Abstract
Background
Reported rates of the incidence of lymph node metastasis in soft tissue sarcoma vary considerably. Many are based on single-institution series and small patient populations. Certain sarcoma subtypes, including synovial sarcoma, have been associated with a higher risk of lymph node involvement. Most single centers have insufficient numbers of patients to assess lymph node metastasis accurately, but larger national databases may allow a more accurate estimation.
Questions/purposes
We queried a large national database and asked the following questions: (1) What proportion of patients with soft tissue sarcoma have lymph node metastasis and distant metastasis? (2) What histologic subtypes are associated with increased risk of nodal metastasis? (3) What is the impact of lymph node metastases and histologic subtype on survival? (4) Does lymph node excision improve survival of patients with soft tissue sarcoma?
Methods
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program is a national database that covers a geographic cross-section representing approximately 28% of the US population across demographic groups. Using the SEER database, we identified 15,525 adults diagnosed with histologically confirmed soft tissue sarcoma from 2004 to 2013. Proportions of patients with lymph node or distant metastases were calculated using descriptive statistics. Overall survival was computed using the Kaplan-Meier method. Multivariate analysis was performed using Cox proportional hazard regression to calculate the association of lymph node metastasis with overall survival while controlling for patient age, sex, race, tumor size, and tumor location.
Results
A total of 820 of 15,525 patients had lymph node metastasis at the time of diagnosis, yielding an overall proportion of 5.3% (95% confidence interval [CI], 4.9%–5.6%). Histologic subtypes that most frequently developed nodal metastasis were rhabdomyosarcoma, clear cell sarcoma, epithelioid sarcoma, and myxoid/round cell liposarcoma. Despite frequent reports regarding its association with lymph node metastasis, the proportion of patients with lymph node metastasis among 885 patients with synovial sarcoma (4.2%) was not different from the proportion with nodal metastasis in the overall soft tissue sarcoma population. For all soft tissue sarcomas, distant metastatic disease was present at diagnosis in 1869 (12%) patients (95% CI, 11.5%–12.6%). After controlling for relevant covariates, lymph node metastasis was associated with poorer overall survival (hazard ratio [HR], 1.34; 95% CI, 1.22–1.48; p < 0.001) as was distant metastasis (HR, 2.87; 95% CI, 2.66–3.09; p < 0.001). When comparing the subgroup of patients with positive lymph nodes, lymphadenectomy in conjunction with local excision/limb salvage was associated with the highest overall 5-year survival (HR, 0.46; 95% CI, 0.31–0.67; p < 0.001).
Conclusions
In clarifying the overall proportion of patients with soft tissue sarcoma with nodal metastases, the current study indicates that lymph node metastases occur at a higher proportion than previous studies have suggested and that synovial sarcoma is not associated with a higher risk of lymphatic spread compared with soft tissue sarcoma overall. Patients with lymph node metastases are associated with poorer survival than those without metastases. Further investigation is needed to clarify the apparent improved overall survival after lymphadenectomy in the setting of nodal metastasis from soft tissue sarcoma.
Level of Evidence
Level II, prognostic study.
Introduction
Lymph node metastasis in soft tissue sarcoma is thought to be a relatively uncommon event in a rare disease [3, 11]. Challenges to the study and understanding of this process include the low disease incidence, heterogeneity of histologic subtypes, and varied treatment approaches [8-10, 20]. Early reviews by Weingrad and Rosenberg [22] and Mazeron and Suit [13] of small, single-institution retrospective studies reported lymph node metastases in 8.2% and 10.8% of patients, respectively. However, analysis of a larger institutional database by Fong et al. [8] found that only 2.6% of patients with soft tissue sarcoma developed lymph node metastases. Several reports have suggested that the proportion of synovial sarcomas with lymph node metastases is as high as 44% [5, 12, 13, 17, 21]. The prognostic implication of lymphatic metastasis is unclear with reports of 5-year overall survival from diagnosis of lymph node metastasis ranging from 12.8% [5] to 34% [8].
The staging of soft tissue sarcomas, particularly regarding the presence of metastatic spread, may have a substantial influence on the overall treatment strategy, potentially altering the field of radiation or adding systemic therapies. Efforts to understand the overall percentage of patients with soft tissue sarcoma who develop lymph node metastases, as well as histologic subtypes that are more or less likely to undergo lymphatic spread, may influence the decision for sentinel node biopsy or further advanced imaging such as PET. The current study aims to further clarify the prognostic importance of lymph node metastasis and the benefit of lymphadenectomy for metastatic disease to improve patient counseling and drive informed treatment decisions regarding sarcoma care.
We queried a large national database and asked the following questions: (1) What proportion of patients with soft tissue sarcoma have lymph node metastasis and distant metastasis? (2) What histologic subtypes are associated with increased risk of nodal metastasis? (3) What is the impact of histologic subtype and lymph node metastases on survival? (4) Does lymph node excision improve survival of patients with soft tissue sarcoma?
Materials and Methods
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database provides a mechanism to study cancer in the United States at a population level [14]. The SEER database comprises 18 geographic registries, which record every case of cancer within their respective regions, regardless of insurance status, demographic group, or anatomic site of origin [15]. Collectively, these registries cover approximately 28% of the US population and aid in the study of low-incidence malignancies such as soft tissue sarcoma [15, 16]. Because this database is linked to numerous registries, including the National Death Index, Social Security, the Centers for Medicare & Medicaid Services, and hospital registries, only 1.1% are lost to data capture within 5 years [18].
