Abstract
Background
Colonoscopy [CS] is the standard for assessing disease activity in ulcerative colitis [UC], although invasive and poorly tolerated. Bowel ultrasound [BUS] may be a valid alternative in UC patients; however, the comparative accuracy between BUS and CS is unknown.
Methods
Consecutive patients with UC were prospectively assessed by CS and BUS. Colonic wall thickening [CWT >3 mm], colonic wall flow at power Doppler [CWF], colonic wall pattern [CWP], and presence of lymph nodes evaluated at BUS were compared with CS. The endoscopic activity was assessed according to the Mayo endoscopic sub-score [0–3]. All BUS investigations were performed by two independent gastroenterologists and the kappa statistic for agreement was calculated. Ultrasonography-based criteria (Humanitas Ultrasound Criteria [HUC]) were developed.
Results
A total of 53 UC patients [56% with left-sided colitis, 19% with pancolitis] were prospectively enrolled. Of these, 22 patients had mucosal healing [Mayo endoscopic sub-score 0–1] and 31 patients were in endoscopic activity. CWT, CWF, hypoechogenic CWP and the presence of lymph nodes significantly correlated with endoscopic activity [p < 0.05]. CWT [p = 0.01] and CWF [p = 0.09] were independent predictors for endoscopic activity. The HUC developed are: [i] the presence of a CWF and CWT > 3 mm; or [ii] the absence of a CWF and CWT > 4.43 mm. They showed high accuracy for the detection of disease activity [sensitivity 0.71, specificity 1.00]. The interobserver agreement for BUS was excellent [kappa 0.86].
Conclusions
BUS is a non-invasive, easy-to-use tool to manage UC patients in clinical practice. HUC were very accurate in assessing disease activity in UC patients.
Keywords: Bowel ultrasound, inflammatory bowel disease, imaging, non-invasive technique
1. Introduction
Ulcerative colitis [UC] is a chronic idiopathic inflammatory disorder that causes mucosal inflammation of the colon and is characterised by a relapsing-remitting clinical course.1 Activity and severity of inflammation influence management and treatment modality of UC patients.2 Ileocolonoscopy [CS] is considered the first-line procedure for the assessment of UC.2 Mucosal healing, defined by a Mayo score of 0–1, is recommended as the therapeutic goal in clinical practice.3 However, CS is an invasive procedure that may increase the risk of bowel perforation, particularly in case of severe flare.4,5 In addition, it causes discomfort and repeated colonoscopies are not well accepted by patients. Bowel ultrasound [BUS] is a well tolerated, non-invasive, non-radiating, cheap, easy-to-use tool to manage UC patients in clinical practice. Up to now, there are only few data evaluating accuracy of BUS in assessing disease activity and severity of UC.6–8 The comparative accuracy of BUS versus CS, as reference standard, in assessing disease activity and severity is not yet well known.
The aim of this study was prospectively to assess the diagnostic accuracy of BUS in detecting disease activity and severity in adult patients with UC, comparing with CS, as the reference standard, and to develop non-invasive quantitative criteria (Humanitas Ultrasound Criteria [HUC]) of disease activity based on BUS findings.
2. Methods
2.1. Study population and examinations
Consecutive adult patients with established diagnosis of UC [for at least 6 months], seen in a tertiary referral centre [Humanitas Research Hospital, Milan, Italy] between September 2015 and November 2017, requiring routine investigations by CS, were prospectively assessed by CS and BUS within 1 week, irrespective of disease activity. The examinations were performed with a standard video endoscope [Fujinon, Japan] by an expert endoscopist with at least 7 years of experience, who was blinded to the findings of BUS; whereas BUS was performed by two independent gastroenterologists experienced in US [each with at least 6 years of experience], unaware of the results of the other diagnostic procedures.
In order to be able to compare different procedures, the ileocolonic tract visualised at BUS was divided into four segments: ileum, caecum-ascending colon [including ileo-caecal valve], transverse colon, descending-sigmoid colon. Treatment was kept stable in the interval between CS and BUS. All patients gave their informed consent for this study. Exclusion criteria were pregnancy, any contraindication to full CS [e.g. intolerance to preparation, severe flare], disease limited to the rectum [less than 15 cm at CS], and concomitant participation in other clinical trials.
All patients meeting inclusion criteria and none of the exclusion criteria, underwent complete clinical assessment. The disease was considered clinically active if the partial Mayo score [PMS] was higher than 2.9 In a subgroup of 34 patients, blood and stool samples were obtained in the same week as BUS, for cell blood counts, C-reactive protein [CRP], and faecal calprotectin [FC] measurements.
