Abstract
In the current study, we sought to determine the significance of the ghrelin system in Prader-Willi Syndrome (PWS). PWS is characterized by hypotonia and difficulty feeding in neonates and hyperphagia and obesity beginning later in childhood. Other features include low GH, neonatal hypoglycemia, hypogonadism, and accelerated mortality. Although the hyperphagia and obesity in PWS have been attributed to elevated levels of the orexigenic hormone ghrelin, this link has never been firmly established, nor have ghrelin’s potentially protective actions to increase GH secretion, blood glucose, and survival been investigated in a PWS context. In the current study, we show that placing Snord116del mice modeling PWS on ghrelin-deficient or ghrelin receptor [GH secretagogue receptor (GHSR)]–deficient backgrounds does not impact their characteristically reduced body weight, lower plasma IGF-1, delayed sexual maturation, or increased mortality in the period prior to weaning. However, blood glucose was further reduced in male Snord116del pups on a ghrelin-deficient background, and percentage body weight gain and percentage fat mass were further reduced in male Snord116del pups on a GHSR-deficient background. Strikingly, 2 weeks of daily administration of the GHSR agonist HM01 to Snord116del neonates markedly improved survival, resulting in a nearly complete rescue of the excess mortality owing to loss of the paternal Snord116 gene. These data support further exploration of the therapeutic potential of GHSR agonist administration in limiting PWS mortality, especially during the period characterized by failure to thrive.
Prader-Willi Syndrome (PWS) is a genetic disorder with an estimated prevalence of 1 in 10,000 to 1 in 30,000, resulting from sporadic loss of, or failure to, express a set of paternally expressed genes within a 5- to 6-Mb segment of chromosome 15 (1). These include several protein-coding genes, genes that generate small nucleolar RNAs [such as Snord116 (PWCR/HBII-85), which likely work by modifying other RNAs], and some antisense transcripts (1, 2). The hallmark symptom of PWS is hyperphagia, which develops on average at ∼8 years of age, is fueled by an unrelenting appetite, is manifested by several food-seeking behaviors, and usually results in obesity despite careful monitoring (2–6). Although hyperphagia develops in nearly all individuals with PWS and is a driver of significant morbidity and mortality, it is only one facet of PWS.
Hypotonia is another central feature of PWS. It is most prominent in neonates, causing decreased movement, lethargy, weak crying, and a poor sucking reflex (2). The poor sucking reflex and lethargy contribute to feeding difficulty and failure to thrive, often necessitating feeding tube placement for adequate nutrition (2). With time, the hypotonia improves, permitting unassisted eating, although hypotonia-linked morbidity—including recurrent childhood respiratory infections (thought to be contributed to by poor respiratory muscle strength), decreased muscle bulk and tone, choking on food, and prevalent scoliosis—persists (2, 7, 8). In childhood, many developmental milestones are delayed and learning disabilities are evident, as are several disruptive behavioral problems (2). Endocrinopathies also are present, including but not limited to GH deficiency [which contributes to hypotonia, short stature, increased adiposity, reduced lean mass, and behavioral disturbances (2, 3)], a predisposition to hypoglycemia in young children (9), and hypogonadism (2). Mental health issues associated with PWS include depression, anxiety, and high pain threshold, among others (10, 11).
Those morbidities contribute to an elevated mortality rate, estimated at 3% per year (12, 13). Febrile illnesses are the most common cause of death in children with PWS (2). In adults, mortality stems mainly from complications of hyperphagia and obesity, with most affected individuals not living past their early 40s (2). Although there have been improvements in PWS treatment during the last few years in response to more inclusive multidisciplinary treatment plans, more widespread use of GH replacement therapy, as well as increased access of families to PWS resources, challenges in the day-to-day management of PWS remain extremely high, as do the morbidity and mortality associated with the syndrome.
A key barrier to advancing PWS management is a gap in the understanding of signals directing the abnormal physiology and behaviors characteristic of PWS. A promising lead has been the hormone ghrelin, the levels of which have long been known to be high in PWS (14–25). In particular, the hyperphagia and obesity of PWS have in the past been associated with the acylated form of ghrelin (acyl-ghrelin) owing to its known potent orexigenic, food reward–inducing, and adiposity-promoting actions in non-PWS models, which are mediated via its interaction with its receptor GH secretagogue receptor (GHSR) (26, 27). High plasma ghrelin in PWS has been reported in several clinical trials, most positively correlating elevated total ghrelin or elevated acyl-ghrelin with the overeating and obesity of PWS (14–24). The magnitude of this elevation is nearly equivalent in adults and children affected by PWS, with the initial 2002 studies describing threefold to 4.5-fold higher fasting plasma ghrelin in adults with PWS than in matched controls with obesity (14, 15) and threefold to fourfold higher plasma ghrelin reported in obese children with PWS than in body mass index–matched children with nonsyndromic obesity or with obesity due to MC4R or leptin mutations (16–18, 22). In young children with PWS, high ghrelin precedes the development of obesity, with one study interpreting this finding as making it unlikely that high ghrelin is responsible for the switch to the hyperphagic nutritional phases in PWS (22). Another study, however, proposed that early high ghrelin may prime the brain for the eventual occurrence of obesity (17), as is supported by work suggesting a key role for proper expression of ghrelin for normal development of hypothalamic feeding circuits (28). Furthermore, in adults with PWS, blunted or absent postprandial ghrelin reductions, which may increase overall eating, have been noted (15, 19).
Of importance, although the many aforementioned studies support a causative role for high acyl-ghrelin in the hyperphagia and obesity of PWS, this link has never been definitively established. Additionally, ghrelin’s known actions to increase lean mass and muscle strength (29, 30), GH secretion (26, 31), blood glucose (26, 32–34), and survival (33, 35, 36) or its endogenous antidepressant and anxiolytic actions (37–42), all of which would be beneficial in PWS, have not been investigated in the context of PWS. For the current study, we have used the Snord116del preclinical mouse model of PWS crossed to mice lacking ghrelin, crossed to mice lacking GHSR, or administered a GHSR agonist to investigate the functional significance of the ghrelin system in PWS, with a focus on the first month of life, prior to the time of weaning.
Materials and Methods
Mouse models
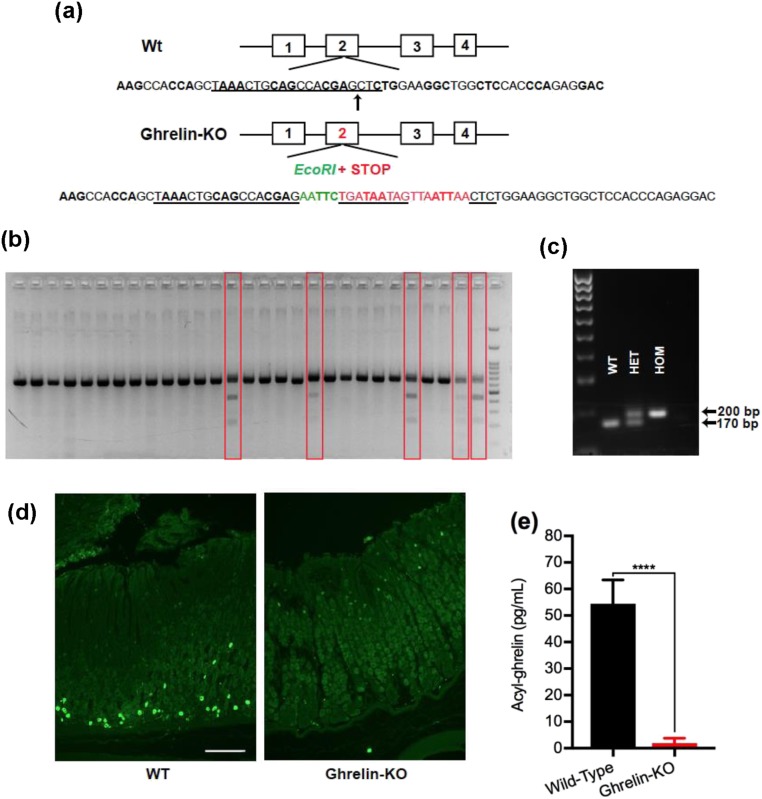
Three different mouse models were used to generate study mice: (i) Snord116del mice [B6(Cg)-Snord116tm1.1Uta/J; stock008149] developed by the Francke laboratory (43), which were obtained from The Jackson Laboratory (Bar Harbor, ME). Snord116del study mice and wild-type littermates were generated by crossing male Snord116del mice (that first had been backcrossed six to nine generations to C57BL/6N mice, depending on the study) to female C57BL/6N mice. (ii) GHSR-null mice (44), which have been maintained in the laboratory on a C57BL/6N background for >13 years. GHSR-null mice are unresponsive to administered acyl-ghrelin, resistant to diet-induced obesity, and are hypoglycemic upon severe caloric restriction (32, 44, 45). (iii) A new line of ghrelin-knockout (KO) mice, which was generated by targeted insertion of multiple stop codons along with an EcoRI site into the coding sequence (exon 2) of the endogenous Ghrl gene using CRISPR-Cas9 technology, as follows (46) [Fig. 1(a)]: short guide RNA designed to direct the Cas9 enzyme to the cleavage site was cloned into the PX459 vector (Addgene, Cambridge, MA). The vector was transformed into competent Escherichia coli (New England Biolabs, Ipswich, MA), which was then placed on an ampicillin selection agar plate overnight at 37°C. After verifying the sequence using U6 sequencing primers, a clone was picked and incubated overnight at 37°C in Luria-Bertani broth containing ampicillin while shaking at 250 rpm. The plasmid DNA was then purified from E. coli using the ZymoPURE Midiprep kit (Zymo Research, Irvine, CA) and transfected into the mouse Neuro2A cell line (American Type Culture Collection, Manassas, VA) using Lipofectamine 3000 transfection reagent (Thermo Fisher Scientific, Waltham, MA). RNA-guided nuclease activity at the target site in the ghrelin gene was confirmed by the presence of EcoRI digestion products. Thereafter, the insert along with the short guide RNA and Cas9 mRNA were microinjected into C57BL/6N zygotes. Resulting progeny were screened for the presence of the modified (KO) ghrelin allele by PCR using the primers “Ghrl R” and “Ghrl L” (47) to amplify a region of the gene flanking the insertion site, followed by assessment of the size of the PCR amplicon and its ability to be digested with EcoRI. Of the 54 mice obtained, 5 harbored the ghrelin-KO allele [Fig. 1(b)]. Ghrelin-KO lines were developed from two different founders (“GKO1” and “GKO3”) by crossing them to C57BL/6N mice and then crossing resulting heterozygotes to each other when needed to generate ghrelin-KO mice (harboring two copies of the ghrelin-KO allele) and wild-type littermates (harboring two copies of the wild-type ghrelin allele). These genotypes were viable, born with the expected Mendelian distribution, and confirmed by PCR amplification of genomic DNA using the primers Ghrl R and Ghrl L [Fig. 1(c)].
Figure 1.
Generation and validation of the novel ghrelin-KO mouse lines GKO1 and GKO3. (a) Schematic of the derivation of ghrelin-KO mice using CRISPR-Cas9 genome editing to insert an EcoR1 site (to facilitate screening for the edited gene) and multiple STOP codons into exon 2 of the Ghrl gene (at the insertion site marked by an arrow). The sequence targeted by the short guide RNA appears in black with underlining. The green-colored nucleotides represent the inserted EcoR1 sequence. The red-colored nucleotides with underlining represent three sequential in-frame STOP codons. The red-colored nucleotides without underlining indicate additional out-of-frame STOP codons. (b) Gel image showing separation of genotyping PCR products from potential ghrelin-KO founders (red boxes) and nontargeted mice, after EcoR1 digestion. (c) PCR analysis of genomic DNA obtained by tail biopsies of representative mice derived from crosses of mice heterozygous for the ghrelin-KO allele, demonstrating identification of mice with two copies of the wild-type Ghrl allele (WT), mice homozygous for the knockout Ghrl allele (HOM), and heterozygotes (HET). (d) Immunohistochemistry of representative stomach sections from 3- to 4-mo-old WT and ghrelin-KO littermates of the GKO3 line. The green color represents ghrelin immunoreactivity. Scale bar, 100 μm. (e) Plasma acyl-ghrelin levels from 28-d-old nonfasted WT and GKO3 littermates. n = 9 to 10 (combination of males and females). Data are expressed as mean ± SEM. ****P < 0.0001, by a Student unpaired t test.
