Abstract
Background
Antibiotic prophylaxis is a common but controversial practice for clean soft tissue procedures of the hand, such as carpal tunnel release or trigger finger release. Previous studies report no substantial reduction in the risk of surgical site infection (SSI) after antibiotic prophylaxis, yet are limited in power by low sample sizes and low overall rates of postoperative infection.
Questions/Purposes
Is there evidence that antibiotic prophylaxis decreases the risk of SSI after soft tissue hand surgery when using propensity score matching to control for potential confounding variables such as demographics, procedure type, medication use, existing comorbidities, and postoperative events?
Methods
This retrospective analysis used the Truven Health MarketScan® databases, large, multistate commercial insurance claims databases corresponding to inpatient and outpatient services and outpatient drug claims made between January 2007 and December 2014. The database includes records for patients enrolled in health insurance plans from self-insured employers and other private payers. Current Procedural Terminology codes were used to identify patients who underwent carpal tunnel release, trigger finger release, ganglion and retinacular cyst excision, de Quervain’s release, or soft tissue mass excision, and to assign patients to one of two cohorts based on whether they had received preoperative antibiotic prophylaxis. We identified 943,741 patients, of whom 426,755 (45%) were excluded after meeting one or more exclusion criteria: 357,500 (38%) did not have 12 months of consecutive insurance enrollment before surgery or 1 month of enrollment after surgery; 60,693 (6%) had concomitant bony, implant, or incision and drainage or débridement procedures; and 94,141 (10%) did not have complete data. In all, our initial cohort consisted of 516,986 patients, among whom 58,201 (11%) received antibiotic prophylaxis. Propensity scores were calculated and used to create cohorts matched on potential risk factors for SSI, including age, procedure type, recent use of steroids and immunosuppressive agents, diabetes, HIV/AIDs, tobacco use, obesity, rheumatoid arthritis, alcohol abuse, malnutrition, history of prior SSI, and local procedure volume. Multivariable logistic regression before and after propensity score matching was used to test whether antibiotic prophylaxis was associated with a decrease in the risk of SSI within 30 days after surgery.
Results
After controlling for patient demographics, hand procedure type, medication use, existing comorbidities (eg, diabetes, HIV/AIDs, tobacco use, obesity), and postoperative events through propensity score matching, we found that the risk of postoperative SSI was no different between patients who had received antibiotic prophylaxis and those who had not (odds ratio, 1.03; 95% CI, 0.93-1.13; p = 0.585).
Conclusions
Antibiotic prophylaxis for common soft tissue procedures of the hand is not associated with reduction in postoperative infection risk. While our analysis cannot account for factors that are not captured in the billing process, this study nevertheless provides strong evidence against unnecessary use of antibiotics before these procedures, especially given the difficulty of conducting a randomized prospective study with a sample size large enough to detect the effect of prophylaxis on the low baseline risk of infection.
Level of evidence:
Level III, therapeutic study
Introduction
Clinical practice guidelines in orthopaedic surgery recommend the use of antibiotic prophylaxis before some procedures, including total joint replacement, closed hip fracture surgery, spine surgery, open fracture treatment, and internal fixation [5, 6, 24, 28]. However, antibiotic use in clean, soft tissue hand surgery does not have the same level of support. Prior studies have found no relationship between risk of surgical site infection (SSI) and antibiotic prophylaxis for procedures such as carpal tunnel release, trigger finger release, ganglion cyst excision, surgery for de Quervain’s tenosynovitis, and mass excision [7, 16, 17, 27, 32]. Routine use of antibiotics therefore may not decrease the risk of infection, but nevertheless can contribute to antibiotic resistance and other unexpected consequences, such as Clostridium difficile-related colitis.
Previous studies regarding antibiotic prophylaxis in hand surgery have several limitations, however. First, the low risk of infection—previously reported to be as low as 0.5%—and potentially small treatment effect require very large sample sizes for adequate statistical power [17, 32]. Moreover, multicenter reviews may not fully capture infections that were treated outside the institutions included in the study, or may not produce results generalizable to institutions outside the study area. Finally, subjects in previous studies were not randomized to treatment groups, and instead received antibiotic prophylaxis based on surgeon discretion [27, 32]. If not corrected for during analysis, this nonrandom treatment assignment can lead to selection bias when estimating treatment effect [12].