We accessed the SEER database using SEER*Stat, version 8.2.1, software (National Cancer Institute, Bethesda, MD, USA). Search criteria were all adults (> 17 years of age) diagnosed with histologically confirmed soft tissue sarcoma from 2004 to 2013. A total of 17,070 patients were identified. After we excluded patients with missing lymph node data, 15,525 patients (91%) remained. For the SEER database, lymph node involvement is determined by clinical, surgical, or pathologic determination of the presence or absence of lymphatic metastasis.
Demographic and clinical factors of interest included patient age, sex, race, histologic subtype, tumor grade, tumor size, and tumor location. We considered surgical and radiation therapy in this analysis because chemotherapy data are unavailable in the SEER database.
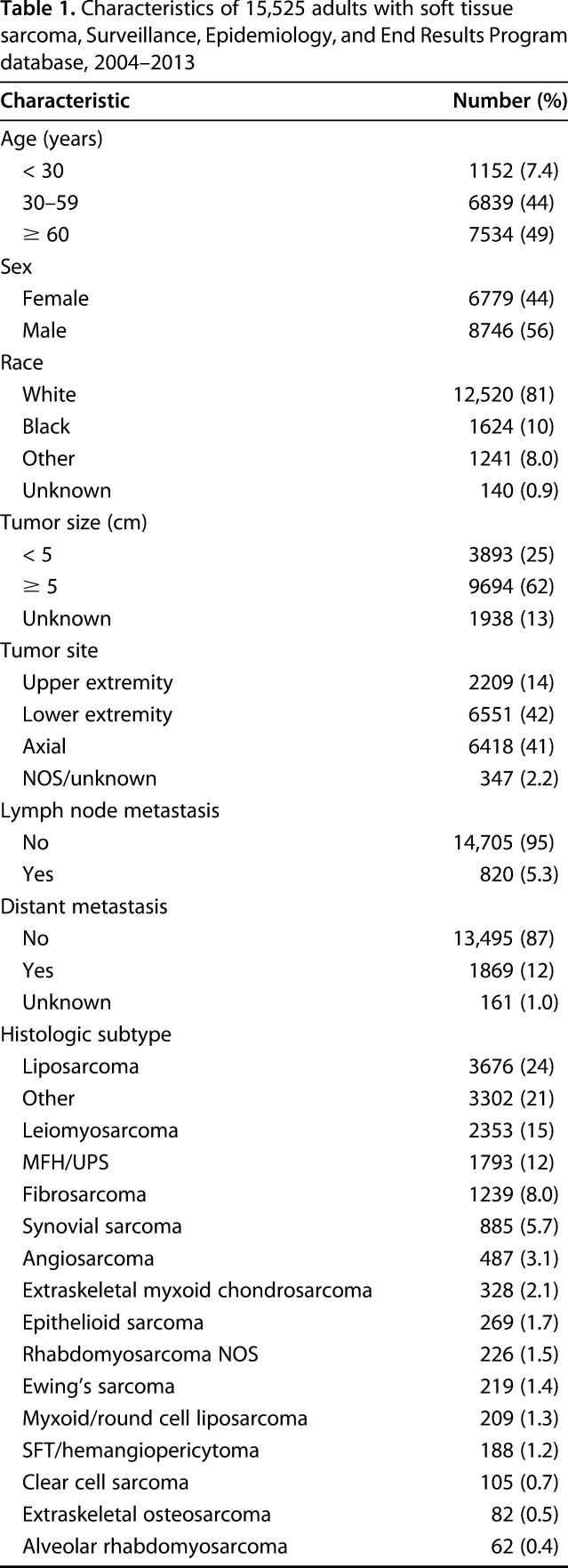
We found that soft tissue sarcoma was more common in white patients (81%; 95% confidence interval [CI], 80%–81%) than in those of other races (19%; 95% CI, 19%–20%; p < 0.001). A greater proportion of patients were male (56%; 95% CI, 56%–57%) than female (44%; 95% CI, 43%–44%; p < 0.001; Table 1). Tumors occurred more frequently in the lower extremities (42%; 95% CI, 41%–43%) than in the upper extremities (14%; 95% CI, 14%–15%; p < 0.001), with 56% occurring in the appendicular skeleton. Tumors were more likely to be ≥ 5 cm at the time of diagnosis (62%; 95% CI, 62%–63%) than to be < 5 cm (25%; 95% CI, 24%–26%; p < 0.001). However, tumor size was not recorded in the SEER database for 12% of the patients in our study population. The most common histologic subtype was liposarcoma (24%). There were 820 patients with documented lymph node involvement. Of these, 764 patients had complete surgical records and were included in the subgroup analysis to assess for the effect of lymphadenectomy. The SEER database does not capture indications for primary or lymph node surgery, so the patient- or physician-specific factors influencing the decision about whether to undertake lymphadenectomy could not be assessed in this analysis.
Table 1.
