Abstract
Background
d-cycloserine is used to treat multidrug-resistant tuberculosis. Its efficacy, contribution in combination therapy, and best clinical dose are unclear, also data on the d-cycloserine minimum inhibitory concentration (MIC) distributions is scant.
Methods
We performed a systematic search to identify pharmacokinetic and pharmacodynamic studies performed with d-cycloserine. We then performed a combined exposure-effect and dose fractionation study of d-cycloserine in the hollow fiber system model of tuberculosis (HFS-TB). In parallel, we identified d-cycloserine MICs in 415 clinical Mycobacterium tuberculosis (Mtb) isolates from patients. We utilized these results, including intracavitary concentrations, to identify the clinical dose that would be able to achieve or exceed target exposures in 10000 patients using Monte Carlo experiments (MCEs).
Results
There were no published d-cycloserine pharmacokinetics/pharmacodynamics studies identified. Therefore, we performed new HFS-TB experiments. Cyloserine killed 6.3 log10 colony-forming units (CFU)/mL extracellular bacilli over 28 days. Efficacy was driven by the percentage of time concentration persisted above MIC (%TMIC), with 1.0 log10 CFU/mL kill achieved by %TMIC = 30% (target exposure). The tentative epidemiological cutoff value with the Sensititre MYCOTB assay was 64 mg/L. In MCEs, 750 mg twice daily achieved target exposure in lung cavities of 92% of patients whereas 500 mg twice daily achieved target exposure in 85% of patients with meningitis. The proposed MCE-derived clinical susceptibility breakpoint at the proposed doses was 64 mg/L.
Conclusions
Cycloserine is cidal against Mtb. The susceptibility breakpoint is 64 mg/L. However, the doses likely to achieve the cidality in patients are high, and could be neurotoxic.
Keywords: minimum inhibitory concentrations, cidal activity, tuberculous meningitis, neurotoxicity, Monte Carlo simulations
d-cycloserine was discovered by 2 independent teams as a secondary metabolite of Streptomyces orchidaceus in 1954 [1, 2]. Results of its first clinical use were published a year later [3]. d-cycloserine is a World Health Organization (WHO) group C second-line agent, whose main role is in treatment of multidrug-resistant tuberculosis (MDR-TB). Neuropsychiatric toxicity is common, especially psychosis and seizures, which are encountered in 10%–50% of patients [4]. Because these adverse events are possibly concentration-dependent, it will be imperative to identify doses that could kill Mycobacterium tuberculosis (Mtb) at concentrations below those associated with toxicity. Here, we performed a systematic analysis to identify the pharmacokinetics (PK) and pharmacodynamics (PD) of d-cycloserine as related to dosing, and if there were gaps to design studies to fill them.
The mechanisms of action of d-cycloserine, an analogue of d-alanine, are unclear, but there are likely multiple targets, and several resistance mechanisms have been described to date [5–10]. However, the question of what d-cycloserine adds to the current MDR-TB treatment regimens remains. In one murine MDR-TB study, d-cycloserine 300 mg/kg/day for 5 months had no effect on lung or spleen Mtb burden as monotherapy, and in combination with moxifloxacin did not add any effect to moxifloxacin monotherapy [11]. The possible lack of effect in animal models has been attributed by others to the rapid elimination of d-cycloserine from mice, and potentially to antagonism from naturally abundant d-alanine in mice and guinea pigs [12]. To avoid use of animal sera that may contain d-alanine, we examined the efficacy of d-cycloserine in the hollow fiber system model of tuberculosis (HFS-TB), an extracellular model, which utilizes Middlebrook 7H9 broth and relies on l-glutamic acid as the α-amino acid. The HFS-TB has been used extensively to study many first- and second-line agents, with good translational accuracy to patient outcomes [13–16].
METHODS
Systematic Review
Details and steps on our systematic review of d-cycloserine pharmacokinetics and PK/PD studies were as given in the introduction to this supplement [17]. The search terms were used to query PubMed and Web of Science, with date of last search of 13 September 2017. In the search terms detailed in the introduction, “drug name” was “cycloserine” OR “d-cycloserine”, while “alternative drug name” was “SC-49088.”
Materials, Organisms, and Reagents
Mtb H37Ra (American Type Culture Collection [ATCC] 25177) was utilized in HFS-TB experiments, as explained in detail elsewhere [18]. d-cycloserine and niacin (internal standard) were purchased from Sigma-Aldrich (St Louis, Missouri). Hollow fiber cartridges were obtained from FiberCell (Frederick, Maryland). The BACTEC 960 mycobacterial growth indicator tube (MGIT) system (BD, Franklin Lakes, New Jersey) was utilized for monitoring growth and time-to-positivity (TTP).
Minimum Inhibitory Concentrations of Laboratory Strains
The d-cycloserine minimum inhibitory concentrations (MICs) for Mtb H37Ra (ATCC 25177) and H37Rv (ATCC 27294) were determined using 4 methods: Sensititre MYCOTB plate, macrobroth dilution, as well as the 1% indirect proportion method using Middlebrook 7H10, and MGIT [19–21]. For the latter 3 methods, the concentrations 0, 0.5, 1, 2, 4, 8, 16, 32, and 64 mg/L were tested. Next, we examined the microbial kill effect of different d-cycloserine concentrations against extracellular Mtb in test tubes and intracellular Mtb in infected THP-1 cells that were activated for 72 hours with 100 nM of phorbol 12-myristate 13-acetate 12-well plates, and coincubated with drug, using protocols described previously [19, 20].
MICs in MDR-TB and Extensively Drug-resistant TB Clinical Isolates From 4 Countries
First, we performed a literature search to identify any d-cycloserine MIC distributions. Next, a total of 415 pretreatment Mtb isolates cultured from patients enrolled in observational cohorts or from laboratory surveillance studies were cultured and species confirmed by DNA probe [22–30]. MIC testing was performed with the Sensititre MYCOTB plate (Trek Diagnostic Systems, Cleveland, Ohio) at the mycobacterial laboratories of Siriraj Hospital, Mahidol University in Bangkok, Thailand; the International Centre for Diarrhoeal Disease Research in Dhaka, Bangladesh; Kilimanjaro Clinical Research Institute in Moshi, Tanzania; and the Irkutsk Clinical Tuberculosis Hospital in Irkutsk, Russian Federation. MIC data from these Mtb isolates have been previously published in studies of comparative drug-susceptibility methodologies [22–30]. MYCOTB plate results were performed in batches, as previously described [31]. Growth was evaluated visually with a manual viewer at 10–21 days by 2 independent technicians. The MIC was recorded as the lowest antibiotic concentration that prevented visible growth. The H37Rv laboratory strain ATCC 27294 was used for quality control, in each batch. The upper end of the phenotypically wild-type MIC distribution was identified as the tentative epidemiological cutoff (ECOFF) [32].
