Abstract
Background
Linezolid exhibits remarkable sterilizing effect in tuberculosis; however, a large proportion of patients develop serious adverse events. The congener tedizolid could have a better side-effect profile, but its sterilizing effect potential is unknown.
Methods
We performed a 42-day tedizolid exposure-effect and dose-fractionation study in the hollow fiber system model of tuberculosis for sterilizing effect, using human-like intrapulmonary pharmacokinetics. Bacterial burden was examined using time to positivity (TTP) and colony-forming units (CFUs). Exposure-effect was examined using the inhibitory sigmoid maximal kill model. The exposure mediating 80% of maximal kill (EC80) was defined as the target exposure, and the lowest dose to achieve EC80 was identified in 10000-patient Monte Carlo experiments. The dose was also examined for probability of attaining concentrations associated with mitochondrial enzyme inhibition.
Results
At maximal effect, tedizolid monotherapy totally eliminated 7.1 log10 CFU/mL Mycobacterium tuberculosis over 42 days; however, TTP still demonstrated some growth. Once-weekly tedizolid regimens killed as effectively as daily regimens, with an EC80 free drug 0- to 24-hour area under the concentration–time curve-to-minimum inhibitory concentration (MIC) ratio of 200. An oral tedizolid of 200 mg/day achieved the EC80 in 92% of 10000 patients. The susceptibility breakpoint was an MIC of 0.5 mg/L. The 200 mg/day dose did not achieve concentrations associated with mitochondrial enzyme inhibition.
Conclusions
Tedizolid exhibits dramatic sterilizing effect and should be examined for pulmonary tuberculosis. A tedizolid dose of 200 mg/day or 700 mg twice a week is recommended for testing in patients; the intermittent tedizolid dosing schedule could be much safer than daily linezolid.
Keywords: optimal dose, intermittent dosing, pharmacokinetics/pharmacodynamics, time to positivity, susceptibility breakpoint
Linezolid has been shown to have dramatic sterilizing effect in patients with tuberculosis (TB), even when it is the only effective drug in the treatment of patients with extensively drug-resistant TB (XDR-TB) [1–3]. Unfortunately, this efficacy comes at the cost of high toxicity rates, encountered in >35% of patients treated with the standard doses [1, 3, 4]. Recently, a new oxazolidinone, tedizolid (formerly DA-7157; prodrug DA-7218), was licensed for use against gram-positive bacterial skin and soft tissue infections [5]. In the hollow fiber system model of intracellular pulmonary Mycobacterium avium disease, tedizolid maximal kill (Emax) was higher than with linezolid [6, 7]. Similarly, for intracellular Mycobacterium tuberculosis (Mtb) infection that is typical of disseminated pediatric disease and comprises up to 20% of cavitary bacillary subpopulations in adult-type disease, tedizolid at optimal exposures had >4 log10 colony-forming units (CFU)/mL Mtb kill compared to linezolid optimal exposure after 4 weeks in the hollow fiber system model of tuberculosis (HFS-TB) [8]. However, in adult-type tuberculosis, 80% of bacteria are extracellular [9]. Here, we investigated the efficacy of tedizolid against extracellular semidormant bacteria at pH 5.8, whose kill defines sterilizing effect [10–12].
In theory, tedizolid has several advantages over linezolid in the treatment of chronic pneumonias. First, the epithelial lining fluid concentration (ELF)–to-plasma ratio and the alveolar macrophage, 0- to 24-hour area under the concentration–time curve (AUC0–24)–to-plasma ratio are 40-fold and 20-fold for tedizolid vs 0.14-fold and 3.3-fold for linezolid, respectively [13, 14]. However, the true extents of penetration into TB cavity lesion of each drug are unknown. On the other hand, while linezolid is only 30% protein bound, tedizolid is 90% protein bound, which could reduce the potency of tedizolid [14]. Moreover, the effect of an acidic pH on tedizolid efficacy is unknown. Here, we utilized the HFS-TB to mimic the human intrapulmonary concentration–time profile of tedizolid to identify optimal exposures for Mtb sterilizing effect under these conditions [15–18]. We then used these results to identify the dose of tedizolid that could be used for treatment of tuberculosis. This approach has been found to be accurate in identifying clinical doses, exposures, and response later shown in tuberculosis patients [16–19].
METHODS
First, we searched PubMed for all tedizolid minimum inhibitory concentration (MIC) studies published up to 31 December 2017. The following Medical Subject Heading (MeSH) terms and strategy were used: “minimum inhibitory concentration” OR “MIC” OR “susceptibility” AND “Mycobacterium tuberculosis” AND “tedizolid” OR “DA-7218” OR “TR-701”. Next, we searched for tedizolid tuberculosis pharmacokinetic/pharmacodynamic studies using the MeSH headings “tedizolid” and “tuberculosis.” There was no exclusion of articles by language.
