Abstract
Background
Effects of high-dose radiation using protons and photons on bone are relatively unexplored, but high rates of insufficiency fractures are reported, and the causes of this are incompletely understood. Imaging studies with pre- and postradiation scans can help one understand the effect of radiation on bone.
Questions/purposes
The purpose of this study was to assess the effects of high-dose radiation on the trabecular density of bone in the sacrum using CT-derived Hounsfield units (HU).
Methods
Between 2009 and 2015, we treated 57 patients (older then 18 years) with sacral chordoma. Fourteen (25%) of them were treated with radiation only. The general indication for this approach is inoperability resulting from tumor size. Forty-two (74%) patients were treated with transverse sacral resections and high-dose radiotherapy (using either protons or photons or a combination) before surgery and after surgery. During this time period, our indication for this approach generally was symptomatic sacral chordoma in which resection would prevent further growth and reasonable sacrifice of nerve roots was possible. Of those patients, 21 (50%) had CT scans both before and after radiation treatment. We used HU as a surrogate for bone density. CT uses HU to derive information on tissue and bone quantity. A recent study presented reference HU values for normal (mean 133 ± 38 HU), osteoporotic (101 ± 25 HU), and osteopenic bone (79 ± 32 HU). To adjust for scanning protocol-induced changes in HU, we calculated the ratio between bone inside and outside the radiation field rather than using absolute values. To assess the effect of radiation, we tested whether there was a difference in ratio (sacrum/L1) before and after radiation. A control measurement was performed (L2/L1) and also tested for a difference before and after radiation. Statistical analyses were performed using the paired t-test.
Results
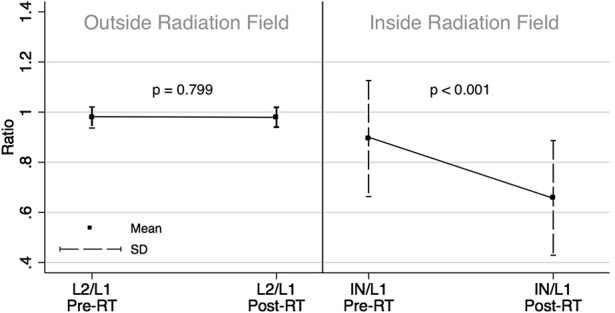
The effects of radiation appeared confined to the intended field, because the bone density outside the treated field was not observed to decrease. The ratio of HU (a surrogate for bone density) in L2 relative to L1 did not change after radiotherapy (preradiation mean: 0.979 ± 0.009, postradiation mean: 0.980 ± 0.009, mean difference outside the radiation field: -0.001, 95% confidence interval [CI], -0.009 to 0.007, p = 0.799). The ratio of HU within the radiation field relative to L1 decreased after radiotherapy (preradiation mean: 0.895 ± 0.050, postradiation mean: 0.658 ± 0.050, mean difference inside the radiation field: 0.237, 95% CI, 0.187-0.287, p < 0.001), suggesting the bone density stayed the same outside the radiation field but decreased inside the radiation field.
Conclusions
Trabecular bone density decreased after high-dose radiation therapy in a small group of patients with sacral chordoma. High-dose radiation is increasingly gaining acceptance for treating sacral malignancies; further long-term prospective studies using calibrated CT scanners and preferably bone biopsies are needed.
Level of Evidence
Level IV, therapeutic study.
Introduction
Photon radiation (as generated by modalities like radiographs) has traditionally been used for radiotherapy and has a role in treating malignancies as primary or (neo)adjuvant treatment. Advances have been made in the delivery of photon radiation to spare surrounding tissues and allow for higher dosages. Intensity-modulated photon radiation therapy is increasingly being used for treatment of challenging bone sarcomas of the axial skeleton. The reason for this is the higher conformality of dose and sparing of normal tissue [4]. Because radiotherapy for chordoma often requires high doses [5, 41] in close proximity to sensitive normal tissues, protons are a good treatment option. The reason for the use of protons rather than photons is the superior dose distribution that can be achieved with protons [19]. Protons deposit little energy in tissue until near the end of the proton range where the residual energy is lost over a short distance, resulting in a steep rise in the absorbed dose known as the Bragg peak [27]. The use of proton radiation has proven to be a safe [1, 15, 17, 42] and useful adjuvant to surgery [12, 38]. DeLaney et al. [13] studied the long-term oncologic outcomes after a combination of proton beam radiation with surgery and reported promising results with local control for primary spine tumors of 84% (of which 58% were chordomas) at 8 years followup. Definitive high-dose radiation using photon and proton radiation rendered good results for patients with medically inoperable or otherwise unresected mobile spine or sacrococcygeal chordomas, as reported by Chen et al. [10]. The study reported chordoma-specific survival of 96% and 82%, local progression-free survival of 90% and 80%, and metastases-free survival of 87% and 76% at 3 and 5 years, respectively.