Hollow Fiber System Model of Tuberculosis
The construction details of the HFS-TB have been described before [16, 33–35]. HFS-TB conditions for log-phase growth Mtb for d-cycloserine are described in detail in the introduction to this supplement [17]. Mtb was inoculated into 10 HFS-TB units, and treated with d-cycloserine doses initiated after 24 hours, to mimic a half-life of 10 hours. d-cycloserine was administered daily to 7 HFS-TB units to achieve peak concentrations of 0, 13, 55, 96, 180, 219, and 257 mg/L whereas 3 of the HFS-TB units were dosed once every week with the lowest, third-lowest, and fourth-lowest daily doses given cumulatively (ie, daily dose times 7 given as single dose each week) to break the co-linearity between the PK/PD indices that would otherwise occur with dose escalation. Treatment duration was for 28 days. The central compartment was sampled for d-cycloserine concentrations at 0, 1, 6, 11, 21, 23.5, 48, 72, 96, 120, 144, and 168 hours after the last dose. d-cycloserine concentrations in these samples were measured using the assay described in the Supplementary Methods. The peripheral compartment was sampled on days 0, 3, 5, 7, 10, 14, 21, and 28 for estimation of Mtb burden using MGIT TTP and colony-forming units (CFU) on Middlebrook 7H10 agar [19, 20, 35]. d-cycloserine–resistant colonies were captured by cultures on agar supplemented with 3 times the d-cycloserine MIC.
Pharmacokinetic and Pharmacodynamic Modeling
Drug concentrations measured in the central compartments of HFS-TB were modeled using ADAPT-5 software. The pharmacokinetic models were used to calculate the 0- to 24-hour area under the concentration–time curve (AUC0–24) and percentage of the 24-hour dosing interval that concentration persisted above MIC (%TMIC), peak concentration to MIC (Cmax/MIC), and AUC0–24/MIC, which were modeled for microbial kill and resistance as outlined in the introduction to this supplement [17]. Optimal exposures were defined as either (1) the exposure associated with 80% of maximal kill (EC80), (2) the exposure associated with cidal effect (1.0 log10 CFU/mL kill below day 0), or (3) the lowest exposure associated with suppression of acquired drug resistance (ADR), which are standard PK/PD definitions [36, 37].
Monte Carlo Experiments for Dose Selection
The rationale and steps for Monte Carlo experiments (MCEs) are described in the introduction to this supplement [17]. We utilized MCEs to determine the proportion of 10000 patients treated with d-cycloserine doses of 250 mg, 500 mg, 750 mg, 1 g, and 1.5 g each day who would achieve the target exposure [17, 18]. For d-cycloserine population pharmacokinetics, we used the results of Alsultan et al (contributed to us by Dr Charles Peloquin) based on 130 patients who had MDR-TB, as well by Chang et al, shown in Table 1 [38, 39]. For lung cavity penetration ratios of d-cycloserine, we used the mean ± standard deviation lung cavity-to-serum penetration ratios of 0.063 ± 0.026 among those who had detectable cycloserine cavitary concentrations [40]. The penetration of d-cycloserine into cerebrospinal fluid (CSF) is about 80%–100% of concurrent serum concentrations in inflamed meninges; case reports suggest that the clearance from subarachnoid space may be slower than in serum [41, 42]. Thus, we utilized a CSF-to-serum penetration ratio of 1.0, essentially the same concentrations as in the blood.
Table 1.
d-Cycloserine Pharmacokinetic Parameters and Variances
| Parameter | Parameters Used in Subroutine PRIOR, Mean ± SD |
10000 Simulated Subjects, Mean ± SD |
|---|---|---|
| Clearance, L/h | 1.16 ± 0.73 | 1.14 ± 0.82 |
| Volume, L | 10.50 ± 3.15 | 10.50 ± 1.79 |
| Absorption constant, h-1 | 5.40 ± 2.11 | 5.40 ± 1.58 |
| TLag, h | 0.46 ± 0.45 | 0.47 ± 0.67 |
| Peak, mg/L, for 250 mg | … | 22.25 ± 3.73 |
Abbreviations: SD, standard deviation; TLag, Time lag.