Mtb H37Rv (American Type Culture Collection strain number 27294) was purchased from American Type Culture Collection (Manassas, Virginia). Prior to each experiment, Mtb from stock was grown as described in a number of previous publications [20–22]. Tedizolid, the active moiety of the prodrug tedizolid phosphate, was synthesized by BOC Sciences (Shirley, New York). Hollow fiber cartridges were purchased from FiberCell Systems Inc (Frederick, Maryland). The BACTEC 960 mycobacterial growth tube indicator system (MGIT) and supplies were purchased from Becton Dickinson (Franklin Lakes, New Jersey).
The tedizolid MIC was identified using the MGIT and macrobroth dilution methods [23]. In sterilizing effect HFS-TB experiments, Mtb was transformed from logarithmic growth phase (log-phase growth) to semidormant bacteria state using the methods described elsewhere [18, 24]. Twenty milliliters of the semidormant Mtb culture was inoculated into the peripheral compartment of each of the 22 HFS-TB units conditioned with media acidified to pH 5.8 using citric acid [18]. The systems either had daily administration of tedizolid AUC0–24 exposures of 6, 12, 24, 31, 78, 95, and 143 mg × hour/L or once a week administration with cumulative weekly exposures of 95, 124, and 424 mg × hour/L. The nontreated control systems received no drug treatment. There were 2 HFS-TB replicates per dose. On day 0 of the study, central compartments were sampled at 0 hour (before administration of the drug) and at 1, 6, 10, 18, 21, and 23.5 hours after drug administration to measure the tedizolid concentration [6]. We sampled the peripheral compartment of each HFS-TB on days 0, 7, 14, 21, 28, 35, and 42 and processed the samples as described in previous publication to enumerate the total Mtb burden [18, 20–22]. To determine the tedizolid-resistant subpopulations Middlebrook 7H10 agar was supplemented with 3 times the tedizolid MIC and incubated for up to 6 weeks before CFUs were counted.
To measure tedizolid concentrations in samples obtained from the central compartment of each HFS-TB, we used a well-validated method, described in detail previously [6, 8]. The observed concentrations were then modeled using ADAPT 5 software [25]. Steps used for pharmacokinetic modeling were as described in the past [18, 21, 26]. The relationship between effective concentration (EC) and bacterial burden was modeled using the inhibitory sigmoid Emax model in ADAPT 5 and in GraphPad Prism version 7 (La Jolla, California) software. We used 2 readouts of bacterial burden: Mtb log10 CFU/mL, and time to positivity (TTP) in days. The pharmacokinetics/pharmacodynamics (PK/PD) parameter, either AUC0–24/MIC ratio, or peak concentration to MIC (Cmax/MIC), or percentage time concentration persists above MIC (%TMIC), or trough/MIC was examined vs bacterial burden using the inhibitory sigmoid Emax model, and the model with the highest r2 was chosen as linked to outcome. We defined the exposure associated with 80% of maximal kill (EC80) as the target exposure to be achieved for Emax.
We performed Monte Carlo experiments (MCEs) of 10000 adult patients with TB to identify the minimal dose of tedizolid that could achieve or exceed the EC80. For population pharmacokinetic parameter estimates entered in subroutine PRIOR of ADAPT, we used values identified from the study of Flanagan et al [27]. The parameters (between individual variability as % coefficient of variation) were an absorption constant of 1.99 hour-1 (194%), clearance of 6.69 L/hour (30%), central volume of 69.0 L (18%), peripheral volume of 13.6 L (18%), and an intercompartmental clearance of 0.96 L/hour (30%). The ELF-to-plasma ratio of 40 and protein binding of 90% were used to calculate a free drug penetration ratio from plasma of 4 [14]. For the tedizolid MIC distribution, we used results of our literature search, which identified the study by Vera-Cabrera et al [28]. We examined the target attainment probability of how well the dose of 50 mg, 100 mg, and 200 mg would achieve the EC80 in the lung of patients with TB, at each MIC. Cumulative fraction of response was then summated over the MIC distribution, as discussed elsewhere in this supplement [24].
RESULTS
In the literature search we identified only 1 relevant study, performed in mice by us [29]. The study did not perform dose-ranging experiments for tedizolid, or dose-scheduling experiments, but had examined the drug at a single dose in combination with bedaquiline and pretomanid. There were no publications of tedizolid use in TB patients. In the second literature search for the largest distribution of tedizolid MICs in Mtb, we found the study by Vera-Cabrera et al in 2006 of 95 clinical Mtb isolates plus H37Rv, in which MICs were identified using microbroth dilution assay concentrations of 0.015–64 mg/L: the MICs for 50% of the isolates (MIC50) and 90% of the isolates (MIC90) were 0.25 mg/L and 0.5 mg/L, respectively [28]. In experiments with our laboratory strain of Mtb H37Rv, we identified an MIC of 0.25 mg/L with both MGIT and microbroth dilution assays, on 2 separate occasions for each assay.