GKO3 was used in the current study. It was validated by assessing ghrelin immunoreactivity within the gastric mucosa of three subjects as compared with wild-type littermates, as follows: anesthetized mice were transcardially perfused with diethyl pyrocarbonate–treated PBS followed by 10% neutral buffered formalin. Stomachs were isolated, flushed out with PBS, fixed for 4 to 6 hours at 4°C with formalin, transferred to PBS overnight at 4°C, paraffin embedded, and sectioned at 8 µm. The paraffin sections were mounted on Superfrost Plus glass slides (Thermo Fisher Scientific), de-waxed with xylenes, rehydrated using graded concentrations of ethanol, and then washed several times in PBS. The sections next were blocked for 2 hours at room temperature using 3% donkey serum with PBT (0.3% Triton X-100 in PBS), incubated overnight at room temperature with goat polyclonal anti-ghrelin antibody [catalog no. sc-10368; Santa Cruz Biotechnology, Dallas, TX; RRID: AB_2232479 (48)] diluted 1:1000 in the blocking solution, washed with PBS, incubated with Alexa Fluor 488® donkey anti-goat IgG [catalog no. A-11055; Thermo Fisher Scientific; RRID: AB_2534102 (49)] for 2 hours at room temperature, and then coverslipped with Vectashield hardset antifade mounting medium [catalog no. H-1400; Vector Laboratories, Burlingame, CA; RRID: AB_2336787 (50)]. Ghrelin immunoreactivity was observed in the expected distribution (51) within the gastric mucosa of wild-type mice, but none was observed in GKO3 mice [Fig. 1(d)]. As further validation, acyl-ghrelin levels were measured in nonfasted 28-day-old GKO3 mice and found to be barely detectable as compared with wild-type littermates [Fig. 1(e)]. Notably, other ghrelin-KO lines have been used to investigate the effects of ghrelin on blood glucose, body weight, and other parameters (36, 52–57).
To generate Snord116del mice on a ghrelin-KO or GHSR-null background and their littermate controls, we crossed Snord116del mice (that had been backcrossed once to C57BL/6N mice) to GKO3 or GHSR-null mice, and then crossed resulting males carrying one Snord116del allele and one ghrelin-KO allele or one GHSR-null allele to females homozygous, heterozygous, or wild-type for the ghrelin-KO allele or for the GHSR-null allele, respectively. Study animals, including Snord116del/ghrelin-KO mice (carrying a paternally inherited Snord116del allele plus two ghrelin-KO alleles) and their control genotypes [wild-type mice (carrying two wild-type Snord116 alleles plus two wild-type ghrelin alleles), ghrelin-KO mice (carrying two wild-type Snord116 alleles plus two ghrelin-KO alleles), and Snord116del mice (carrying a paternally inherited Snord116del allele plus two wild-type ghrelin alleles)] and Snord116del/GHSR-null mice (carrying a paternally inherited Snord116del allele plus two GHSR-null alleles) and their control genotypes (wild-type mice, GHSR-null mice, and Snord116del mice), were born with the expected Mendelian distribution. All mice used in the current studies were derived from litters with 6 to 12 pups noted at birth.
Mice were housed at room temperature (21.5°C to 22.5°C) using a 12-hour light/12-hour dark cycle and were provided ad libitum access to water and regular chow [2016 Teklad global 16% protein rodent diet (Envigo, Huntingdon, United Kingdom); 3 kcal/g; 12% kcal from fat]. All pups were marked with identifying foot tattoos at birth and ear tagged at 14 days of age in those experiments extending beyond day 14. All animal procedures were approved by the Institutional Animal Care and Use Committee of the UT Southwestern Medical Center.
Body weights, body composition, and body length
Body weight was measured daily (HM01 administration study), every other day, and/or upon collection of blood. Percentage body fat mass and percentage lean mass were calculated by dividing fat mass or lean mass, as determined using an EchoMRI-100™ (Echo Medical Systems, Houston, TX), by body weight as measured on the same day using a standard scale. Body (nose-to-anus) length was measured using a protractor.
Genotyping protocols
Genomic DNA was isolated from tail snips using the REDExtract-N-Amp™ tissue PCR kit (MilliporeSigma, Burlington, MA) and GoTaq® Green master mix (Promega, Madison, WI). The primer sets used to detect the Snord116del and wild-type Snord116 alleles, the ghrelin-KO and wild-type ghrelin alleles, the GHSR-null and wild-type GHSR alleles, and the Sry gene (which amplifies the Y chromosome and was used in some studies to determine the sex of neonatal pups) are as found in Zigman and Rodriguez (47).
Plasma hormone and blood glucose levels
Blood was collected from the trunk following live decapitation (of 7-, 14-, or 28-day-old mice) for hormone analysis or by tail bleed (of 14-, 17-, 18-, or 28-day-old mice) for blood glucose measurement. For assessment of plasma acyl-ghrelin and total ghrelin, blood was collected into ice-cold EDTA-coated microtubes containing the protease inhibitor p-hydroxymercuribenzoic acid (final concentration 1 mM). The samples were immediately centrifuged at 4°C at 1500g for 15 minutes, and hydrochloric acid was added to the supernatant to achieve a final concentration of 0.1 N (for stabilization of acyl-ghrelin). Processed samples were stored at −80°C in small aliquots until analysis using ELISA kits for acyl-ghrelin and total ghrelin (catalog nos. EZRGRA-90K and EZRGRT-91K; MilliporeSigma). For measurement of IGF-1, blood was collected into EDTA-coated microtubes and analyzed with the AssayMax mouse IGF-1 kit (catalog no. EMI1001-1; Assaypro, St. Charles, MO). The endpoint calorimetric assays were performed using a BioTek PowerWave XS microplate spectrophotometer (BioTek Instruments, Winooski, VT) and KC4 junior software (BioTek Instruments). Blood glucose levels were measured using the Bayer Contour (for Fig. 4 only; Bayer, Whippany, NJ) or the ReliOn Confirm (Walmart, Bentonville, AK) blood glucose monitoring systems.
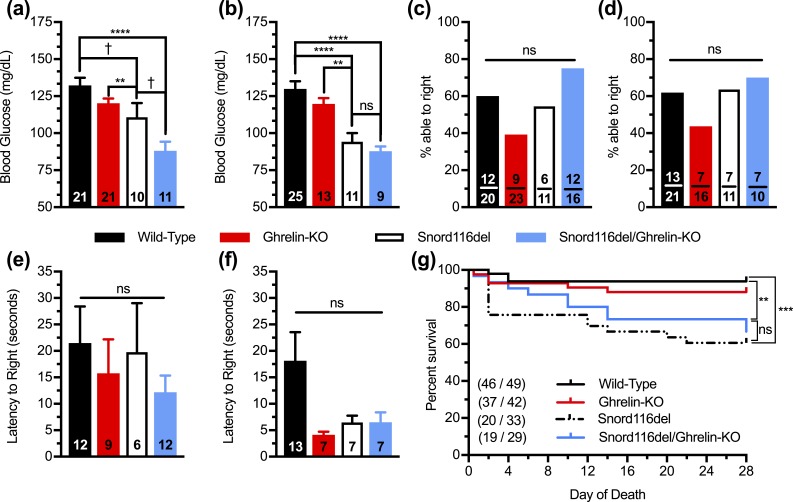
Figure 4.
Effects of ghrelin deletion on blood glucose, muscle strength, and survival of Snord116del mice. (a and b) Blood glucose levels of (a) male and (b) female 17-d-old pups. n = 9 to 25, as indicated within the bars. (c–f) Performance in surface righting task of 5-d-old pups, as indicated by percentage of pups able to attain the upright position within 60 s [(c) males, (d) females] and the time needed to attain the upright position within 60 s [(e) males, (f) females]. n = 6 to 13 pups able to right themselves out of n = 10 to 23 pups tested, as indicated within the bars. (g) Survival curves of pups during the first 28 d of life. n = 19 to 46 survivors out of n = 29 to 49 pups. (Of note, the sexes of many of the neonates were not assessed prior to their demise, and thus these survival data include a combined population of males and females.) Curves were calculated by the Kaplan-Meier method. **P < 0.01, ***P < 0.001, ****P < 0.0001, †0.05 ≤ P < 0.1, effect of genotype as assessed (a–f) by one-way ANOVA, followed by a Tukey post hoc analysis or (g) by comparisons among groups using the Mantel-Cox log-rank test. ns, not significant.
Assessment of sexual maturity
Female mice were checked daily from 23 days of age onward, until vaginal opening was observed, as described in Caligioni (58).
Surface righting task
Guided by a published protocol (59), forceps were used to place 5- to 6-day-old mice on their backs on the bench top. The time taken for the mice to return to the upright position (all four paws on the bench top) was determined and recorded as “latency.” If an animal did not right itself within 60 seconds, it was noted to have failed to achieve the upright position.
Quantitative RT-PCR
Hypothalami were extracted from anesthetized 1-week-old mice. Total RNA was isolated using the guanidinium thiocyanate–phenol/chloroform extraction method by addition of RNA STAT-60 (Amsbio, Cambridge, MA) and quantified using a Nanodrop spectrophotometer (Thermo Fisher Scientific). One microgram of total RNA was treated with ribonuclease-free deoxyribonuclease (Roche, Indianapolis, IN), and complementary DNA was synthesized using SuperScript III (Invitrogen, Carlsbad, CA). Gene-specific primers (47) were as used in previous publications or obtained as Assays-on-Demand (Thermo Fisher Scientific). Quantitative PCR was performed using an Applied Biosystems ViiA 7 real-time PCR system (Thermo Fisher Scientific) with either TaqMan primer/probe or SYBR Green chemistry. The reaction mixture for TaqMan quantitative PCR contained 2.0 μL of reverse-transcribed RNA, 250 nM of each primer, 250 nM probe (or 0.7 μL of the Assay-on-Demand mix), and 3.5 μL of 2× TaqMan Gene Expression Master Mix (Thermo Fisher Scientific). Reaction amounts used for SYBR Green quantitative PCR were identical except that 3.5 μL of 2× iTaq Universal SYBR Green Supermix (Bio-Rad, Hercules, CA) and 175 nM of each primer were used. The mRNA levels were normalized to an invariant control gene (18S ribosomal RNA) and are represented by the comparative threshold cycle (ΔΔCt) method relative to the wild-type control mice.
HM01 administration study
Snord116del mice and their wild-type littermates were randomly assigned to receive either the GHSR agonist HM01 or saline beginning at 1 day of age until 14 days of age. HM01 (Helsinn Healthcare, Lugano, Switzerland) (30 µg/g body weight; 10 µL/g body weight of a 3 µg/µL HM01 solution dissolved in saline) or saline (10 µL/g body weight) was administered subcutaneously once daily between 0900 and 1000 hours. Blood glucose determination and blood collection to assay IGF-1 occurred 1 hour after injection on day 14.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, San Diego, CA) or NCSS 2007 (NCSS Statistical Software, Kaysville, UT). Data are expressed as mean ± SEM. The specific tests used for each analysis are indicated in the figure legends. If unequal variance among groups was detected by a Bartlett test, data were log transformed before analysis, as required. Outliers were detected by a Grubb test. A P value <0.05 was considered statistically significant, and values of 0.05 ≤ P < 0.1 were considered to be evidence of statistical trends.