Characteristics of 15,525 adults with soft tissue sarcoma, Surveillance, Epidemiology, and End Results Program database, 2004–2013
Statistical Analysis
Statistical analysis was performed using R software (R Foundation for Statistical Computing, Vienna, Austria). Proportions of patients with lymph node and distant metastasis were calculated using descriptive statistics. Overall survival and disease-specific mortality were computed by the Kaplan-Meier method and the log-rank test with Bonferroni correction for multiple comparisons. Multivariable analysis was performed using Cox proportional hazard regression to calculate the effect of positive lymph nodes on prognosis while controlling for the effects of patient age, sex, race, tumor grade, tumor size, tumor location, surgery, and radiation therapy. Multivariable analysis was then performed on the subgroup of 764 patients with positive lymph nodes and known surgical status to calculate the effect of lymphadenectomy on overall survival. Statistical significance was determined using an α error of 0.05.
Results
At the time of diagnosis, 820 of 15,525 patients (5.3%; 95% CI, 4.9%–5.6%) had lymph node metastasis and 1869 of 15,525 patients (12%; 95% CI, 11.5%–12.6%) had distant metastasis. Of these, 286 patients with nodal metastases underwent biopsy or excision and were presumed to have histologic confirmation. The remaining 534 patients with positive nodes were coded in SEER as having undergone no surgery (509 patients) or as having unknown surgery status (25 patients) and are presumed to have been clinically or radiographically determined to have lymph node metastasis. When accounting for patients with incomplete data, the most conservative estimate would result from presuming that every patient with an unknown lymph node status had no nodal disease, in which case the overall proportion with nodal metastasis would be 4.8%. Alternatively, if every patient with unknown lymph node status were positive for nodal metastasis, the overall rate of lymphatic spread would be 13.9%.
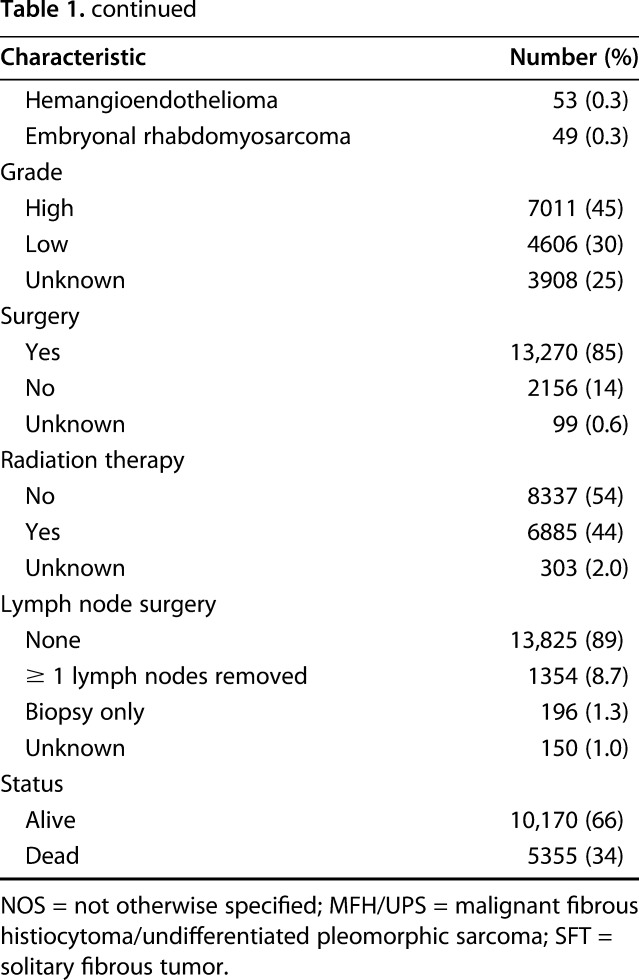
We found a clear association between histologic subtype and the proportion of patients with lymph node metastasis. Compared with the overall proportion of patients presenting with lymph node metastasis (5.3%; 95% CI, 4.9%–5.6%), adults with alveolar rhabdomyosarcoma had a higher proportion of lymph node metastasis (55% [34 of 62]; 95% CI, 42%–68%; p < 0.001). Patients with embryonal rhabdomyosarcoma (27% [13 of 49]; 95% CI, 15%–41%; p < 0.001) and rhabdomyosarcoma not otherwise specified (21% [47 of 226]; 95% CI, 16%–27%; p < 0.001) also had higher rates of lymph node metastasis than did the overall population. Because this study included only adults, however, there were relatively small numbers of patients with rhabdomyosarcoma. Other histologic subtypes with higher proportions of nodal metastasis than the overall population were clear cell sarcoma (23% [23 of 105]; 95% CI, 14%–31%; p < 0.001) and epithelioid sarcoma (20% [55 of 269]; 95% CI, 16%–26%; p < 0.001). Interestingly, 19% (40 of 209; 95% CI, 14%–25%; p < 0.001) of patients with myxoid/round cell liposarcoma developed lymph node metastasis (Table 2), although this subtype is not commonly considered to be in the high-risk group. Liposarcoma (1.2% [45 of 3676]; 95% CI, 0.9%–1.6%; p < 0.001) and fibrosarcoma (1.4% [17 of 1239]; 95% CI, 0.8%–2.2%; p < 0.001) were associated with a lower risk of lymph node metastasis than the overall rate for patients with soft tissue sarcoma in this analysis. The proportion of patients with synovial sarcoma with nodal metastasis was 4.2% (37 of 885; 95% CI, 3.0%–5.7%), which was not statistically significantly lower than the overall proportion among patients with all soft tissue sarcomas combined (5.3% [820 of 15,525]; 95% CI, 4.9%–5.6%; p = 0.176).
Table 2.