Tedizolid pharmacokinetics achieved in the HFS-TB was best described using a 1-compartment pharmacokinetic model, based on Akaike information criteria, Bayesian information criteria, and parsimony. The pharmacokinetic model predicted vs observed concentrations were as shown in Supplementary Figure 1. The clearance was 0.013 ± 0.008 L/hour, and volume was 0.249 ± 0.24 L, which translates to a half-life of 13.2 hours.
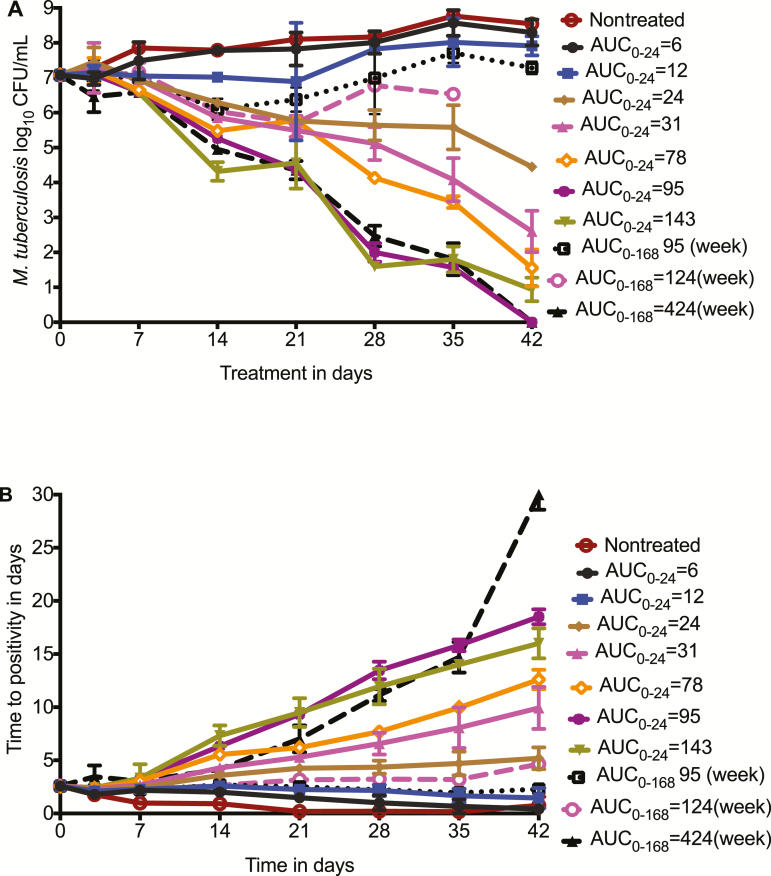
Time-kill curves for the different tedizolid total concentrations (AUCs) are shown in Figure 1. Figure 1A shows AUC vs response for each dosing schedule, starting with the CFU/mL readout. For the daily dosing schedule, exposures between an AUC0–24 of 31 mg × hour/L and 78 mg × hour/L marked the transition to a steep sterilizing effect curve. For the largest once-weekly dose, which is the cumulative weekly (ie, 168 hours) AUC0–168 of 424 mg × hour/L, equivalent to a daily AUC0–24 of 60 mg × hour/L, the microbial kill fell below limits of detection for the CFU/mL assay by day 42, which would indicate complete eradication of Mtb in the HFS-TB replicates. However, using the more sensitive TTP readout, shown in Figure 1B, there was still growth of Mtb in those systems, demonstrating that extinction of the bacterial population had not been achieved with monotherapy in 42 days. Nevertheless, the same pattern of sterilizing effect seen with CFU/mL was seen with TTP. Intermittent dosing was effective; the HFS-TB replicates treated with tedizolid AUC0–168 of 424 mg × hour/L administered once a week had the highest TTP of all at the end of the experiment, which means that intermittently administered tedizolid can achieve dramatic sterilizing effect.
Figure 1.
Tedizolid time-kill curves in the hollow fiber system model. Tedizolid doses are shown as area under the concentration–time curve either daily or for the whole week in the once-weekly doses. It can be seen that even with a once-weekly dosing schedule, a steep sterilizing effect slope was achieved. A, Shows the results as colony-forming units (CFU)/mL. B, Shows results using time to positivity, which, because of greater sensitivity of assay, demonstrates that no systems were totally sterilized by day 42, unlike the CFU/mL results. Abbreviations: AUC, area under the concentration–time curve; CFU, colony-forming units.