Results
Preclinical models
For this study, we used the Snord116del mouse model of PWS (43, 60). Snord116del mice lack the paternal Snord116 small nucleolar RNA cluster within the PWS critical region, as a result of germline whole-body deletion of the Snord116 gene (60). Notably, a report of a human subject with a microdeletion limited to the Snord116 gene cluster described many similarities to humans with the more usual chromosomal deletions (of the entire PWS critical region) typical of PWS (61), suggesting a key role for loss of paternal Snord116 in PWS pathogenesis. These similarities include neonatal hypotonia and poor sucking requiring tube feeding, development of hyperphagia, excessive weight gain and obesity in early childhood, GH deficiency, short body length, incomplete pubertal development, typical psychiatric and behavioral manifestations, central hypothyroidism, mild cognitive impairment, developmental delay, facies reminiscent of PWS, and thick saliva. Subjects with limited deletions of Snord116 that extend into the nearby SNURF–SNRPN and/or Snord115 genes exhibit a similar phenotype (62–65). Multiple key PWS features also are recapitulated within the Snord116del mouse model, particularly those that manifest in the neonatal/young childhood period. Similar to PWS, these mice are reported to exhibit growth delay (reduced body weight and reduced body length) and an impaired GH pathway (60, 66). Also, as observed in many older children with PWS, Snord116del mice show delayed sexual maturation (60). Thus, and together with additional supporting evidence from the current study (see below), it is our assessment that the Snord116del line is a good model for PWS in the neonatal/young childhood period prior to the development of hyperphagia and obesity.
In contrast, mice lacking paternal Snord116 do not, in our assessment, adequately represent the most prominent phenotypes of the adult period, namely marked hyperphagia and obesity. In particular, although adult Snord116del mice exhibit prolonged eating bouts (suggesting impaired satiation), increased food intake during a fast/refeed protocol, and hyperphagia, they do not develop obesity and the hyperphagia reported is slight, is present only by comparing relative food intake to their reduced body weights, and has not been recapitulated in all studies (60, 66–68). Additionally, mice with biallelic Snord116 deletion do not develop obesity, although they do exhibit increased body weight gain after weaning (69, 70). Only in a subset of mice in which mediobasal hypothalamic-selective Snord116 deletion was achieved at 10 weeks of age did a combination of both frank hyperphagia and obesity subsequently develop (68). Importantly, opposite to PWS, which is associated with reduced energy expenditure, adult Snord116del mice and adult mice with biallelic Snord116 deletion display increased energy expenditure when housed at room temperature, which likely contributes to their lack of obesity (60, 69, 70).
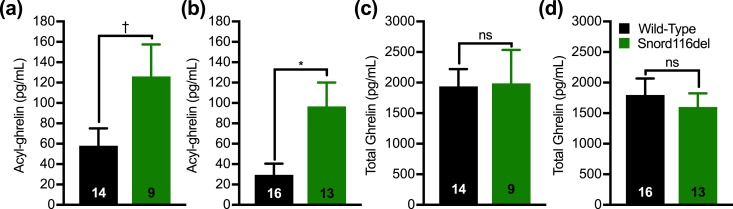
Also lacking from the Snord116del mouse model is frank hypotonia, although adult mice were reported to display a motor learning deficit (60). Similar to PWS, however, Snord116del mice show anxiety-like behavior and decreased bone mineral density (60, 71). Also similar to PWS, ghrelin is elevated in Snord116del mice. In particular, total ghrelin was reported to be twofold to threefold higher in fed and 24 hour–fasted 11-month-old Snord116del mice than in wild-type controls (60). In another report, total ghrelin was ∼1.7-fold higher in fasted “adult” male Snord116del mice (66). In the current study, we measured plasma acyl-ghrelin and total ghrelin in 28-day-old Snord116del mice and wild-type littermates, confirming higher levels of acyl-ghrelin in the Snord116del groups (Fig. 2).
Figure 2.
Plasma acyl-ghrelin and total ghrelin levels in Snord116del mice. (a and b) Plasma acyl-ghrelin and (c and d) total ghrelin from 28-d-old nonfasted (a and c) male and (b and d) female wild-type and Snord116del littermates. n = 9 to 16, as indicated within the bars. Data are expressed as mean ± SEM. *P < 0.05, †0.05 ≤ P < 0.1, by a Student unpaired t test. ns, not significant.
Of particular interest to the current study is the recent work from Burnett et al. (66) in which induced pluripotent stem cell–derived neurons from three human subjects with the most usual genetic form of PWS and from one human subject with a microdeletion of the Snord116 gene (as well as the flanking Snord109A and IPW genes) were shown to display markedly downregulated expression of prohormone convertase 1/3 (PC1/3) and of a transcription factor (NHLH2), which regulates PC1/3 expression. Reduced NHLH2 and/or PC1/3 expression also was observed in a number of tissues from Snord116del mice, including hypothalamus, islets, and stomach (66) [although reduced hypothalamic expression of NHLH2 and PC1/3 was not confirmed in a separate study of Snord116del mice (68)]. As a result, impaired prohormone processing of proinsulin, pro-GH–releasing hormone, and proghrelin were observed, and thus, for instance, there was an increased ratio of proghrelin/ghrelin in stomach lysates from Snord116del mice (66). Also of note, the stomach ghrelin mRNA level in Snord116del mice was nearly doubled (66). Furthermore, acylated forms of both mature ghrelin and proghrelin were found to be detectable by standard ELISA kits (including the one used in the current study) (66), although the exact amounts of acyl-ghrelin vs the acylated form of proghrelin (acyl-proghrelin) in circulation remain enigmatic. Thus, it has been proposed that the hyperghrelinemia of PWS reflects both increased ghrelin transcription and inefficient PC1/3-mediated processing of proghrelin to mature ghrelin.
Not only do we assess Snord116del mice in the current study, but we also crossed Snord116del mice with a new line of ghrelin-KO mice and separately with GHSR-null mice (44) to generate Snord116del mice lacking either ghrelin or GHSR and their respective control littermates. The mice were studied during the first 4 weeks of life, prior to weaning.
Studies with Snord116del/ghrelin-KO mice
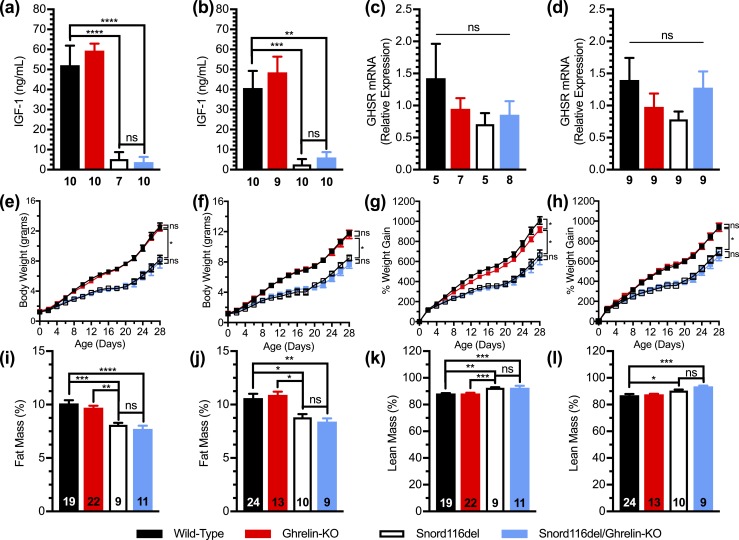
We first studied Snord116del mice on a ghrelin-KO background (Snord116del/ghrelin-KO) together with three control littermate groups (see “Materials and Methods”). Plasma IGF-1 in nonfasted 1-week-old Snord116del mice was ∼6.5% to 10% of that in wild-type littermates. This is lower than previously reported for 4-week-old and 8-week-old Snord116del mice, in which circulating IGF-1 was 39% to 57% of that in wild-type mice (60, 66–68). Plasma IGF-1 was unaffected by ghrelin deletion [Fig. 3(a) and 3(b)]. Hypothalamic levels of GHSR mRNA expression in 1-week-old male and female wild-type, ghrelin-KO, Snord116del, and Snord116del/ghrelin-KO mice were similar [Fig. 3(c) and 3(d)].
Figure 3.
Effects of ghrelin deletion on plasma IGF-1, hypothalamic GHSR mRNA, body weight, body weight gain, and body composition of Snord116del mice. (a–d) Plasma IGF-1 levels and hypothalamic GHSR mRNA expression of 1-wk-old (a and c) male and (b and d) female mice. n = 4 to 10, as indicated beneath the bars. (e–h) Body weights of (e) male and (f) female Snord116del/ghrelin-KO pups and three control littermate groups (wild-type, ghrelin-KO, and Snord116del) during the first 28 d of life, and body weight gain of (g) male and (h) female pups during the first 28 d of age. n = 11 to 24. (i–l) Percentage fat mass of male (i) and female (j) 28-d-old pups and percentage lean mass of the same (k) male and (l) female pups. n = 9 to 24, as indicated within the bars. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, by (a–h) repeated measures two-way ANOVA, followed by a Tukey post hoc analysis or (i–l) effect of genotype as assessed by one-way ANOVA, followed by a Tukey post hoc analysis. ns, not significant.
During the first 28 days of life, body weights and body weight gain of both male and female Snord116del mice were lower as compared with wild-type littermates, as had been reported previously (60, 68) [Fig. 3(e)–3(h)]. These reductions in Snord116del mice were not impacted by ghrelin deletion. Male ghrelin-KO mice did exhibit a slightly reduced body weight gain during the neonatal period as compared with wild-type mice [Fig. 3(g)]. In comparison with 28-day-old wild-type mice, similarly aged Snord116del mice (both males and females) exhibited reduced percentage fat mass and increased percentage lean mass; there were no significant differences between Snord116del mice and Snord116del/ghrelin-KO mice [Fig. 3(i)–3(l)].
We examined blood glucose, considering the predisposition toward hypoglycemia in young children with PWS as well as a high prevalence of diabetes in obese individuals with PWS (9). Regarding hypoglycemia, a recent chart review of 95 children with PWS reported that 13% had documented hypoglycemia, 11% had recurrent episodes, and 7% had their first hypoglycemic episode by the first day of life, prompting the investigators to propose increased blood glucose monitoring of infants with PWS (9). Especially concerning is the potential for this hypoglycemia to be underdiagnosed given its potential to facilitate hypotonia, feeding difficulty, and impaired neurocognitive function (9). In the present study, mean nonfasted blood glucose on postnatal day 17 was lower in both male and female Snord116del mice as compared with wild-type littermates [Fig. 4(a) and 4(b)]. Notably, it was even lower in Snord116del/ghrelin-KO male mice, although it remained within the normal range [Fig. 4(a)].
We also checked mice for hypotonia, which although prominent in PWS, was not obvious in Snord116del mice when checked previously using a wire-hanging test (60). Five-day-old mice performed the surface righting task equivalently regardless of genotype [Fig. 4(c)–4(f)].
Additionally, we assessed mortality, which, as mentioned, is increased in individuals with PWS, and also is increased in certain mouse models of PWS (72). Although not previously described for Snord116del mice, they exhibited a mortality during the first 28 days of life of 39%, which is increased as compared with 6% for wild-type littermates [Fig. 4(g)]. There was no significant effect of ghrelin deletion on survival of the Snord116del mice [Fig. 4(g)].
