Abstract
Background
To determine participants’ human immunodeficiency virus (HIV) risk, the Australian preexposure prophylaxis (PreEPX) trial used 6 eligibility criteria derived from the US Centers for Disease Control and Prevention PrEP guidelines. Participants who fulfilled no eligibility criteria could be enrolled if clinically assessed to need PrEP. This study evaluated whether PREPX eligibility criteria correlated with biological HIV risk markers—namely, syphilis, anorectal chlamydia, or anorectal gonorrhea (sexually transmitted infections [STIs]).
Methods
We calculated adjusted odds ratios (aORs) to assess whether eligibility criteria predicted STI diagnoses at enrollment.
Results
We included 1774 participants, of whom 10.2% tested positive for STIs. Eligibility criteria predicted STI diagnoses as follows: (1) aOR 2.5 (95% confidence interval [CI], 1.4–4.4) for condomless anal intercourse (CLAI) with an HIV-positive regular sexual partner (RSP) with detectable viral load; (2) aOR 1.8 (95% CI, 1.3–2.5) for receptive CLAI with casual sexual partners; (3) aOR 1.8 (95% CI, 1.3–2.5) for previous STIs; (4) aOR 2.1 (95% CI, 1.4–3.0) for methamphetamine use; (5) aOR 0.8 (95% CI, .6–1.1) for unsuccessful condom use; and (6) aOR 1.0 (95% CI, .7–1.4) for insertive CLAI when uncircumcised. Of participants enrolled outside eligibility criteria, 7.1% had STIs.
Conclusions
Eligibility criteria 1–4 predicted diagnoses of STIs, but eligibility criteria 5 and 6 did not. Our findings support the use of PrEP eligibility criteria recommended in current guidelines. Participants enrolled outside the eligibility criteria had substantial prevalence of STIs, suggesting that people who request PrEP but do not fulfill eligibility criteria may nonetheless need PrEP.
Keywords: men who have sex with men, gay and bisexual men, syphilis, gonorrhea, chlamydia
The PrEPX trial used eligibility criteria based on the US Centers for Disease Control and Prevention PrEP guidelines. We evaluated whether these eligibility criteria correlated with biological human immunodeficiency virus (HIV) risk markers—namely, syphilis, anorectal chlamydia, and anorectal gonorrhea.
In Australia, human immunodeficiency virus (HIV) is largely concentrated among men who have sex with men (MSM), with almost three quarters of new HIV diagnoses occurring as a result of male-to-male sexual exposure [1]. Human immunodeficiency virus preexposure prophylaxis (PrEP) is the ongoing use of antiretroviral medication to prevent HIV infection in an HIV-negative person who is at ongoing risk of HIV infection. Currently, the most-studied PrEP regimen consists of once-daily coformulated tenofovir and emtricitabine. Several large trials have convincingly demonstrated both the efficacy and safety of PrEP in reducing HIV transmission in MSM [2, 3], with an estimated HIV risk reduction of up to 99% if a PrEP user is optimally adherent [4].
The PrEPX study sought to provide PrEP to 2600 people at high risk of HIV in Victoria, Australia, with the primary aim of reducing HIV transmissions in Victorian MSM by 30% and in the entire Victorian population by 25%. To maximize the cost-effectiveness and public health benefit of PrEP, international guidelines recommend the use of eligibility criteria to select participants who are at risk of HIV. The PrEPX study used eligibility criteria adapted from the Australian PrEP guidelines [5], which, in turn, were based on the 2014 US Centers for Disease Control and Prevention (CDC) guidelines [6]. These eligibility criteria consisted of self-reported behavioral risk markers for HIV, and in this analysis, we aimed to assess whether these behavioral markers correlated with known biological markers for HIV risk—namely, incident syphilis or anorectal infection with chlamydia or gonorrhea [7–11]. To this end, we assessed the prevalence of these sexually transmitted infections (STIs) at enrollment in participants who fulfilled each of the PrEP eligibility criteria to assess how well each eligibility criterion correlated with biological markers of HIV risk.