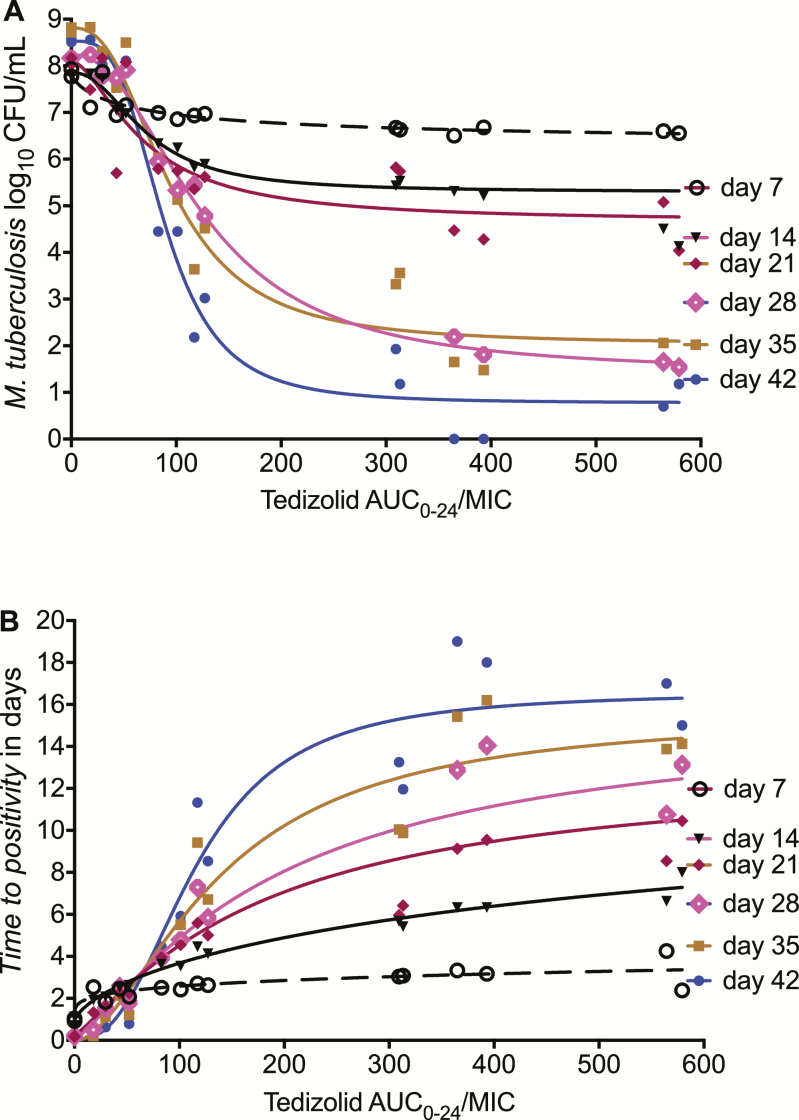
Supplementary Table 1 shows that the highest r2 for PK/PD index vs bacterial burden based on the inhibitory sigmoid Emax model fits. The table shows that whether bacterial burden was expressed as log10 CFU/mL or TTP, the PK/PD index linked with efficacy was the AUC0–24/MIC ratio. Thus, unequivocally, tedizolid free AUC0–24/MIC was the PK/PD driver for sterilizing effect. The relationship between AUC0–24/MIC and log10 CFU/mL burden is shown in Figure 2A. The EC50 varied from an AUC0–24/MIC of 70.22 on day 7 to 125.7 on day 28, consistent with observations with other antituberculosis drugs in the past [20, 30]. The relationship between AUC0–24/MIC and bacterial burden at end of study, on day 42, was described by the equation:
Figure 2.
The relationship between tedizolid exposure and bacterial burden. Inhibitory sigmoid maximal kill modeling for each of the weekly sampling days, chosen because the most intermittent dose was once every 7 days. A, Results showing colony-forming units/mL inhibition with increasing area under the concentration–time curve (AUC)/minimum inhibitory concentration. B, On the other hand, time to positivity (TTP) decreases with increased bacterial burden, so that the “inhibition” is upside down with higher TTP with increasing AUC. This is reflected with a negative Hill slope (H) in the resultant equations. Abbreviations: AUC, area under the concentration–time curve; CFU, colony-forming units; MIC, minimum inhibitory concentration.
Based on this, the EC80, we calculated a free (f) AUC0–24/MIC of 134. The relationship between TTP and fAUC0–24/MIC is shown in Figure 2B. Day 42 results revealed an EC50 of 166 (95% confidence interval [CI], 78.08–153.9), and an H of 2.53 (95% CI, .08–5.13), which translates to an EC80 of 200 (r2 = 0.93). This latter fAUC0–24/MIC of 200 was adopted as the target exposure for optimal sterilizing effect.
