculoskeletal tumor board meeting. All relevant radiology and pathology were reviewed to confirm the diagnosis before proceeding with the final treatment plan. If the CNB tissue from either the home institution or outside hospital was deemed nondiagnostic after review at the tumor board, the patient underwent subsequent open biopsy. No patients with nondiagnostic CNB tissue from an outside institution had a CNB repeated at the home institution; all underwent subsequent open biopsy.
For this study, each case was reviewed for age, sex, type of biopsies performed, biopsy pathologic diagnosis, definitive surgical procedure performed, and final surgical resection pathologic diagnosis. Biopsy complications including bleeding, infection, fracture, readmission, and nerve injury were recorded from inpatient and outpatient chart review. In addition to CNB and resection pathology diagnoses, cases were reviewed for the presence of open biopsies and their resultant pathologic diagnoses.
CNBs were classified as effective or ineffective. Effective CNBs provided adequate information to indicate appropriate surgical treatment without the patient undergoing further open biopsy. Ineffective CNB did not provide adequate information and therefore these patients had an open biopsy to establish the diagnosis or underwent an inappropriate surgery.
CNBs were then further defined as accurate or inaccurate. Accurate CNBs were CNBs categorized as effective, which also yielded pathology that was consistent with the subsequent definitive surgical pathologic diagnosis. Inaccurate CNB yielded pathology that was not consistent with the subsequent definitive surgical pathologic diagnosis. Ineffective biopsies were not included in this calculation because there was inadequate tissue to make such a determination.
Observer and analytical bias was addressed by an independent review of the pathology database by five authors (VH, ZDCB, DJ, SDZ, BVK). All conflicting diagnoses were reviewed to ensure accurate labeling. The reviewing authors were not involved in any of the diagnostic or surgical procedures, and data analysis was performed by authors similarly removed from interventional procedures.
The sensitivity and specificity of CNB and the associated 95% confidence intervals (CIs) were calculated based on the diagnostic CNBs. In addition, positive predictive value (PPV) and negative predictive values (NPV) of obtaining a malignant diagnosis from a CNB were calculated. The NPV was calculated twice, once not including nondiagnostic biopsy results and once including nondiagnostic biopsy results. All calculations were performed using Microsoft Excel 2016 (Microsoft, Redmond, WA, USA).
Results
Is CNB Effective (provided adequate information to indicate appropriate surgical treatment without further open biopsy) as an Initial Diagnostic Test for ABC?
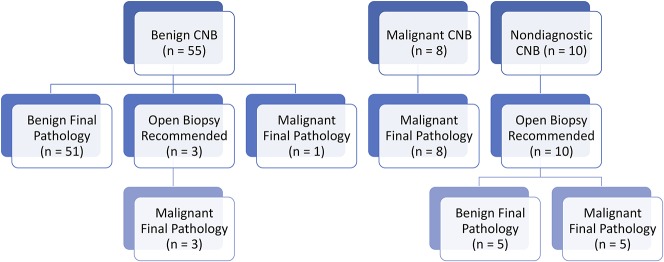
The CNBs were found to be very effective. Fifty-nine (81%) CNBs were classified as effective, whereas 14 (19%) were classified as ineffective. On final pathology, 56 (77%) cases reviewed in this series were benign, whereas 17 (23%) were malignant (Table 2). Of the 73 CNBs, 55 (77%) were read as benign, 51 (93%) of which were benign on final diagnosis, whereas four (7%) were malignant on final diagnosis (Fig. 2). Three of four of the CNBs that were read as benign and ended up malignant on final diagnosis were recommended for obtaining further tissue before definitive surgery because of areas of concerning histology, whereas one patient was taken for definitive surgery with a presumed benign diagnosis (ABC) that was malignant on final pathology (telangiectatic osteosarcoma). Eight CNBs were read as malignant, all of which were malignant on final diagnosis. The remaining 10 CNBs were nondiagnostic and these patients underwent open biopsy (five benign and five malignant). Thus, of the 73 CNBs, five (7%) patients had subsequent open biopsy to confirm a benign diagnosis, whereas eight (11%) were followed by open biopsy resulting in a malignant diagnosis. One patient with a nondiagnostic CNB underwent open biopsy followed by definitive treatment for a benign lesion in the same surgery. This resulted in 13 CNBs in patients who underwent further open biopsy to confirm the diagnosis and indicate appropriate surgical treatment.