METHODS
Selection of Participants and Enrollment Criteria
Sample size calculations for the PrEPX study can be found in the Supplementary Appendix of this paper, and the PrEPX protocol has been published in full elsewhere [12]. In brief, to join the PrEPX trial, participants needed to preregister on the PrEPX website. Once the trial opened for enrollment, preregistered participants were sent an invitation to enroll and were advised to attend a participating clinic for assessment by a clinician, who would undertake an HIV risk assessment and medical safety assessment in accordance with the PrEPX protocol. Participants were eligible to enroll in PrEPX if they had no medical contraindications to PrEP use, and if they self-reported behavioral risk of HIV in the preceding 3 months, and if they foresaw an ongoing risk of HIV over the upcoming 3 months. Men who have sex with men were considered to be at risk of HIV if they fulfilled 1 of the following eligibility criteria, all relating to the 3 months preceding enrollment into PrEPX:
Criterion 1: A regular male sexual partner who is HIV-positive, and who is not on treatment and/or with a detectable viral load, with whom condoms were not consistently used.
Criterion 2: Any receptive condomless anal intercourse with a casual male partner who is HIV-positive or has an unknown HIV status.
Criterion 3: A previous diagnosis of anorectal gonorrhea, anorectal chlamydia, or infectious syphilis in the last 3 months.
Criterion 4: Methamphetamine use.
Criterion 5: More than 1 episode of anal intercourse when correct and consistent condom use was not achieved. For example, a history of broken condoms or slipped off condoms.
Criterion 6: More than 1 episode of insertive condomless anal intercourse where the HIV serostatus of their partner(s) was not known, or the partner was HIV positive and not on antiretroviral treatment, and the study participant is uncircumcised.
At the enrollment visit, to determine participants’ eligibility to join the trial, clinicians completed an online enrollment questionnaire that included questions on what eligibility criteria were fulfilled by the patient. Multiple eligibility criteria could be selected, based on what risks the participant reported. Participants who fulfilled none of the above-listed eligibility criteria could still be enrolled by their treating clinician under an exemption clause, if their clinician felt that it was in the participant’s best interest to start PrEP. For these participants, clinicians recorded in free text the reason why the patient was enrolled under the exemption clause.
Prior to the commencement of PrEPX, people in Victoria were able to access PrEP in 2 ways. First, the Australian Therapeutic Goods Administration allows people to self-import medication for personal use if they have a valid prescription from an Australian-registered medical practitioner. People accessing PrEP this way would order it through the Internet from an overseas pharmacy. Second, in early 2014 a small PrEP demonstration project named the VicPrEP study, in which PrEP was provided to 115 participants, commenced in Victoria. The VicPrEP study neared completion as the PrEPX study commenced. The PrEPX study allowed enrollment by participants who were accessing PrEP through either self-importation or as part of the VicPrEP study. Because preexisting PrEP use may affect responses to the PrEPX eligibility criteria, we conducted a separate analysis of responses from participants who were PrEP-naive at the time of enrollment.
Enrollment into the PrEPX study ceased on 1 April 2018; this analysis included participants who enrolled between 26 July 2016 and 23 December 2016, inclusive.
Collection of Data on Sexually Transmitted Infections
At their enrollment visit, participants were asked to undergo full STI screening, including HIV serology, syphilis serology, and testing for chlamydia and gonorrhea by first-pass urine, an anal swab, and an oropharyngeal swab. This was recommended for all participants, irrespective of a history of anal sex or oral sex, in accordance with the Australian STI testing guidelines for MSM [13]. A new diagnosis of infectious syphilis was defined as a newly positive specific syphilis serology test (enzyme immunoassay [EIA], Treponema pallidum particle agglutination assay [TPPA], or Treponema pallidum haemagglutination assay [TPHA]), or a 4-fold rise in rapid plasma reagin (RPR) titer in someone with a previous history of syphilis, or a positive polymerase chain reaction test on a swab of a syphilis chancre. Laboratory testing for Chlamydia trachomatis and Neisseria gonorrhoeae was by nucleic acid amplification testing (NAAT) of urine and swabs, because NAAT has been shown to be twice as sensitive as culture at the anorectum, and five times as sensitive as culture at the oropharynx at detecting Neisseria gonorrhoeae [14].
Sexually transmitted infection results were extracted from the medical records of participating clinics and pathology services using data extraction software (GRHANITE). Not every PrEPX clinic had data extraction capabilities, and hence this study only included data from those clinics whose data were extractable.