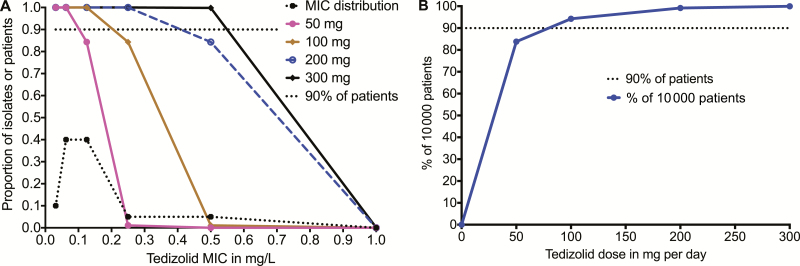
We, performed 10000-patient MCE to identify the dose best able to achieve the EC80fAUC0–24/MIC of 200, based on the MIC distribution of Vera-Cabrera et al [28]. With the clinical dose of 200 mg/day, we identified a serum mean ± standard deviation AUC0–24 mg/L of 31.0 ± 6.6 in the 10000 patient simulation, which similar to the steady-state serum AUC0–24 mg/L values of 29.2 ± 6.2 and 25.6 ± 8.4 reported for this dose to the US Food and Drug Administration (http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205435s000lbl.pdf). This validates that our MCE-identified concentrations that are clinically meaningful [31]. Figure 3A shows that for the doses of 100 mg/day, target attainment probability (TAP) was 100% in patients with Mtb isolates that had MIC ≤0.125 mg/L, and then fell below 90% one tube dilution higher. In patients treated with tedizolid 200 mg/day, the TAP was 100% until the highest MIC of 0.5 mg/L, at which point it fell to 84%. The cumulative fraction of response, which is the proportion of patients achieving EC80, calculated by taking an expectation over the MIC distribution, is shown for each dose in Figure 3B. The figure shows that the cumulative fraction of response in patients treated with tedizolid 100 and 200 mg/day was 46.63% and 92.17%, respectively. This means that 200 mg/day tedizolid is the dose to be explored for sterilizing effect in patients. Alternatively, the dose could be given as 700 mg twice a week or 1400 mg once a week and would still achieve the same cumulative fraction of response as the 200 mg/day.
Figure 3.
Target attainment of different doses in Monte Carlo simulations. A, Target attainment probabilities (TAPs) of the different oral tedizolid doses at each minimum inhibitory concentration (MIC). The MIC distribution ranged from 0.125–0.5 mg/L for 95 isolates [28]. This MIC distribution and the high epithelial lining fluid concentration–to-plasma ratios [14] were advantageous with regard to high TAP. At the dose of 200 mg a day, the TAP falls from 100% at an MIC of 0.25 mg/L to 84% at an MIC of 0.5 mg/L. B, After summation at each MIC, the cumulative fraction of response for each dose on a “normogram” reveals that the dose of 200 mg would achieve sterilizing effect exposure target in >92% of patients and a dose of 300 mg per day would achieve the sterilizing effect exposure in 99.9% of 10000 patients. Abbreviation: MIC, minimum inhibitory concentration.
DISCUSSSION
There is need for newer compounds that have sterilizing effect in patients with drug-susceptible TB, MDR-TB, and XDR-TB, with several antibiotics being repurposed for that use [3, 32–36]. Oxazolidinones, in the form of linezolid, have demonstrated great promise in that direction. Here, we provide evidence that the congener tedizolid has good sterilizing effect even as monotherapy in the HFS-TB. In comparison to first-line antituberculosis drugs in the same model system, the sterilizing effect kill rates in the same system were better than isoniazid, pyrazinamide, ethambutol, and standard-dose rifampin as monotherapy [18, 21, 37–39]. In the accompanying article, we have shown that tedizolid also had good efficacy against intracellular Mtb, which means that tedizolid may be effective against different bacillary subpopulations encountered in pulmonary cavities and in children with disseminated disease [8]. Thus, at a minimum, tedizolid could be able to replace linezolid in MDR-TB and XDR-TB regimens. However, clinical verification of this sterilizing effect is still required.
Second, we identified the optimal exposure of tedizolid for sterilizing effect, which was an AUC0–24/MIC of 200, or a cumulative weekly AUC0–168/MIC of 1400. This would also be optimal for intracellular Mtb kill, based on the target exposure AUC0–24/MIC of 188 we identified for that subpopulation in separate experiments [8]. Based on MCE, we identified the oral tedizolid dose of 200 mg a day as the candidate for clinical trials. Since this is an AUC/MIC-driven drug, intermittent therapy such as 700 mg twice a week would be as effective as daily doses, allowing for intermittent tedizolid regimens. Indeed, our experiments demonstrated efficacy even with once-weekly dosing. This would allow combination of this drug for intermittent therapy regimens, without compromising efficacy. Intermittent dosing is a great advantage for TB treatment programs.