RESULTS
Systematic Analysis Findings
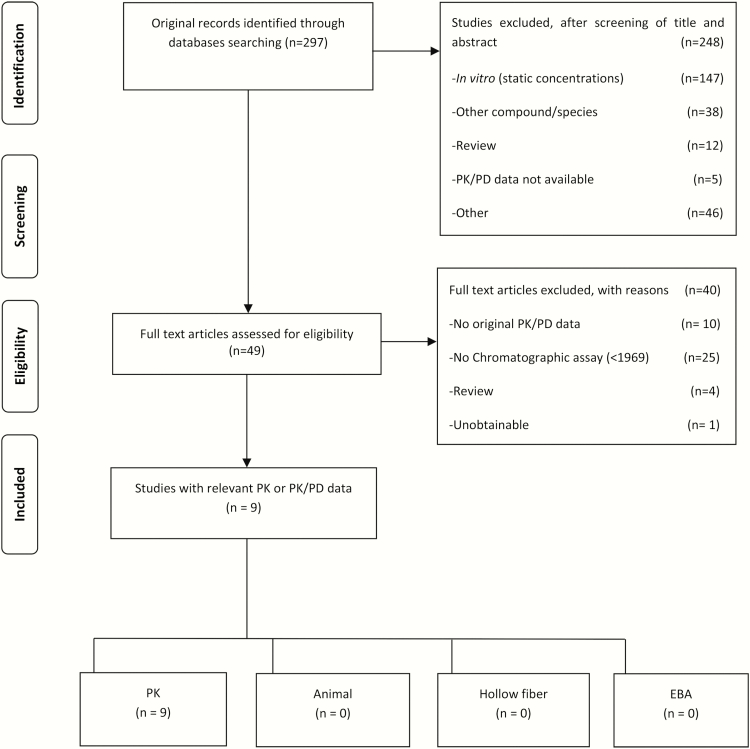
Figure 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram summarizing literature search findings for d-cycloserine, which shows that hitherto there have been no PK/PD studies performed in preclinical models. There were 9 pharmacokinetic studies; 2 were duplicates, leading to 7 studies shown in Supplementary Table 1 [26, 28, 38, 43–46]. Doses of cycloserine used in the pharmacokinetic studies ranged from 250 mg twice a day to 500 mg twice a day. Only 3 studies assessed multiple drug concentration measurements over 1 dosing interval (ie, >1 sample) [38, 47, 48]. In 2 studies, only the mean concentration time curves were shown, which could not be analyzed further. Only Chang et al performed a population PK analysis in 98 time–concentration data points in 14 patients treated with 250–500 mg twice daily [38]. The mean parameter estimates (interindividual variability as percentage coefficient of variation) of 1.38L/h (22.3%) for clearance, and 10.5L (35.1%) for volume of distribution [38]. As regards to formulation, 2 noncompartmental pharmacokinetic analyses reported concentrations of cycloserine after administration of terizidone [46, 47]. No study has yet explored the relationship between concentrations such as peak or AUC0–24 of cycloserine and clinical outcomes such as cure or relapse.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. Abbreviations: EBA, early bactericidal activity; PD, pharmacodynamic; PK, pharmacokinetic.
d-Cycloserine MICs in MDR-TB and Extensively Drug-resistant TB Clinical Isolates
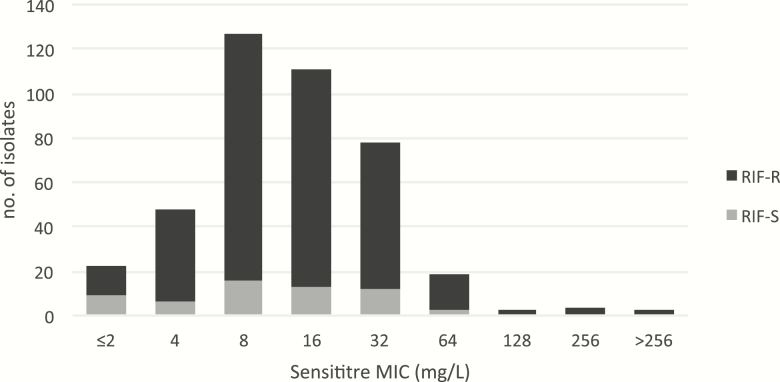
In the literature, and from current studies, the MICs of the H37Ra laboratory stain varied between 4 and 8 mg/L depending on the method used, which was comparable to the figures for H37Rv, shown in Supplementary Table 2. However, because only the Sensititre assay had been used in 4 laboratories to measure the MICs of a total of 415 clinical isolates (Figure 2), we adopted the MIC of 8 mg/L for our PK/PD work and simulations. The tentative ECOFF for Sensititre was found to be 64 mg/L [32, 49].
Figure 2.
Sensititre d-cycloserine minimum inhibitory concentration (MIC) distribution for 415 clinical isolates. Using the MIC distribution of 415 clinical isolates from 4 countries, including rifampicin-susceptible and -resistant isolates, 64 mg/L was chosen as the tentative epidemiological cutoff for d-cycloserine using the Sensititre method based on visual inspection [49]. No (tentative) ECOFFs are available for other media [32]. Abbreviations: ECOFF, epidemiological cutoff; MIC, minimum inhibitory concentration; RIF-R, rifampicin resistant; RIF-S, rifampicin susceptible.
d-Cycloserine Concentration Effect Against Extracellular and Intracellular Mtb
Following 7 days of coincubation with extracellular log-phase growth Mtb, d-cycloserine achieved a maximal kill (Emax) of 5.13 ± 0.28 log10 CFU/mL and a concentration mediating 50% of maximal kill (EC50) of 5.44 ± 0.54 mg/L (r2 = 0.97); maximal kill below stasis (stasis = day 0 bacterial burden) was 4.61 log10 CFU/mL. After 7 days of coincubation with intracellular Mtb, the Emax was 2.55 ± 0.06 log10 CFU/mL and the EC50 was 6.87 ± 0.29 mg/L (r2 = 0.99); maximal kill below stasis was only 1.59 log10 CFU/mL. Thus, d-cycloserine maximal kill of intracellular Mtb was >1000-fold (ie, 3.02 log10) less than for extracellular Mtb, and was also less potent (EC50 is higher). d-cycloserine had no effect on the viability of adherent THP-1 cells.
PK/PD of d-Cycloserine Microbial Kill in the HFS-TB
A 1-compartment model with first-order input and elimination best described the HFS-TB pharmacokinetic parameters, based on Akaike information criteria (AIC). The observed vs model-predicted concentrations are shown in Supplementary Figure 1A. Supplementary Figure 1B and 1C shows the modeled and observed concentration-time profiles, which demonstrate that our dose fractionation exercise was successful.
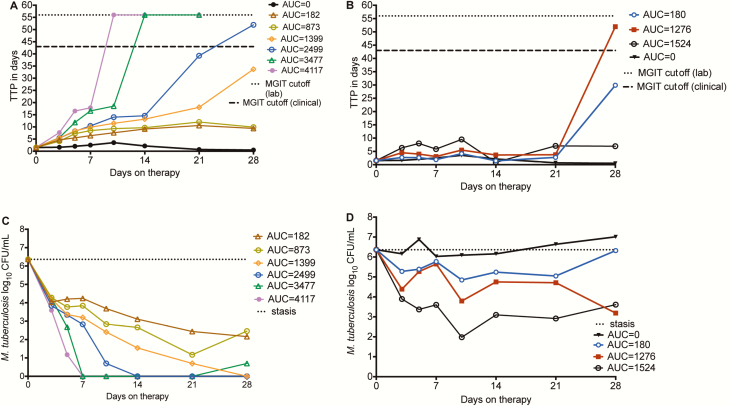
Time-kill curves are shown in Figure 3A for daily therapy dosing schedule (exposures shown as AUC0–24) vs TTP as a measure of bacterial burden. Some concentrations administered as daily schedule achieved negative bacterial burden by day 14, demonstrating that kill rates of extracellular Mtb by d-cycloserine are high. Figure 3B shows that the once-a-week dosing schedule led to generally slower kill rates than daily dosing schedule. Figure 3C and 3D demonstrates the same pattern, based on log10 CFU/mL. The PK/PD index linked to microbial kill was chosen based on AIC scores, as shown in Table 2. The table shows that while AUC0–24/MIC had the best AIC score on day 7, in subsequent weeks %TMIC had the best score. At the end of the study, the relationship between %TMIC and bacterial burden was:
| (1) |
Figure 3.