Table 2.
Biopsy categorization
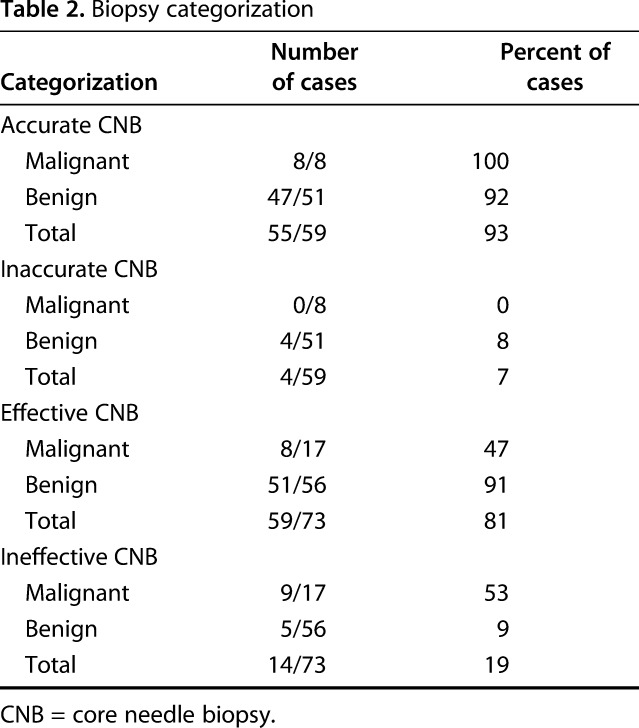
Fig. 2.
Flow diagram showing CNB results and ensuing open biopsy and final surgical pathology results for all 73 cases.
Is CNB Accurate (pathology consistent with the subsequent definitive surgical pathologic diagnosis) in Differentiating Between Benign Lesions Such as Primary or Secondary ABCs and Malignant Radiolucent Lesions Such as Telangiectatic Osteosarcoma?
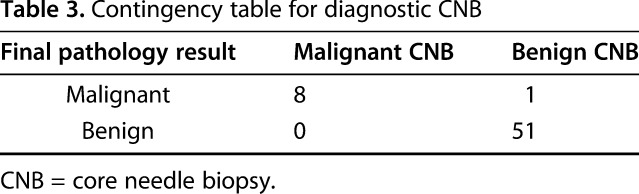
The sensitivity and specificity for detecting malignancy using CNB were both high. The 60 diagnostic CNBs had a sensitivity of 0.89 (95% CI, 0.51-0.99) and specificity of 1.0 (95% CI, 0.91-1.0) for identifying malignant tumors (Table 3). The PPV was 1.0 (95% CI, 0.58-1.0; eight of eight) and the NPV was 0.98 (95% CI, 0.88-0.99; 51 of 52) excluding nondiagnostic results and 0.82 (95% CI, 0.70-0.90; 51 of 62) including nondiagnostic results. The CNBs were also found to be highly accurate. In total, 55 of 59 (93%) of effective CNBs were classified as accurate.
Table 3.
Contingency table for diagnostic CNB
What Are the Complications of CNB?
There were no complications associated with the 73 CNBs reviewed in this series. Twelve patients underwent an additional procedure (12 CNBs that had subsequent open biopsy without concurrent definitive treatment with one additional patient receiving an open biopsy and definitive treatment concurrently), none of which were associated with complications.
Is There Any Difference in the Effectiveness or Accuracy of CNB Performed at Outside Institutions When Compared With a Referral Center?