Studies with Snord116del/GHSR-null mice
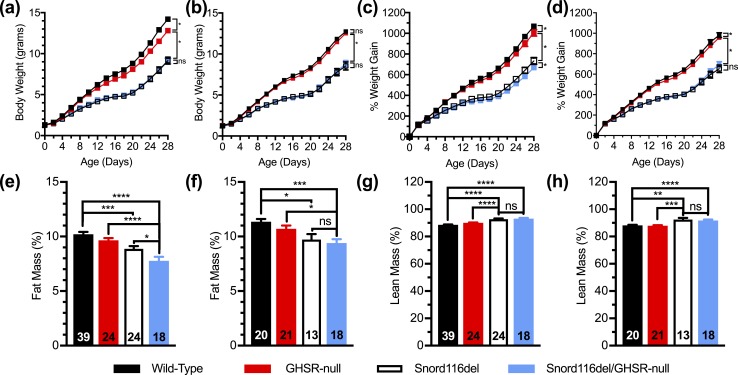
We next examined Snord116del mice on a GHSR-null background (Snord116del/GHSR-null) together with three control littermate groups. Similar to the findings with the Snord116del/ghrelin-KO cohort, both male and female Snord116del mice gained less body weight during the course of their initial 28 days of life than did wild-type littermates [Fig. 5(a)–(d)]. GHSR deletion slightly reduced the body weights of male wild-type mice [Fig. 5(a)] and slightly reduced percentage body weight gain for both male wild-type mice and male Snord116del mice [Fig. 5(d)]. There also was an effect of GHSR deletion to slightly further lower the percentage fat mass observed in 28-day-old male Snord116del mice [Fig. 5(e)]. This was not accompanied by an increase in percentage lean mass [Fig. 5(g)] nor did 28-day-old female Snord116del/GHSR-null mice exhibit differences in body composition from their Snord116del littermates [Fig. 5(f) and 5(h)].
Figure 5.
Effects of GHSR deletion on body weight, body weight gain, and body composition of Snord116del mice. (a–d) Body weights of (a) male and (b) female Snord116del/GHSR-null pups and three control littermate groups (wild-type, GHSR-null, and Snord116del) during the first 28 d of life, and body weight gain of (c) male and (d) female pups during the first 28 d of age. n = 19 to 43. (e–h) Percentage fat mass of (e) male and (f) female 28-d-old pups and percentage lean mass of the same (g) male and (h) female pups. n = 18 to 39, as indicated within the bars. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, (a–d) by repeated measures two-way ANOVA, followed by a Tukey post hoc analysis or (e–h) effect of genotype as assessed by one-way ANOVA, followed by a Tukey post hoc analysis. ns, not significant.
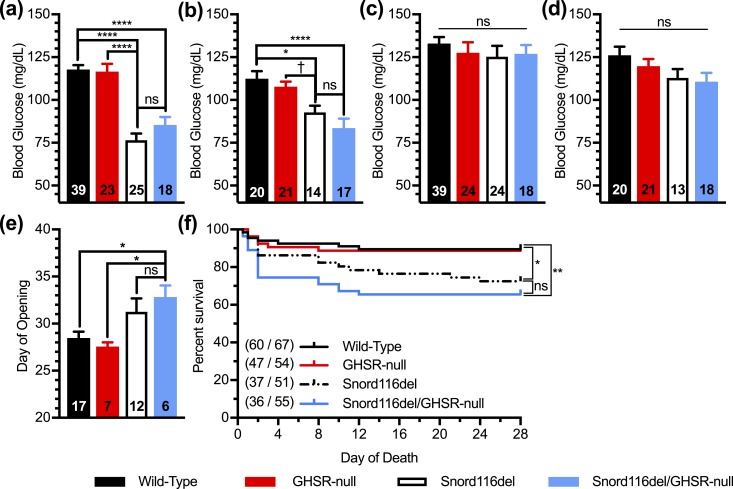
Nonfasted blood glucose levels in this cohort were checked on postnatal days 18 and 28. Just as observed in 17-day-old Snord116del mice in the Snord116del/ghrelin-KO cohort [Fig. 4(a) and 4(b)], the 18-day-old Snord116del mice from the Snord116del/GHSR-null cohort exhibited reduced blood glucose levels as compared with wild-type mice [Fig. 6(a) and 6(b)]. There was no effect of GHSR deletion to further lower blood glucose levels. At 28 days of age, these genotype-specific blood glucose differences were not apparent [Fig. 6(c) and 6(d)].
Figure 6.
Effects of GHSR deletion on blood glucose, sexual maturation, and survival of Snord116del mice. (a–d) Blood glucose levels of (a) male and (b) female 18-d-old pups and (c) male and (d) female 28-d-old pups. n = 14 to 39, as indicated within the bars. (e) Day of age of vaginal opening. n = 6 to 12, as indicated within the bars. (f) Survival curves of pups during the first 28 d of life. n = 36 to 60 survivors out of n = 51 to 67 pups. (Of note, the sexes of many of the neonates were not assessed prior to their demise, and thus these survival data include a combined population of males and females.) Curves were calculated by the Kaplan-Meier method. *P < 0.05, **P < 0.01, ****P < 0.0001, †0.05 ≤ P < 0.1, effect of genotype as assessed (a–e) by one-way ANOVA, followed by a Tukey post hoc analysis or (f) with comparisons among groups using the Mantel-Cox log-rank test. ns, not significant.
We also assessed sexual maturation in females. Whereas wild-type mice and GHSR-null mice exhibited vaginal opening on average on days 28 and 27, respectively, Snord116del mice displayed vaginal opening on average on day 31 [Fig. 6(e)], typical of the delay that had previously been reported (60), and that is similar to the deficiencies in sexual maturation observed in human subjects with PWS. Although Snord116del/GHSR-null mice exhibited vaginal opening on average 2 days later (on day 33), this was not significantly different than for Snord116del mice [Fig. 6(e)].
We again observed increased mortality in Snord116del mice during the first 28 days of life [27% in Snord116del mice as compared with 10% in wild-type mice; Fig. 6(f)]. Similar to that observed for ghrelin deletion [Fig. 4(g)], there was no significant effect of GHSR deletion on survival of the Snord116del mice [Fig. 6(f)].
Administration of GHSR agonist
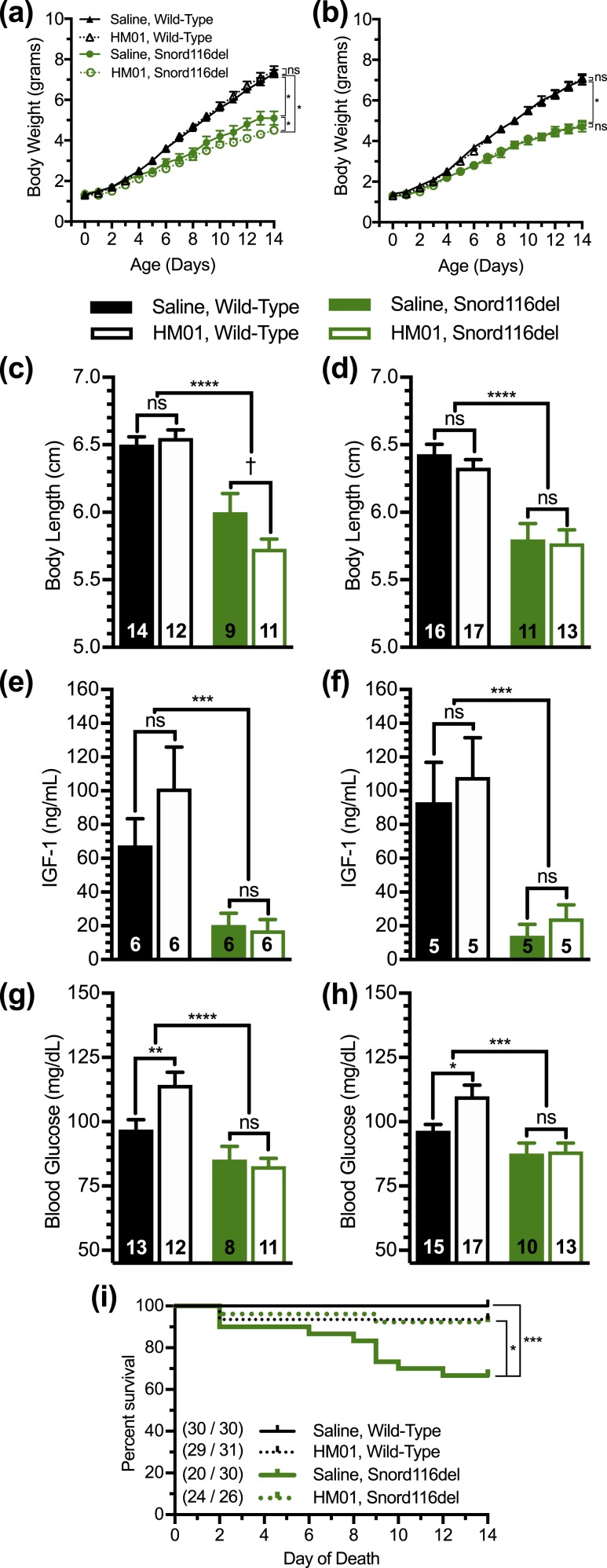
Finally, given the report of inefficient processing of proghrelin to bioactive ghrelin in induced pluripotent stem cell–derived neurons from subjects with PWS and in Snord116del mice (66), we sought to determine whether administration of the GHSR agonist HM01 could reduce morbidity and mortality in Snord116del neonates. HM01 is a partial GHSR agonist (with an Emax, or rather, maximum effect = 78% of the peak effect of acyl-ghrelin) that exhibits high GHSR binding affinity, high bioavailability, and high central nervous system permeability (73–75). Similar to acyl-ghrelin, HM01 activates intracellular calcium signaling in GHSR-containing cells, activates neurons of the arcuate nucleus and area postrema, promotes food intake, raises body weight, enhances gastrointestinal motility, and, in tumor models, attenuates cachexia (73–76). HM01, which has a longer plasma half-life than does acyl-ghrelin (74), was administered subcutaneously daily for 14 days to Snord116del neonates and wild-type littermates beginning at 1 day of age. This age range was chosen given the higher mortality observed in the above studies in Snord116del mice during the first 2 weeks of life than during weeks 3 to 4 [Figs. 4(g) and 6(f)]. Whereas the body weights of wild-type pups were unaffected by HM01 administration as compared with saline administration, the body weights of male Snord116del littermates administered HM01 were slightly lower than those given saline by the end of the 14-day protocol; HM01 did not affect body weights of female Snord116del pups [Fig. 7(a) and 7(b)]. Similarly, whereas the body (nose-to-anus) lengths upon completion of the 14-day protocol for wild-type pups were unaffected by HM01 administration as compared with saline administration, the body lengths of male Snord116del pups [which were significantly reduced as compared with wild-type littermates, as expected (60, 66)] administered HM01 were slightly lower than those given saline; HM01 did not affect body lengths of female Snord116del pups [Fig. 7(c) and 7(d)]. The end-of-study plasma IGF-1 levels of Snord116del mice were lower than those of wild-type mice, but they were unaffected by HM01 administration, in both male and female pups [Fig. 7(e) and 7(f)]. Although HM01 acutely raised blood glucose in both male and female wild-type mice when measured 1 hour following the final injection of the 14-day protocol, it had no effect on blood glucose in the Snord116del neonates [Fig. 7(g) and 7(h)].
Figure 7.
Effect of HM01 treatment on the phenotype of Snord116del pups. (a and b) Body weights of (a) male and (b) female Snord116del pups and wild-type littermates injected daily for 14 d with either HM01 or saline beginning on day 1 of age. n = 11 to 18. (c and d) Body lengths, (e and f) 1-h postinjection plasma IGF-1 levels, and (g and h) 1-h postinjection blood glucose levels in (c, e, and g) male and (d, f, and h) female pups on the final day of the injection protocol. n = 5 to 17, as indicated within the bars. (i) Survival curves of pups during the first 14 d of life. n = 20 to 30 survivors out of n = 26 to 31 pups. (By study design, no pups that died in the first 24 h of life were randomized to treatment, and thus they were excluded from the survival curves.) Curves were calculated by the Kaplan-Meier method. *P < 0.05, ***P < 0.001, ****P < 0.0001, †0.05 ≤ P < 0.1, by repeated measures two-way ANOVA, followed by (a and b) a Tukey post hoc analysis; effects of genotype and treatment as assessed by two-way ANOVA, followed by (c–h) a Tukey post hoc analysis; or (i) by comparisons among groups using the Mantel-Cox log-rank test. ns, not significant.