d-cycloserine microbial kill in the hollow fiber system model of tuberculosis. A, Time to positivity (TTP) as a measure of bacterial burden when d-cycloserine doses were administered daily; the doses are shown as the 0- to 24-hour area under the concentration–time curve (AUC0–24) values. TTP increases as bacterial burden decreases. The highest 2 doses with an area under the concentration–time curve (AUC) of 182 and 273 mg × h/L achieved TTP >56 days, and thus negative culture. The TTP >56 days is a more stringent cutoff point for negative cultures compared to the 42 days used in the clinic, though this varies between clinical laboratories. B, The once-weekly regimens did worse, with TTPs only increasing after 3 weeks; doses are shown as AUC0–24 values. C, When microbial kill was measured using colony-forming units (CFU)/mL, the 2 highest doses achieved negative cultures by day 7, unlike what was seen with TTP. The CFU/mL assay is less sensitive at lower bacterial burdens. D, Based on CFU/mL, the once-weekly dosing schedules demonstrated microbial kill during the first 10 days, then failed, with regrowth after 21 days. The kill slopes were less steep compared to the daily dosing schedule. Doses are shown as AUC0–24 values. AUC, area under the concentration–time curve; CFU, colony-forming units; MGIT, mycobacterial growth indicator tube; TTP, time to positivity.
Table 2.
Akaike Information Criteria for Pharmacokinetic/Pharmacodynamic Indices on Different Sampling Days in the Hollow Fiber System Model of Tuberculosis
| PK/PD Index | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|
| Microbial kill | ||||
| AUC0–24/MIC | 20.22 | 25.82 | 29.99 | 30.85 |
| Peak/MIC | 35.03 | 36.00 | 36.39 | 37.69 |
| %TMIC | 29.96 | 23.53 | 18.47 | 20.69 |
| Resistance log10 CFU/mL | ||||
| AUC0–24/MIC | –8.37 | –0.35 | 9.30 | 13.44 |
| Peak/MIC | 7.42 | 12.35 | 14.81 | 16.89 |
| %TMIC | 11.62 | 5.69 | 8.94 | –0.23 |
Values in bold indicate the PK/PD parameter with the lowest AIC scores for microbial kill and ADR on the different sampling days.
Abbreviations: %TMIC, percentage of time concentration persisting above the minimum inhibitory concentration; AUC0–24, 0- to 24-hour area under the concentration–time curve; CFU, colony-forming units; MIC, minimum inhibitory concentration; PK/PD, pharmacokinetic/pharmacodynamic.
where the EC50 is %TMIC of 40.25. From equation 1, we calculated the %TMIC associated with stasis as 20%; that mediating 1.0 log10 CFU/mL kill (cidal) was 30%, while EC80 was a %TMIC of 64%.
Evolution of Resistance in the HFS-TB
The change in size of the d-cycloserine–resistant subpopulation with treatment duration is shown in Supplementary Figure 2A and 2B. AIC scores for PK/PD parameter vs the size of the ADR are also shown in Table 2, which shows that the PK/PD index linked to resistance “wobbled” from AUC0–24/MIC during the first 2 weeks to %TMIC by the end of the experiment [50]. The relationship between %TMIC and size of the d-cycloserine–resistant subpopulation, on day 28 (Supplementary Figure 2C) was:
| (2) |
From equation 2, we calculated the %TMIC associated with complete ADR suppression as 100%.
Monte Carlo Experiments to Identify d-Cycloserine Clinical Doses
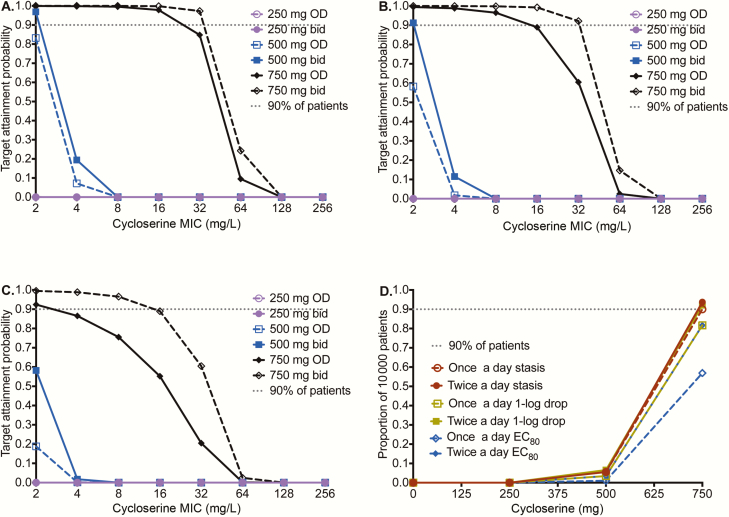
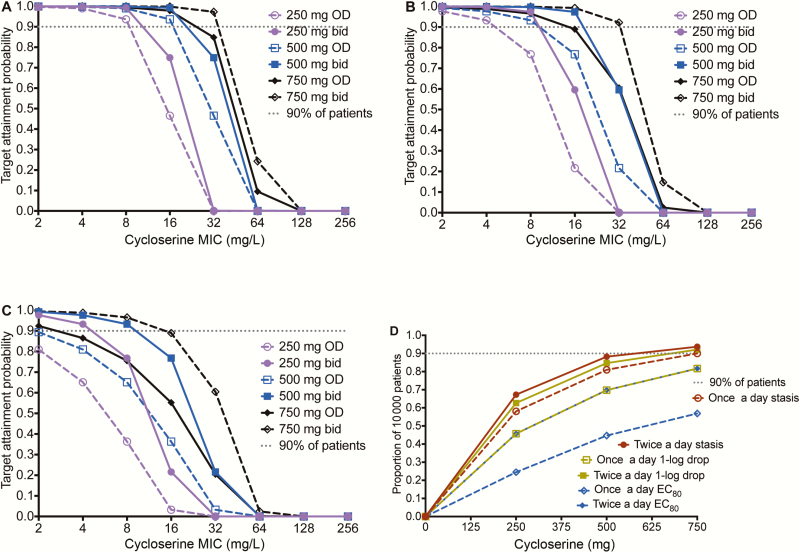
First we examined how well doses would achieve the exposure that suppresses ADR (%TMIC = 100%); we abandoned the exercise as even doses of 3000 mg a day performed poorly. Table 1 compares the MCE-derived PK parameters to those in patients, an internal validation step. Figure 4 shows the performance of several different doses and schedules at achieving (1) %TMIC associated with stasis, (2) %TMIC associated with cidal effect (1.0 log10 CFU/mL kill), and (3) the EC80, in pulmonary tuberculosis cavities. Figure 4A–C shows that all doses performed poorly, reflecting the uniformly poor penetration of d-cycloserine into the pulmonary cavity. Based on the poorer efficacy and lower potency against intracellular Mtb demonstrated earlier, performance of doses would even be worse against intracellular bacilli. The performance of all doses fell when examined for the ability to achieve %TMIC associated with 1.0 log10 CFU/mL drop (cidal effect) in Figure 4B and fell even more in achieving the EC80 target. In the highest dose tested, of 750 mg twice a day, the target attainment probability (TAP) fell below 90% at an MIC of 64 mg/L for cidal effect and 32 mg/L for EC80 target. Figure 4D shows that the dose best able to achieve cidal effect was 750 mg twice a day. Based on this, the dose of 750 mg twice a day was chosen as at least being able to achieve cidal effect inside most patients’ pulmonary cavities.