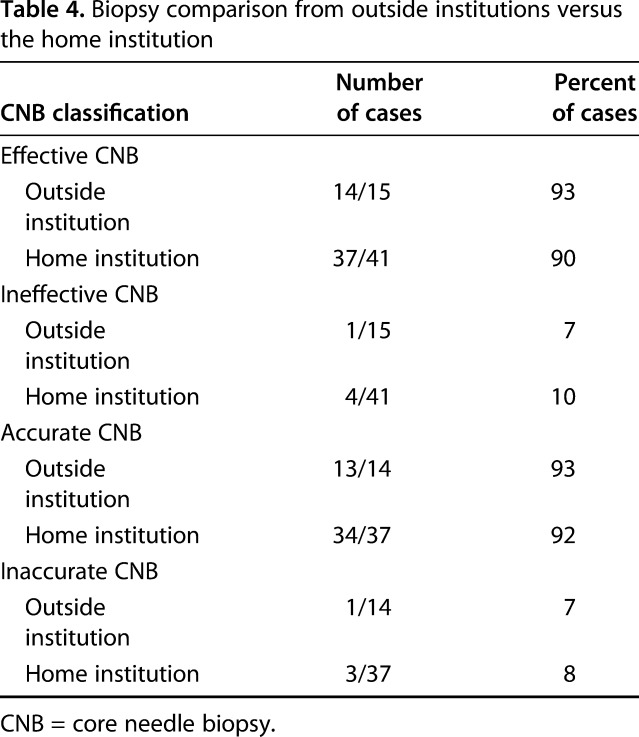
When examining only benign final pathology diagnoses at the home institution, 41 CNBs were performed, 90% (37 of 41) of which were classified as effective and 92% (34 of 37) of which were classified as accurate. Fifteen CNBs were performed at outside institutions, all with benign diagnoses on final pathology. Of these, 14 of 15 were classified as effective and 13 of 14 were classified as accurate (Table 4). Thus, the numbers available, we saw no difference in effectiveness (90% [37 of 41 versus 14 of 15]; odds ratio, 0.97 [95% CI, 0.41-2.27], p = 0.94) or accuracy (92% [34 of 37 versus 13 of 14]; odds ratio, 0.87 [95% CI, 0.08-9.16], p = 0.91) between CNBs performed at our facility and those referred to our center from outside.
Table 4.
Biopsy comparison from outside institutions versus the home institution
Discussion
When faced with a patient presenting with an aggressive radiolucent bone tumor, obtaining a pathologic diagnosis before definitive surgical management is critical. At times, benign radiolucent lesions such as ABCs may present with an aggressive appearance on imaging and raise the suspicion of a malignant process. Because ABCs and bone sarcomas have different biologies and treatments, it is important to make a correct diagnosis before treating a patient. Although CNBs are routinely used to diagnose most bone lesions where it is deemed necessary to obtain a tissue diagnosis, some treating physicians still prefer open biopsy for the diagnosis of ABC given the critical importance of accurate pathologic diagnosis. Less invasive methods such as FNAB have been previously studied but have accuracy rates deemed too low to be acceptable [4, 13]. Although still controversial in radiolucent tumors such as ABC, CNB is an accepted method of procuring tissue for pathologic diagnosis in solid tumors. CNB has a number of advantages over traditional open biopsy, including cost-effectiveness, decreased pain, less intraoperative risk of adjacent body compartment violation, and ability to target specific areas within heterogeneous tumors through image guidance [2, 5, 6, 10, 12, 14, 22, 23]. Although prior studies have called into question the accuracy of CNB in the diagnosis of cystic tumors [11], no previous study has specifically evaluated using CNB to differentiate between ABCs and malignant radiolucent lesions such as telangiectatic osteosarcoma. In this study, CNBs had a sensitivity of 0.89 and specificity of 1.0 for identifying malignant tumors (Table 2). The PPV was 1.0 and the NPV was 0.98 excluding nondiagnostic results and 0.82 including nondiagnostic results. CNB was determined to be effective in 81% of cases, where a further open biopsy was not deemed to be indicated before appropriate definitive surgical intervention. The accuracy of CNB from effective biopsies was determined to be 93%. In the 73 cases examined in which CNB was performed, there were no complications associated with the CNB. Finally, there was no difference in the efficacy or accuracy of CNBs performed at outside institutions when compared with the home institution (93% versus 90% efficacy and 92% versus 93% accuracy, respectively).