We also compared the frequency of STIs in our participants with the frequency of the same STIs among HIV-negative MSM who attended the general STI clinic at the Melbourne Sexual Health Centre (MSHC) during the same time period. These aggregate data were extracted from the clinic’s internal database by MSHC research staff.
Statistical Analyses
For this analysis, we created a single binary variable to signify a diagnosis of syphilis, anorectal chlamydia, and/or anorectal gonorrhea because these STIs are known markers of HIV risk [7–10]. This binary variable (labelled “STI”) was positive if participants tested positive for anorectal chlamydia or anorectal gonorrhea or had a diagnosis of a new syphilis infection, even if they had not completed full testing for these STIs. The binary STI variable was negative if participants had completed full testing for these STIs and tested negative for all of them. Participants who did not test positive for any of these STIs and who did not complete full testing for STIs were treated as missing data.
We considered the fulfillment of each eligibility criterion to be a statistically independent event. Using logistic regression analyses, we calculated crude odds ratios (ORs) to assess how strongly each eligibility criterion predicted positivity of the binary STI variable at enrollment. Because participants could fulfill multiple eligibility criteria, we adjusted odds ratios (aORs) for fulfillment of the other eligibility criteria. Because our analyses were intended to only measure the predictive value of each eligibility criterion on participants’ “STI” risk, we did not adjust our analyses for other confounders, such as age.
Ethics and Consent
Ethics approval was obtained from the Alfred Hospital Ethics Committee, Melbourne, Australia (no. 100/16). Participants signed a consent form that detailed what data would be collected.
RESULTS
Demographics and Condom Use
Two thousand eight male participants enrolled at clinics with extractable STI data, of which 1774 participants had sufficient STI data to create the binary STI variable, as described. Two hundred thirty-four men were excluded from the analysis for having missing STI data. Compared with excluded participants, participants who were included in this analysis were slightly older (33 y vs 35 y; P = .03 by Mann-Whitney test) but had a similar frequency of consistent condom use for anal sex with casual partners (33.1% vs 30.3%; P = .54 by χ2 test).
Sexually Transmitted Infections
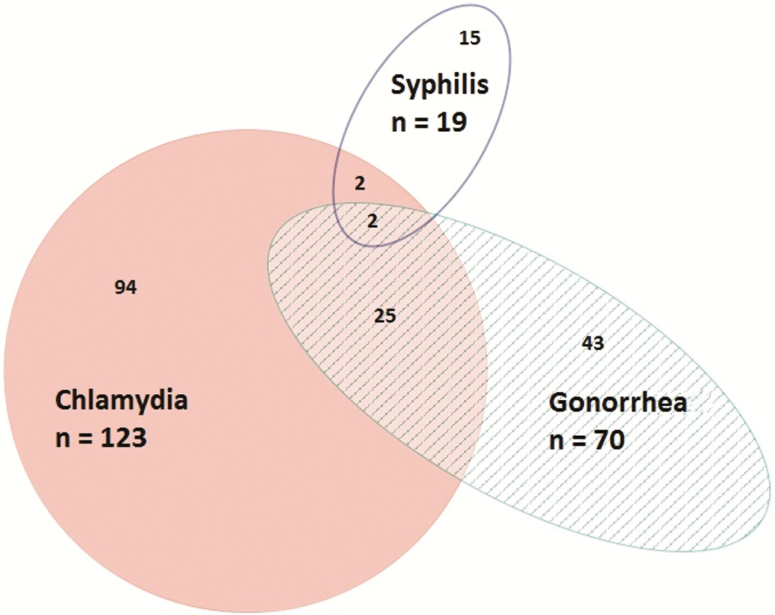
Of the 1774 participants included in the STI analysis, 181 (10.2%) tested newly positive for syphilis, anorectal gonorrhea, and/or anorectal chlamydia at enrollment. All 1774 participants were tested for syphilis by serology, of whom 19 (1.1%) had a result consistent with newly diagnosed infectious syphilis. One thousand seven hundred seventy-two men were tested for anorectal chlamydia, of whom 123 (6.9%) tested positive. One thousand seven hundred sixty-six men were tested for anorectal gonorrhea, of whom 70 (4.0%) tested positive. The distribution of these STIs is illustrated in Figure 1.
Figure 1.