Therefore, we asked: is there evidence that antibiotic prophylaxis decreases the risk of SSI after soft tissue hand surgery when using propensity score matching to control for potential confounding variables such as demographics, procedure type, medication use, existing comorbidities, and postoperative events?
Patients and Methods
Data and Study Cohort
We conducted a retrospective analysis using administrative insurance claims data from the Truven Health MarketScan® Commercial and Medicare Supplemental Databases (Truven Health Analytics, an IBM Company, Ann Arbor, MI, USA). The database includes records for more than 50 million individuals across the United States who received private health insurance from self-insured employers and other private health plans between January 2007 and December 2014. Claims reflect services provided to enrollees, their spouses, and their dependents in inpatient and outpatient settings, and include outpatient pharmacy claims. Procedures and diagnoses were identified in the data using Current Procedural Terminology (CPT) codes and ICD-9-Clinical Modification (ICD-9-CM) codes, respectively.
Patients treated with any of the following soft tissue hand procedures were assessed for inclusion: carpal tunnel release; trigger finger release; ganglion or retinacular cyst excision; de Quervain’s release; or soft tissue mass excision. The earliest instance of a procedure was considered for patients who may have had multiple operations. Patients were excluded if they did not have at least 1 month of insurance enrollment after surgery or 12 months of consecutive insurance enrollment before surgery. Moreover, we excluded patients who underwent concomitant bony, implant, or incision and drainage or débridement procedures on the day of treatment to limit analysis to clean soft tissue procedures. Only patients with complete data were considered.
A two-tailed, z-test power analysis for logistic regression was conducted using G*Power software (Version 3.1.9.3; University of Dusseldorf, Dusseldorf, Germany) [14] to determine the minimum sample size needed to detect a very small effect size similar to that reported by Tosti et al. [32]. Based on their study, we assumed that the probabilities of SSI were 0.8% and 0.5% without and with antibiotics, respectively. A minimum total sample size of 79,910 patients is needed to detect the corresponding effect size with 90% power at a significance level of 0.05, given a low correlation with other covariates and a prophylaxis rate of 15%, which falls in the range of previously reported values [7, 32].
The Research and Compliance Office of Stanford University deemed the study exempt from human studies review.
Cohort Characteristics
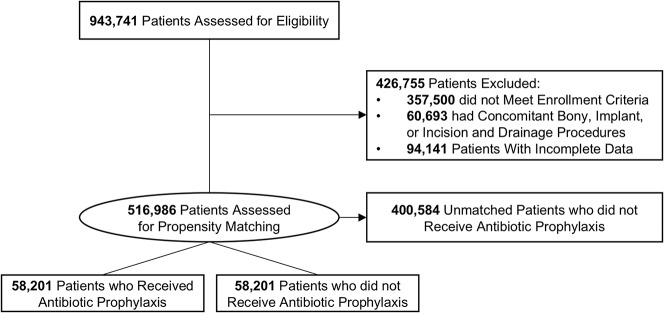
We identified 943,741 patients who underwent one of the selected soft tissue hand procedures. Of these 943,741 individuals, 426,755 (45%) were deemed ineligible owing to exclusion criteria: 357,500 (38%) did not meet continuous enrollment criteria; 60,693 (6%) had concomitant bony, implant, or incision and drainage or débridement procedures; and 94,141 (10%) did not have complete data. Overall, 516,986 patients were included in the initial study cohort before propensity score matching (Fig. 1). Carpal tunnel release was the most represented of all procedures in the initial study cohort at 48% (250,613 of 516,986).
Fig. 1.
The initial study cohort included 516,986 patients before propensity score matching. Treatment and control cohorts matched using propensity scores consisted of 58,201 individuals each. Patients may be excluded on the basis of multiple criteria. All included patients were assessed in univariable and multivariable logistic regression.