Frequency of lymph node metastasis by histologic subtype in 15,525 adults diagnosed with soft tissue sarcoma, Surveillance, Epidemiology, and End Results Program database, 2004–2013
The 5-year overall survival rate for the study population was 61% (95% CI, 50%–62%; Fig. 1). As expected, lymph node metastasis was associated with lower 5-year overall survival. Patients with positive lymph nodes at the time of diagnosis had a 5-year overall survival rate of 20% (95% CI, 17%–24%; p < 0.001; Fig. 2). Similarly, patients with distant metastatic disease at diagnosis had a lower 5-year overall survival rate of 11% (95% CI, 9.5%–13%; p < 0.001; Fig. 3). When controlling for patient age, sex, race, tumor size, and tumor location on multivariate analysis, lymph node metastasis was associated with a higher mortality rate (hazard ratio [HR], 1.3; 95% CI, 1.2–1.5; p < 0.001) as was distant metastatic disease (HR, 2.9; 95% CI, 2.7–3.1; p < 0.001; Table 3). As demonstrated in other studies, tumor grade, tumor size, and axial location are all associated with 5-year overall survival. The current analysis appears to suggest prognostic significance for patient age, sex, and race (Table 3), the investigation of which is outside the scope of the current study.
Fig. 1.
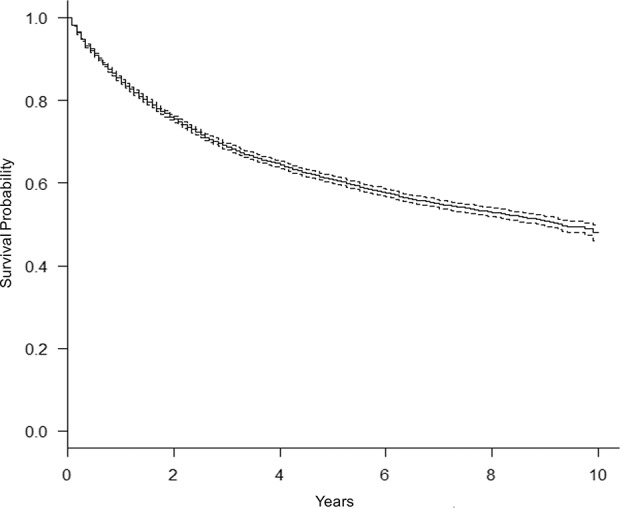
This graph shows a product limit survival estimate for 15,525 adults with soft tissue sarcoma using data from the SEER program database, 2004–2013.
Fig. 2.
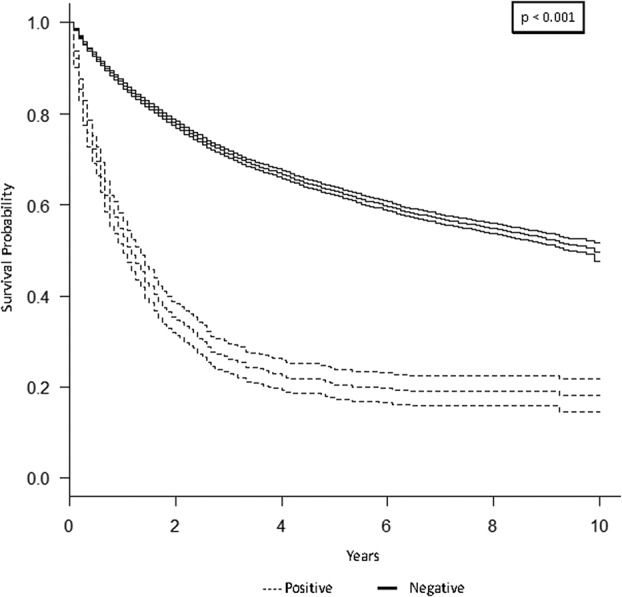
This graph shows a Kaplan-Meier product limit survival curve for lymph node (LN) metastasis among 15,525 adults with soft tissue sarcoma compared with those without LN metastases using data from the SEER program database, 2004–2013.
Fig. 3.
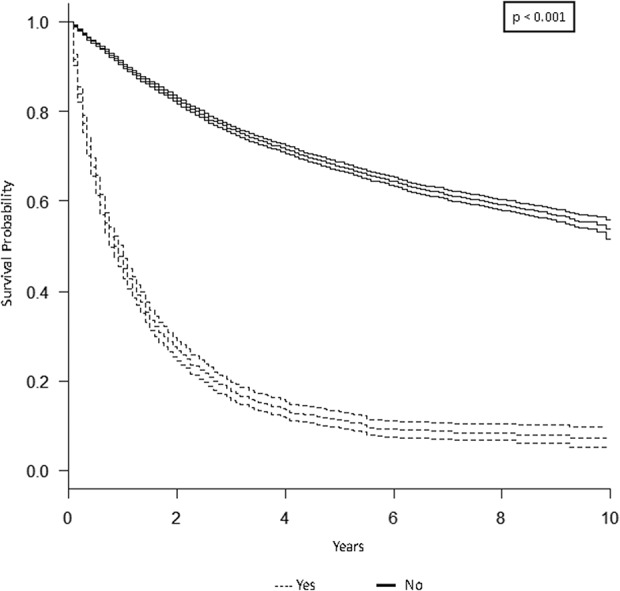
This graph shows a Kaplan-Meier product limit survival curve for distant metastasis among 15,525 adults with soft tissue sarcoma compared with those with no metastases or unknown status using data from the SEER program database, 2004–2013.