2.2. Endoscopic findings
The endoscopic activity was evaluated by CS, according to the Mayo endoscopic sub-score: 0 = normal or inactive disease; 1 = erythema, decreased vascular pattern, mild friability; 2 = absent vascular pattern, erosions; and 3 = spontaneous bleeding, ulcerations. Mucosal healing was defined by an absolute Mayo endoscopic sub-score of 0 or 1.9 Mayo endoscopic sub-score was calculated globally and per segment. The extent of disease was defined according to the Montréal classification into ulcerative left-sided colitis [up to the splenic flexure] and extensive colitis.2 CSs were performed after standard bowel preparation by administration of 4 L of polyethylene glycol [PEG].
2.3. BUS findings
All patients underwent BUS 3 days before or after CS. BUS was performed after a 6–8 h fast, using an Aloka Arietta V60 with convex [5-1 MHz] and microconvex probes [4–8 MHz]. Neither preparation nor contrast were used. The entire abdomen was systematically scanned starting from the right iliac fossa. The following parameters, selected based on a literature search, were evaluated. Colonic wall thickening [CWT] [normal values up to 3 mm] was measured in longitudinal and transverse sections, from the interface between the mucosa and the lumen to the interface between the serosa and the muscle layer. A mean of three measurements for section was taken; colonic wall pattern [CWP] that could be [0] normal, multilayered, [1] prevalently hypoechogenic, [2] prevalently hyperechogenic, [3] lost; and colonic wall flow [CWF] [defined as absence [0] or presence [1] of blood signals at power Doppler]. Also the presence of enlarged mesenteric lymph nodes [short axis > 5 mm] and mesenteric hypertrophy [defined as the presence of a hyperechoic area surrounding the pathological intestinal tract] was investigated. These parameters were evaluated in each colonic segment.6,7,10 The worst segment was taken into account.
2.4. Statistical analysis
Descriptive statistics of the baseline data are presented as medians (interquartile range [IQR]), or as percentages when appropriate. Differences in qualitative BUS findings were tested using the χ2 test. The Wilcoxon test was used to compare differences in quantitative variables. A logistic regression analysis was performed. The presence of endoscopically active disease was the outcome variable [or dependent variable] [i.e. a binomial variable taking the value 1 if Mayo score ≥ 2, and the value 0 if Mayo score < 2]. All the BUS parameters described [CWT, CWF, CWP, the presence of enlarged mesenteric lymph nodes] and FC values were employed as explanatory variables [or independent variables]. Univariable analysis was used to identify candidate predictors. Then, a multivariable model was fitted using a ‘backwards elimination procedure’. All variables with p < 0.05 were retained in the model. The correlation between CS and BUS extension variables was evaluated with the Spearman’s correlation coefficient [r], and the respective p-value.
Sensitivity, specificity, accuracy, positive predicitive value [PPV], and negative predictive value [NPV] of BUS were calculated with a 95% confidence interval [CI], using CS as reference standard. Data were compared using the McNemar test. Using the receiver operating characteristic [ROC] curve, the best cut-off value of FC for distinguishing between endoscopic activity [Mayo score ≥ 2] and endoscopic remission [Mayo score < 2] was found and the diagnostic accuracy of BUS parameters and/or FC was calculated with a 95% confidence interval [CI], using CS as reference standard. Data were analysed using the McNemar test.
Interobserver agreement [regarding the paired evaluations of BUS] was assessed with kappa statistic. K values less than 0.20 represented a poor agreement; values between 0.21 and 0.40 a fair agreement; values between 0.41 and 0.60 a moderate agreement; values between 0.61 and 0.80 a good agreement; and values more than 0.80 an excellent agreement.
2.4.1. Development of the Humanitas Ultrasound Criteria [HUC]
Based on our findings in the comparison between BUS and CS, we aimed to build up non-invasive quantitative ultrasound-based criteria to identify patients with active UC, defined as patients with a Mayo endoscopic sub-score ≥ 2. We used the coefficients derived from the multivariable analysis to develop these criteria and we identified by a ROC curve analysis the best cut-off for disease activity in terms of sensitivity and specificity.
2.5. Statistical power
Since this was a pilot study, no sample size calculation was performed, but we assumed that at least 50 patients would be enough to address all the outcomes of the studies and to detect significant differences among the procedures, based on similar studies by Rimola et al.11,12p-Values less than 0.05 were considered to be statistically significant. Stata software was used for all statistical analyses [Stata Corp., College Station, TX, USA]. The study was performed according to Good Clinical Practice guidelines and was approved by our Institutional Review Board.