Figure 4.
Performance of different d-cycloserine doses for pulmonary disease. d-cycloserine minimum inhibitory concentrations (MICs) based on the Sensititre assay were used. A, For the stasis target (ie, exposure at which there is no kill or growth compared to day 0), the target attainment probabilities (TAPs) are shown for doses of 250–750 mg at 2 dosing schedules. There is clear separation of performance by the 750-mg doses (shown in black) from the rest of the doses. At 750 mg daily, TAP falls at MICs of ≥32 mg/L. B, For cidal activity, the same pattern is shown, except that now there is also separation of TAPs between 750 mg once daily and 750 mg twice daily. At the latter dose and schedule, the MIC above which TAP falls below 90% is 32 mg/L. C, For the target of 80% of maximal kill (EC80), even the dose if 750 mg twice a day would fall below 90% at around 32 mg/L. D, When an expectation was taken over the entire MIC range, the dose of 750 mg twice daily was able to achieve the exposure target of stasis, cidal effect, and EC80, in 93%, 92%, and 81% of 10000 patients, respectively. Abbreviations: bid, twice daily; EC80, 80% of maximal kill; MIC, minimum inhibitory concentration; OD, once daily.
If we bargained to get good microbial kill in the meninges, based on the better penetration of d-cycloserine into subarachnoid space, in exchange for possible increased neurotoxicity, then target attainment in tuberculous meningitis was as shown in Figure 5. A 500 mg twice a day achieved a cumulative fraction of response of 88.2% for the stasis target, 84.7% for cidal effect, and 69.8% for the EC80 target. While the target is still shy of the 90% target attainment, it is still acceptable performance when balanced against possible increase neurotoxicity at higher doses.
Figure 5.
Target attainment probability (TAP) of various d-cycloserine doses in meningitis. Shown are TAP values for 10000 patients with tuberculous meningitis (TBM) and extracavitary compartments of tuberculosis. d-cycloserine has better penetration into cerebrospinal fluid (CSF) than lung cavities. A, The TAP for stasis target exposure demonstrated good performance for the dose of 500 mg twice daily, and only fell below 90% at a minimum inhibitory concentration (MIC) of 32 mg/L, and for the highest dose at an MIC of 64 mg/L. B, For the cidal exposure target, 500 mg twice daily and 750 mg twice daily achieved good TAP up to an MIC of 32 mg/L, such that the majority of patients with meningitis would be expected to achieve exposures that have cidal effect in CSF at these doses. This means that as long as MICs were <64 mg/L, cidal effect was achieved. Given the paucibacillary nature of TBM, this is thought to be the best pharmacokinetic/pharmacodynamic exposure target for meningitis. C, Even with good penetration into the subarachnoid space, the 80% of maximal kill was more difficult to achieve at all doses for MICs ≥16 mg/L. D, Cumulative fraction of patients who achieve responses for each dose. The dose of 500 mg twice daily falls just short of 90% for the cidal effect, but is proposed for use in TBM. Abbreviations: bid, twice daily; EC80, 80% of maximal kill; MIC, minimum inhibitory concentration; OD, once daily.
DISCUSSION
First, we found a tentative ECOFF value of 64 mg/L based on the Sensititre MYCOTB assay (Figure 2). In MCEs, at the proposed dose of 750 mg twice a day for pulmonary tuberculosis, the TAP falls below 90% at an MIC of 64 mg/L for the cidal effect target (Figure 4). This means that for d-cycloserine both the PK/PD-based approach and the tentative ECOFF are in agreement. Based on both, we propose a susceptibility breakpoint of 64 mg/L.