Table 3.
Results of multivariate analysis for overall survival
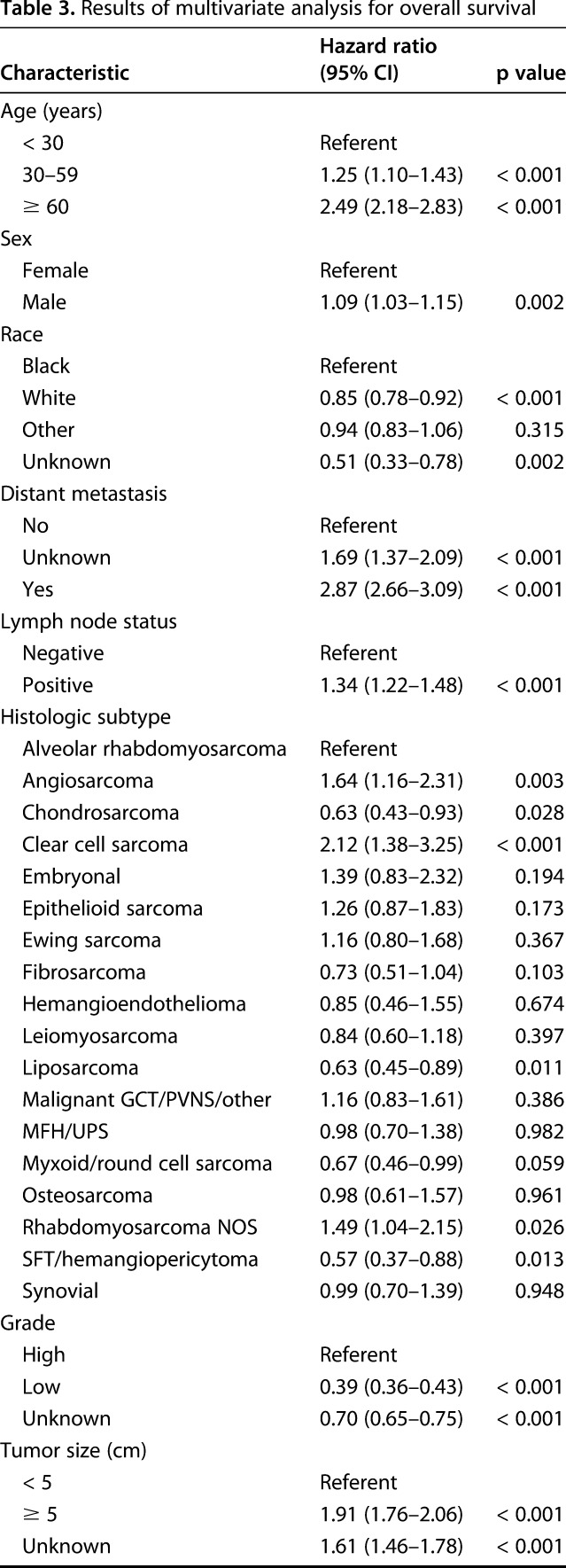
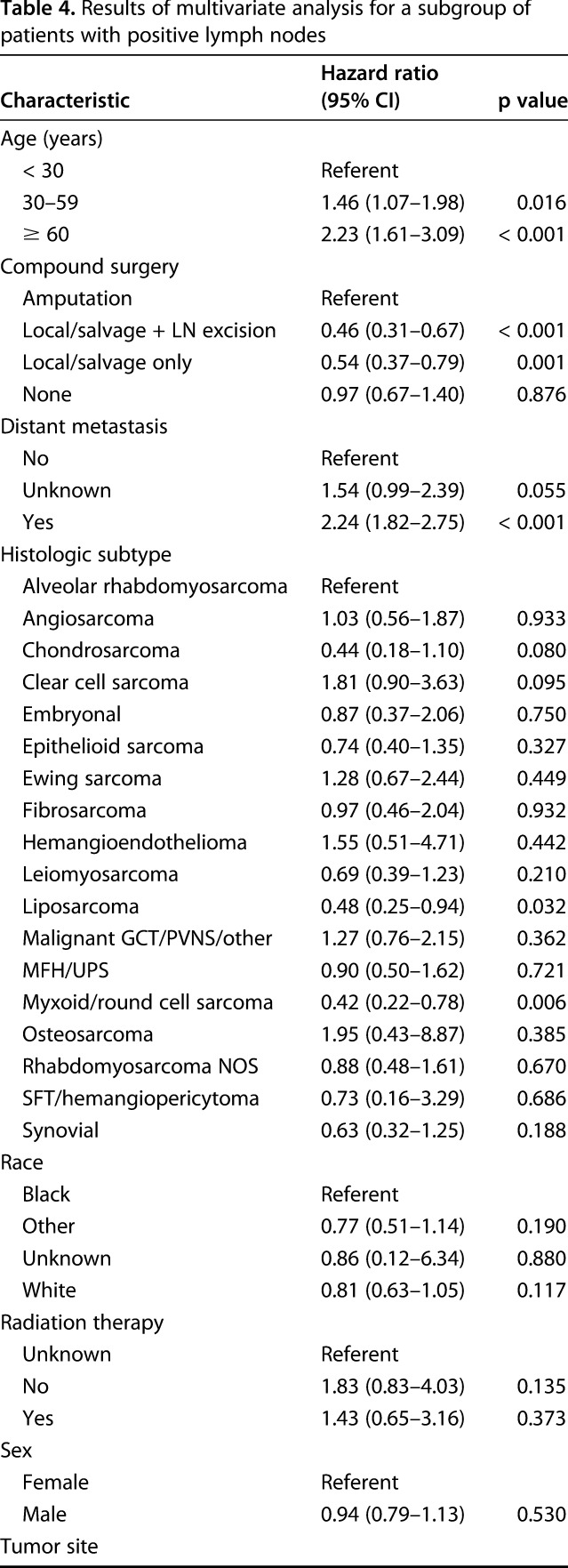
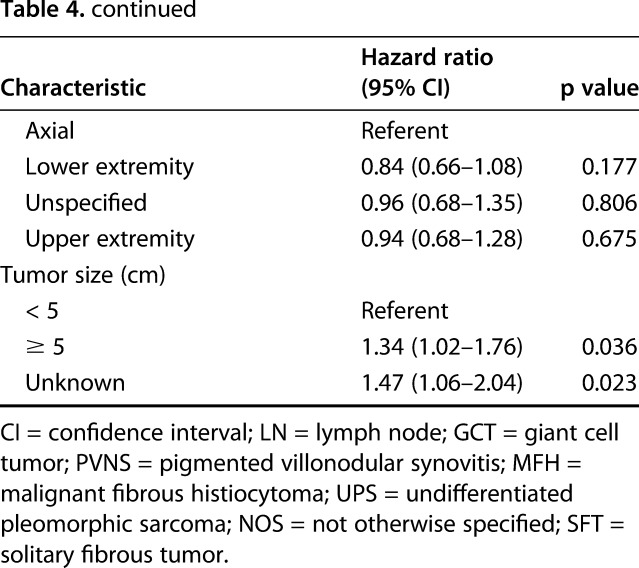
In a subgroup analysis of 764 patients with positive lymph nodes and known surgical status, lymphadenectomy in conjunction with local excision/limb salvage was performed in 190 patients (25%) and was associated with the best overall 5-year survival (HR, 0.46; 95% CI, 0.31–0.67; p < 0.001; Table 4).
Table 4.
Results of multivariate analysis for a subgroup of patients with positive lymph nodes
Discussion
Soft tissue sarcoma is a collection of rare malignancies, which presents inherent challenges to risk stratification and our understanding of this heterogeneous group of diseases. In an analysis of the risk, prognostic importance, and surgical treatment considerations regarding nodal metastases, the current analysis demonstrated that 5.3% of all adults with soft tissue sarcomas will develop lymph node metastases. Nodal involvement was associated with histologic subtype and overall survival. In patients who develop lymph node metastasis, this analysis demonstrates an association between lymphadenectomy and overall survival.
This study is limited by its retrospective approach and missing lymph node status for some patients who would be otherwise appropriate for inclusion. Although data for female and nonwhite patients have been shown to be less complete than those for other patients in large cancer databases, the SEER database has been shown to be representative of the general cancer population of the United States and to have a higher degree of completeness than other cancer registries[1]. It is impossible to know the reasons for not examining or recording node status in these patients. Given the nature of the database, there may be differences in individual or institutional use of investigation for lymphatic disease. The SEER database coding guidelines allow for clinical, surgical, or pathologic adjudication of the presence of nodal metastasis. The true incidence of lymph node metastasis in soft tissue sarcoma is otherwise difficult to determine. Physical examination may show nodal sites of disease. PET can detect regional and distant lymph node involvement, although it is not routinely performed as part of sarcoma staging. Sentinel node biopsy is commonly performed in the staging of rhabdomyosarcoma and epithelioid sarcoma but is most commonly performed in tertiary cancer centers or in the setting of a clinical trial, potentially excluding many patients in the SEER database. Interestingly, the utility of sentinel node biopsy, even for histologic subtypes that are presumed to be at increased risk for nodal metastasis, remains to be fully clarified [12].