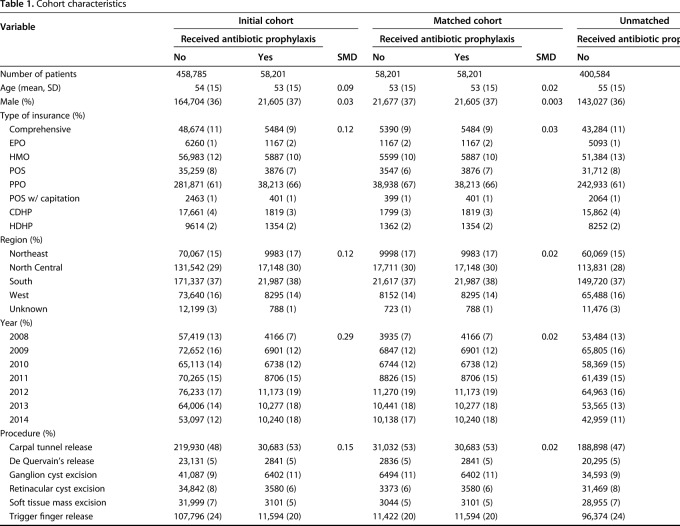
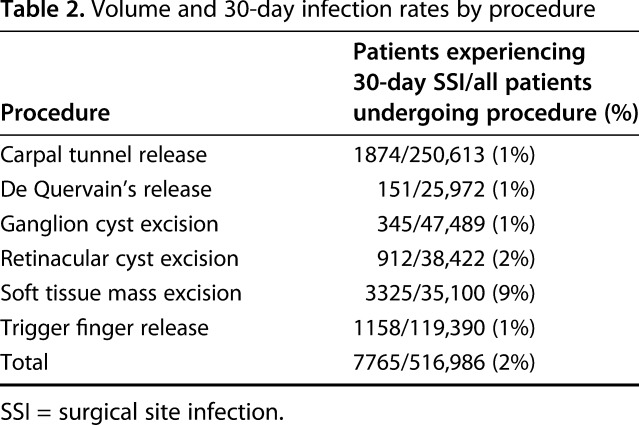
Of all 516,986 patients included in the initial study cohort, 58,201 (11%) received intravenous antibiotic prophylaxis on the day of the procedure (Table 1). The overall 30-day SSI rate was 1.5% (6933 of 458,785) for patients who did not receive prophylaxis and 1.4% (832 of 58,201) for patients who did. Across the entire initial cohort, the 30-day SSI rate was highest in patients with soft tissue mass excision at 9% (3325 of 35,100) and lowest in those with de Quervain’s release at 1% (151 of 25,972) (Table 2).
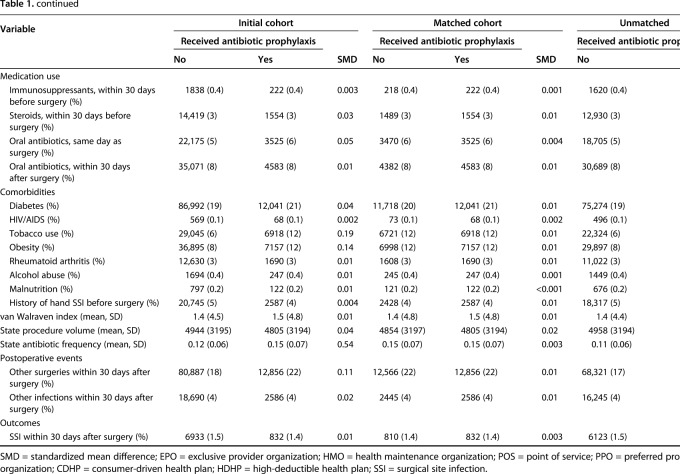
Table 1.
Cohort characteristics
Table 2.
Volume and 30-day infection rates by procedure
Explanatory Variables
We examined whether the risk of SSI within 30 days after soft tissue hand surgery was decreased after antibiotic prophylaxis. Prophylaxis was identified by claims made for intravenous antibiotics on the day of surgery. Patients were further characterized by demographics (age, sex, insurance plan type), geographic region (Northeast, North Central, South, West), year of treatment, and procedure type.
Additionally, specific covariates were captured to control for potential effects on treatment assignment or outcome. Covariates representing diagnoses and procedures in the year before surgery included history of diabetes, HIV/AIDs, tobacco use, obesity, rheumatoid arthritis, alcohol abuse, malnutrition, and prior hand SSIs. A patient’s relative comorbidity burden in the year before surgery was further assessed using the van Walraven formulation of the Elixhauser index, which represents 30 comorbidities as a single numeric score that describes overall association with mortality [34]. Use of prescription steroids or immunosuppressive agents within 30 days before the procedure was captured to identify patients who potentially were in an immunosuppressed state. To account for postoperative factors that could affect SSI risk, we also assessed use of oral antibiotics on and within 30 days after the procedure, and the occurrence of unrelated procedures or infection events during the 30-day postoperative observation period. Finally, potential geographic differences in antibiotic prophylaxis tendencies were assessed as state- and year-specific prophylaxis frequency and overall soft tissue hand procedure volume.