3. Results
3.1. Patients
A total of 53 consecutive UC patients, irrespective of disease activity and current therapy, were included in the study. Baseline characteristics and clinical data of the study population are presented in Table 1. In all: 22 patients [41%] were endoscopically in remission [Mayo score 0–1]; 31 patients [59%] were in endoscopic activity [Mayo score 2–3]; 30 patients [56%] had left-sided ulcerative colitis; and 10 patients [19%] had an extensive ulcerative colitis, as evaluated by CS. All patients performed CS and BUS within 7 days. Figures 1 and 2 illustrate examples of CS and BUS for two UC patients.
Table 1.
Characteristics of patients at inclusion in the study [n = 53]
| Characteristic | Median [interquartile range] or percentage and range |
|---|---|
| Female | 21 [40] |
| Age at diagnosis | 32.29 [22.90–41.42] |
| Age at inclusion | 46.41 [31.90–54.96] |
| Disease duration [years] | 7.19 [2.85–19.33] |
| Disease extent at diagnosis | |
| ▪E2 Left sided | 31 [58] |
| ▪E3 Extensive | 22 [42] |
| Concomitant treatmentsa | |
| ▪Steroids | 9 [17] |
| ▪Immunosuppressants | 1 [2] |
| ▪Biologic therapyb | 12 [23] |
| Smoking | |
| ▪Past | 14 [26] |
| ▪Active | 3 [6] |
| Partial Mayo score [PMS] | 2 [0–5] |
| PMS > 2 | 21 [40] |
| CRP [mg/L] | 5.0 [1.75–8.30] |
| Calprotectin [µg/g] | 422.5 [99.0–1414.0] |
| Mayo endoscopic sub-score | |
| ▪0 | 13 [24] |
| ▪1 | 9 [17] |
| ▪2 | 12 [23] |
| ▪3 | 19 [36] |
| Disease extent at colonoscopy at inclusion | |
| ▪E2 Left-sided | 30 [56] |
| ▪E3 Extensive | 10 [19] |
CRP, C-reactive protein.
** All patients took mesalazine; * 8 patients were given infliximab, 4 vedolizumab
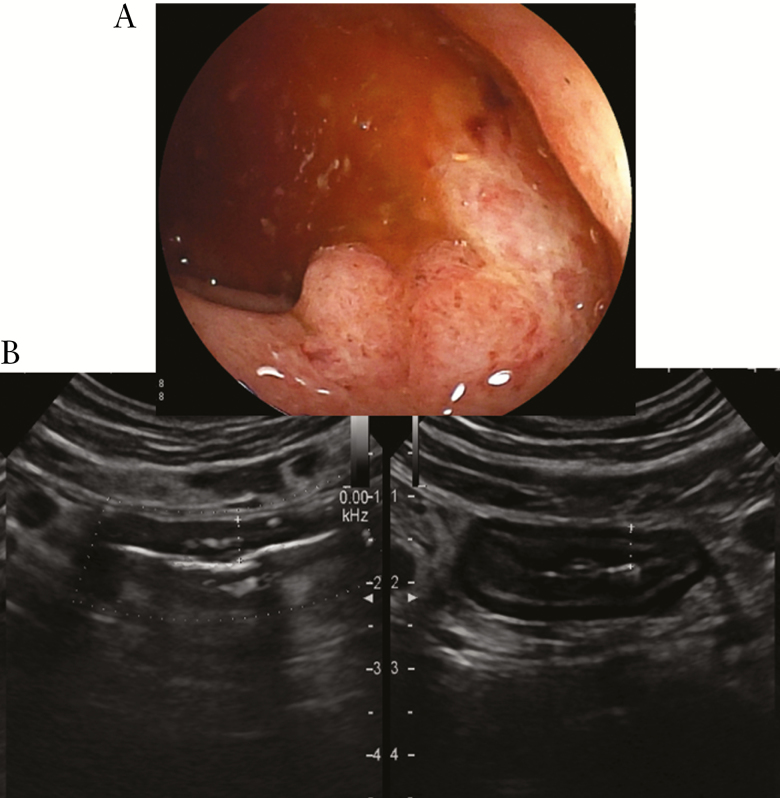
Figure 1.