The distribution of histologic subtypes of soft tissue sarcomas would be expected to differ between adults and children, and the current analysis was limited to adults. Although there is little reason to believe that the risk of lymph node metastasis would differ according to patient age, we would expect a larger representation of patients with rhabdomyosarcoma among children and adolescents, which could skew the overall proportion of patients with nodal metastasis across the population of patients with soft tissue sarcomas. Therefore, we felt that excluding children and adolescents would more accurately represent the adult sarcoma population as a whole. In contrast to adults, a larger proportion of pediatric patients with sarcoma may be treated within large multicenter cooperative group studies, which may dictate surveillance and treatment strategies and may provide more granular detail on the management of nodal metastases in their respective cohorts.
We found that 5.3% of patients in the SEER database had lymph node metastases and 12% had distant metastases. Only 9% of patients were missing data on lymph node status. Exclusion of these patients with incomplete data yielded a proportion with nodal metastasis of 5.3%. Previous studies of soft tissue sarcoma have varied widely in their reported incidence of lymph node metastasis [3, 7, 13, 17, 22]. Even in our most conservative estimation, if all patients with unknown lymph node status were presumed to be negative for nodal disease, the proportion would be 4.8%, which is still markedly greater than the largest single-institution analysis [8]. This difference likely reflects improved collection of data on these uncommon events in a rare tumor with the use of a large national database. Although our proportions are nearly double those suggested in some reports, this relatively low rate is unlikely to alter current surveillance protocols for soft tissue sarcomas. However, our findings can help physicians better advise patients and their families regarding their risk of nodal spread of disease.