Despite the advantages in local control, insufficiency fractures of irradiated bone pose a serious problem [25]. A recent paper published by Osler et al. [26] reported sacral insufficiency fractures in 76% of patients treated with a combination of high sacrectomy and high-dose radiation and 22% of the patients treated with definitive high-dose radiation; overall 47% of patients treated had a fracture. Management of radiation-induced fractures has been studied to some extent in long bones [20]. A high risk of nonunion and prolonged healing is common when treating these fractures and are therefore thought to be difficult to treat [7]. Interestingly, a recent paper by Imai et al. [17] with a median of 62 months of followup did not report any fractures after treating 133 patients with sacral chordoma with high-dose carbon-ion radiation alone, although they did not comment on the fracture risk specifically.
The gold standard for measuring bone mineral density in vivo is by dual-energy x-ray absorptiometry (DEXA) [6, 21]. In a finite element modeling study, CT scans have proven to be accurate in predicting fracture patterns and failure loads [34]. CT uses Hounsfield units (HU) to derive information on tissue and bone quantity. HU are the standardized attenuation coefficient of tissue and represent tissue on a scale of -1000 defined for air and 0 defined for distilled water at room temperature and pressure [33]. Whereas DEXA and other quantitative CT scans require the use of a calibration phantom, routine clinical CT scans have HU information readily available without additional costs or radiation. Several studies have successfully used HU in assessing bone mineral density and osteoporosis [18, 28, 32, 33, 39]. However, this has not yet been done in patients undergoing high-dose radiation of the sacrum although fractures are a big problem. By using the knowledge of the relationship between HU and bone density from osteoporosis studies, we look at this specific problem in a new way.
The aim of this study was to assess the effects of high-dose radiation on the trabecular density of the sacrum. To do this, we present a novel method of assessing changes in trabecular bone density using routine clinical CT scans done pre- and postradiation acquired using different imaging and contrast protocols.
Patients and Methods
This study was approved by our institutional review board. The current study is a retrospective series of patients with sacral chordoma treated with a combination of surgery and radiation at our institution between May 2009 and December 2015. Patients were excluded if they were younger than 18 years of age, if no pre- and postradiation CT was available, or if the radiation scheduled deviated from our institution’s protocol (preoperative course of 50.4 Gy and postoperative course of 19.8 Gy) [13].
Over this time period, we treated 57 patients (older then 18 years) with sacral chordoma. Fourteen (25%) of them were treated with radiation only. The main indication for this approach is inoperability resulting from tumor size. Forty-two (74%) patients were treated with transverse sacral resections and high-dose radiotherapy (using either protons or photons or a combination) before surgery and after surgery. During this time period, our indications for this approach generally were symptomatic sacral chordoma in which resection would prevent further growth and the potential negative effects of the tumor outweigh the consequences of the nerve root sacrifice. In seven (16%) patients, there was no preradiation scan available and in 12 (28%) patients, the postradiation imaging was done using MRI, not CT. Four (9%) patients received < 50.4 Gy preoperative radiation. Twenty-one (50%) patients met our inclusion criteria. The study exclusions were unlikely to cause selection bias, because the main reasons for exclusion generally were unrelated to patient or tumor factors.
The mean age (± SD) was 51 ± 15 years (range, 22-73 years; Table 1). Of the 21, six (29%) were women and 15 (71%) were men. The most cephalad sacral vertebrae involved with tumor was S1 in three (14%) patients, S2 in five (24%) patients, S3 in 10 (48%) patients, S5 in two (10%) patients, and the coccyx in one (5%) patient. Eight measurements were done in S1 (38%), 10 were done in S2 (48%), two were done in S4 (10%), and one was done in S5 (5%). Although fractures in the lower sacral area are rare, it is bone with a similar structure also receiving high dosages or radiation and therefore interesting to include in this study.
Table 1.