Left-sided ulcerative colitis. At colonoscopy [CS], absent vascular pattern, erythema, erosions, and rare ulcers [Mayo 3] [A]. At bowel ultrasound [BUS], longitudinal and transverse sections of sigmoid colon with a 5-mm wall thickening and presence of blood signals at power Doppler. Some lymph nodes are present surrounding the pathological intestinal tract [B].
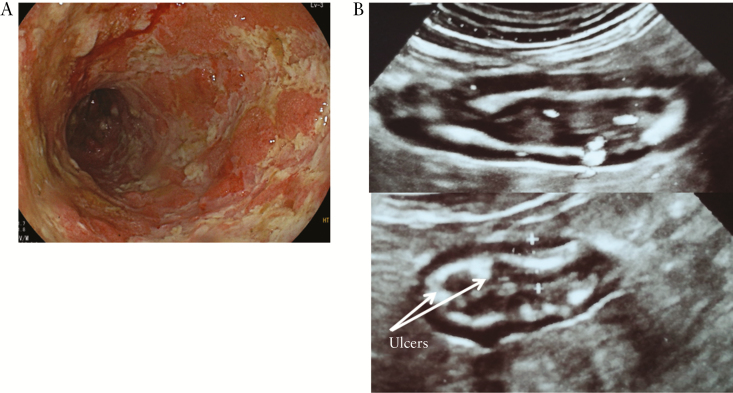
Figure 2.
Left-sided ulcerative colitis. At colonoscopy [CS], absent vascular pattern, marked erythema, friability, spontaneous bleeding, and ulcerations [Mayo 3] [A]. At bowel ultrasound [BUS], longitudinal and transverse sections of sigmoid colon with a 6-mm wall thickening and presence of blood signals at power Doppler. Arrows indicate ulcers [B].
3.2. BUS findings compared with CS
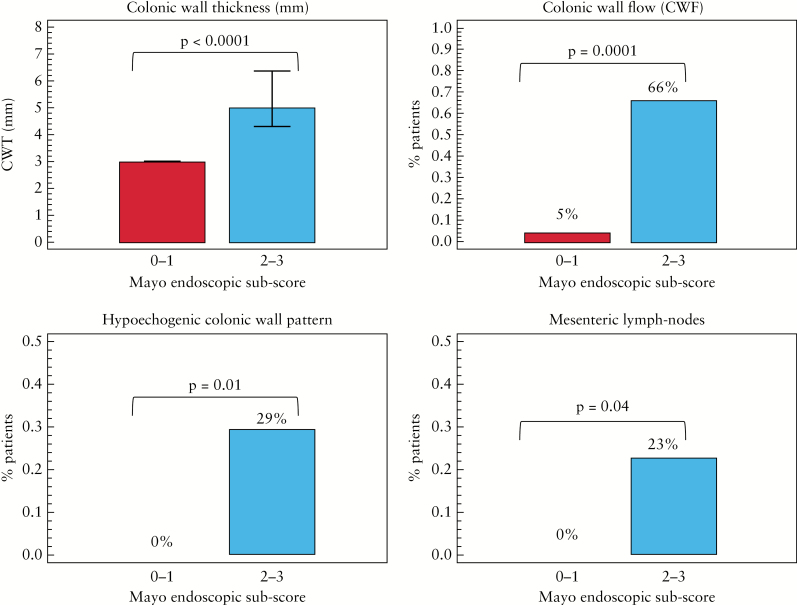
In Figure 3 are shown the BUS parameters that correlated with the Mayo endoscopic sub-score with significant differences between patients in endoscopic remission [Mayo 0–1] and patients in endoscopic activity [Mayo 2–3]. Median values of CWT in patients with endoscopic remission were 3.0 mm [IQR 3.0 to 3.0] compared with 5.0 mm [IQR 4.3 to 6.4] in patients with endoscopic active disease [p < 0.0001]. CWF was present in 5% [1/20 patients] of patients in endoscopic remission versus 66% [18/27] of patients in endoscopic activity [p = 0.0001]. Hypoechogenic or lost CWP was present in 0% [0/22 patients] of patients in endoscopic remission versus 29% [9/31] of patients in endoscopic activity [p = 0.01]. Enlarged mesenteric lymph nodes were present in 0% [0/22] of patients in endoscopic remission compared with 23% [7/31] of patients in endoscopic activity [p = 0.04]. The multivariable analysis identified CWT (per 1-mm increase, odds ratio [OR]: 4.05, 95% CI: 1.37–11.9, p = 0.01) and CWF [OR: 7.99, 95% CI: 0.67–94.4, p = 0.09] as independent predictors for endoscopic activity. Disease extent evaluated by BUS significantly correlated with the extension at CS [r 0.660, 95% CI: 0.474–0.790, p < 0.0001]. The sensitivity, specificity, accuracy, PPV, and NPV of BUS compared with CS [as the reference standard] in assessing endoscopically active disease [Mayo 2–3] in UC are represented in Table 2. The sensitivity and specificity of CWT > 3 mm were 89% and 87%, respectively. The three false-negative cases were referred to patients with mild erosions localised in the distal tract of sigma. No false-negative outcome was recorded in patients with severe endoscopic lesions [such as the presence of ulcers]. The three false-positive cases were patients with Mayo 1. The maximum wall thickness was 4.4 mm and there was never presence of blood signals at power Doppler. A CWT > 3 mm with a positive CWF had a specificity of 100% in assessing endoscopic activity. Finally, the interobserver agreement between the two operators for BUS was 86%, indicating an excellent agreement.