Second, d-cycloserine had impressive kill rates against Mtb in the HFS-TB, which rivaled some of the first-line compounds and fluoroquinolones [18, 36, 51]. Thus the drug is not “static,” as has been traditionally thought. Instead, the limitation of this drug stems from its poor lung cavitary penetration in patients. Another problem is the poor kill of intracellular Mtb, which constitutes about 20% of all bacteria in lung cavities [52]. As a result, we propose 750 mg twice a day for pulmonary tuberculosis. While our dose findings are MCE-based, and thus require clinical confirmation, it is interesting that we found that 500 to 750 mg a day would achieve the target of EC80 in 1%–56% of patient’s in lung cavities. In 1962, Rivera and Browning treated 90 patients with 500 mg d-cycloserine plus 3 g pyrazinamide each day; sputum conversion plus disappearance of cavity was achieved in only 11% of patients [53]. Similarly, Epstein and colleagues treated patients with pulmonary disease with 1000–1500 mg/day of d-cycloserine [54]. In patients without prior treatment, the culture conversion occurred in 11% on isoniazid-cycloserine combination compared with 13% on d-cycloserine monotherapy; in drug-resistant tuberculosis, 33% achieved negative cultures on solid agar. Thus, at high doses, 1000–1500 mg/day cure was achieved in 10%–30% of patients with pulmonary tuberculosis, which is consistent with our MCE.
Third, d-cycloserine has good CSF penetration, which likely explains the high rates of neurotoxicity. If we made the Faustian bargain to treat tuberculous meningitis with the potentially neurotoxic d-cycloserine, at the high doses of 500 mg twice a day that we propose, kill rates would be equivalent to those of fluoroquinolones. However, it could be that the neuropsychiatric problems would be worse during treatment for a disease that is by definition neurotoxic. Vitamin B6 could be prescribed concurrently with the d-cycloserine to minimize toxicity, with some experts recommending 50 mg of pyridoxine for every 250 mg of d-cycloserine [53]. However, human dosing trials of d-cycloserine and pyridoxine in combination with standard tuberculous meningitis regimens have not been performed, and the effectiveness of this approach as yet unproven.
There are some limitations to our study. First, we did not examine d-cycloserine in combination with other drugs for synergistic effects, which could potentially lower the exposures of d-cycloserine needed. Second, in contrast to our work with gatifloxacin, levofloxacin, and ethionamide, we had no clinical data to validate the doses or susceptibility breakpoints we identified [18, 51, 55]. Third, we relied on the Sensititre assay, which is not endorsed by WHO and could differ from conventional media, to define a tentative ECOFF. As an example, the MGIT-derived MIC was systematically 1-tube dilution lower than MYCOTB-derived for our quality control isolate, which could affect the PK/PD target exposures, and hence optimal dose. These limitations mean that the accuracy of our d-cycloserine dose choices remains to be prospectively confirmed in a clinical study.
In summary, d-cycloserine has cidal effects against Mtb, provided optimal exposures are achieved. In practice, given the poor penetration into human tuberculous cavities, the drug could be effective to some extent in pulmonary disease but at high doses. The drug should be given at least twice daily to optimize exposure and should preferably be used in the intensive phase of treatment due to its poor intracellular and thus sterilizing efficacy. On the other hand, d-cycloserine likely could add to effectiveness of regimens to treat tuberculous meningitis and pulmonary tuberculosis outside cavities, at doses of about 500 mg twice a day.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. T. G. and D. D designed the laboratory studies. J.-W. C. A., N. S., and M. G. G. S. designed and performed the systematic analysis and literature review. T. G., D. D., and M. L. C. performed the hollow fiber studies. C. U. K. and T. S. provided critical feedback and editing on MIC distributions, ECOFF, clinical breakpoints, and MIC methods. H. M. provided information on pharmacokinetics of cycloserine, especially as terizidone. D. D wrote the first draft of the manuscript. P. S. L. performed drug concentration assays. T. K. performed DNA extraction. T. G. performed PK/PD modeling and MCEs. K. D. and T. G. performed the clinical study that identified d-cycloserine penetration into lung cavities. S. G. M., S. B., S. F., O. O., S. P., E. R. H., and S. K. H. identified MICs in the MDR-TB clinical studies and took part in the d-cycloserine population pharmacokinetics study. D. D., S. K. H., and T. G. wrote the manuscript. All authors edited and contributed to the final version of the manuscript.
Acknowledgments. We would like to thank Dr Charles Peloquin (University of Florida) for allowing us use his group’s ongoing population PK study. We acknowledge the work of Dr Onno W. Akkerman, Dr Mathieu S. Bolhuis, and Samiksha Ghimire (University of Groningen, University Medical Center Groningen) on the systematic review.
Financial support. This work was supported by the Baylor Research Institute, Dallas, Texas (to T. G.) and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant numbers R01AI116155 to H. M. and T. G. and U01AI115594 to S. K. H.). C. U. K. is a research associate at Wolfson College, University of Cambridge.
Supplement sponsorship. This supplement is sponsored by the Baylor Institute of Immunology Research of the Baylor Research Institute.