Also, we assessed mortality, recording deaths noted upon starting the HM01 vs saline injections (and not those that occurred prior to the first injection on day 1 of life). Mortality was greater in saline-treated Snord116del pups than in saline-treated wild-type littermates [Fig. 7(i)], just as had been observed in the previous two cohorts that received no treatments [Fig. 4(g) and 6(f)]. Strikingly, HM01 administration markedly reduced the mortality of Snord116del mice from 33% in the saline-treated group to just 8% [Fig. 7(i)]. The mortality of HM01-treated Snord116 neonates during the 14-day treatment period was nearly identical to that of HM01-treated wild-type neonates and not significantly different to that of saline-treated wild-type neonates [Fig. 7(i)].
Discussion
In this study, we used the Snord116del mouse model to investigate the functional significance of the ghrelin system in PWS, with a focus on modeling the neonatal period of the syndrome. Similar to previous studies, Snord116del pups exhibited reduced body weights, altered body composition (reduced percentage fat mass and increased percentage lean mass), reduced body lengths, reduced plasma IGF-1, and delayed sexual maturation, all of which emulate important clinical manifestations of PWS. Similar to the previous reports of elevated plasma ghrelin in older Snord116del mice and in human subjects with PWS, 28-day-old Snord116del neonates exhibited elevated plasma acyl-ghrelin (although plasma total ghrelin was equivalent to that of wild-type littermates). Although not previously reported in the Snord116del model, Snord116del pups 14 to 18 days of age exhibited relative hypoglycemia, which is reminiscent of the frank hypoglycemia described in many young children with PWS. Also, although not previously reported in the Snord116del model, Snord116del mice exhibited decreased survival during their first 28 days of life, similar to the decreased survival observed throughout the lifespan of human subjects with PWS.
Heading into this study, we had hypothesized that elevated acyl-ghrelin may be protective in the setting of PWS, just as has been proposed (77) to explain the behavior and functional role of the endogenous ghrelin system in rodent models of severe caloric restriction, cancer cachexia, and chronic psychosocial stress (33, 35–37, 78–81). Those latter three conditions are all associated with high plasma acyl-ghrelin, and rodent models of those conditions experience negative outcomes as a result of experimental induction of deficient or blocked ghrelin signaling, including reduced GH and life-threatening hypoglycemia, exaggerated reduction in lean mass and accelerated death, and exaggerated depressive-like behavior, respectively (35, 37, 77, 81). Were the ghrelin system to work similarly in PWS, secretion of acyl-ghrelin might rise, for instance, so as to engage its known orexigenic, glucoregulatory, muscle strength–promoting, and prosurvival actions. In turn, these actions presumably would help protect against what would otherwise be more severe forms of neonatal growth delay, failure to thrive, hypoglycemia, and hypotonia and more prevalent and accelerated mortality.
However, our current data indicate that ghrelin deletion and GHSR deletion only minimally affected the phenotypes associated with paternal Snord116 deletion. Only in male Snord116del/ghrelin-KO pups, but not in similarly aged female Snord116del/ghrelin-KO mice or in Snord116del/GHSR-null mice, was the relative hypoglycemia associated with paternal Snord116 deletion further exaggerated. Also, only in male Snord116del/GHSR-null pups, but not in similarly aged female Snord116del/GHSR-null mice or in Snord116del/ghrelin-KO mice, were the reductions in percentage body weight gain and percentage fat mass associated with paternal Snord116 deletion further exaggerated. Perhaps these limited effects suggest that the endogenous ghrelin system does not serve in a major protective capacity in PWS. Alternatively, perhaps genetic deletion of ghrelin and GHSR do not impact the effects of what could be already low plasma acyl-ghrelin levels in PWS. By way of explanation, the reported inefficiency of acyl-proghrelin processing to mature acyl-ghrelin (66) might result in low acyl-ghrelin levels in PWS [despite an overall high combined amount of plasma acyl-ghrelin plus acyl-proghrelin, as detected using available antisera (66)]. Low baseline plasma acyl-ghrelin presumably could already negatively affect metabolism, survival, and other features of PWS, such that complete removal of ghrelin or GHSR expression, as achieved by crossing the Snord116del mice onto ghrelin-KO or GHSR-null backgrounds, possibly would not further impact these processes in the setting of PWS. To test such a theory, the finding of decreased PC1/3 activity in PWS would need to be confirmed (66, 68) and, more importantly, a way to accurately measure plasma acyl-ghrelin (in which acyl-ghrelin could be distinguished from acyl-proghrelin) would need to be developed.
In contrast to the limited effects of placing Snord116del mice on ghrelin-KO or GHSR-null backgrounds, there was a striking effect of GHSR agonist administration to reduce the excess mortality observed in Snord116del neonates. In particular, this excess mortality was nearly completely rescued using the once daily, 2-week-long HM01 administration protocol. This survival-promoting effect of the pharmacologic GHSR agonist administration presumably is the result of a more robust engagement of GHSRs, which might be low at baseline in PWS due to inefficient processing of acyl-proghrelin (see discussion above). Certainly, in three mouse models with absent or markedly reduced levels of acyl-ghrelin [GOAT-KO mice (which lack the enzyme responsible for acylating ghrelin species), ghrelin-KO mice, and mice with ghrelin cell-selective deletion of β1-adrenergic receptors], replacement of acyl-ghrelin using continuous subcutaneous infusions prevented the severe hypoglycemia that developed over a week-long, daily exposure to just 40% of usual daily calories, while also rescuing the accompanying excess mortality (33, 35). Alternatively, assuming that the elevated plasma acyl-ghrelin detected in Snord116del mice does accurately reflect increased levels of acyl-ghrelin, it is still possible for the survival-promoting effect of HM01 observed in the Snord116del neonates to occur as a result of boosting already high engagement of GHSRs. Indeed, plasma acyl-ghrelin is elevated in several rodent models of cachexia and in patients with cachexia (81–90). Despite this, treatment with acyl-ghrelin or GHSR agonists limits drops in, or elevates, body weight and food intake, abrogates atrophy of hind leg muscles and reduced grip strength, and improves survival in several cachexia rodent models (30, 81, 91–94). The traditional herbal medicine rikkunshito, which stimulates acyl-ghrelin secretion, has similar effects in cachexia models, whereas in contrast, GHSR antagonist administration worsens anorexia and hastens death (81). In cancer cachexia patients, GHSR agonists increase lean body mass, handgrip strength, appetite, and quality of life (29, 95).
The effect of HM01 to markedly improve survival of Snord116del pups stands in contrast to its effects on blood glucose. Although nonfasted Snord116del mice 14 to 18 days of age exhibited lower blood glucose levels than did wild-type littermates, HM01 acutely raised blood glucose only in wild-type mice, but not in Snord116del mice. The effect in wild-type mice aligns with a substantial literature using non-PWS models supporting essential glucoregulatory roles for the ghrelin system, including an overall effect of acyl-ghrelin to frankly raise blood glucose or prevent life-threatening falls in blood glucose (36, 77). However, the absence of a blood glucose-raising effect of HM01 in the Snord116del pups (although such an effect is observed in wild-type controls) suggests an element of relative resistance to HM01’s glucose-raising capacity, as well as a separation of its glucoregulatory vs survival-promoting functions in the setting of PWS.
Exactly how HM01 was able to rescue the increased mortality of the Snord116del mice is not yet apparent, although it does not appear to involve improvements in body weight, body length, IGF-1, or blood glucose. Its survival-inducing effects, however, are reminiscent of another set of studies involving oxytocin administration to the Magel2-deficient mouse model of PWS (96, 97). Similar to Snord116del mice, these Magel2-deficient mice, which lack the paternal Magel2 gene within the PWS critical region, exhibit a high neonatal mortality (97). This high mortality (42% die before the mice reach postnatal day 2) is associated with a suckling/feeding deficit and can be prevented by a single subcutaneous injection of oxytocin 3 to 5 hours following birth (97). Daily oxytocin administration to Magel2-deficient pups during the first week of life also rescues their increased mortality and additionally restores their aberrant social behavior and spatial learning deficits when assessed 4 months later (96). Of interest, a link between acyl-ghrelin and oxytocin has been examined in the literature, including studies that indicate effects of acyl-ghrelin to directly activate oxytocin neurons and release oxytocin and effects of oxytocin to both modulate acyl-ghrelin activity and stimulate ghrelin release (98–102). Thus, although a relative oxytocin deficiency has been reported for PWS (97, 103), it may be reasonable for future studies to investigate whether oxytocin might contribute to HM01’s effects on survival as observed in the present study in Snord116del pups.
Of note, and as described in more detail above, although the neonatal growth delay, as well as the increased mortality and relative hypoglycemia in Snord116del mice, highlights their utility as a representative model for the overall phenotype present in the very young with PWS, a limitation of the Snord116del model is that it does not faithfully recapitulate the marked hyperphagia or obesity of later stages of PWS, nor does it appropriately model the hypotonia of PWS. Thus, although the possibility remains that ghrelin may impact hyperphagia, obesity, hypotonia-related morbidity, and other phenotypes characteristic of PWS, the Snord116del model is likely not the optimal way to definitively confirm such potential associations. Notwithstanding these caveats of the Snord116del model, advanced age Snord116del mice previously were shown to exhibit blunted responses to the acute anorexigenic effects of GHSR antagonists and a GHSR inverse agonist (67), suggesting that other, non-ghrelin–related mechanisms dictate their eating behaviors. Such was also the conclusion of two clinical trials in which octreotide administration to adolescents or adults with PWS was shown to blunt ghrelin release without effects on appetite, food intake, and/or body mass [albeit those trials were limited by small subject number and likely off-target suppression by octreotide or somatostatin of the release of other relevant feeding-related hormones (104, 105)]. In their study, Burnett et al. (66) argue that impaired prohormone processing of one or more of the several neuropeptides known to mediate food intake and energy expenditure—such as α-MSH, oxytocin, or BDNF (as opposed to the peripheral hormone ghrelin)—likely accounts for the hyperphagia and obesity of PWS.
It is also relevant to discuss the potential impact of the unacylated form of ghrelin (unacyl-ghrelin) in the setting of PWS. Both acyl-ghrelin and unacyl-ghrelin are secreted from ghrelin cells; degradation of secreted acyl-ghrelin likely also contributes to the circulating plasma unacyl-ghrelin pool (77). Although most studies agree that unacyl-ghrelin does not bind to GHSR, it nonetheless has been shown in some studies to exhibit some, presumably GHSR-independent, biological actions, including effects to reduce food intake and/or block the orexigenic effect of acyl-ghrelin (106–111). These effects on food intake are less consistently documented than the GHSR-dependent orexigenic effects of acyl-ghrelin. Nonetheless, with the potential anorexigenic actions of unacyl-ghrelin as a backdrop, some studies support the proposals that high plasma unacyl-ghrelin levels and corresponding low plasma acyl-ghrelin/unacyl-ghrelin ratios in infants with PWS contribute to the failure-to-thrive phenotype at this stage, whereas the switch to excessive weight gain in PWS coincides with an observed increase in the plasma acyl-ghrelin/plasma unacyl-ghrelin ratio (21, 25). Whether or not increased levels of endogenous unacyl-ghrelin contribute to the failure-to-thrive phenotype in youngsters with PWS, its purported anorexigenic actions have been taken advantage of pharmacologically in a recently reported clinical trial. AZP-531, an unacyl-ghrelin analog, was shown in a 2-week-long, proof-of-concept multicenter, randomized, double-blind, placebo-controlled trial of 47 adult patients with PWS to significantly improve food-related behaviors and reduce appetite (112). Alternatively, a recent clinical trial of intranasal oxytocin delivery to infants with PWS demonstrated not only improved oral feeding and social skills with oxytocin administration, but also a significant increase in plasma acyl-ghrelin, which the investigators implied may contribute to the overall efficacy of the compound (by counterbalancing the high unacyl-ghrelin, which was unaltered by oxytocin) (103).