A recent analysis of patients with metastatic sarcoma noted that more than 88% of patients with metastatic disease developed metastasis to only one site [19]. In that report, however, the proportion of patients with metastatic sarcoma to the lung was 4.6 times higher than the proportion of patients with metastasis to lymph nodes. The current study from the SEER database, in contrast, suggests that the relative proportion of metastasis to lymph nodes is greater. The reason for this discrepancy is unclear and may be related to different methods of identifying patients with nodal involvement.
Consistent with previous studies [1, 8, 12], we reported substantial variation in the risk of nodal metastasis among histologic subtypes of soft tissue sarcoma. Tumors with an increased nonspindle cell component tend to display an increased frequency of extrapulmonary metastases, including lymph node metastases [1, 12]. This observation is reflected in our analysis, in which rhabdomyosarcoma, clear cell sarcoma, epithelioid sarcoma, and myxoid/round cell liposarcoma exhibited the highest proportions with reported lymph node involvement. Historically, synovial sarcoma has been included in this group of sarcomas with nodal metastatic tendencies. A few single-institution studies [5, 12, 13, 21] have suggested that synovial sarcoma may be associated with increased risk of lymph node metastasis with a recent review by Nelen et al. [17] suggesting nodal involvement in 10%. Among 42 patients with synovial sarcoma in a recently published prospective study, lymph node metastasis was noted in 7% [1] compared with 4.2% of 885 patients with synovial sarcoma in our study, which was not higher than the overall proportion for all soft tissue sarcomas. It is unclear why the proportions of patients with nodal metastasis in myxoid/round cell liposarcoma and synovial sarcoma differ from those in prior reports. This may reflect a referral bias of advanced cases of synovial sarcomas at the academic centers that published many of the prior series on this disease, but there is no clear explanation as to why that would be reflected only in synovial sarcoma, whereas the overall sarcoma population shows higher proportions with nodal involvement. This may indicate that advanced nodal surveillance for synovial sarcoma may be unwarranted, and it helps support the increasingly recognized extrapulmonary pattern of metastasis in myxoid/round cell liposarcoma.
This study’s results confirm an association between the histologic subtype or soft tissue sarcoma and overall survival. The 5-year overall survival was lower in patients who developed lymph node metastasis than in those who did not. Prior reports have suggested that the 5-year overall survival rate of patients with soft tissue sarcoma with lymph node metastasis was approximately 13% to 34% [5, 8], which is consistent with our finding of 20% 5-year overall survival.
In our investigation, the highest overall survival for patients with lymph node metastases was in those who underwent excision of the primary tumor with lymphadenectomy. The role of lymphadenectomy in the treatment of soft tissue sarcoma has been debated [2-4, 6]. Although we are unable to determine causality in this study, and the proportion with nodal metastasis was still relatively low across the entire cohort, this association may reflect better survival in patients who undergo a more aggressive approach to tumor control. However, this may also reflect selection bias, because those deemed to be healthier or with a lesser metastatic burden may be more likely to undergo aggressive surgical excision. Furthermore, the SEER database is unable to establish whether the addition of chemotherapy can explain this unexpected finding. There were no differences in 5-year overall survival between patients who had only lymph node biopsy compared with those who did not undergo lymph node biopsy. This may indicate a low yield of biopsy or nodal sampling in the overall soft tissue sarcoma population. A recent prospective trial of a subset of soft tissue sarcoma histologic types showed a strong correlation between positive sentinel lymph node biopsy and decreased survival [1]. Although these findings support the potential role for lymphadenectomy in the setting of nodal metastasis, further investigation is necessary to fully elucidate its clinical benefit. However, the current analysis suggests that properly selected patients may be appropriate for lymphadenectomy for management of lymph node metastasis from soft tissue sarcoma.
We conclude that, in contrast to previous reports [1, 12], synovial sarcoma does not seem to be associated with a higher risk of lymph node metastasis compared with that of soft tissue sarcoma overall. These results suggest that the need for sampling or excision of asymptomatic lymph nodes in synovial sarcoma may be lower than previously thought. These data might be useful when considering which patients with given histologic subtypes should be considered for lymph node biopsy or sentinel lymph node biopsy. Traditionally, these approaches are used more commonly in patients who have a perceived higher likelihood to develop lymph node metastases, which often includes those with synovial sarcoma. Our findings suggest that patients with synovial sarcoma may not be at as great a risk as previously thought. This information may be useful for clinicians when considering whether to sample regional lymph nodes. Further investigation into the molecular characteristics of metastatic soft tissue sarcoma may lead to better risk stratification and may influence which patients receive nodal sampling. Although our data cannot definitively determine a clinical benefit of lymphadenectomy in patients with soft tissue sarcoma with lymph node metastasis, our findings support further investigation into this issue.
Acknowledgments
We thank Rachel Box, Jenni Weems, and Eileen Martin for their outstanding editorial assistance in the preparation of this manuscript.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, have funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
This work was performed at the Feinstein Institute for Medical Research, Northwell Health System, Manhasset, NY, USA.