Demographics (n = 21)
A combination of proton-based radiation and surgery was used to treat chordoma. Radiation included 70.2 Gy of photon/proton radiation divided into a 5-week preoperative course of 50.4 Gy and a 2-week postoperative boost of 19.8 Gy [13]. We were only able to analyze the effect of the preoperative radiation course of 50.4 Gy because CT scans were made before and after the preoperative radiation course, and not before and after the postoperative radiation course. Preradiation scans were available because all patients undergo CT scan of the lumbosacral spine for radiotherapy treatment planning. Areas of interest (including target volumes and organs at risk) were contoured using MIM Software (MIM Software Inc, Cleveland, OH, USA) and three-dimensional (3-D) conformal photon or proton radiotherapy plans were generated using RayStation (RaySearch Laboratories, Stockholm, Sweden) or in-house Pencil Beam Scanning treatment planning systems, respectively. Postradiation scans were available because patients routinely underwent a preoperative CT scan of the lumbosacral spine for restaging and surgical planning purposes.
The total dose of the preoperative course of radiation was 50.4 Gy, which was delivered using only photons for four patients, only protons for three patients, and a combination of photons and protons for 14 patients. Typically a combination of 19.8 Gy of photons and 30.6 Gy of protons was used. We did include all patients because the total dose of radiation was similar in all patients. In our institution, proton radiation is limited, so to conserve resources, a combination of proton and photon radiation is used. This way we are able to treat more patients with protons, especially children who need all proton therapy because they cannot tolerate high dosages of photon radiation. A mean of 23.9 ± 16.5 Gy of photons and 26.5 ± 16.5 Gy of protons was used. The mean time between the pre- and postradiation scans was 97 ± 11 days. The mean time between the end of radiation and the postradiation scan was 38 ± 10 days.
The preradiation images were acquired using a GE scanner (GE, Milwaukee, WI, USA) applying the standard algorithm: helical mode, 2.5-mm slice thickness, a voltage of 140 kV, and a current set automatically. The intravenous contrast medium injection was performed with a delay of 60 seconds and a flow rate of 2.5 mL/s. Additionally, some patients were given 900 mL oral contrast medium 45 to 60 minutes before scanning. Postradiation images were acquired using a GE scanner applying the standard algorithm: helical mode, 5-mm slice thickness, a voltage of 100 or 120 kV, and a current set automatically. The intravenous contrast medium injection was performed with a delay of 70 seconds and a flow rate of 2.0 mL/s.
In this retrospective study, the preradiation CT scans and postradiation CT scans were done using a different scanner and different imaging and contrast protocols. To adjust for these differences, we first converted the HU values for scans made using a voltage of 100 or 140 kV to the HU values associated with 120-kV scans (for conversion formulas, see Appendix, Supplemental Digital Content 1). Second, we tested whether differences in HU were attributed to scanning protocols. Two measurements were performed (in L1 and L2) outside the radiation field (L1 preradiation mean: 182 ± [SD] 52 HU, L1 postradiation mean: 197 ± 54 HU, p < 0.003, same region of interest [ROI] with a mean volume of 3.9 ± 1.4 mL and L2 preradiation mean: 176 ± 52 HU, L2 postradiation mean: 191 ± 55 HU, p = 0.002, same ROI with a mean volume of 4.4 ± 1.8 mL; Table 2). The difference in increase in HU in L1 and L2 was tested and no difference was found (mean increase in L1: 15 HU, 95% confidence interval [CI], 5.7-25 HU; mean increase in L2: 15 HU, 95% CI, 6.3-25 HU; p = 0.867). This increase was attributed to the differences in scanning protocols. Subsequently, we calculated the ratio of the value outside the radiation field relative to the reference value and the value inside the radiation field relative to the reference value, eliminating any change caused by the scanning protocol that we did not already correct for. Any difference found in the measurement would most likely be caused by the radiation because there were only a few weeks between the two scans.
Table 2.
Absolute Hounsfield units pre- and postradiation
Trabecular bone density was assessed by measuring the HU [18, 28, 32, 33, 39] in a vertebra outside of the radiation field (ie, L2) and inside the radiation field (the exact vertebra was dependent on the extent of the radiation field and tumor location). A reference value was measured in the L1 vertebra. Using MIM Software (MIM Software Inc), a volumetric ROI was created within the cortices of the vertebra and thus including only trabecular bone by using a 3-D brush. This allowed us to calculate the mean HU of that specific volume. Instead of taking the mean of one two-dimensional ROI in the sagittal plane and one in the transverse plane [3, 37], this technique effectively does multiple measurements in all three planes and calculates a mean of HU in a volume. This reduces the impact of errors in single measurements. Contrast accumulation in the vertebral venous plexus might influence the HU measurements. Therefore, the ROI was manually created by following the inner border of the cephalad, caudal, and ventral cortices of only the ventral two-thirds of the vertebra for lumbar vertebrae (Fig. 1) and sacral vertebrae (Fig. 2), excluding the vertebral venous plexus from the ROI. Using the autoalignment function, the pre- and postradiation CT scans were fused, which allowed measuring the exact same volumetric ROI in the same vertebrae on both CT scans of each patient.