Proportional Venn diagram of the distribution of newly diagnosed syphilis and anorectal chlamydia and anorectal gonorrhea in participants who were included in our binary “STI” variable (n = 1774). One hundred eighty-one (10.2%) participants tested positive for at least 1 sexually transmitted infection (STI). Presented numbers are absolute numbers of diagnoses.
To provide some context for the STI risk experienced by HIV-negative MSM in Melbourne during the same time period, the general STI clinic at the Melbourne Sexual Health Centre conducted 7324 consultations for HIV-negative MSM; of these, 5020 MSM completed testing for anal STIs and syphilis, and 716 (14.3%) tested positive for anal chlamydia, anal gonorrhea, or infectious syphilis: 92 men (1.8%) had infectious syphilis, 399 men (7.9%) had anorectal chlamydia, and 292 men (5.8%) had anorectal gonorrhea.
Associations Between Sexually Transmitted Infection Positivity and Eligibility Criteria
After adjusting for enrollment under the other eligibility criteria, the diagnosis of an STI was most strongly predicted by recent condomless anal sex with an HIV-positive regular sexual partner (aOR, 2.5; 95% confidence interval [CI], 1.4–4.4; P = .002) followed by methamphetamine use (aOR, 2.1; 95% CI, 1.4–3.0; P < .001), receptive condomless anal sex with a casual sexual partner (aOR, 1.8; 95% CI, 1.3–2.5; P = .001), and an STI diagnosis in the 3 months prior (aOR, 1.8; 95% CI, 1.2–2.5; P = .003). The diagnosis of an STI at enrollment was not predicted by a history of broken or slipped-off condoms (P = .21) or condomless insertive anal sex with a casual partner (P = .96). Those enrolled under the exemption clause were less likely to be diagnosed with an STI at enrollment (crude OR, 0.6; 95% CI, .40–.90) (Table 1), compared with those who fulfilled 1 of the eligibility criteria.
Table 1.
Number of Participants Who Fulfilled Each of the 6 Eligibility Criteria and Corresponding Positivity of the Sexually Transmitted Infection Variable, and Logistic Regression Analyses of Associations Between Each Eligibility Criterion and Sexually Transmitted Infection Positivity
| Participants (n = 1774) | STI Positivea | |||
|---|---|---|---|---|
| No. (%) | No. (%) | OR (95% CI) | aORb (95% CI) | |
| Criterion 1: CLAI with an HIV-positive male regular sexual partner with a detectable viral load | ||||
| Yes | 78 (4.4) | 17 (21.8) | 2.6** (1.5–4.6) | 2.5** (1.4–4.4) |
| No | 1696 (95.6) | 164 (9.7) | 1.0 (referent) | 1.0 (referent) |
| Criterion 2: Receptive CLAI with a casual sexual partner | ||||
| Yes | 876 (49.4) | 115 (13.1) | 1.9*** (1.4–2.6) | 1.8** (1.3–2.5) |
| No | 898 (50.6) | 66 (7.3) | 1.0 (referent) | 1.0 (referent) |
| Criterion 3: Previous diagnosis of anorectal gonorrhea, anorectal chlamydia, or infectious syphilis | ||||
| Yes | 288 (16.2) | 49 (17.0) | 2.1*** (1.5–3.0) | 1.8** (1.2–2.5) |
| No | 1486 (83.8) | 132 (8.9) | 1.0 (referent) | 1.0 (referent) |
| Criterion 4: Methamphetamine use | ||||
| Yes | 290 (16.3) | 54 (18.6) | 2.4*** (1.7–3.5) | 2.1*** (1.4–3.0) |
| No | 1484 (83.7) | 127 (8.6) | 1.0 (referent) | 1.0 (referent) |
| Criterion 5: Broken condoms or slipped-off condoms | ||||
| Yes | 502 (28.3) | 48 (9.6) | 0.9 (.6–1.3) | 0.8 (.6–1.1) |
| No | 1272 (71.7) | 133 (10.5) | 1.0 (referent) | 1.0 (referent) |
| Criterion 6: Insertive CLAI with a casual sexual partner, when the participant is uncircumcised | ||||
| Yes | 652 (36.8) | 76 (11.7) | 1.3 (.9–1.7) | 1.0 (.7–1.4) |
| No | 1122 (63.2) | 105 (9.4) | 1.0 (referent) | 1.0 (referent) |
| Enrolled despite not meeting any of the above eligibility criteria | ||||
| Yes | 368 (20.7% | 26 (7.1) | 0.6* (.4–.9) | N/A |
| No | 1406 (79.3) | 155 (11.0) | 1.0 (referent) | N/A |
All eligibility criteria relate to the 3 months prior to enrollment. The sum of participants enrolled under each criterion is greater than the total number of participants because participants could be enrolled under multiple criteria.Enrollment under the exemption clause (“None”) is not included in the multivariate analysis because these enrollments were mutually exclusive to enrollments under the other criteria.