Figure 3.
Changes in bowel ultrasound [BUS] parameters according to endoscopic activity.
Table 2.
Performance of bowel ultrasound [BUS] compared with colonoscopy [CS] in assessing endoscopic activity in ulcerative colitis [UC] [95% confidence interval]: per-patient analysis
| Sensitivity % | Specificity % | Accuracy % | PPV % | NPV % | |
|---|---|---|---|---|---|
| CWT > 3 mm | 89 [73–97] | 87 [67–97] | 88 [76–95] | 89 [73–97] | 87 [67–97] |
| CWF | 72 [51–88] | 95 [77–99] | 82 [69–92] | 94 [74–99] | 75 [55–89] |
| CWT > 3 mm and CWF | 68 [46–85] | 100 | 83 [69–92] | 100 | 73 [54–87] |
PPV, positive predictive value; NPV, negative predictive value; CWT, colonic wall thickening; CWF, colonic wall flow
3.3. BUS and FC in combination compared with CS
In the subgroup of 34 patients for whom FC measurements were available, using the ROC curve, FC value > 101 µg/g had a sensitivity of 100%, and specificity of 67%, with an area under the curve [AUC] of 0.833 [95% CI: 0.666–0.938] for endoscopically active disease. Diagnostic accuracy of BUS parameters and/or FC value > 101 µg/g is shown in Table 3. The presence of a CWT > 3 mm or of a calprotectin value > 101 µg/g had a sensitivity of 100% in assessing endoscopic activity. The combined presence of CWT > 3 mm, FC value > 101 µg/g, and the CWF had a specificity of 100%.
Table 3.
Diagnostic accuracy of bowel ultrasound [BUS] and/or faecal calprotectin [FC] compared with colonoscopy [CS] in assessing endoscopic activity in a subgroup of 34 patients with ulcerative colitis [UC] [95% confidence interval]: per-patient analysis
| Sensitivity % | Specificity % | Accuracy % | PPV % | NPV % | |
|---|---|---|---|---|---|
| CWT > 3 mm or FC > 101 µg/g | 100 | 53 [26–78] | 79 [62–91] | 73 [52–88] | 100 |
| CWT > 3 mm + FC > 101 µg/g | 84 [60–96] | 93 [68–99] | 88 [72–96] | 94 [71–99] | 82 [56–96] |
| CWT > 3 mm + FC > 101 µg/g + CWF | 66 [40–86] | 100 | 81 [64–93] | 100 | 71 [47–88] |
PPV, positive predictive value; NPV, negative predictive value; CWT, colonic wall thickening; CWF, colonic wall flow.