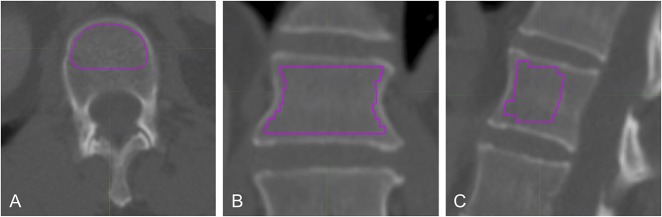
Fig. 1A-C.
Presented here are CT images of axial (A), coronal (B), sagittal (C) views of the second lumbar vertebral body. A volumetric ROI was placed within the cortices of the vertebral body. The dorsal third of the vertebral body was avoided to minimize impact of contrast accumulation in the vertebral venous plexus on HU measurements.
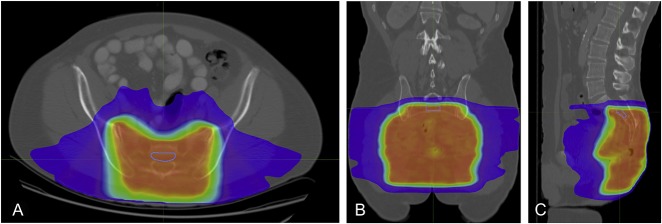
Fig. 2 A-C.
Presented here are CT images of axial (A), coronal (B), and sagittal (C) views of the second sacral vertebral body. A volumetric ROI was placed within the cortices of the vertebral body. The isodose lines were used to ensure that the measurements were done in bone that received the complete dose of radiation.
The gold standard for measuring bone mineral density and assessing fracture risk is the DEXA scan [21]. A recent study showed that HU can be used as an alternative method for determining regional bone mineral density at no additional cost to the patient and therefore can aid in the fracture risk assessment [33]. The same study provides reference HU values for normal (mean 133 ± 38 HU), osteoporotic (101 ± 25 HU), and osteopenic bone (79 ± 32 HU).
Continuous data are presented as means with SDs. Categorical data are presented as frequencies with percentages. The ratio data were tested for normality using the Shapiro-Wilk test. After confirming the normality of the data, we performed the paired t-test to compare pre- and postradiation ratios. A p value of < 0.05 was considered significant. Statistical analysis and building of the graphs were done using Stata Version 12.0 (Stata Corp, College Station, TX, USA).
Results
The effects of radiation appeared confined to the intended field, because the bone density outside the treated field was not observed to decrease. The ratio of L2 relative to L1 did not change after radiotherapy (preradiation mean: 0.979 ± 0.009, postradiation mean: 0.980 ± 0.009, mean difference outside the radiation field: -0.001, 95% CI, -0.009 to 0.007, p = 0.799; Table 3). The ratio of the measurement within the radiation field relative to L1 decreased after radiotherapy (preradiation mean: 0.895 ± 0.050, postradiation mean: 0.658 ± 0.050, mean difference inside the radiation field: 0.237, 95% CI, 0.187-0.287, p < 0.001; Table 3). This suggests that the bone density stayed the same outside the radiation field but decreased inside the radiation field (Fig. 3).
Table 3.
Ratios pre- and postradiotherapy
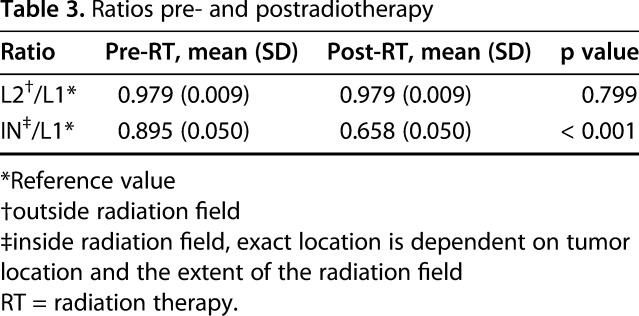
Fig. 3.
Presented here is a graphic representation of the dramatic effect of radiation on bone density. To correct contrast-induced changes in HU, we performed an additional measurement in L1 as a reference value. Both the measurements inside the radiation field and outside the radiation field are presented as mean ratios that are relative to the reference measurement in L1.