As a concluding thought, based on the data presented in the present study, we now propose that enhancing GHSR signaling via administration of GHSR agonists similar to HM01 may represent an efficacious new treatment of young individuals with PWS, with an overall goal of enhancing survival. This strategy would benefit not only from GHSR engagement of GH-dependent pathways (reminiscent of the GH replacement regimens currently widely used for the treatment of PWS), but also from GHSR engagement of GH-independent pathways through which acyl-ghrelin is also known to act. Although the data in the current study do not address a causative role for elevated acyl-ghrelin in the hyperphagia and obesity of older individuals with PWS, even if such were to be the case, a therapeutic strategy could be envisioned in which GHSR agonist administration could be used early in PWS to enhance survival prior to the start of the hyperphagic nutritional phase.
Acknowledgments
We thank Connor Lawrence and Sydney Lawrence for technical assistance with animal breeding and blood and tissue collection; Dr. Robert Hammer from the UT Southwestern Medical Center Transgenic Technology Core Facility for help in generating the mouse lines for these studies; John Shelton from the UT Southwestern Medical Center Histo Pathology Core for assistance with paraffin embedding, tissue sectioning, and histology; Dr. Syann Lee from the UT Southwestern Mouse Metabolic Phenotyping Core for providing access to the EchoMRI-100 for body composition analyses; Dr. Joyce Repa for providing sequences for some primers; Dr. Perry Bickel from UT Southwestern Endocrinology for providing access to the quantitative RT-PCR system; and Robert Northrup from Helsinn Healthcare SA for helpful discussions.
Financial Support: This work was supported primarily through research grants from the Foundation for Prader-Willi Research; the Diana and Richard C. Strauss Professorship in Biomedical Research; the Mr. and Mrs. Bruce G. Brookshire Professorship in Medicine; the Kent and Jodi Foster Distinguished Chair in Endocrinology, in Honor of Daniel Foster, MD; and institutional funds from the UT Southwestern Medical Center (to J.M.Z.), with additional support from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (Grants R01 DK103884-03S1 to J.M.Z., R01 DK114036 to C.L., and F32 DK103449 to C.C.L.), the American Heart Association (Grant AHA 16SDG27260001 to C.L.), the UT Southwestern Medical Student Summer Research Program (to H.L.V.), and the Hilda and Preston Davis Foundation Postdoctoral Fellowship Program in Eating Disorders Research (to B.K.M.).
Author Contributions: J.A.R., E.C.B., B.K.M., C.P., and J.M.Z. contributed to the experimental concept and design. J.A.R., E.C.B., B.K.M., S.O.-L., H.F.R., P.V., N.P.M., D.G., H.L.V., C.C.L., K.S. and C.L. performed experiments. J.A.R., E.C.B., B.K.M., and J.M.Z. analyzed and critically discussed the data. J.A.R., E.C.B., B.K.M., S.O.-L. and J.M.Z. created figures and tables for publication. C.P. supplied the HM01 compound and reviewed the manuscript. J.M.Z. wrote the manuscript with input from J.A.R., B.K.M., E.C.B., and S.O.-L.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- acyl-ghrelin
acylated form of ghrelin
- acyl-proghrelin
acylated form of proghrelin
- GHSR
GH secretagogue receptor
- KO
knockout
- PC1/3
prohormone convertase 1/3
- PWS
Prader-Willi Syndrome
- unacyl-ghrelin
unacylated form of ghrelin
References
- 1. Resnick JL, Nicholls RD, Wevrick R; Prader-Willi Syndrome Animal Models Working Group . Recommendations for the investigation of animal models of Prader-Willi syndrome. Mamm Genome. 2013;24(5–6):165–178. [DOI] [PubMed] [Google Scholar]
- 2. Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012;14(1):10–26. [DOI] [PubMed] [Google Scholar]
- 3. Miller JL. Approach to the child with Prader-Willi syndrome. J Clin Endocrinol Metab. 2012;97(11):3837–3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldstone AP. Prader-Willi syndrome: advances in genetics, pathophysiology and treatment. Trends Endocrinol Metab. 2004;15(1):12–20. [DOI] [PubMed] [Google Scholar]
- 5. Holm VA, Cassidy SB, Butler MG, Hanchett JM, Greenswag LR, Whitman BY, Greenberg F. Prader-Willi syndrome: consensus diagnostic criteria. Pediatrics. 1993;91(2):398–402. [PMC free article] [PubMed] [Google Scholar]
- 6. Rodriguez JA, Zigman JM. Hypothalamic loss of Snord116 and Prader-Willi syndrome hyperphagia: the buck stops here? J Clin Invest. 2018;128(3):900–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shim JS, Lee SH, Seo SW, Koo KH, Jin DK. The musculoskeletal manifestations of Prader-Willi syndrome. J Pediatr Orthop. 2010;30(4):390–395. [DOI] [PubMed] [Google Scholar]
- 8. de Lind van Wijngaarden RF, de Klerk LW, Festen DA, Duivenvoorden HJ, Otten BJ, Hokken-Koelega AC. Randomized controlled trial to investigate the effects of growth hormone treatment on scoliosis in children with Prader-Willi syndrome. J Clin Endocrinol Metab. 2009;94(4):1274–1280. [DOI] [PubMed] [Google Scholar]
- 9. Harrington RA, Weinstein DA, Miller JL. Hypoglycemia in Prader-Willi syndrome. Am J Med Genet A. 2014;164(5):1127–1129. [DOI] [PubMed] [Google Scholar]
- 10. Skokauskas N, Sweeny E, Meehan J, Gallagher L. Mental health problems in children with Prader-Willi syndrome. J Can Acad Child Adolesc Psychiatry. 2012;21(3):194–203. [PMC free article] [PubMed] [Google Scholar]
- 11. Sinnema M, Boer H, Collin P, Maaskant MA, van Roozendaal KE, Schrander-Stumpel CT, Curfs LM. Psychiatric illness in a cohort of adults with Prader-Willi syndrome. Res Dev Disabil. 2011;32(5):1729–1735. [DOI] [PubMed] [Google Scholar]
- 12. Whittington JE, Holland AJ, Webb T, Butler J, Clarke D, Boer H. Population prevalence and estimated birth incidence and mortality rate for people with Prader-Willi syndrome in one UK Health Region. J Med Genet. 2001;38(11):792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Einfeld SL, Kavanagh SJ, Smith A, Evans EJ, Tonge BJ, Taffe J. Mortality in Prader-Willi syndrome. Am J Ment Retard. 2006;111(3):193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS, Schwartz MW, Basdevant A, Weigle DS. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med. 2002;8(7):643–644. [DOI] [PubMed] [Google Scholar]
- 15. DelParigi A, Tschöp M, Heiman ML, Salbe AD, Vozarova B, Sell SM, Bunt JC, Tataranni PA. High circulating ghrelin: a potential cause for hyperphagia and obesity in Prader-Willi syndrome. J Clin Endocrinol Metab. 2002;87(12):5461–5464. [DOI] [PubMed] [Google Scholar]
- 16. Haqq AM, Farooqi IS, O’Rahilly S, Stadler DD, Rosenfeld RG, Pratt KL, LaFranchi SH, Purnell JQ. Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader-Willi syndrome. J Clin Endocrinol Metab. 2003;88(1):174–178. [DOI] [PubMed] [Google Scholar]
- 17. Feigerlová E, Diene G, Conte-Auriol F, Molinas C, Gennero I, Salles JP, Arnaud C, Tauber M. Hyperghrelinemia precedes obesity in Prader-Willi syndrome. J Clin Endocrinol Metab. 2008;93(7):2800–2805. [DOI] [PubMed] [Google Scholar]
- 18. Bizzarri C, Rigamonti AE, Luce A, Cappa M, Cella SG, Berini J, Sartorio A, Müller EE, Salvatoni A. Children with Prader-Willi syndrome exhibit more evident meal-induced responses in plasma ghrelin and peptide YY levels than obese and lean children. Eur J Endocrinol. 2010;162(3):499–505. [DOI] [PubMed] [Google Scholar]
- 19. Giménez-Palop O, Giménez-Pérez G, Mauricio D, González-Clemente JM, Potau N, Berlanga E, Trallero R, Laferrère B, Caixàs A. A lesser postprandial suppression of plasma ghrelin in Prader-Willi syndrome is associated with low fasting and a blunted postprandial PYY response. Clin Endocrinol (Oxf). 2007;66(2):198–204. [DOI] [PubMed] [Google Scholar]
- 20. Goldstone AP, Patterson M, Kalingag N, Ghatei MA, Brynes AE, Bloom SR, Grossman AB, Korbonits M. Fasting and postprandial hyperghrelinemia in Prader-Willi syndrome is partially explained by hypoinsulinemia, and is not due to peptide YY3-36 deficiency or seen in hypothalamic obesity due to craniopharyngioma. J Clin Endocrinol Metab. 2005;90(5):2681–2690. [DOI] [PubMed] [Google Scholar]
- 21. Kuppens RJ, Diène G, Bakker NE, Molinas C, Faye S, Nicolino M, Bernoux D, Delhanty PJ, van der Lely AJ, Allas S, Julien M, Delale T, Tauber M, Hokken-Koelega AC. Elevated ratio of acylated to unacylated ghrelin in children and young adults with Prader-Willi syndrome. Endocrine. 2015;50(3):633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kweh FA, Miller JL, Sulsona CR, Wasserfall C, Atkinson M, Shuster JJ, Goldstone AP, Driscoll DJ. Hyperghrelinemia in Prader-Willi syndrome begins in early infancy long before the onset of hyperphagia. Am J Med Genet A. 2015;167(1):69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Senda M, Ogawa S, Nako K, Okamura M, Sakamoto T, Ito S. The glucagon-like peptide-1 analog liraglutide suppresses ghrelin and controls diabetes in a patient with Prader-Willi syndrome. Endocr J. 2012;59(10):889–894. [DOI] [PubMed] [Google Scholar]
- 24. Purtell L, Sze L, Loughnan G, Smith E, Herzog H, Sainsbury A, Steinbeck K, Campbell LV, Viardot A. In adults with Prader-Willi syndrome, elevated ghrelin levels are more consistent with hyperphagia than high PYY and GLP-1 levels. Neuropeptides. 2011;45(4):301–307. [DOI] [PubMed] [Google Scholar]
- 25. Beauloye V, Diene G, Kuppens R, Zech F, Winandy C, Molinas C, Faye S, Kieffer I, Beckers D, Nergårdh R, Hauffa B, Derycke C, Delhanty P, Hokken-Koelega A, Tauber M. High unacylated ghrelin levels support the concept of anorexia in infants with Prader-Willi syndrome. Orphanet J Rare Dis. 2016;11(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Müller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, Casanueva FF, D’Alessio D, Depoortere I, Geliebter A, Ghigo E, Cole PA, Cowley M, Cummings DE, Dagher A, Diano S, Dickson SL, Diéguez C, Granata R, Grill HJ, Grove K, Habegger KM, Heppner K, Heiman ML, Holsen L, Holst B, Inui A, Jansson JO, Kirchner H, Korbonits M, Laferrère B, LeRoux CW, Lopez M, Morin S, Nakazato M, Nass R, Perez-Tilve D, Pfluger PT, Schwartz TW, Seeley RJ, Sleeman M, Sun Y, Sussel L, Tong J, Thorner MO, van der Lely AJ, van der Ploeg LH, Zigman JM, Kojima M, Kangawa K, Smith RG, Horvath T, Tschöp MH. Ghrelin. Mol Metab. 2015;4(6):437–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perello M, Dickson SL. Ghrelin signalling on food reward: a salient link between the gut and the mesolimbic system. J Neuroendocrinol. 2015;27(6):424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steculorum SM, Collden G, Coupe B, Croizier S, Lockie S, Andrews ZB, Jarosch F, Klussmann S, Bouret SG. Neonatal ghrelin programs development of hypothalamic feeding circuits. J Clin Invest. 2015;125(2):846–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garcia JM, Boccia RV, Graham CD, Yan Y, Duus EM, Allen S, Friend J. Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials. Lancet Oncol. 2015;16(1):108–116. [DOI] [PubMed] [Google Scholar]