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; CLAI, condomless anal intercourse; HIV, human immunodeficiency virus; N/A, not applicable; OR, odds ratio; STI, sexually transmitted infection.
aPositivity of dependent binary sexually transmitted infection variable signifies a new diagnosis of syphilis, anorectal chlamydia, or anorectal gonorrhea at enrollment.
bAdjusted for enrollment under the other eligibility criteria.
*P < .05; **P < .01; ***P < .001.
Participants Who Were Preexposure Prophylaxis–Naive at Enrollment
Of the 1774 included participants, 568 (32.0%) were using PrEP prior to enrollment, either as part of the VicPrEP study (n = 25) or through self-importation (n = 543). Compared with participants who were already using PrEP, participants who were PrEP-naive were more likely to enroll under the exemption clause (14.1% vs 23.9%; P < .001) and were less likely to have a recent history of an HIV-positive regular sexual partner (6.7% vs 3.3%; P < .01), receptive condomless anal sex with a casual partner (60.7% vs 44.0%; P < .001), a recent diagnosis of an STI (21.0% vs 14.0%; P < .001), or insertive condomless anal sex with a casual partner (45.4% vs 32.7%; P < .001). There was no reported difference in methamphetamine use or recent broken or slipped-off condoms (Table 2).
Table 2.
Enrollment of Participants New to Preexposure Prophylaxis Compared With Those Already Using Preexposure Prophylaxis
| Existing PrEP participants (n = 568) | New to PrEP participants (n = 1206) | |
|---|---|---|
| No. (%) | No. (%) | Difference (95% CI) |
| Criterion 1: CLAI with a HIV-positive male regular sexual partner with a detectable viral load | ||
| 38 (6.7) | 40 (3.3) | 3.4%** (1.2–6.0) |
| Criterion 2: Receptive CLAI with a casual sexual partner | ||
| 345 (60.7) | 531 (44.0) | 16.7%*** (11.7–21.6) |
| Criterion 3: Previous diagnosis of rectal gonorrhea, rectal chlamydia, or infectious syphilis | ||
| 119 (21.0) | 169 (14.0) | 7.0%*** (3.11–11.1) |
| Criterion 4: Methamphetamine use | ||
| 100 (17.6) | 190 (15.8) | 1.8% (−1.9 to 5.7) |
| Criterion 5: Broken condoms or slipped-off condoms | ||
| 162 (28.5) | 340 (28.2) | 0.3% (−4.2 to 5.0) |
| Criterion 6: Insertive CLAI with a casual sexual partner, when the participant is uncircumcised | ||
| 258 (45.4) | 394 (32.7) | 12.7%*** (7.7–17.7) |
| Enrolled despite not meeting any of the above eligibility criteria | ||
| 80 (14.1) | 288 (23.9) | 9.8%*** (5.9–13.5) |
All eligibility criteria relate to the 3 months prior to enrollment.
Abbreviations: CI, confidence interval; CLAI, condomless anal intercourse HIV, human immunodeficiency virus; PReP, preexposure prophylaxis.
**P < .01; ***P < .001, calculated by χ2 test.
Compared with participants who were already using PrEP, participants who were PrEP-naive were less likely to have an STI (anal chlamydia, anal gonorrhea and/or syphilis) at enrollment (12.3% vs 9.2%; P = .04). After adjusting for enrollment under the other eligibility criteria, for participants who were PrEP-naive at enrollment, the diagnosis of an STI was most strongly predicted by a recent history of condomless anal sex with an HIV-positive regular partner (aOR, 4.6; 95% CI, 2.2–9.7) followed by methamphetamine use (aOR, 2.4; 95% CI, 1.5–3.8), receptive condomless anal sex with a casual partner (aOR, 1.8; 95% CI, 1.2–2.7), and a recent diagnosis of an STI (aOR, 1.7; 95% CI, 1.0–2.8). The diagnosis of an STI was not predicted by a history of broken or slipped-off condoms or of condomless insertive anal sex with a casual partner. Those enrolled under the exemption clause were less likely to be diagnosed with an STI at enrollment (6.3% vs 10.1% STI positivity; crude OR, 0.6; 95% CI, .4–1.0) (Supplementary Table 1).