Potential conflicts of interest. C. U. K. is a consultant for the Foundation for Innovative New Diagnostics which involves work for the Cepheid Inc., Hain Lifescience and the WHO. C. U. K. is an advisor to GenoScreen and QuantuMDx Group Ltd. The Bill & Melinda Gates Foundation, Janssen Pharmaceutica, and PerkinElmer covered C. U. K.’s travel and accommodation to present at meetings. The European Society of Mycobacteriology awarded C. U. K. the Gertrud Meissner Award, which is sponsored by Hain Lifescience. The Global Alliance for TB Drug Development Inc. and Otsuka Novel Products GmbH have supplied C.U.K. with antibiotics for in vitro research. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hidy PHH, Hodge EB, Young VV, et al. Structure and reactions of cycloserine. J Am Chem Soc 1955; 77:2345–6. [Google Scholar]
- 2. Kuehl FA, Wolf FJ, Trenner NR, et al. D-4-amino-3-isoxazolidinone, a new antibiotic. J Am Chem Soc 1955; 77:2344–5. [Google Scholar]
- 3. Boyd LJ, Epstein IG, Nair KG. The treatment of human tuberculosis with cycloserine: a year’s progress. Antibiot Annu 1955; 3:141–7. [PubMed] [Google Scholar]
- 4. Takiguchi K, Uezato A, Itasaka M, et al. Association of schizophrenia onset age and white matter integrity with treatment effect of D-cycloserine: a randomized placebo-controlled double-blind crossover study. BMC Psychiatry 2017; 17:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hwang TJ, Wares DF, Jafarov A, Jakubowiak W, Nunn P, Keshavjee S. Safety of cycloserine and terizidone for the treatment of drug-resistant tuberculosis: a meta-analysis. Int J Tuberc Lung Dis 2013; 17:1257–66. [DOI] [PubMed] [Google Scholar]
- 6. Nakatani Y, Opel-Reading HK, Merker M, et al. Role of alanine racemase mutations in Mycobacterium tuberculosis D-cycloserine resistance. Antimicrob Agents Chemother 2017; 61. doi: 10.1128/AAC.01575-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feng Z, Barletta RG. Roles of Mycobacterium smegmatis D-alanine:D-alanine ligase and D-alanine racemase in the mechanisms of action of and resistance to the peptidoglycan inhibitor D-cycloserine. Antimicrob Agents Chemother 2003; 47:283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prosser GA, de Carvalho LP. Metabolomics reveal D-alanine:D-alanine ligase as the target of D-cycloserine in Mycobacterium tuberculosis. ACS Med Chem Lett 2013; 4:1233–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lambert MP, Neuhaus FC. Mechanism of D-cycloserine action: alanine racemase from Escherichia coli W. J Bacteriol 1972; 110:978–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Desjardins CA, Cohen KA, Munsamy V, et al. Genomic and functional analyses of Mycobacterium tuberculosis strains implicate ald in D-cycloserine resistance. Nat Genet 2016; 48:544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fattorini L, Tan D, Iona E, et al. Activities of moxifloxacin alone and in combination with other antimicrobial agents against multidrug-resistant Mycobacterium tuberculosis infection in BALB/c mice. Antimicrob Agents Chemother 2003; 47:360–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoeprich PD. Alanine: cycloserine antagonism. VI. Demonstration of D-alanine in the serum of guinea pigs and mice. J Biol Chem 1965; 240:1654–60. [PubMed] [Google Scholar]
- 13. Pasipanodya JG, Nuermberger E, Romero K, Hanna D, Gumbo T. Systematic analysis of hollow fiber model of tuberculosis experiments. Clin Infect Dis 2015; 61(Suppl 1):S10–7. [DOI] [PubMed] [Google Scholar]
- 14. Gumbo T, Pasipanodya JG, Nuermberger E, Romero K, Hanna D. Correlations between the hollow fiber model of tuberculosis and therapeutic events in tuberculosis patients: learn and confirm. Clin Infect Dis 2015; 61(Suppl 1):S18–24. [DOI] [PubMed] [Google Scholar]
- 15. Gumbo T, Pasipanodya JG, Romero K, Hanna D, Nuermberger E. Forecasting accuracy of the hollow fiber model of tuberculosis for clinical therapeutic outcomes. Clin Infect Dis 2015; 61(Suppl 1):S25–31. [DOI] [PubMed] [Google Scholar]
- 16. Gumbo T, Lenaerts AJ, Hanna D, Romero K, Nuermberger E. Nonclinical models for antituberculosis drug development: a landscape analysis. J Infect Dis 2015; 211(Suppl 3):S83–95. [DOI] [PubMed] [Google Scholar]
- 17. Gumbo T, Alffenaar JWC. An introduction to pharmacokinetics/pharmacodynamics methods and scientific evidence base for dosing of second-line tuberculosis drugs. Clin Infect Dis 2018; 67(Suppl 3):S267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deshpande D, Pasipanodya JG, Srivastava S, et al. Gatifloxacin pharmacokinetics/pharmacodynamics-based optimal dosing for pulmonary and meningeal multidrug-resistant tuberculosis. Clin Infect Dis 2018; 67(Suppl 3):S293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deshpande D, Srivastava S, Pasipanodya JG, et al. Linezolid for infants and toddlers with disseminated tuberculosis: first steps. Clin Infect Dis 2016; 63:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deshpande D, Srivastava S, Chapagain M, et al. Ceftazidime-avibactam has potent sterilizing activity against highly drug-resistant tuberculosis. Sci Adv 2017; 3:e1701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Springer B, Lucke K, Calligaris-Maibach R, Ritter C, Böttger EC. Quantitative drug susceptibility testing of Mycobacterium tuberculosis by use of MGIT 960 and EpiCenter instrumentation. J Clin Microbiol 2009; 47:1773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pholwat S, Heysell S, Stroup S, Foongladda S, Houpt E. Rapid first- and second-line drug susceptibility assay for Mycobacterium tuberculosis isolates by use of quantitative PCR. J Clin Microbiol 2011; 49:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mpagama SG, Heysell SK, Ndusilo ND, et al. Diagnosis and interim treatment outcomes from the first cohort of multidrug-resistant tuberculosis patients in Tanzania. PLoS One 2013; 8:e62034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mpagama SG, Houpt ER, Stroup S, et al. Application of quantitative second-line drug susceptibility testing at a multidrug-resistant tuberculosis hospital in Tanzania. BMC Infect Dis 2013; 13:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Banu S, Rahman SM, Khan MS, et al. Discordance across several methods for drug susceptibility testing of drug-resistant Mycobacterium tuberculosis isolates in a single laboratory. J Clin Microbiol 2014; 52:156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mpagama SG, Ndusilo N, Stroup S, et al. Plasma drug activity in patients on treatment for multidrug-resistant tuberculosis. Antimicrob Agents Chemother 2014; 58:782–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heysell SK, Ahmed S, Ferdous SS, et al. Quantitative drug-susceptibility in patients treated for multidrug-resistant tuberculosis in Bangladesh: implications for regimen choice. PLoS One 2015; 