Outcome Variables
The primary outcome assessed was SSI occurring within 30 days after surgery. An SSI was defined as a record of either an infection-related procedure or a diagnosis of infection during the 30-day postoperative observation period. We assumed that claims were submitted for all instances of SSIs, and that absence of a claim during the observation period reflected an absence of infection.
Statistical Methods
Summary statistics were represented as frequencies for categorical variables and means and standard deviations for continuous variables. Differences in covariate distributions between treatment and control cohorts were quantified using a chi-square test for categorical variables and a t-test for continuous variables. The effect of antibiotic prophylaxis on the risk of postoperative SSI was tested using multivariable logistic regression before and after propensity score matching of treatment and control cohorts, and was represented as an odds ratio (OR) with 95% CIs and a significance level of 0.05. Data extraction and manipulation were performed using SAS (Version 9.4; SAS Institute, Cary, NC, USA), and further statistical analysis was performed using R (Version 3.4.2; R Foundation for Statistical Computing, Vienna, Austria). We used the MatchIt R package for propensity score analysis [18].
Propensity Score Matching
The large and diverse sample afforded by this dataset allows for generalizable results and adequate statistical power to detect a small effect size. To limit potential selection bias, we used propensity score matching to create a treatment and a control cohort that were balanced with respect to variables that could influence the risk for SSIs and the likelihood (or “propensity”) of receiving antibiotic prophylaxis, an approach often used in studies with nonrandom treatment assignment [12]. A propensity score is a single number that reflects a patient’s probability of receiving antibiotic prophylaxis based on the value of measured covariates that could influence SSI risk or treatment assignment, and is generated using multivariable logistic regression [4, 11, 30]. Matching generated a treatment cohort of patients who had received prophylaxis and a control cohort of patients who had not received prophylaxis, and was conducted using the nearest-neighbor method in which each patient in the treatment cohort was matched one-to-one with the patient in the control cohort with the closest propensity score. Treated patients were matched to a corresponding control patient in order of descending propensity score. Patients in the control cohort who were not matched were not included in analyses conducted after matching.
We evaluated the quality of matching by assessing whether the treatment and control cohorts became more similar (“balanced”) across the set of measured covariates after matching. Balance for each covariate was quantified as a standardized mean difference (SMD), which assesses the distance between the means of the covariate in each of the two groups, normalized by sample variance for continuous variables or by the prevalence of each variable level for noncontinuous variables [2]. Cohorts were considered balanced across a given covariate if the SMD was less than 0.1, a common benchmark in propensity score matching studies [25]. Matching was repeated using stricter calipers, which limit the permissible difference in propensity scores between matched patients to improve the closeness of match.
Before matching, the patients who were treated and untreated were unbalanced across numerous covariates, including plan type, geographic region, treatment year, procedure type, tobacco use, obesity, state-specific annual prophylaxis frequency, and the occurrence of other, unrelated procedures during the 30 days after hand surgery. Propensity score matching yielded 58,201 patients in each of the treated and untreated cohorts, for a total sample size of 116,402.
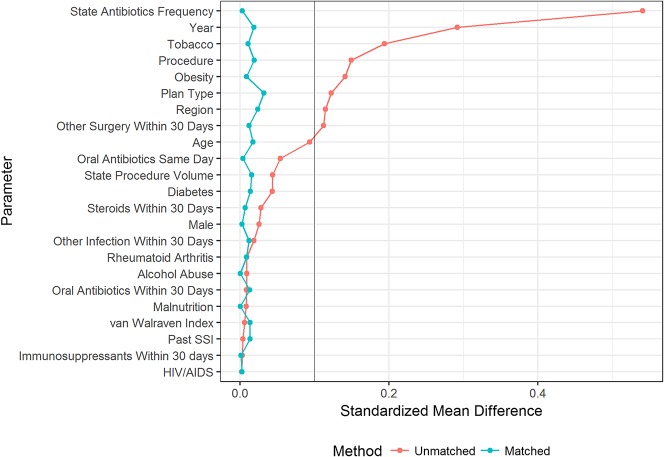
Prophylaxis rates ranged from 3% (1751 of 51,699) to 26% (13,260 of 51,699) in patients with first-decile and tenth-decile propensity scores, respectively. Compared with lower propensity score deciles, higher deciles—corresponding to an increased likelihood of receiving antibiotic prophylaxis—were characterized by the following: younger age; different distributions of patients across insurance plan type and geographic region; later year of treatment; a higher proportion of patients who underwent carpal tunnel release; increasing use of same-day oral antibiotics; history of diabetes, tobacco use, and obesity; different annual state procedure volume and prophylaxis frequency; and a higher proportion of patients who underwent other, unrelated procedures during the 30 days after hand surgery. Matching improved balance across most covariates and reduced the SMD across all covariates to less than 0.1 (Fig. 2).