DISCUSSION
In the PrEPX study, participants who fulfilled eligibility criteria 1–4 had a significantly higher risk of having either anorectal chlamydia, anorectal gonorrhea, or syphilis than those not fulfilling these criteria. These criteria consisted of having an HIV-positive regular partner with a detectable viral load, having condomless anal intercourse with casual partners, having previously been diagnosed with an STI, and using methamphetamines. Syphilis and anorectal STIs are markers of potential HIV risk [7–9, 11], and hence these results suggest that these PrEPX eligibility criteria accurately select participants who are at high risk of HIV. The 2017 Australian PrEP guidelines include these four eligibility criteria as signifying high risk of HIV acquisition [15], and the US CDC guidelines use similar criteria to indicate that PrEP is recommended [6]; our results support the use of these criteria in the guidelines. Participants enrolled under criteria 5 and 6 had a lower risk of these STIs compared with the other criteria, but nevertheless these STIs were common in men who fulfilled these criteria (9.6%–11.7%). The 2017 Australian PrEP guidelines classify eligibility criteria 5 and 6 as having a medium risk of HIV infection, rather than high risk, and our results support this classification [15]. Finally, participants who were enrolled despite not fulfilling any PrEP eligibility criteria were found nonetheless to have a substantial prevalence of syphilis and anorectal STIs at enrollment (7.1%), albeit lower than those participants who did fulfill eligibility criteria, suggesting that they were at substantial risk of HIV. These findings highlight that a person who requests PrEP is likely to be at risk of HIV and support the recommendation that clinicians should have the discretion to prescribe PrEP for individuals who do not meet formal eligibility criteria, as is permitted by the 2017 Australian PrEP guidelines [15].
Our subanalysis of participants who were PrEP-naive at enrollment found similar associations between eligibility criteria and STIs compared with our overall analysis, thus confirming the relevance of these results to people who are PrEP-naive.
Compared with participants who were PrEP-naive, participants who were taking PrEP at enrollment were more likely to report a recent STI, more likely to report recent condomless anal sex with casual sexual partners, and more likely to be diagnosed with an STI at their baseline visit. Participants who were already using PrEP may have felt less need to use condoms because they felt protected by PrEP. Such a change in behavior has been termed “risk compensation” and was observed in VicPrEP, which saw a decline in condom use and a rise in STIs over the 12 months after PrEP commencement [16]. This potential change in behavior in PrEPX study participants would have increased participants’ risk of bacterial STIs at baseline.
The main limitation of this study is that we did not have complete data on STI positivity, with missing STI data for 11.7% of enrolled participants. Another limitation of this analysis is that the obtained odds ratios reflect a patient’s HIV/STI risk if they fulfill a single enrollment criterion. If a patient fulfills multiple enrollment criteria, then their HIV/STI risk is a combination of the odds ratios for each fulfilled criterion, after adjustment for effect modification. We have not provided odds ratios for every possible combination of eligibility criteria; hence the results cannot be used to accurately calculate the HIV/STI risk of an individual patient who fulfills multiple enrollment criteria, other than to say that their risk would be larger than the largest odds ratio among the fulfilled enrollment criteria. However, the aim of this analysis was to assess whether the eligibility criteria listed in PrEP guidelines accurately predict HIV/STI risk. This analysis was not intended to be used as an assessment tool to calculate an individual patient’s HIV/STI risk. A final limitation of this analysis is that we used STI prevalence data collected at enrollment rather than STI incidence data collected at follow-up, and STI incidence would perhaps be a more accurate indicator of recent risk. However, STI incidence at follow-up would be influenced by the fact that participants had started PrEP and would therefore reflect participants’ prospective risk rather than their risk at PrEPX enrollment [16].