Discussion
Currently sacrectomy is a standard approach for sacral chordoma. Promising results have been reported when using high-dose radiation as an adjuvant treatment. The effects of high-dose radiation using protons and photons on bone are relatively unexplored, but a high risk of insufficiency fractures has been reported [26]. We aimed to assess the effects of high-dose radiation on the trabecular density of bone in the sacrum using CT-derived HU. We observed a decrease in trabecular bone density of radiated bone in patients with sacral chordoma using retrospectively collected CT scans. Using a reference value outside of the radiation field for each patient, we were able to control for heterogeneity between the pre- and postradiation CT scans. Some patients—such as those undergoing sacrectomy—will receive radiation doses even greater than the dose we studied here. That being said, it is especially important to consider the loss of trabecular bone density.
This study has several limitations. First is the use of clinical CT scans in which images were obtained using different imaging and contrast protocols. Changes in HU measurement cannot completely be attributed to radiation, but also the differences in the methods used to obtain the images. This explains the increase in HU outside the radiation field, an area where no change is expected. We also measured a difference in HU inside the radiation field; interestingly, this was not an increase but a decrease in HU. This means that even with the increase in HU caused by a difference in scanning protocol, the decrease in HU inside the radiation field was big enough that a decrease was still measured. Nevertheless, we accounted for differences in voltages used by converting HU values using a conversion formula (shown in Appendix, Supplemental Digital Content 1). We normalized HU values to a reference value outside of the radiation field for each patient and tested a difference in ratios rather than the absolute HU values. Ideally, the pre- and postradiation would have been done using the same scanner and scanning protocol. However, the method we used did allow us to isolate the change in HU not caused by the difference in scanner or scanning protocol. In this case, the most likely factor causing the decrease in density in between the two CT scans was the radiation. Second, we were not able to calibrate our measurements to bone mineral density because phantoms are not routinely used for clinical CT scans. This makes it difficult to compare our results with studies reporting on bone mineral density (ie, studies using DEXA scans or phantoms). In routine clinic, no phantoms or DEXA scans are used. Our study does present a method that allows using routinely made scans for measuring bone density and might be of value as a first step in recognizing potential clinical problems, in this case the high rate of insufficiency fractures observed in patients treated for sacral chordoma.
In addition, almost half of the patients treated for the diagnosis of interest were not included because a pre- or postradiation CT scan was not available. This might introduce selection bias, although this is unlikely because scans were not missing because of any specific patient or treatment characteristic. Rather, practical issues with obtaining the old scans prevented us from including these patients. Fourth, the sacral level that was used to measure density was dependent on the level of the tumor. Although not all sacral vertebrae have the same density [37], they have similar biology and were therefore grouped together. We did not compare different levels within one patient; we were able to measure the exact same location within one patient before and after radiation. Last, not all patients received a similar amount of proton and photon radiation. We were therefore not able to analyze the effect of photon or proton radiation separately. Most likely a combination of both protons and photons will be used in the foreseeable future given the rarity of proton centers. We did however make sure patients received a similar total dose of radiation. Potentially the effect of photon radiation on bone might be greater because proton radiation can be delivered with more precision as a result of its biologic behavior [22].
Our results show that the high-dose combined proton and photon radiotherapy decreases trabecular bone mineral density within a matter of weeks. When comparing our raw results with reference values [33], it becomes clear that the bone inside the radiation field changed from being within the range of normal bone (mean 133 ± 38 HU) to within the lower range of osteoporotic bone (101 ± 25 HU). Outside the radiation field the bone stayed within the range of normal bone. A recent study looking at bone mineral density changes of thoracic and lumbar vertebral bodies in patients treated with chemoradiation (using chemotherapy and photons only) for abdominal tumors found similar results [39]. The authors reported decreased bone mineral density after 4 to 8 months and after 9 to 12 months. However, gemcitabine was used in 57% of the patients in their study. Gemcitabine has been associated with myelosuppression [11, 35, 36]. A recent animal study reported myelosuppressive therapies to increase inflammation and directly contribute to bone loss [29], indicating a potential contribution of the chemotherapy to the decreased bone mineral density. Although the authors state that they controlled for this by performing the same measurement in patients treated with chemotherapy alone (and found no difference), they do not state which chemotherapies were given to the control group. In addition, the effect of the combination of radiation and chemotherapy on bone is unknown.