There are a number of limitations to this study, including its retrospective design and the lack of a control group. Because the study period was two decades and spans the careers of two orthopaedic oncologists, the decision to proceed with CNB was undoubtedly subjective. Despite the guidelines that are detailed in the Methods, clinical and radiographic findings concerning for malignancy used by both the orthopaedic oncologists and physicians on the musculoskeletal tumor board may have subtly evolved over time, influencing which patients subsequently underwent CNB. As our center became more comfortable with CNB, it may be the case that more patients underwent CNB for diagnosis. This may have impacted the results of this study; because a broader selection of patients underwent CNB instead of open biopsy, it is likely that more nondiagnostic results were obtained. Unfortunately, these limitations are difficult to address, because an ABC is a rare diagnosis and telangiectatic osteosarcoma even rarer still. We are able to compare our results with related CNB studies examining radiolucent lesions as a frame of reference, but to date no other study has looked specifically at the use of CNB to diagnose ABC and telangiectatic osteosarcoma. Complications were obtained through inpatient and outpatient chart review, which may have missed complications that developed after patient discharge, yet all patients continued to be followed after discharge, because they underwent a definitive open procedure an average of 1.6 months after CNB. The home institution is a major academic sarcoma referral center, so the rate of telangiectatic osteosarcoma is higher than at other hospitals. In addition, simple ABCs without concerning features in patients who were taken to the operating room without CNB are not captured in this study. As a consequence, this study may not be generalizable to all settings. Despite this, the authors feel that this study does provide important information regarding the utility of CNB in the diagnosis of these atypical cystic bone tumors.
Is CNB Effective (provided adequate information to indicate appropriate surgical treatment without further open biopsy) as an Initial Diagnostic Test for ABC?
Our efficacy of 81% in diagnosing radiolucent lesions suspected to be ABC is higher than another group that demonstrated that CNB was 71% effective in diagnosing radiolucent lesions [11]. Previously published studies have also shown that FNAB, which has been abandoned as a diagnostic test for ABC, is approximately 70% effective [4]. This is comparable to the previously published study on CNB but lower than the data published in this study. The decreased efficacy of FNAB may be the result of the fact that FNAB cytology of ABC is largely nonspecific, and image guidance enables the procurement of cores of tissue from areas of greater cellularity when a CNB is performed, leading to a higher likelihood of diagnosis.
Is CNB Accurate (pathology consistent with the subsequent definitive surgical pathologic diagnosis) in Differentiating Between Benign Lesions Such as Primary or Secondary ABCs and Malignant Radiolucent Lesions Such as Telangiectatic Osteosarcoma?
Although no other study of which we are aware has looked specifically at the diagnostic accuracy of CNB for differentiating between benign ABC and malignant processes such as telangiectatic osteosarcoma, our accuracy of 93% is comparable to another group that demonstrated 95% accuracy when using CNB to differentiate between benign and malignant radiolucent lesions [11]. These results are also in line with previous work demonstrating accuracy between 74% and 89% when using CNB to diagnose musculoskeletal neoplasms [1, 6, 9, 18, 23, 24]. In addition, although the number of cases included is low for this type of calculation (n = 60), it may be useful to note the sensitivity (0.89), specificity (1.0), PPV (1.0), and NPV (0.98 or 0.82) for identifying malignant lesions using CNB. Although four CNBs that were initially read as benign resulted in malignant final pathology, three were recommended for open biopsy before definitive surgical management. These three lesions had areas of more atypical histology and, on review at our tumor board, in discussion with musculoskeletal radiologists, pathologists, and orthopaedic oncologists, final recommendations for open biopsy were made before definitive surgery. This highlights the seminal importance of a multidisciplinary musculoskeletal tumor board. Prior studies examining CNB have also discussed the value of clinical and radiologic correlation with the biopsy result by experienced musculoskeletal physicians in a multidisciplinary setting [1, 23]. Through comprehensive review, biopsy results that do not “fit” the rest of the story can be identified and further inquiry can be recommended. It is important for institutions without these resources to recognize this critical component of the diagnostic workup and send CNB tissue from patients with atypical clinical findings to a larger center that does have these capabilities.
What Are the Complications of CNB?