Fig. 2.
To assess the improvement in covariate balance owing to propensity score matching, the standardized mean difference between cohorts across covariates was calculated before and after matching. Cohorts were considered balanced with respect to a given covariate if the standardized mean difference was less than 0.1 (demarcated in the image with a vertical line), a common benchmark in propensity score matching studies. SSI = surgical site infection.
Results
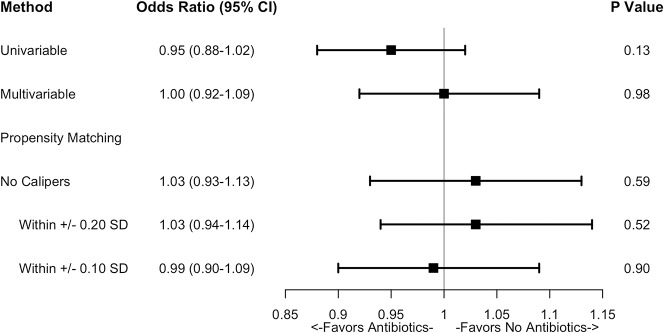
After controlling for relevant confounding variables such as demographics, hand procedure type, medication usage, existing comorbidities (eg, diabetes, HIV/AIDs status, tobacco use, obesity), and unrelated postoperative events through propensity score matching, we found that the risk of postoperative SSI was no different between patients who had received antibiotic prophylaxis and those who had not (OR, 1.03; 95% CI, 0.93-1.13; p = 0.585), a result that was maintained after applying various caliper requirements to the matching algorithm (Fig. 3).
Fig. 3.
The effect of antibiotic prophylaxis on the risk of surgical site infection within 30 days after surgery was assessed using unadjusted univariable logistic regression and multivariable regression before and after propensity score matching. Propensity score matching was repeated using several caliper requirements, which limit the permissible difference in propensity scores between matched patients to improve the closeness of match. Statistical significance was assessed at a significance level of p = 0.05.
Discussion
Previous studies have cast doubt on the effectiveness of antibiotic prophylaxis in soft tissue hand surgery, showing no differences in the risk of postoperative SSI with its use [7, 17, 26, 32]. Given low overall SSI rates, however, these studies may not be adequately powered to detect a small treatment effect from prophylaxis. We therefore aimed to revisit the hypothesis that antibiotic prophylaxis does not decrease SSI risk, using an administrative claims database to maximize sample size and propensity score matching to reduce selection bias that could otherwise result from nonrandom treatment assignment. Our analysis confirmed our hypothesis and therefore supports previously reported findings that prophylaxis does not reduce the risk of postoperative SSI.
Antibiotic prophylaxis will likely remain an important component of surgical workup in certain situations, such as in open trauma with wound contamination and in bony injury [10, 19]. The validity of this indication may be inferred from our data, which showed that the 30-day SSI rate after soft tissue mass excision procedures exceeded those of the other procedures studied; billing codes corresponding to these mass excision procedures encompass excision of tumor or vascular malformations, but sometimes may be used in the excision of pyogenic granulomas, which can be associated with superficial skin infections. Even with the inclusion of patients undergoing this procedure, however, antibiotic prophylaxis was not found to have an effect in reducing the risk of postoperative SSI. Despite this result, we found that antibiotic prophylaxis was still being used in more than 10% of our study cohort, in line with the range of 10% to 48% reported by Tosti et al. [32].