A cross-sectional study of 19 patients treated with surgical excision and radiotherapy for soft tissue extremity sarcomas had bone density measured using a DEXA scan. The authors concluded that radiation does not routinely decrease bone density [14]. These results have to be interpreted with care because no preradiation scans were done. Results were based on comparisons with the contralateral limb depending on the site of radiation. Furthermore, the bones included were not homogenous and included only long bones, which have more cortical bone (nine femura, three humerii, four radii, and three tibiae) and are less sensitive to damage or we are less able to detect a change in bone density when compared with trabecular bone [2].
Based on recent animal studies, fragility of radiated bone is attributed to early postradiation activation of existing osteoclasts resulting in a decrease of the trabecular bone and thus the trabecular bone mineral density. Trabecular bone that is lost after early radiation-induced resorption is not regenerated as a result of the lack of a scaffold to guide osteoblasts [24]. Osteoclast progenitors reside in marrow and are known to be radiosensitive. Depletion of osteoclast progenitors may be attributed to long-term decreased loss of osteoclasts [16, 31, 40]. This may ultimately lead to uncoupling of the bone resorption and formation. This allows long-term unopposed appositional bone growth, which eventually may lead to increased bone mineral density of trabecular and cortical bone combined, cortical thickening, decreased remodeling, and accumulation of poor-quality matrix [24]. Historically it is suggested that bone loss is mediated by the damage done to mesenchymal stem cells resulting from radiation [8, 9]. Osteoblasts are short-lived cells that need constant replenishing; damage to their mesenchymal progenitors may result in long-term bone damage. These are all mechanisms for radiation to contribute to bone fragility and an increased fracture risk. The question arises whether preventive stabilization is necessary. At our institution, lumbosacral reconstruction is routinely done in patients with an osteotomy higher than at the S2 to S3 level in patients who received high-dose radiation. Despite this, insufficiency fractures in patients with reconstructions are still seen. To more definitively determine whether preventive stabilization is necessary, studies should be done to determine whether the remaining bone is strong enough for screws to hold and also to look at bone quality over the longer term after high-dose radiation to the sacrum. In the osteoporosis literature, surgical techniques have been described, mentioning that osteoporotic bone quality might lead to the risk of screw loosening [23, 30]. This being said, osteoporosis and radiation-induced loss of bone are a result of different pathophysiologies and comparisons should be made with care.
This study sheds light on the short-term effect of the radiation, but not on the long-term results. It has been suggested that at higher dosages, the lack of osteoclasts results in uncoupling of the bone resorption and formation. This allows long-term unopposed appositional bone growth, which eventually may lead to increased bone mineral density of trabecular and cortical bone combined, decreased remodeling, and accumulation of poor-quality matrix [24]. The poor-quality bone may not be well suited for fixation.
We observed that trabecular bone density decreased after high-dose radiation therapy in a small group of patients with sacral chordoma; specifically, trabecular density decreased within weeks of the end of radiation in patients who received a dosage of 50.4 Gy. High-dose radiation is increasingly gaining acceptance for treating malignancy of the sacrum; these results should be taken into consideration when planning treatment involving high-dose radiation. To fully comprehend the effect of high-dose radiation on bone and be able to guide clinical care, long-term prospective studies using calibrated CT scanners and preferably bone biopsies need to be conducted.
Footnotes
Each author certifies that neither he, nor any member of his immediate family, have funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Massachusetts General Hospital, Boston, MA, USA.