Third, we have found that toxicity of oxazolidinones such as linezolid is AUC driven with an inhibitory concentration (IC50) for mitochondrial inhibition of 94 mg × hour/L; however, tedizolid AUC0–24 of 90 mg × hour/L was not associated with such a mitochondrial enzyme inhibition signature [8, 40]. The tedizolid dose of 200 mg a day or 700 mg twice a week achieves an AUC of 90 mg × hour/L over 3.5 days, lower than tedizolid AUC0–24 of 90 mg × hour/L each day that did not generate a mitochondrial toxicity signal [8]. Song et al and Brown et al have proposed that linezolid toxicity is driven by trough concentration [41, 42]. In a recent study, Milosevic et al demonstrated rapid reversal of tedizolid toxic effects upon discontinuous administration and found that an intermittent dosing schedule was what contributed to the drug’s lower toxicity [40]. If so, our proposed twice-weekly dosing schedules could be advantageous as regards to safety without compromising efficacy. However, the clinical safety of our proposed tedizolid dosing scheme over the longer durations of therapy that are used to treat tuberculosis still needs to be established.
Finally, our MCEs allow us to establish a proposed tedizolid clinical susceptibility breakpoint, which was an MIC of 0.5 mg/L at the dose of 200 mg/day. This value is virtually the same as that identified using both clinical response, epidemiologic cutoff values, and PK/PD approaches in a variety of mundane gram-positive cocci [43]. We propose the same as that tentative clinical breakpoint in TB. The approach that uses the HFS-TB followed by MCE has had a good track record in identifying MICs above which patients fail combination therapy in tuberculosis [17, 31, 44–46]. Thus, there is a good probability that this will be the final clinical breakpoint.
Our study has its own limitations. The first limitation is use of only 1 laboratory strain of Mtb in the HFS-TB experiments. However, in the accompanying article, tedizolid was also tested in HFS-TB of H37Ra, while MICs were also identified in Mtb CDC1551, Mtb SS18b, and HN878, which were also within the range of MIC distributions of the clinical isolates identified in our literature search [8, 28]. Indeed, the MIC distribution we identified in our literature search means that tedizolid is likely to be effective against >90% of clinical strains. The second limitation is that we did not detect any tedizolid resistance in the current experiment. This could either be that no drug resistance arose, or more likely that our assay of tedizolid 3 times the MIC on Middlebrook agar did not work. However, despite these limitations, our data are adequate for demonstrating that tedizolid has excellent sterilizing effect against Mtb.
In conclusion, we identified the optimal exposure target of tedizolid for sterilizing activity against Mtb, the susceptibility breakpoint for the optimal dose, and the possibility of intermittent dosing without compromising efficacy. Clinical trials have been designed to combine a tedizolid once-daily 200-mg dose, and a once-weekly dose regimen in combination with other antibiotics with a long half-life in the treatment of MDR-TB, XDR-TB, and drug-susceptible TB.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was provided by the National Institute of Allergy and Infectious Diseases of the National Institutes for Health (grant number R56 AI111985).