- 30. Chen JA, Splenser A, Guillory B, Luo J, Mendiratta M, Belinova B, Halder T, Zhang G, Li YP, Garcia JM. Ghrelin prevents tumour- and cisplatin-induced muscle wasting: characterization of multiple mechanisms involved. J Cachexia Sarcopenia Muscle. 2015;6(2):132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. [DOI] [PubMed] [Google Scholar]
- 32. Chuang JC, Sakata I, Kohno D, Perello M, Osborne-Lawrence S, Repa JJ, Zigman JM. Ghrelin directly stimulates glucagon secretion from pancreatic α-cells. Mol Endocrinol. 2011;25(9):1600–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mani BK, Osborne-Lawrence S, Vijayaraghavan P, Hepler C, Zigman JM. β1-Adrenergic receptor deficiency in ghrelin-expressing cells causes hypoglycemia in susceptible individuals. J Clin Invest. 2016;126(9):3467–3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dezaki K, Sone H, Yada T. Ghrelin is a physiological regulator of insulin release in pancreatic islets and glucose homeostasis. Pharmacol Ther. 2008;118(2):239–249. [DOI] [PubMed] [Google Scholar]
- 35. Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL, Brown MS. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA. 2010;107(16):7467–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li RL, Sherbet DP, Elsbernd BL, Goldstein JL, Brown MS, Zhao TJ. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. J Biol Chem. 2012;287(22):17942–17950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11(7):752–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, Zigman JM. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest. 2011;121(7):2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Walker AK, Rivera PD, Wang Q, Chuang JC, Tran S, Osborne-Lawrence S, Estill SJ, Starwalt R, Huntington P, Morlock L, Naidoo J, Williams NS, Ready JM, Eisch AJ, Pieper AA, Zigman JM. The P7C3 class of neuroprotective compounds exerts antidepressant efficacy in mice by increasing hippocampal neurogenesis. Mol Psychiatry. 2015;20(4):500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chuang JC, Zigman JM. Ghrelin’s roles in stress, mood, and anxiety regulation. Int J Pept. 2010;2010:460549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jensen M, Ratner C, Rudenko O, Christiansen SH, Skov LJ, Hundahl C, Woldbye DP, Holst B. Anxiolytic-like effects of increased ghrelin receptor signaling in the amygdala. Int J Neuropsychopharmacol. 2016;19(5):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spencer SJ, Emmerzaal TL, Kozicz T, Andrews ZB. Ghrelin’s role in the hypothalamic-pituitary-adrenal axis stress response: implications for mood disorders. Biol Psychiatry. 2015;78(1):19–27. [DOI] [PubMed] [Google Scholar]
- 43. Ding F, Prints Y, Dhar MS, Johnson DK, Garnacho-Montero C, Nicholls RD, Francke U. Lack of Pwcr1/MBII-85 snoRNA is critical for neonatal lethality in Prader-Willi syndrome mouse models. Mamm Genome. 2005;16(6):424–431. [DOI] [PubMed] [Google Scholar]
- 44. Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115(12):3564–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang Q, Liu C, Uchida A, Chuang JC, Walker A, Liu T, Osborne-Lawrence S, Mason BL, Mosher C, Berglund ED, Elmquist JK, Zigman JM. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol Metab. 2014;3(1):64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zigman JM, Rodriguez JA. Data from: Oligonucleotide primer sequences. UT Southwestern Institutional Repository 2018. Deposited 6 September 2018.http://hdl.handle.net/2152.5/5772.
- 48.RRID:AB_2232479.
- 49.RRID:AB_2534102.
- 50.RRID:AB_2336787.
- 51. Sakata I, Nakano Y, Osborne-Lawrence S, Rovinsky SA, Lee CE, Perello M, Anderson JG, Coppari R, Xiao G, Lowell BB, Elmquist JK, Zigman JM. Characterization of a novel ghrelin cell reporter mouse. Regul Pept. 2009;155(1–3):91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sun Y, Butte NF, Garcia JM, Smith RG. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology. 2008;149(2):843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, Moncrieffe M, Thabet K, Cox HJ, Yancopoulos GD, Wiegand SJ, Sleeman MW. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci USA. 2004;101(21):8227–8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wortley KE, del Rincon JP, Murray JD, Garcia K, Iida K, Thorner MO, Sleeman MW. Absence of ghrelin protects against early-onset obesity. J Clin Invest. 2005;115(12):3573–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Abizaid A, Mineur YS, Roth RH, Elsworth JD, Sleeman MW, Picciotto MR, Horvath TL. Reduced locomotor responses to cocaine in ghrelin-deficient mice. Neuroscience. 2011;192:500–506. [DOI] [PubMed] [Google Scholar]
- 56. Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschöp MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9(3):381–388. [DOI] [PubMed] [Google Scholar]
- 57. Sun Y, Asnicar M, Saha PK, Chan L, Smith RG. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab. 2006;3(5):379–386. [DOI] [PubMed] [Google Scholar]
- 58. Caligioni CS. doi: 10.1002/0471142301.nsa04is48. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009;48(1):A.4I.1–A.4I.8. [DOI] [PMC free article] [PubMed]
- 59. Lone AM, Leidl M, McFedries AK, Horner JW, Creemers J, Saghatelian A. Deletion of PREPl causes growth impairment and hypotonia in mice. PLoS One. 2014;9(2):e89160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ding F, Li HH, Zhang S, Solomon NM, Camper SA, Cohen P, Francke U. SnoRNA Snord116 (Pwcr1/MBII-85) deletion causes growth deficiency and hyperphagia in mice. PLoS One. 2008;3(3):e1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bieth E, Eddiry S, Gaston V, Lorenzini F, Buffet A, Conte Auriol F, Molinas C, Cailley D, Rooryck C, Arveiler B, Cavaille J, Salles JP, Tauber M. Highly restricted deletion of the SNORD116 region is implicated in Prader-Willi Syndrome. Eur J Hum Genet. 2015;23(2):252–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sahoo T, del Gaudio D, German JR, Shinawi M, Peters SU, Person RE, Garnica A, Cheung SW, Beaudet AL. Prader-Willi phenotype caused by paternal deficiency for the HBII-85 C/D box small nucleolar RNA cluster. Nat Genet. 2008;40(6):719–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Duker AL, Ballif BC, Bawle EV, Person RE, Mahadevan S, Alliman S, Thompson R, Traylor R, Bejjani BA, Shaffer LG, Rosenfeld JA, Lamb AN, Sahoo T. Paternally inherited microdeletion at 15q11.2 confirms a significant role for the SNORD116 C/D box snoRNA cluster in Prader-Willi syndrome. Eur J Hum Genet. 2010;18(11):1196–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim SJ, Miller JL, Kuipers PJ, German JR, Beaudet AL, Sahoo T, Driscoll DJ. Unique and atypical deletions in Prader-Willi syndrome reveal distinct phenotypes. Eur J Hum Genet. 2012;20(3):283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. de Smith AJ, Purmann C, Walters RG, Ellis RJ, Holder SE, Van Haelst MM, Brady AF, Fairbrother UL, Dattani M, Keogh JM, Henning E, Yeo GS, O’Rahilly S, Froguel P, Farooqi IS, Blakemore AI. A deletion of the HBII-85 class of small nucleolar RNAs (snoRNAs) is associated with hyperphagia, obesity and hypogonadism. Hum Mol Genet. 2009;18(17):3257–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Burnett LC, LeDuc CA, Sulsona CR, Paull D, Rausch R, Eddiry S, Carli JF, Morabito MV, Skowronski AA, Hubner G, Zimmer M, Wang L, Day R, Levy B, Fennoy I, Dubern B, Poitou C, Clement K, Butler MG, Rosenbaum M, Salles JP, Tauber M, Driscoll DJ, Egli D, Leibel RL. Deficiency in prohormone convertase PC1 impairs prohormone processing in Prader-Willi syndrome. J Clin Invest. 2017;127(1):293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lin D, Wang Q, Ran H, Liu K, Wang Y, Wang J, Liu Y, Chen R, Sun Y, Liu R, Ding F. Abnormal response to the anorexic effect of GHS-R inhibitors and exenatide in male Snord116 deletion mouse model for Prader-Willi syndrome. Endocrinology. 2014;155(7):2355–2362. [DOI] [PubMed] [Google Scholar]
- 68. Polex-Wolf J, Lam BY, Larder R, Tadross J, Rimmington D, Bosch F, Cenzano VJ, Ayuso E, Ma MK, Rainbow K, Coll AP, O’Rahilly S, Yeo GS. Hypothalamic loss of Snord116 recapitulates the hyperphagia of Prader-Willi syndrome. J Clin Invest. 2018;128(3):960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Qi Y, Purtell L, Fu M, Lee NJ, Aepler J, Zhang L, Loh K, Enriquez RF, Baldock PA, Zolotukhin S, Campbell LV, Herzog H. Snord116 is critical in the regulation of food intake and body weight. Sci Rep. 2016;6(1):18614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Qi Y, Purtell L, Fu M, Sengmany K, Loh K, Zhang L, Zolotukhin S, Sainsbury A, Campbell L, Herzog H. Ambient temperature modulates the effects of the Prader-Willi syndrome candidate gene Snord116 on energy homeostasis. Neuropeptides. 2017;61:87–93. [DOI] [PubMed] [Google Scholar]
- 71. Khor EC, Fanshawe B, Qi Y, Zolotukhin S, Kulkarni RN, Enriquez RF, Purtell L, Lee NJ, Wee NK, Croucher PI, Campbell L, Herzog H, Baldock PA. Prader-Willi critical region, a non-translated, imprinted central regulator of bone mass: possible role in skeletal abnormalities in Prader-Willi syndrome. PLoS One. 2016;11(1):e0148155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim Y, Lee HM, Xiong Y, Sciaky N, Hulbert SW, Cao X, Everitt JI, Jin J, Roth BL, Jiang YH. Targeting the histone methyltransferase G9a activates imprinted genes and improves survival of a mouse model of Prader-Willi syndrome. Nat Med. 2017;23(2):213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Naitou K, Mamerto TP, Pustovit RV, Callaghan B, Rivera LR, Chan AJ, Ringuet MT, Pietra C, Furness JB. Site and mechanism of the colokinetic action of the ghrelin receptor agonist, HM01. Neurogastroenterol Motil. 2015;27(12):1764–1771. [DOI] [PubMed] [Google Scholar]