In the present study, there were no complications associated with CNB in all 73 patients. Prior data on CNB are consistent with this study and report only rare complications (0% to 1%) [1, 11, 17]. Open biopsy has similar hazards, including infection, bleeding, and tumor contamination, although almost certainly in greater frequency compared with CNB [8, 16, 23].
Is There Any Difference in the Accuracy or Effectiveness of CNB Performed at Outside Institutions When Compared With a Referral Center?
The accuracy and efficacy of CNB at outside institutions were the same compared with the home institution (93% versus 90% efficacy and 92% versus 93% accuracy, respectively). This indicates that despite concerns about variability in technique when performing CNB, there was no difference in the ability of outside hospitals to obtain sufficient tissue when compared with our home institution. All 15 of the CNBs at outside institutions were for benign final pathologic diagnoses. When comparing the results with the home institution, only benign final pathologic diagnoses were chosen, because this was believed to be a more appropriate comparison. Unfortunately, there were very few CNBs from outside institutions and none for malignant final pathologic diagnoses, making more robust analysis difficult.
Unlike FNAB, CNB is an accurate and effective initial diagnostic test to differentiate between radiolucent benign and malignant bone tumors, providing an efficient and less invasive alternative to open biopsy. CNB was shown to be associated with no complications noted in the 73 performed. Finally, there was no difference in the efficacy or accuracy of CNBs performed at outside institutions when compared with the home institution, indicating that the CNB technique can be reliably replicated by other centers. We believe it is ideal if there is an experienced team of musculoskeletal radiologists, pathologists, and orthopaedic oncologists who can obtain samples and interpret the results within the radiologic and clinical context of the patient and appropriately evaluate a situation in which the pathology does not “fit” with the presentation. These scenarios, albeit uncommon, may lead to an open biopsy to avoid treating a malignant tumor with a procedure intended for a benign lesion. We do caution that each center should be comfortable with their diagnostic accuracy, particularly when using less invasive methods of ABC treatment such as sclerotherapy or other nonsurgical means to treat ABCs.
Footnotes
One of the authors (NMB) has or may receive payments or benefits from Onkos (Parsippany, NJ, USA) not related to this work. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number 5K08AR069112-01 (NMB).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Altuntas AO, Slavin J, Smith PJ, Schlict SM, Powell GJ, Ngan S, Toner G, Choong PFM. Accuracy of computed tomography guided core needle biopsy of musculoskeletal tumours. ANZ J Surg. 2005;75:187–191. [DOI] [PubMed] [Google Scholar]
- 2.Anderson MW, Temple HT, Dussault RG, Kaplan PA. Compartmental anatomy: relevance to staging and biopsy of musculoskeletal tumors. AJR Am J Roentgenol. 1999;173:1663–1671. [DOI] [PubMed] [Google Scholar]
- 3.Copley L, Dormans JP. Benign pediatric bone tumors. Evaluation and treatment. Pediatr Clin North Am. 1996;43:949–966. [DOI] [PubMed] [Google Scholar]
- 4.Creager AJ, Madden CR, Bergman S, Geisinger KR. Aneurysmal bone cyst. Am J Clin Pathol. 2007;128:740–745. [DOI] [PubMed] [Google Scholar]
- 5.Dollahite HA, Tatum L, Moinuddin SM, Carnesale PG. Aspiration biopsy of primary neoplasms of bone. J Bone Joint Surg Am. 1989;71:1166–1169. [PubMed] [Google Scholar]