10:e0116795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heysell SK, Moore JL, Peloquin CA, Ashkin D, Houpt ER. Outcomes and use of therapeutic drug monitoring in multidrug-resistant tuberculosis patients treated in Virginia, 2009–2014. Tuberc Respir Dis (Seoul) 2015; 78:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heysell SK, Ogarkov OB, Zhdanova S, et al. Undertreated HIV and drug-resistant tuberculosis at a referral hospital in Irkutsk, Siberia. Int J Tuberc Lung Dis 2016; 20:187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee J, Armstrong DT, Ssengooba W, et al. Sensititre MYCOTB MIC plate for testing Mycobacterium tuberculosis susceptibility to first- and second-line drugs. Antimicrob Agents Chemother 2014; 58:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hall L, Jude KP, Clark SL, et al. Evaluation of the Sensititre MycoTB plate for susceptibility testing of the Mycobacterium tuberculosis complex against first- and second-line agents. J Clin Microbiol 2012; 50:3732–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. World Health Organization. Technical report on critical concentrations for drug susceptibility testing of medicines used in the treatment of drug-resistant tuberculosis. Geneva, Switzerland: Global TB Programme, 2018. Available at: http://apps.who.int/iris/bitstream/10665/260470/1/WHO-CDS-TB-2018.5-eng.pdf?ua=1. Accessed 24 August 2018. [Google Scholar]
- 33. Gumbo T, Louie A, Deziel MR, Parsons LM, Salfinger M, Drusano GL. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J Infect Dis 2004; 190:1642–51. [DOI] [PubMed] [Google Scholar]
- 34. Gumbo T, Louie A, Liu W, et al. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob Agents Chemother 2007; 51:2329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deshpande D, Srivastava S, Nuermberger E, Pasipanodya JG, Swaminathan S, Gumbo T. A faropenem, linezolid, and moxifloxacin regimen for both drug-susceptible and multidrug-resistant tuberculosis in children: FLAME path on the milky way. Clin Infect Dis 2016; 63:S95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gumbo T, Angulo-Barturen I, Ferrer-Bazaga S. Pharmacokinetic-pharmacodynamic and dose-response relationships of antituberculosis drugs: recommendations and standards for industry and academia. J Infect Dis 2015; 211(Suppl 3):S96–106. [DOI] [PubMed] [Google Scholar]
- 37. Ambrose PG, Bhavnani SM, Rubino CM, et al. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it’s not just for mice anymore. Clin Infect Dis 2007; 44:79–86. [DOI] [PubMed] [Google Scholar]
- 38. Chang MJ, Jin B, Chae JW, et al. Population pharmacokinetics of moxifloxacin, cycloserine, p-aminosalicylic acid and kanamycin for the treatment of multi-drug-resistant tuberculosis. Int J Antimicrob Agents 2017; 49:677–87. [DOI] [PubMed] [Google Scholar]
- 39. Alsultan AN, Neely M, Alghamdi W, et al. Population pharmacokinetics of cycloserine. In: 10th International Workshop on Clinical Pharmacology of Tuberculosis Drugs, Atlanta, GA, 2017. [Google Scholar]
- 40. Dheda K, Lenders L, Magombedze G, et al. Drug penetration gradients associated with acquired drug resistance in tuberculosis patients [manuscript published online ahead of print 7 June 2018]. Am J Respir Crit Care Med 2018. doi:10.1164/rccm.201711-2333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Donald PR. Cerebrospinal fluid concentrations of antituberculosis agents in adults and children. Tuberculosis (Edinb) 2010; 90:279–92. [DOI] [PubMed] [Google Scholar]
- 42. DeVincenzo JP, Berning SE, Peloquin CA, Husson RN. Multidrug-resistant tuberculosis meningitis: clinical problems and concentrations of second-line antituberculous medications. Ann Pharmacother 1999; 33:1184–8. [DOI] [PubMed] [Google Scholar]
- 43. Lee SH, Seo KA, Lee YM, et al. Low serum concentrations of moxifloxacin, prothionamide, and cycloserine on sputum conversion in multi-drug resistant TB. Yonsei Med J 2015; 56:961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Han M, Jun SH, Lee JH, Park KU, Song J, Song SH. Method for simultaneous analysis of nine second-line anti-tuberculosis drugs using UPLC-MS/MS. J Antimicrob Chemother 2013; 68:2066–73. [DOI] [PubMed] [Google Scholar]
- 45. Hung WY, Yu MC, Chiang YC, et al. Serum concentrations of cycloserine and outcome of multidrug-resistant tuberculosis in northern Taiwan. Int J Tuberc Lung Dis 2014; 18:601–6. [DOI] [PubMed] [Google Scholar]
- 46. Court R, Wiesner L, Stewart A, et al. Steady state pharmacokinetics of cycloserine in patients on terizidone for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2018; 22:30–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zítková L, Tousek J. Pharmacokinetics of cycloserine and terizidone. A comparative study. Chemotherapy 1974; 20:18–28. [DOI] [PubMed] [Google Scholar]
- 48. Yew WW, Cheung SW, Chau CH, et al. Serum pharmacokinetics of antimycobacterial drugs in patients with multidrug-resistant tuberculosis during therapy. Int J Clin Pharmacol Res 1999; 19:65–71. [PubMed] [Google Scholar]
- 49. European Committee on Antimicrobial Susceptibility Testing. MIC distributions and the setting of epidemiological cutoff (ECOFF) values EUCAST SOP 10.0, 17 November 2017. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/EUCAST_SOPs/EUCAST_SOP_10.0_MIC_distributions_and_ epidemiological_cut-off_value__ECOFF__setting_20171117.pdf. Accessed 24 August 2018.
- 50. Musuka S, Srivastava S, Siyambalapitiyage Dona CW, et al. Thioridazine pharmacokinetic-pharmacodynamic parameters “wobble” during treatment of tuberculosis: a theoretical basis for shorter-duration curative monotherapy with congeners. Antimicrob Agents Chemother 2013; 57:5870–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Deshpande D, Pasipanodya JG, Mpagama SG, et al. Levofloxacin pharmacokinetics/pharmacodynamics, dosing, susceptibility breakpoints, and artificial intelligence in the treatment of multidrug-resistant tuberculosis. Clin Infect Dis 2018; 67(Suppl 3):S312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eum SY, Kong JH, Hong MS, et al. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest 2010; 137:122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rivera E, Browning RH. Re-treatment therapy with cycloserine-pyrazinamide. Response in cavity closure and disappearance of tubercle bacilli from sputum. Am Rev Respir Dis 1962; 86:937. [DOI] [PubMed] [Google Scholar]
- 54. Epstein IG, Nair KG, Boyd LJ. The treatment of human pulmonary tuberculosis with cycloserine: progress report. Dis Chest 1956; 29:241–57. [DOI] [PubMed] [Google Scholar]
- 55. Deshpande D, Pasipanodya JG, Mpagama SG, et al. Ethionamide pharmacokinetics/pharmacodynamics-derived dose, the role of minimum inhibitory concentrations in clinical outcome, and the resistance arrow of time, in multidrug-resistant tuberculosis. Clin Infect Dis 2018; 67(Suppl 3):S283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.