3.4. Development of the Humanitas ultrasound criteria
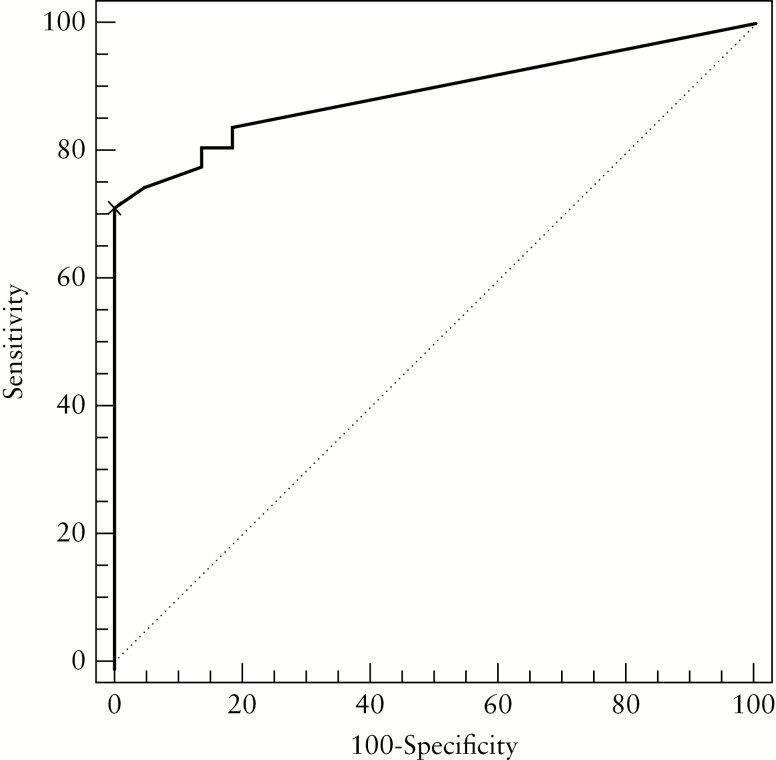
The BUS parameters related to endoscopic activity of UC were included in the enquiry Humanitas Ultrasound Criteria [HUC] [1.4 x CWT [mm] + 2 × CWF]. FC was significant [p = 0.03] in the univariable analysis, but not in the multivariable analysis [p = 0.97]. The multivariable model finally included only CWT and CWF. ROC analysis set an enquiry HUC ≥ 6.3 as a threshold that discriminated patients with active UC versus non-active UC [Figure 4]. Sensitivity and specificity for the proposed cut-off were 0.71 [95% CI: 0.52–0.86] and 1.00 [95% CI: 0.84–1.0], respectively, with an AUC of 0.891 [95% CI: 0.775–0.959]. The combination of HUC > 6.2 and FC > 101 had a sensitivity of 1.00 [95% CI: 0.82–1.00] and a specificity of 0.67 [95% CI: 0.38–0.88], with an AUC of 0.833 [95% CI: 0.666–0.938]. The comparison of the ROC curves regarding HUC alone and HUC + FC showed non-significant differences between the two approaches [p = 0.490; Supplementary Figure 1, available as Supplementary data at ECCO-JCC online]. Finally, the Humanitas Ultrasound Criteria [HUC] developed for the detection of disease activity were: [i] the presence of a CWF, and CWT > 3 mm; or [ii] the absence of a CWF, and CWT > 4.43 mm.
Figure 4.
Receiver operating characteristic [ROC] curve analysis measuring sensitivity and specificity of the Humanitas Ultrasound Criteria [HUC]. An HUC ≥ 6.3 [black cross] identifies the best cut-off to discriminate active versus non active ulcerative colitis [UC].
4. Discussion
This prospective study compared the diagnostic accuracy of BUS versus CS, in assessing disease activity and severity in UC. CS is considered the first-line procedure for the assessment of UC, and mucosal healing, defined by a Mayo score of 0 or 1, is recommended as the therapeutic goal in clinical practice.2,3 Patients with UC undergo repeated CS to assess the activity and severity of lesions and to monitor the response to treatment. However, CS is an invasive procedure, poorly tolerated by patients. In addition, it is not always practicable during a severe flare because of the risk of perforation or clinical worsening.4,5 Compared with CS, BUS has the advantage of not requiring any preparation, of being non-invasive, less costly, well tolerated, and easily repeatable, representing a valuable tool in the management of patients with UC. Whereas the role of BUS for assessing the small bowel and complications in Crohn’s disease is well known,13 in UC we have only scarce and retrospective data.6–8 Maconi et al. reported in 30 UC patients a significant correlation between the degree of CWT at BUS and endoscopic activity of disease, both before and after treatment.6 Parente et al. evaluated the response to treatment in 83 UC patients and found a good agreement and an excellent agreement between endoscopic and BUS findings at 3 and 15 months, respectively.8
We prospectively compared the accuracy of BUS versus CS [as the reference standard], in a blinded fashion, in 53 UC adult patients, irrespective of activity of disease and current therapy. CWT, CWF, hypoechogenic or lost CWP and the presence of enlarged lymph nodes significantly correlated with the endoscopic activity [Mayo sub-score ≥ 2; p < 0.05; Figure 3]. At the multivariable analysis, only CWT and CWF showed to be independent predictors for endoscopic activity by binary logistic regression analysis. These parameters were used to build up non-invasive ultrasonography-based criteria (Humanitas Ultrasound Criteria [HUC] [1.4 x CWT in mm + 2 × CWF [dichotomous value, present = 1, absent = 0]) to assess and measure disease activity in our UC cohort. Our ROC curve analysis identified a score ≥ 6.3 as an indicator of endoscopic activity [Mayo sub-score ≥ 2], with a specificity of 100%. When we combined the HUC with FC, the accuracy of HUC did not change significantly [p = 0.490; Supplementary Figure 1], suggesting that HUC alone may be able to catch all patients with endoscopic remission and more than 70% of patients with active disease. Rather than complementary to faecal calprotectin, our data suggest that HUC (either the presence of a CWF, and CWT > 3 mm [4.2/1.4] or the absence of a CWF, and CWT > 4.43 [6.2/1.4]) may be a sensitive and specific alternative in discriminating active from non-active disease.,