Supplement sponsorship. This supplement is sponsored by the Baylor Institute of Immunology Research of the Baylor Research Institute.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lee M, Lee J, Carroll MW, et al. . Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med 2012; 367:1508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coleman MT, Chen RY, Lee M, et al. . PET/CT imaging reveals a therapeutic response to oxazolidinones in macaques and humans with tuberculosis. Sci Transl Med 2014; 6:265ra167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sotgiu G, Centis R, D’Ambrosio L, et al. . Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur Respir J 2012; 40:1430–42. [DOI] [PubMed] [Google Scholar]
- 4. Sotgiu G, Centis R, D’Ambrosio L, Spanevello A, Migliori GB; International Group for the Study of Linezolid. Linezolid to treat extensively drug-resistant TB: retrospective data are confirmed by experimental evidence. Eur Respir J 2013; 42:288–90. [DOI] [PubMed] [Google Scholar]
- 5. Shorr AF, Lodise TP, Corey GR, et al. . Analysis of the phase 3 ESTABLISH trials of tedizolid versus linezolid in acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 2015; 59:864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deshpande D, Srivastava S., Pasipanodya JG, Lee PS, Gumbo T. Tedizolid is highly bactericidal in the treatment of pulmonary Mycobacterium avium-complex disease. J Antimicrob Chemother 2017; 72(Suppl 2):i30–5. [DOI] [PubMed] [Google Scholar]
- 7. Deshpande D, Srivastava S, Pasipanodya J, Gumbo T. Linezolid as treatment of pulmonary Mycobacterium avium disease. J Antimicrob Chemother 2017; 72(Suppl 2):i24–9. [DOI] [PubMed] [Google Scholar]
- 8. Deshpande D, Srivastava S, Nuermberger E, et al Multiparameter responses to tedizolid monotherapy and moxifloxacin combination therapy models of children with intracellular tuberculosis. Clin Infect Dis 2018; 67(Suppl 3):S337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eum SY, Kong JH, Hong MS, et al. . Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest 2010; 137:122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mitchison DA. The action of antituberculosis drugs in short-course chemotherapy. Tubercle 1985; 66:219–25. [DOI] [PubMed] [Google Scholar]
- 11. McCune R, Lee SH, Deuschle K, McDermott W. Ineffectiveness of isoniazid in modifying the phenomenon of microbial persistence. Am Rev Tuberc 1957; 76:1106–9. [DOI] [PubMed] [Google Scholar]
- 12. McDermott W, Tompsett R. Activation of pyrazinamide and nicotinamide in acidic environments in vitro. Am Rev Tuberc 1954; 70:748–54. [DOI] [PubMed] [Google Scholar]
- 13. Conte JE Jr, Golden JA, Kipps J, Zurlinden E. Intrapulmonary pharmacokinetics of linezolid. Antimicrob Agents Chemother 2002; 46:1475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Housman ST, Pope JS, Russomanno J, et al. . Pulmonary disposition of tedizolid following administration of once-daily oral 200-milligram tedizolid phosphate in healthy adult volunteers. Antimicrob Agents Chemother 2012; 56:2627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chigutsa E, Pasipanodya JG, Visser ME, et al. . Impact of nonlinear interactions of pharmacokinetics and MICs on sputum bacillary kill rates as a marker of sterilizing effect in tuberculosis. Antimicrob Agents Chemother 2015; 59:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gumbo T, Pasipanodya JG, Nuermberger E, Romero K, Hanna D. Correlations between the hollow fiber model of tuberculosis and therapeutic events in tuberculosis patients: learn and confirm. Clin Infect Dis 2015; 61(Suppl 1):S18–24. [DOI] [PubMed] [Google Scholar]
- 17. Gumbo T, Pasipanodya JG, Romero K, Hanna D, Nuermberger E. Forecasting accuracy of the hollow fiber model of tuberculosis for clinical therapeutic outcomes. Clin Infect Dis 2015; 61(Suppl 1):S25–31. [DOI] [PubMed] [Google Scholar]
- 18. Gumbo T, Dona CS, Meek C, Leff R. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob Agents Chemother 2009; 53:3197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pasipanodya JG, Nuermberger E, Romero K, Hanna D, Gumbo T. Systematic analysis of hollow fiber model of tuberculosis experiments. Clin Infect Dis 2015; 61(Suppl 1):S10–7. [DOI] [PubMed] [Google Scholar]
- 20. Musuka S, Srivastava S, Siyambalapitiyage Dona CW, et al. . Thioridazine pharmacokinetic-pharmacodynamic parameters “wobble” during treatment of tuberculosis: a theoretical basis for shorter-duration curative monotherapy with congeners. Antimicrob Agents Chemother 2013; 57:5870–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Srivastava S, Pasipanodya JG, Meek C, Leff R, Gumbo T. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J Infect Dis 2011; 204:1951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Srivastava S, Sherman C, Meek C, Leff R, Gumbo T. Pharmacokinetic mismatch does not lead to emergence of isoniazid- or rifampin-resistant Mycobacterium tuberculosis but to better antimicrobial effect: a new paradigm for antituberculosis drug scheduling. Antimicrob Agents Chemother 2011; 55:5085–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clinical and Laboratory Standards Institute. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard. Wayne, PA: CLSI, 2011. [PubMed] [Google Scholar]