References
- 1.Andreou D, Boldt H, Werner M, Hamann C, Pink D, Tunn PU. Sentinel node biopsy in soft tissue sarcoma subtypes with a high propensity for regional lymphatic spread: results of a large prospective trial. Ann Oncol. 2013;24:1400–1405. [DOI] [PubMed] [Google Scholar]
- 2.Baratti D, Pennacchioli E, Casali PG, Bertulli R, Lozza L, Olmi P, Collini P, Radaelli S, Fiore M, Gronchi A. Epithelioid sarcoma: prognostic factors and survival in a series of patients treated at a single institution. Ann Surg Oncol. 2007;14:3542–3551. [DOI] [PubMed] [Google Scholar]
- 3.Behranwala KA, A'Hern R, Omar AM, Thomas JM. Prognosis of lymph node metastasis in soft tissue sarcoma. Ann Surg Oncol. 2004;11:714–719. [DOI] [PubMed] [Google Scholar]
- 4.Blazer DG, 3rd, Sabel MS, Sondak VK. Is there a role for sentinel lymph node biopsy in the management of sarcoma? Surg Oncol. 2003;12:201–206. [DOI] [PubMed] [Google Scholar]
- 5.Daigeler A, Kuhnen C, Moritz R, Stricker I, Goertz O, Tilkorn D, Steinstraesser L, Steinau HU, Lehnhardt M. Lymph node metastases in soft tissue sarcomas: a single center analysis of 1,597 patients. Langenbecks Arch Surg. 2009;394:321–329. [DOI] [PubMed] [Google Scholar]
- 6.de Visscher SA, van Ginkel RJ, Wobbes T, Veth RP, Ten Heuvel SE, Suurmeijer AJ, Hoekstra HJ. Epithelioid sarcoma: still an only surgically curable disease. Cancer. 2006;107:606–612. [DOI] [PubMed] [Google Scholar]
- 7.Deenik W, Mooi WJ, Rutgers EJ, Peterse JL, Hart AA, Kroon BB. Clear cell sarcoma (malignant melanoma) of soft parts: a clinicopathologic study of 30 cases. Cancer. 1999;86:969–975. [PubMed] [Google Scholar]
- 8.Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kane JM, Finley JW, Driscoll D, Kraybill WG, Gibbs JF. The treatment and outcome of patients with soft tissue sarcomas and synchronous metastases. Sarcoma. 2002;6:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis JJ, Brennan MF. Soft tissue sarcomas. Curr Probl Surg. 1996;33:817–872. [PubMed] [Google Scholar]
- 11.Loya AC, Prayaga AK, Arora A, Sundaram C, Rao IS, Uppin SG, Raju GS, Surath A, Rajappa RS. Lymph node metastasis of soft tissue tumors: a cytomorphologic study. Acta Cytol. 2007;51:153–160. [DOI] [PubMed] [Google Scholar]
- 12.Maduekwe UN, Hornicek FJ, Springfield DS, Raskin KA, Harmon DC, Choy E, Rosenberg AE, Nielsen GP, DeLaney TF, Chen YL, Ott MJ, Yoon SS. Role of sentinel lymph node biopsy in the staging of synovial, epithelioid, and clear cell sarcomas. Ann Surg Oncol. 2009;16:1356–1363. [DOI] [PubMed] [Google Scholar]
- 13.Mazeron JJ, Suit HD. Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer. 1987;60:1800–1808. [DOI] [PubMed] [Google Scholar]
- 14.National Institutes of Health, National Cancer Institute. Surveillance, epidemiology, and end results program. Available at: http://seer.cancer.gov/data/seerstat/nov2012/#. Accessed August 25, 2016.
- 15.National Institutes of Health, National Cancer Institute. Data flow in the SEER registries. Available at: http://seer.cancer.gov/about/factsheets/. Accessed August 25, 2016.
- 16.National Institutes of Health, National Cancer Institute. SEER as a research resource. Available at: seer.cancer.gov/about/factsheets/. Accessed August 25, 2016.
- 17.Nelen SD, Vogelaar FJ, Gilissen F, Van der Linden JC, Bosscha K. Lymph node metastasis after a soft tissue sarcoma of the leg: a case report and a review of the literature. Case Rep Surg. 2013;2113:930361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinheiro PS, Morris CR, Liu L, Bungum TJ, Altekruse SF. The impact of follow-up type and missed deaths on population-based cancer survival studies for Hispanics and Asians. J Natl Cancer Inst Monogr. 2014;2014:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savina M, Le Cesne A, Blay JY, Ray-Coquard I, Mir O, Toulmonde M, Cousin S, Terrier P, Ranchere-Vince D, Meeus P, Stoeckle E, Honoré C, Sargos P, Sunyach MP, Le Péchoux C, Giraud A, Bellera C, Le Loarer F, Italiano A. Patterns of care and outcomes of patients with METAstatic soft tissue SARComa in a real-life setting: the METASARC observational study. BMC Med. 2017;15:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skinner KA, Eilber FR. Soft tissue sarcoma nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am. 1996;5:121–127. [PubMed] [Google Scholar]
- 21.Wagner W, Willich N, Rube C, Prott FJ, Micke O. [Treatment results of soft tissue sarcomas in adults] [in German]. Strahlenther Onkol. 1994;170:91–99. [PubMed] [Google Scholar]
- 22.Weingrad DN, Rosenberg SA. Early lymphatic spread of osteogenic and soft-tissue sarcomas. Surgery. 1978;84:231–240. [PubMed] [Google Scholar]