References
- 1.Allen AM, Pawlicki T, Dong L, Fourkal E, Buyyounouski M, Cengel K, Plastaras J, Bucci MK, Yock TI, Bonilla L, Price R, Harris EE, Konski AA. An evidence based review of proton beam therapy: the report of ASTRO's emerging technology committee. Radiother Oncol. 2012;103:8–11. [DOI] [PubMed] [Google Scholar]
- 2.Barak MM, Weiner S, Shahar R. The contribution of trabecular bone to the stiffness and strength of rat lumbar vertebrae. Spine (Phila Pa 1976). 2010;35:E1153–1159. [DOI] [PubMed] [Google Scholar]
- 3.Baum T, Muller D, Dobritz M, Rummeny EJ, Link TM, Bauer JS. BMD measurements of the spine derived from sagittal reformations of contrast-enhanced MDCT without dedicated software. Eur J Radiol. 2011;80:e140–145. [DOI] [PubMed] [Google Scholar]
- 4.Bilsky MH, Yamada Y, Yenice KM, Lovelock M, Hunt M, Gutin PH, Leibel SA. Intensity-modulated stereotactic radiotherapy of paraspinal tumors: a preliminary report. Neurosurgery. 2004;54:823-830; discussion 830–831. [DOI] [PubMed] [Google Scholar]
- 5.Bjornsson J, Wold LE, Ebersold MJ, Laws ER. Chordoma of the mobile spine. A clinicopathologic analysis of 40 patients. Cancer. 1993;71:735–740. [DOI] [PubMed] [Google Scholar]
- 6.Blake GM, Fogelman I. The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad Med J. 2007;83:509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon CP, Lin PP, Lewis VO, Yasko AW. Management of radiation-associated fractures. J Am Acad Orthop Surg. 2008;16:541–549. [PubMed] [Google Scholar]
- 8.Cao X, Wu X, Frassica D, Yu B, Pang L, Xian L, Wan M, Lei W, Armour M, Tryggestad E, Wong J, Wen CY, Lu WW, Frassica FJ. Irradiation induces bone injury by damaging bone marrow microenvironment for stem cells. Proc Natl Acad Sci U S A. 2011;108:1609–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandra A, Lan S, Zhu J, Lin T, Zhang X, Siclari VA, Altman AR, Cengel KA, Liu XS, Qin L. PTH prevents the adverse effects of focal radiation on bone architecture in young rats. Bone. 2013;55:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YL, Liebsch N, Kobayashi W, Goldberg S, Kirsch D, Calkins G, Childs S, Schwab J, Hornicek F, DeLaney T. Definitive high-dose photon/proton radiotherapy for unresected mobile spine and sacral chordomas. Spine (Phila Pa 1976). 2013;38:E930–936. [DOI] [PubMed] [Google Scholar]
- 11.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, Roessner M, Gupta S, Sartor AO; TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. [DOI] [PubMed] [Google Scholar]
- 12.DeLaney TF, Liebsch NJ, Pedlow FX, Adams J, Dean S, Yeap BY, McManus P, Rosenberg AE, Nielsen GP, Harmon DC, Spiro IJ, Raskin KA, Suit HD, Yoon SS, Hornicek FJ. Phase II study of high-dose photon/proton radiotherapy in the management of spine sarcomas. Int J Radiat Oncol Biol Phys. 2009;74:732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLaney TF, Liebsch NJ, Pedlow FX, Adams J, Weyman EA, Yeap BY, Depauw N, Nielsen GP, Harmon DC, Yoon SS, Chen YL, Schwab JH, Hornicek FJ. Long-term results of Phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J Surg Oncol. 2014;110:115–122. [DOI] [PubMed] [Google Scholar]
- 14.Dhakal S, Chen J, McCance S, Rosier R, O'Keefe R, Constine LS. Bone density changes after radiation for extremity sarcomas: exploring the etiology of pathologic fractures. Int J Radiat Oncol Biol Phys. 2011;80:1158–1163. [DOI] [PubMed] [Google Scholar]
- 15.Fossati P, Vavassori A, Deantonio L, Ferrara E, Krengli M, Orecchia R. Review of photon and proton radiotherapy for skull base tumours. Rep Pract Oncol Radiother. 2016;21:336–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong B, Oest ME, Mann KA, Damron TA, Morris MD. Raman spectroscopy demonstrates prolonged alteration of bone chemical composition following extremity localized irradiation. Bone. 2013;57:252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai R, Kamada T, Araki N; Working Group for Bone and Soft Tissue Sarcomas. Carbon ion radiation therapy for unresectable sacral chordoma: an analysis of 188 cases. Int J Radiat Oncol Biol Phys. 2016;95:322–327. [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Chung CK, Oh SH, Park SB. Correlation between bone mineral density measured by dual-energy x-ray absorptiometry and Hounsfield units measured by diagnostic CT in lumbar spine. J Korean Neurosurg Soc. 2013;54:384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin WP, Kooy H, Loeffler JS, DeLaney TF. Proton beam therapy. Br J Cancer. 2005;93:849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin PP, Boland PJ, Healey JH. Treatment of femoral fractures after irradiation. Clin Orthop Relat Res. 1998;352:168–178. [PubMed] [Google Scholar]