- 24. Gumbo T, Alffenaar JC. Pharmacokinetics/pharmacodynamics background and methods and scientific evidence base for dosing of second-line tuberculosis drugs. Clin Infect Dis 2018; 67(Suppl 3):S267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. D’Argenio DZ, Schumitzky A, Wang X.. ADAPT 5 user’s guide: pharmacokinetic/pharmacodynamic systems analysis software. Los Angeles: Biomedical Simulations Resource, 2009. [Google Scholar]
- 26. Hall RG 2nd, Swancutt MA, Meek C, Leff RD, Gumbo T. Ethambutol pharmacokinetic variability is linked to body mass in overweight, obese, and extremely obese people. Antimicrob Agents Chemother 2012; 56:1502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Flanagan S, Fang E, Muñoz KA, Minassian SL, Prokocimer PG. Single- and multiple-dose pharmacokinetics and absolute bioavailability of tedizolid. Pharmacotherapy 2014; 34:891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vera-Cabrera L, Castro-Garza J, Rendon A, et al. . In vitro susceptibility of Mycobacterium tuberculosis clinical isolates to garenoxacin and DA-7867. Antimicrob Agents Chemother 2005; 49:4351–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tasneen R, Betoudji F, Tyagi S, et al. . Contribution of oxazolidinones to the efficacy of novel regimens containing bedaquiline and pretomanid in a mouse model of tuberculosis. Antimicrob Agents Chemother 2016; 60:270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 2013; 208:1464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gumbo T, Angulo-Barturen I, Ferrer-Bazaga S. Pharmacokinetic-pharmacodynamic and dose-response relationships of antituberculosis drugs: recommendations and standards for industry and academia. J Infect Dis 2015; 211(Suppl 3):S96–106. [DOI] [PubMed] [Google Scholar]
- 32. Deshpande D, Srivastava S, Chapagain M, et al. . Ceftazidime-avibactam has potent sterilizing activity against highly drug-resistant tuberculosis. Sci Adv 2017; 3:e1701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deshpande D, Srivastava S, Bendet P, et al. . Antibacterial and sterilizing effect of benzylpenicillin in tuberculosis. Antimicrob Agents Chemother 2018; 62:e02232–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Rijn SP, Srivastava S, Wessels MA, van Soolingen D, Alffenaar JC, Gumbo T. Sterilizing effect of ertapenem-clavulanate in a hollow-fiber model of tuberculosis and implications on clinical dosing. Antimicrob Agents Chemother 2017; 61:e02039–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deshpande D, Srivastava S, Nuermberger E, Pasipanodya JG, Swaminathan S, Gumbo T. A faropenem, linezolid, and moxifloxacin regimen for both drug-susceptible and multidrug-resistant tuberculosis in children: FLAME path on the Milky Way. Clin Infect Dis 2016; 63:S95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aung KJ, Van Deun A, Declercq E, et al. . Successful ‘9-month Bangladesh regimen’ for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis 2014; 18:1180–7. [DOI] [PubMed] [Google Scholar]
- 37. Srivastava S, Musuka S, Sherman C, Meek C, Leff R, Gumbo T. Efflux-pump-derived multiple drug resistance to ethambutol monotherapy in Mycobacterium tuberculosis and the pharmacokinetics and pharmacodynamics of ethambutol. J Infect Dis 2010; 201:1225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gumbo T, Louie A, Liu W, et al. . Isoniazid’s bactericidal activity ceases because of the emergence of resistance, not depletion of Mycobacterium tuberculosis in the log phase of growth. J Infect Dis 2007; 195:194–201. [DOI] [PubMed] [Google Scholar]
- 39. Gumbo T, Louie A, Deziel MR, et al. . Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob Agents Chemother 2007; 51:3781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Milosevic TV, Payen VL, Sonveaux P, Muccioli GG, Tulkens PM, Van Bambeke F. Mitochondrial alterations (inhibition of mitochondrial protein expression, oxidative metabolism, and ultrastructure) induced by linezolid and tedizolid at clinically relevant concentrations in cultured human HL-60 promyelocytes and THP-1 monocytes. Antimicrob Agents Chemother 2018; 62: e01599–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Song T, Lee M, Jeon HS, et al. . Linezolid trough concentrations correlate with mitochondrial toxicity-related adverse events in the treatment of chronic extensively drug-resistant tuberculosis. EBioMedicine 2015; 2:1627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brown AN, Drusano GL, Adams JR, et al. . Preclinical evaluations to identify optimal linezolid regimens for tuberculosis therapy. MBio 2015; 6:e01741–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bien PF, Flanafan S, Passarell J, Fiedler-Kelly J Prokocimer P. Determination of susceptibility breakpoints for the novel oxazolidinone tedizolid. In: 25th Annual European Society of Clinical Microbiology and Infectious Diseases, Copenhagen, Denmark, 2015:P1312. [Google Scholar]
- 44. Gumbo T. New susceptibility breakpoints for first-line antituberculosis drugs based on antimicrobial pharmacokinetic/pharmacodynamic science and population pharmacokinetic variability. Antimicrob Agents Chemother 2010; 54:1484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gumbo T, Chigutsa E, Pasipanodya J, et al. . The pyrazinamide susceptibility breakpoint above which combination therapy fails. J Antimicrob Chemother 2014; 69:2420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gumbo T, Pasipanodya JG, Wash P, Burger A, McIlleron H. Redefining multidrug-resistant tuberculosis based on clinical response to combination therapy. Antimicrob Agents Chemother 2014; 58:6111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.