This study has several limitations. As with any claims data analysis, diagnoses or interventions that may act as confounders but were not captured in billing codes could not be accounted for in multivariable and propensity score analysis. Our matching algorithm incorporated a broad range of putative risk factors for SSIs in orthopaedic surgery, spanning from existing comorbidities, to geographic variation in prophylaxis use, to treatment and patient characteristics [7, 13, 15, 21, 22]. It nevertheless is reasonable to suspect that the cohort treated with antibiotic prophylaxis represents patients at higher risk for SSI even after matching—for example, if treatment was provided preferentially to patients with an unmeasured risk factor—which could bias results toward the alternative hypothesis that there is a difference in SSI risk between the treated and untreated cohorts. However, other unobservable factors such as length of surgery, suture material, surgical technique, or timing of antibiotic administration, may be present in both cohorts equally and bias results toward the null hypothesis [9, 20]. Inaccuracies in medical claims coding that lead to misclassification of treatment or outcome also could bias results toward the null hypothesis [33]. While such factors could be mitigated in a randomized, prospective study, we believe that the sample size necessary to detect a very small treatment effect would be difficult to attain in this setting, and that a retrospective analysis that controls for a wide range of potential confounding variables therefore is appropriate. Another limitation of our study is that we did not explore the harms or costs of not using antibiotics; SSIs can negatively affect revision rates, cost, and quality of life, and can lead to reimbursement penalties for providers [3, 23, 35]. Nevertheless, such implications must be weighed against the well-documented adverse effects of routine antibiotic administration, such as pseudomembranous colitis and the increased emergence of community-acquired methicillin-resistant Staphylococcus aureus strains [1, 8, 29, 31] and of antimicrobial resistance more generally [1, 29, 32]. Finally, the external validity of our study is limited by the generalizability of commercial administrative claims data, which may not be representative of other populations, such as individuals who are on Medicare or Medicaid, are uninsured, or are self-pay.
Based on the results from our analysis and on the implications of antibiotics overuse, we conclude that antibiotic prophylaxis for common soft tissue hand procedures is not associated with a reduction in postoperative infection risk. Therefore, health systems may benefit from implementing care pathways that avoid unwarranted or routine use of antibiotics prior to common soft tissue hand surgery.
Acknowledgments
Data for this project were accessed using the Stanford Center for Population Health Sciences (PHS) Data Core. The PHS Data Core is supported by a National Institutes of Health (NIH) National Center for Advancing Translational Science Clinical and Translational Science Award (UL1 TR001085) and by internal Stanford funding. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We thank Eran Bendavid MD, MS (Center for Health Policy and the Center for Primary Care and Outcomes Research, Stanford University) for guidance in study design.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Archer GL. Alteration of cutaneous staphylococcal flora as a consequence of antimicrobial prophylaxis. Rev Infect Dis. 1991;13 Suppl 10:S805–S809. [DOI] [PubMed] [Google Scholar]
- 2.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braitman LE, Rosenbaum PR. Rare outcomes, common treatments: analytic strategies using propensity scores. Ann Intern Med. 2002;137:693–695. [DOI] [PubMed] [Google Scholar]
- 5.Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, Fish DN, Napolitano LM, Sawyer RG, Slain D, Steinberg JP, Weinstein RA; American Society of Health-System Pharmacists (ASHP); Infectious Diseases Society of America (IDSA); Surgical Infection Society (SIS); Society for Healthcare Epidemiology of America (SHEA). Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt). 2013;14:73–156. [DOI] [PubMed] [Google Scholar]
- 6.Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189:395–404. [DOI] [PubMed] [Google Scholar]
- 7.Bykowski MR, Sivak WN, Cray J, Buterbaugh G, Imbriglia JE, Lee WA. Assessing the impact of antibiotic prophylaxis in outpatient elective hand surgery: a single-center, retrospective review of 8,850 cases. J Hand Surg Am. 2011;36:1741–1747. [DOI] [PubMed] [Google Scholar]