The diagnostic accuracy of BUS for endoscopically active disease was 88% and, in the subgroup of 34 subjects for whom FC measurements were available, the sensitivity of CWT > 3 mm or FC value > 101 µg/g for the presence of endoscopic activity [Mayo score 2 or 3] was 100%. In addition, the concomitant presence of CWT > 3 mm and CWF had a specificity of 100%. The value of FC we found is in agreement with data available in literature, showing that FC values between 50 and 100 indicate a quiescent disease, and FC values > 100 the presence of inflammation.14 Also the extent of lesions defined by BUS well correlated with those found by CS [p < 0.0001].
The strong correlation found between BUS and CS makes BUS an easy, non-invasive first-line procedure for assessing disease severity and activity in patients with UC, allowing delay or even avoidance of CS in some circumstances. BUS may therefore be the preferred procedure for monitoring disease course and the short-term treatment response. This has implications not only for reducing risks due to invasiveness of CS and for increasing acceptability to patients, but also for reducing health care costs. The estimated saving in our hospital using BUS instead of CS is €60 per procedure, with an overall saving of €3180 for the entire study population [Supplementary Figure 2, available as Supplementary data at ECCO-JCC online].
This study has several strengths. This is a prospective, controlled study, performed in a blinded fashion in adult patients with established diagnosis of UC, evaluating clinical, endoscopic, imaging, and biological activity at the same time [all assessments were performed within 1 week]; all BUSs were performed by two independent gastroenterologists with an excellent agreement, demonstrating that BUS is a reproducible tool to assess disease activity and to manage UC patients. We also showed that the combination of CWT and CWF alone was highly sensitive and specific for assessing disease activity with excellent correlation with endoscopy. The main limitation of the study is the exclusion of the patients with disease localised to the rectum, since transabdominal bowel ultrasound is not able to assess the rectum. Second, we did not collect FC in all patients; however, it is not likely that this influenced the statistical analysis or the construction of our Humanitas Ultrasound Criteria, since the p-value of 0.97 is far from significance. Furthermore, being an observational study, calprotectin was not centralised. In addition, not all patients performed BUS before CS, but nevertheless there are no clear data on bowel cleaning worsening colitis. Finally, endoscopic activity was evaluated according to Mayo score by just one expert endoscopist.
In conclusion, BUS may represent a useful first-line, non-invasive tool for assessing endoscopic activity, severity, and extent, and may be helpful to determine in a rapid manner whether a significant flare has occurred and to guide the management of UC patients, delaying or avoiding colonoscopy when it is not needed. In addition, BUS may be preferred in clinical practice for monitoring disease course and for assessing short-term treatment response, reducing the necessity of repeated CS, although specific data on monitoring will be needed. Larger multicentre studies are needed to confirm our preliminary findings and to test the reproducibility of BUS and of the HUC.
Funding
No funding was obtained for this project.
Conflict of Interest
MA received consulting fees from Nikkiso Europe and lecture fees from Janssen and Pfizer; GF served as a consultant and a member of Advisory Boards for MSD, Takeda Pharmaceuticals, and Janssen Pharmaceuticals and received lecture fees from Janssen and Pfizer; FF received consulting fees from MSD, Biogen, and Abbvie and lecture fees from Janssen and Pfizer; DG received consultancy fees from Nikkiso GMBH and SOFAR SpA and lecture fees from Janssen and Pfizer; LP-B received consulting fees from Merck, Abbott, Janssen, Genentech, Mitsubishi, Ferring, Norgine, Tillots, Vifor, Shire, Therakos, Pharmacosmos, Pilège, BMS, UCB-pharma, Hospira, Celltrion, Takeda, Boerhinger-Ingelheim, and Lilly, and lecture fees from Merck, Abbott, Janssen, Ferring, Norgine, Tillots, Vifor, Therakos, HAC-pharma; SD has served as a speaker, consultant, and advisory board member for Schering-Plough, Abbott Laboratories, Merck & Co, UCB Pharma, Ferring, Cellerix, Millenium Takeda, Nycomed, Pharmacosmos, Actelion, Alpha Wasserman, Genentech, Grunenthal, Pfizer, Astra Zeneca, Novo Nordisk, Cosmo Pharmaceuticals, Vifor, and Johnson & Johnson; the other authors have no conflicts to declare.