- 74. Karasawa H, Pietra C, Giuliano C, Garcia-Rubio S, Xu X, Yakabi S, Tache Y, Wang L. New ghrelin agonist, HM01 alleviates constipation and L-dopa-delayed gastric emptying in 6-hydroxydopamine rat model of Parkinson's disease. Neurogastroenterol Motil. 2014;26(12):1771–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Villars FO, Pietra C, Giuliano C, Lutz TA, Riediger T. Oral treatment with the ghrelin receptor agonist HM01 attenuates cachexia in mice bearing colon-26 (C26) tumors. Int J Mol Sci. 2017;18(5):986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Borner T, Loi L, Pietra C, Giuliano C, Lutz TA, Riediger T. The ghrelin receptor agonist HM01 mimics the neuronal effects of ghrelin in the arcuate nucleus and attenuates anorexia-cachexia syndrome in tumor-bearing rats. Am J Physiol Regul Integr Comp Physiol. 2016;311(1):R89–R96. [DOI] [PubMed] [Google Scholar]
- 77. Mani BK, Zigman JM. Ghrelin as a survival hormone. Trends Endocrinol Metab. 2017;28(12):843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. McFarlane MR, Brown MS, Goldstein JL, Zhao TJ. Induced ablation of ghrelin cells in adult mice does not decrease food intake, body weight, or response to high-fat diet. Cell Metab. 2014;20(1):54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang Y, Fang F, Goldstein JL, Brown MS, Zhao TJ. Reduced autophagy in livers of fasted, fat-depleted, ghrelin-deficient mice: reversal by growth hormone. Proc Natl Acad Sci USA. 2015;112(4):1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ge X, Yang H, Bednarek MA, Galon-Tilleman H, Chen P, Chen M, Lichtman JS, Wang Y, Dalmas O, Yin Y, Tian H, Jermutus L, Grimsby J, Rondinone CM, Konkar A, Kaplan DD. LEAP2 is an endogenous antagonist of the ghrelin receptor. Cell Metab. 2018;27(2):461–469.e6. [DOI] [PubMed] [Google Scholar]
- 81. Fujitsuka N, Asakawa A, Uezono Y, Minami K, Yamaguchi T, Niijima A, Yada T, Maejima Y, Sedbazar U, Sakai T, Hattori T, Kase Y, Inui A. Potentiation of ghrelin signaling attenuates cancer anorexia-cachexia and prolongs survival. Transl Psychiatry. 2011;1(7):e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. DeBoer MD. Ghrelin and cachexia: will treatment with GHSR-1a agonists make a difference for patients suffering from chronic wasting syndromes? Mol Cell Endocrinol. 2011;340(1):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nagaya N, Uematsu M, Kojima M, Date Y, Nakazato M, Okumura H, Hosoda H, Shimizu W, Yamagishi M, Oya H, Koh H, Yutani C, Kangawa K. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation. 2001;104(17):2034–2038. [DOI] [PubMed] [Google Scholar]
- 84. Itoh T, Nagaya N, Yoshikawa M, Fukuoka A, Takenaka H, Shimizu Y, Haruta Y, Oya H, Yamagishi M, Hosoda H, Kangawa K, Kimura H. Elevated plasma ghrelin level in underweight patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(8):879–882. [DOI] [PubMed] [Google Scholar]
- 85. Uzum AK, Aydin MM, Tutuncu Y, Omer B, Kiyan E, Alagol F. Serum ghrelin and adiponectin levels are increased but serum leptin level is unchanged in low weight chronic obstructive pulmonary disease patients. Eur J Intern Med. 2014;25(4):364–369. [DOI] [PubMed] [Google Scholar]
- 86. Bossola M, Scribano D, Colacicco L, Tavazzi B, Giungi S, Zuppi C, Luciani G, Tazza L. Anorexia and plasma levels of free tryptophan, branched chain amino acids, and ghrelin in hemodialysis patients. J Ren Nutr. 2009;19(3):248–255. [DOI] [PubMed] [Google Scholar]
- 87. Xu Z, Wu W, Zhang X, Liu G. Endogenous ghrelin increases in adriamycin-induced heart failure rats. J Endocrinol Invest. 2007;30(2):117–125. [DOI] [PubMed] [Google Scholar]
- 88. Garcia JM, Garcia-Touza M, Hijazi RA, Taffet G, Epner D, Mann D, Smith RG, Cunningham GR, Marcelli M. Active ghrelin levels and active to total ghrelin ratio in cancer-induced cachexia. J Clin Endocrinol Metab. 2005;90(5):2920–2926. [DOI] [PubMed] [Google Scholar]
- 89. Hanada T, Toshinai K, Date Y, Kajimura N, Tsukada T, Hayashi Y, Kangawa K, Nakazato M. Upregulation of ghrelin expression in cachectic nude mice bearing human melanoma cells. Metabolism. 2004;53(1):84–88. [DOI] [PubMed] [Google Scholar]
- 90. Hanada T, Toshinai K, Kajimura N, Nara-Ashizawa N, Tsukada T, Hayashi Y, Osuye K, Kangawa K, Matsukura S, Nakazato M. Anti-cachectic effect of ghrelin in nude mice bearing human melanoma cells. Biochem Biophys Res Commun. 2003;301(2):275–279. [DOI] [PubMed] [Google Scholar]
- 91. Wang W, Andersson M, Iresjö BM, Lönnroth C, Lundholm K. Effects of ghrelin on anorexia in tumor-bearing mice with eicosanoid-related cachexia. Int J Oncol. 2006;28(6):1393–1400. [PubMed] [Google Scholar]
- 92. Chance WT, Dayal R, Friend LA, Thomas I, Sheriff S. Continuous intravenous infusion of ghrelin does not stimulate feeding in tumor-bearing rats. Nutr Cancer. 2008;60(1):75–90. [DOI] [PubMed] [Google Scholar]
- 93. DeBoer MD, Zhu XX, Levasseur P, Meguid MM, Suzuki S, Inui A, Taylor JE, Halem HA, Dong JZ, Datta R, Culler MD, Marks DL. Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model of cancer cachexia. Endocrinology. 2007;148(6):3004–3012. [DOI] [PubMed] [Google Scholar]
- 94. Tsubouchi H, Yanagi S, Miura A, Matsumoto N, Kangawa K, Nakazato M. Ghrelin relieves cancer cachexia associated with the development of lung adenocarcinoma in mice. Eur J Pharmacol. 2014;743:1–10. [DOI] [PubMed] [Google Scholar]
- 95. Garcia JM, Friend J, Allen S. Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer-related cachexia: a multicenter, randomized, double-blind, crossover, pilot study. Support Care Cancer. 2013;21(1):129–137. [DOI] [PubMed] [Google Scholar]
- 96. Meziane H, Schaller F, Bauer S, Villard C, Matarazzo V, Riet F, Guillon G, Lafitte D, Desarmenien MG, Tauber M, Muscatelli F. An early postnatal oxytocin treatment prevents social and learning deficits in adult mice deficient for Magel2, a gene involved in Prader-Willi syndrome and autism. Biol Psychiatry. 2015;78(2):85–94. [DOI] [PubMed] [Google Scholar]
- 97. Schaller F, Watrin F, Sturny R, Massacrier A, Szepetowski P, Muscatelli F. A single postnatal injection of oxytocin rescues the lethal feeding behaviour in mouse newborns deficient for the imprinted Magel2 gene. Hum Mol Genet. 2010;19(24):4895–4905. [DOI] [PubMed] [Google Scholar]
- 98. Olszewski PK, Bomberg EM, Martell A, Grace MK, Levine AS. Intraventricular ghrelin activates oxytocin neurons: implications in feeding behavior. Neuroreport. 2007;18(5):499–503. [DOI] [PubMed] [Google Scholar]
- 99. Koyama H, Iwakura H, Dote K, Bando M, Hosoda H, Ariyasu H, Kusakabe T, Son C, Hosoda K, Akamizu T, Kangawa K, Nakao K. Comprehensive profiling of GPCR expression in ghrelin-producing cells. Endocrinology. 2016;157(2):692–704. [DOI] [PubMed] [Google Scholar]
- 100. Iwakura H, Ariyasu H, Hosoda H, Yamada G, Hosoda K, Nakao K, Kangawa K, Akamizu T. Oxytocin and dopamine stimulate ghrelin secretion by the ghrelin-producing cell line, MGN3-1 in vitro. Endocrinology. 2011;152(7):2619–2625. [DOI] [PubMed] [Google Scholar]
- 101. Dos Santos RC, Grover HM, Reis LC, Ferguson AV, Mecawi AS. Electrophysiological effects of ghrelin in the hypothalamic paraventricular nucleus neurons. Front Cell Neurosci. 2018;12:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gálfi M, Radács M, Molnár Z, Budai I, Tóth G, Pósa A, Kupai K, Szalai Z, Szabó R, Molnár HA, Gardi J, László FA, Varga C. Ghrelin-induced enhancement of vasopressin and oxytocin secretion in rat neurohypophyseal cell cultures. J Mol Neurosci. 2016;60(4):525–530. [DOI] [PubMed] [Google Scholar]
- 103. Tauber M, Boulanouar K, Diene G, Çabal-Berthoumieu S, Ehlinger V, Fichaux-Bourin P, Molinas C, Faye S, Valette M, Pourrinet J, Cessans C, Viaux-Sauvelon S, Bascoul C, Guedeney A, Delhanty P, Geenen V, Martens H, Muscatelli F, Cohen D, Consoli A, Payoux P, Arnaud C, Salles JP. The use of oxytocin to improve feeding and social skills in infants with Prader-Willi syndrome. Pediatrics. 2017;139(2):e20162976. [DOI] [PubMed] [Google Scholar]
- 104. De Waele K, Ishkanian SL, Bogarin R, Miranda CA, Ghatei MA, Bloom SR, Pacaud D, Chanoine JP. Long-acting octreotide treatment causes a sustained decrease in ghrelin concentrations but does not affect weight, behaviour and appetite in subjects with Prader-Willi syndrome. Eur J Endocrinol. 2008;159(4):381–388. [DOI] [PubMed] [Google Scholar]
- 105. Tan TM, Vanderpump M, Khoo B, Patterson M, Ghatei MA, Goldstone AP. Somatostatin infusion lowers plasma ghrelin without reducing appetite in adults with Prader-Willi syndrome. J Clin Endocrinol Metab. 2004;89(8):4162–4165. [DOI] [PubMed] [Google Scholar]
- 106. Inhoff T, Wiedenmann B, Klapp BF, Mönnikes H, Kobelt P. Is desacyl ghrelin a modulator of food intake? Peptides. 2009;30(5):991–994. [DOI] [PubMed] [Google Scholar]
- 107. Delhanty PJ, Neggers SJ, van der Lely AJ. Des-acyl ghrelin: a metabolically active peptide. Endocr Dev. 2013;25:112–121. [DOI] [PubMed] [Google Scholar]
- 108. Asakawa A, Inui A, Fujimiya M, Sakamaki R, Shinfuku N, Ueta Y, Meguid MM, Kasuga M. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut. 2005;54(1):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chen CY, Inui A, Asakawa A, Fujino K, Kato I, Chen CC, Ueno N, Fujimiya M. Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology. 2005;129(1):8–25. [DOI] [PubMed] [Google Scholar]
- 110. Matsuda K, Miura T, Kaiya H, Maruyama K, Shimakura S, Uchiyama M, Kangawa K, Shioda S. Regulation of food intake by acyl and des-acyl ghrelins in the goldfish. Peptides. 2006;27(9):2321–2325. [DOI] [PubMed] [Google Scholar]
- 111. Inhoff T, Mönnikes H, Noetzel S, Stengel A, Goebel M, Dinh QT, Riedl A, Bannert N, Wisser AS, Wiedenmann B, Klapp BF, Taché Y, Kobelt P. Desacyl ghrelin inhibits the orexigenic effect of peripherally injected ghrelin in rats. Peptides. 2008;29(12):2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Allas S, Caixàs A, Poitou C, Coupaye M, Thuilleaux D, Lorenzini F, Diene G, Crinò A, Illouz F, Grugni G, Potvin D, Bocchini S, Delale T, Abribat T, Tauber M. AZP-531, an unacylated ghrelin analog, improves food-related behavior in patients with Prader-Willi syndrome: A randomized placebo-controlled trial. PLoS One. 2018;13(1):e0190849. [DOI] [PMC free article] [PubMed] [Google Scholar]