- 8.Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326:281–286. [DOI] [PubMed] [Google Scholar]
- 10.Cummings P. Antibiotics to prevent infection in patients with dog-bite wounds: a metaanalysis of randomized trials. Ann Emerg Med. 1994;23:535–540. [DOI] [PubMed] [Google Scholar]
- 11.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 12.Dehejia RH, Wahba S. Propensity score-matching methods for nonexperimental causal studies. Rev Econ Stat. 2002;84:151–161. [Google Scholar]
- 13.Fang A, Hu SS, Endres N, Bradford DS. Risk factors for infection after spinal surgery. Spine (Phila Pa 1976). 2005;30:1460–1465. [DOI] [PubMed] [Google Scholar]
- 14.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. [DOI] [PubMed] [Google Scholar]
- 15.Guild GN, Moore TJ, Barnes W, Hermann C. CD4 count is associated with postoperative infection in patients with orthopaedic trauma who are HIV positive. Clin Orthop Relat Res. 2012;470:1507–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanssen AD, Amadio PC, DeSilva SP, Ilstrup DM. Deep postoperative wound infection after carpal tunnel release. J Hand Surg Am. 1989;14:869–873. [DOI] [PubMed] [Google Scholar]
- 17.Harness NG, Inacio MC, Pfeil FF, Paxton LW. Rate of infection after carpal tunnel release surgery and effect of antibiotic prophylaxis. J Hand Surg Am. 2010;35:189–196. [DOI] [PubMed] [Google Scholar]
- 18.Ho D, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42:1–28. [Google Scholar]
- 19.Hoffman RD, Adams BD. The role of antibiotics in the management of elective and post-traumatic hand surgery. Hand Clin. 1998;14:657–666. [PubMed] [Google Scholar]
- 20.Menovsky T, Bartels RH, van Lindert EL, Grotenhuis JA. Skin closure in carpal tunnel surgery: a prospective comparative study between nylon, polyglactin 910 and stainless steel sutures. Hand Surg. 2004;9:35–38. [DOI] [PubMed] [Google Scholar]
- 21.Moucha CS, Clyburn T, Evans RP, Prokuski L. Modifiable risk factors for surgical site infection. J Bone Joint Surg Am. 2011;93:398–404. [PubMed] [Google Scholar]
- 22.Muilwijk J, van den Hof S, Wille JC. Associations between surgical site infection risk and hospital operation volume and surgeon operation volume among hospitals in the Dutch nosocomial infection surveillance network. Infect Control Hosp Epidemiol. 2007;28:557–563. [DOI] [PubMed] [Google Scholar]
- 23.Munday GS, Deveaux P, Roberts H, Fry DE, Polk HC. Impact of implementation of the Surgical Care Improvement Project and future strategies for improving quality in surgery. Am J Surg. 2014;208:835–840. [DOI] [PubMed] [Google Scholar]
- 24.Norden CW. Antibiotic prophylaxis in orthopedic surgery. Rev Infect Dis. 1991;13 Suppl 10:S842–S846. [DOI] [PubMed] [Google Scholar]
- 25.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. [DOI] [PubMed] [Google Scholar]
- 26.Oriel BS, Chen Q, Itani KM. The impact of surgical hand antisepsis technique on surgical site infection. Am J Surg. 2017;213:24–29. [DOI] [PubMed] [Google Scholar]
- 27.Platt AJ, Page RE. Post-operative infection following hand surgery: guidelines for antibiotic use. J Hand Surg Br. 1995;20:685–690. [DOI] [PubMed] [Google Scholar]
- 28.Prokuski L. Prophylactic antibiotics in orthopaedic surgery. J Am Acad Orthop Surg. 2008;16:283–293. [DOI] [PubMed] [Google Scholar]
- 29.Rizvi M, Bille B, Holtom P, Schnall SB. The role of prophylactic antibiotics in elective hand surgery. J Hand Surg Am. 2008;33:413–420. [DOI] [PubMed] [Google Scholar]
- 30.Rosenbaum PR. Discussing hidden bias in observational studies. Ann Intern Med. 1991;115:901–905. [DOI] [PubMed] [Google Scholar]
- 31.Tacconelli E, De Angelis G, Cataldo MA, Pozzi E, Cauda R. Does antibiotic exposure increase the risk of methicillin-resistant Staphylococcus aureus (MRSA) isolation? A systematic review and meta-analysis. J Antimicrob Chemother. 2008;61:26–38. [DOI] [PubMed] [Google Scholar]
- 32.Tosti R, Fowler J, Dwyer J, Maltenfort M, Thoder JJ, Ilyas AM. Is antibiotic prophylaxis necessary in elective soft tissue hand surgery? Orthopedics. 2012;35:e829–e833. [DOI] [PubMed] [Google Scholar]
- 33.Tyree PT, Lind BK, Lafferty WE. Challenges of using medical insurance claims data for utilization analysis. Am J Med Qual. 2006;21:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–633. [DOI] [PubMed] [Google Scholar]
- 35.Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ. The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol. 2002;23:183–189. [DOI] [PubMed] [Google Scholar]