Author Contributions
SD is the guarantor of the article; MA conceived of and designed the study; DG collected the data; MA, GF, and SB performed the data analysis; MA, GF, and FF drafted the manuscript; PM, M Argollo, LP-B, and SD critically revised the manuscript; all authors approved the final version of the manuscript.
Supplementary Data
Supplementary Figure 1. Comparison between the ROC curves of the Humanitas ultrasound criteria (HUC) (continuous line) and of the HUC + FC (dashed line). No statistical difference was observed (p = 0.49).
Supplementary Figure 2. Cost analysis for colonoscopy (CS) and bowel ultrasound (BUS) using a score where 1 indicates low cost; 2: medium cost; 3: high cost. The cost of CS is almost twice (1.75) that of BUS.
Supplementary Material
References
- 1. Silverberg MS, Satsangi J, Ahmad T, et al. . Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19 [Suppl A]:5A–36A. [DOI] [PubMed] [Google Scholar]
- 2. Magro F, Gionchetti P, Eliakim R, et al. ; European Crohn’s and Colitis Organisation [ECCO]. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017;11:649–70. [DOI] [PubMed] [Google Scholar]
- 3. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. . Selecting Therapeutic taRgets in Inflammatory bowel DiseasE [STRIDE]: determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324–38. [DOI] [PubMed] [Google Scholar]
- 4. Damore LJ II, Rantis PC, Vernava AM III, Longo WE. Colonoscopic perforations. Etiology, diagnosis, and management. Dis Colon Rectum 1996;39:1308–14. [DOI] [PubMed] [Google Scholar]
- 5. Eckardt VF, Gaedertz C, Eidner C. Colonic perforation with endoscopic biopsy. Gastrointest Endosc 1997;46:560–2. [DOI] [PubMed] [Google Scholar]
- 6. Maconi G, Ardizzone S, Parente F, Bianchi Porro G. Ultrasonography in the evaluation of extension, activity, and follow-up of ulcerative colitis. Scand J Gastroenterol 1999;34:1103–7. [DOI] [PubMed] [Google Scholar]
- 7. Antonelli E, Giuliano V, Casella G, et al. . Ultrasonographic assessment of colonic wall in moderate-severe ulcerative colitis: comparison with endoscopic findings. Dig Liver Dis 2011;43:703–6. [DOI] [PubMed] [Google Scholar]
- 8. Parente F, Molteni M, Marino B, et al. . Bowel ultrasound and mucosal healing in ulcerative colitis. Dig Dis 2009;27:285–90. [DOI] [PubMed] [Google Scholar]
- 9. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987;317:1625–9. [DOI] [PubMed] [Google Scholar]
- 10. Civitelli F, Di Nardo G, Oliva S, et al. . Ultrasonography of the colon in pediatric ulcerative colitis: a prospective, blind, comparative study with colonoscopy. J Pediatr 2014;165:78–84.e2. [DOI] [PubMed] [Google Scholar]
- 11. Ordás I, Rimola J, García-Bosch O, et al. . Diagnostic accuracy of magnetic resonance colonography for the evaluation of disease activity and severity in ulcerative colitis: a prospective study. Gut 2013;62:1566–72. [DOI] [PubMed] [Google Scholar]
- 12. Rimola J, Rodriguez S, García-Bosch O, et al. . Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut 2009;58:1113–20. [DOI] [PubMed] [Google Scholar]
- 13. Gomollón F, Dignass A, Annese V, et al. ; ECCO. Third European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016. Part 1: diagnosis and medical management. J Crohns Colitis 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 14. Bressler B, Panaccione R, Fedorak RN, Seidman EG. Clinicians’ guide to the use of fecal calprotectin to identify and monitor disease activity in inflammatory bowel disease. Can J Gastroenterol Hepatol 2015;29:369–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.