- 21.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munzenrider JE, Austin-Seymour M, Blitzer PJ, Gentry R, Goitein M, Gragoudas ES, Johnson K, Koehler AM, McNulty P, Moulton G, et al. Proton therapy at Harvard. Strahlentherapie. 1985;161:756–763. [PubMed] [Google Scholar]
- 23.Oberkircher L, Masaeli A, Bliemel C, Debus F, Ruchholtz S, Kruger A. Primary stability of three different iliosacral screw fixation techniques in osteoporotic cadaver specimens–a biomechanical investigation. Spine J. 2016;16:226–232. [DOI] [PubMed] [Google Scholar]
- 24.Oest ME, Franken V, Kuchera T, Strauss J, Damron TA. Long-term loss of osteoclasts and unopposed cortical mineral apposition following limited field irradiation. J Orthop Res. 2015;33:334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogino I, Okamoto N, Ono Y, Kitamura T, Nakayama H. Pelvic insufficiency fractures in postmenopausal woman with advanced cervical cancer treated by radiotherapy. Radiother Oncol. 2003;68:61–67. [DOI] [PubMed] [Google Scholar]
- 26.Osler P, Bredella MA, Hess KA, Janssen SJ, Park CJ, Chen YL, DeLaney TF, Hornicek FJ, Schwab JH. Sacral insufficiency fractures are common after high-dose radiation for sacral chordomas treated with or without surgery. Clin Orthop Relat Res. 2016;474:766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel S, DeLaney TF. Advanced-technology radiation therapy for bone sarcomas. Cancer Control. 2008;15:21–37. [DOI] [PubMed] [Google Scholar]
- 28.Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med. 2013;158:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quach JM, Askmyr M, Jovic T, Baker EK, Walsh NC, Harrison SJ, Neeson P, Ritchie D, Ebeling PR, Purton LE. Myelosuppressive therapies significantly increase pro-inflammatory cytokines and directly cause bone loss. J Bone Miner Res. 2015;30:886–897. [DOI] [PubMed] [Google Scholar]
- 30.Rommens PM, Hofmann A. Comprehensive classification of fragility fractures of the pelvic ring: recommendations for surgical treatment. Injury. 2013;44:1733–1744. [DOI] [PubMed] [Google Scholar]
- 31.Scheven BA, Wassenaar AM, Kawilarang-de Haas EW, Nijweide PJ. Comparison of direct and indirect radiation effects on osteoclast formation from progenitor cells derived from different hemopoietic sources. Radiat Res. 1987;111:107–118. [PubMed] [Google Scholar]
- 32.Schreiber JJ, Anderson PA, Hsu WK. Use of computed tomography for assessing bone mineral density. Neurosurg Focus. 2014;37:E4. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG. Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am. 2011;93:1057–1063. [DOI] [PubMed] [Google Scholar]
- 34.Silva MJ, Keaveny TM, Hayes WC. Computed tomography-based finite element analysis predicts failure loads and fracture patterns for vertebral sections. J Orthop Res. 1998;16:300–308. [DOI] [PubMed] [Google Scholar]
- 35.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA; TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. [DOI] [PubMed] [Google Scholar]
- 36.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner D, Kamer L, Sawaguchi T, Richards RG, Noser H, Rommens PM. Sacral bone mass distribution assessed by averaged three-dimensional CT models: implications for pathogenesis and treatment of fragility fractures of the sacrum. J Bone Joint Surg Am. 2016;98:584–590. [DOI] [PubMed] [Google Scholar]
- 38.Weber DC, Malyapa R, Albertini F, Bolsi A, Kliebsch U, Walser M, Pica A, Combescure C, Lomax AJ, Schneider R. Long term outcomes of patients with skull-base low-grade chondrosarcoma and chordoma patients treated with pencil beam scanning proton therapy. Radiother Oncol. 2016;120:169–174. [DOI] [PubMed] [Google Scholar]
- 39.Wei RL, Jung BC, Manzano W, Sehgal V, Klempner SJ, Lee SP, Ramsinghani NS, Lall C. Bone mineral density loss in thoracic and lumbar vertebrae following radiation for abdominal cancers. Radiother Oncol. 2016;118:430–436. [DOI] [PubMed] [Google Scholar]
- 40.Wright LE, Buijs JT, Kim HS, Coats LE, Scheidler AM, John SK, She Y, Murthy S, Ma N, Chin-Sinex HJ, Bellido TM, Bateman TA, Mendonca MS, Mohammad KS, Guise TA. Single-limb irradiation induces local and systemic bone loss in a murine model. J Bone Miner Res. 2015;30:1268–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.York JE, Kaczaraj A, Abi-Said D, Fuller GN, Skibber JM, Janjan NA, Gokaslan ZL. Sacral chordoma: 40-year experience at a major cancer center. Neurosurgery. 1999;44:74-79; discussion 79–80. [DOI] [PubMed] [Google Scholar]
- 42.Zagars GK, Ballo MT. Significance of dose in postoperative radiotherapy for soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 2003;56:473–481. [DOI] [PubMed] [Google Scholar]