- 6.Dupuy DE, Rosenberg AE, Punyaratabandhu T, Tan MH, Mankin HJ. Accuracy of CT-guided needle biopsy of musculoskeletal neoplasms. AJR Am J Roentgenol. 2012;171:759–762. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher C, Unni KK, Mertens F. Pathology and genetics of tumours of soft tissue and bone. In: Unni KK, ed. World Health Organization Classification of Tumors. Lyon, France: International Agency for Research on Cancer; 2002:1–427. [Google Scholar]
- 8.Gerrand CH, Rankin K. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am. 1982;64:1121–1127. [PubMed] [Google Scholar]
- 9.Hau M, Kim J, Kattapuram S, Hornicek FJ. Accuracy of CT-guided biopsies in 359 patients with musculoskeletal lesions. Skeletal Radiol. 2002;31:349–353. [DOI] [PubMed] [Google Scholar]
- 10.Hodge JC. Percutaneous biopsy of the musculoskeletal system: a review of 77 cases. Can Assoc Radiol J. 1999;50:121–125. [PubMed] [Google Scholar]
- 11.Jelinek JS, Murphey MD, Welker JA, Henshaw RM, Kransdorf MJ, Shmookler BM, Malawer MM. Diagnosis of primary bone tumors with image-guided percutaneous biopsy: experience with 110 tumors. Radiology. 2002;223:731–737. [DOI] [PubMed] [Google Scholar]
- 12.Kattapuram SV, Rosenthal DI. Percutaneous biopsy of skeletal lesions. AJR Am J Roentgenol. 1991;157:935–942. [DOI] [PubMed] [Google Scholar]
- 13.Layfield LJ, Armstrong K, Zaleski S, Eckardt J. Diagnostic accuracy and clinical utility of fine-needle aspiration cytology in the diagnosis of clinically primary bone lesions. Diagn Cytopathol. 1993;9:168–173. [DOI] [PubMed] [Google Scholar]
- 14.Logan PM, Connell DG, OConnell JX. Image-guided percutaneous biopsy of musculoskeletal tumors: an algorithm for selection of specific biopsy techniques. AJR Am J Roentgenol. 1996;166:137–141. [DOI] [PubMed] [Google Scholar]
- 15.Mahnken A, Nolte-Ernsting C, Wildberger J. Aneurysmal bone cyst: value of MR imaging and conventional radiography. Eur Radiol. 2003;13:1118–1124. [DOI] [PubMed] [Google Scholar]
- 16.Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. For the members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am. 1996;78:656–663. [DOI] [PubMed] [Google Scholar]
- 17.Mitton B, Seeger LL, Eckardt MA. Image-guided percutaneous core needle biopsy of musculoskeletal tumors in children. J Pediatr Hematol Oncol. 2014;36:337–341. [DOI] [PubMed] [Google Scholar]
- 18.Omura MC, Motamedi K, UyBico S. Revisiting CT-guided percutaneous core needle biopsy of musculoskeletal lesions: contributors to biopsy success. AJR Am J Roentgenol. 2011;197:457–461. [DOI] [PubMed] [Google Scholar]
- 19.Park HY, Yang SK, Sheppard WL, Hegde V, Zoller SD, Nelson SD, Federman N, Bernthal NM. Current management of aneurysmal bone cysts. Curr Rev Musculoskelet Med. 2016;9:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rapp TB, Ward JP, Alaia MJ. Aneurysmal bone cyst. J Am Acad Orthop Surg. 2012;20:233–241. [DOI] [PubMed] [Google Scholar]
- 21.Revel MP, Vanel D, Sigal R, Luboinski B. Aneurysmal bone cysts of the jaws: CT and MR findings. J Comput Assist Tomogr. 1992;16:84–86. [DOI] [PubMed] [Google Scholar]
- 22.Schweitzer ME, Gannon FH, Deely DM. Percutaneous skeletal aspiration and core biopsy: complementary techniques. AJR Am J Roentgenol. 1996;166:415–418. [DOI] [PubMed] [Google Scholar]
- 23.Skrzynski MC, Biermann JS, Montag A, Simon MA. Diagnostic accuracy and charge-savings of outpatient core needle biopsy compared with open biopsy of musculoskeletal tumors. J Bone Joint Surg Am. 1996;78:644–649. [DOI] [PubMed] [Google Scholar]
- 24.Yao L, Nelson SD, Seeger LL, Eckardt JJ, Eilber FR. Primary musculoskeletal neoplasms: effectiveness of core-needle biopsy. Radiology. 1999;212:682–686. [DOI] [PubMed] [Google Scholar]
- 25.Ye Y, Pringle LM, Lau AW, Riquelme DN, Wang H. TRE17/USP6 oncogene translocated in aneurysmal bone cyst induces matrix metalloproteinase production via activation of NF-κB. Oncogene. 2010;29:3619–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]