CONCLUSION
Using STI diagnosis data as biological markers of HIV risk, our results indicate that the PrEP eligibility criteria in the Australian PrEP guidelines, based on the PrEP guidelines of the US CDC, accurately selected participants who had substantial risk of HIV. Hence our results support the use of these eligibility criteria in PrEP guidelines. PrEPX participants who enrolled despite not fulfilling any eligibility criteria had a lower prevalence of STIs than participants who did fulfill eligibility criteria, but their STI prevalence was nonetheless substantial. This suggests that a person who requests PrEP but who does not fulfill formal eligibility criteria, may nonetheless be at risk of HIV. This finding supports the Australian PrEP guidelines in recommending that clinicians should have discretion to provide PrEP for patients who do not meet formal eligibility criteria.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. E. J. W. initiated and coordinated the PrEPX study. V. J. C. and E. W. J. conceived this analysis. J. A. curated the data. V. J. C. conducted the data analysis and wrote the first draft of this paper. All authors reviewed the paper for intellectual content.
Acknowledgments. The authors wish to acknowledge PrEPX participants and the participating clinics, all participants of previous PrEP studies, and all animals used in previous studies to evaluate the safety and efficacy of PrEP.
Financial support. The PrEPX study was supported by funding from the Victorian Department of Health and Human Services and the Victorian AIDS Council. V. J. C. receives a stipend from the Research Training Program of the Australian Government’s Department of Education and Training. M. S. is supported by a National Health and Medical Research Council Senior Research Fellowship. C. C. is supported by an National Health and Medical Research Council Early Career fellowship (APP1092160).
Potential conflicts of interest. V. J. C. has received speaker’s fees and conference assistance from Gilead Sciences; speaker’s fees from Merck Sharp & Dohme; and advisory board fees from ViiV Healthcare. M. S. received grants from the Commonwealth Department of Health during the conduct of the study. J. A. received grants from the Commonwealth of Australia, Department of Health, during the conduct of the study. E. P. F. C. has received consulting fees from Gilead Sciences and research funding from Merck Sharp & Dohme and Seqirus Australia. B. P. received grants from Department of Health and Human Services Victorian Government and grants from the Victorian AIDS Council, during the conduct of the study. E. J. W. has received financial support from Gilead Sciences; Abbott Laboratories; Janssen-Cilag; Boehringer Ingelheim; ViiV Healthcare; Alfred Hospital; and Merck Sharp & Dohme. Gilead Sciences donated the study drug to the VicPrEP study (precursor to the PrEPX study). All other authors declare no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. The Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia Annual Surveillance Report 2016. Sydney: The Kirby Institute, UNSW Australia, 2016: The Kirby Institute, UNSW Australia, 2016. [Google Scholar]
- 2. Grant RM, Lama JR, Anderson PL, et al. ; iPrEx Study Team Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016; 387:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4:151ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Australasian Society for HIV Medicine. Australian commentary on the preexposure prophylaxis for the prevention of HIV in the United States—2014 clinical practice guideline Accessed 9 February 2015.
- 6. US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2014 clinical practice guideline. Atlanta, GA: Department of Health and Human Services, 2014. [Google Scholar]
- 7. Solomon MM, Mayer KH, Glidden DV, et al. ; iPrEx Study Team Syphilis predicts HIV incidence among men and transgender women who have sex with men in a preexposure prophylaxis trial. Clin Infect Dis 2014; 59:1020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guy RJ, Spelman T, Stoove M, et al. Risk factors for HIV seroconversion in men who have sex with men in Victoria, Australia: results from a sentinel surveillance system. Sex Health 2011; 8:319–29. [DOI] [PubMed] [Google Scholar]
- 9. Jin F, Prestage GP, Imrie J, et al. Anal sexually transmitted infections and risk of HIV infection in homosexual men. J Acquir Immune Defic Syndr 2010; 53:144–9. [DOI] [PubMed] [Google Scholar]
- 10. Cheung KT, Fairley CK, Read TR, et al. HIV incidence and predictors of incident HIV among men who have sex with men attending a sexual health clinic in Melbourne, Australia. PLoS One 2016; 11:e0156160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Poynten IM, Jin F, Prestage GP, Kaldor JM, Kippax S, Grulich AE. Defining high HIV incidence subgroups of Australian homosexual men: implications for conducting HIV prevention trials in low HIV prevalence settings. HIV Med 2010; 11:635–41. [DOI] [PubMed] [Google Scholar]
- 12. Ryan KE, Mak A, Stoove M, et al. Protocol for an HIV pre-exposure prophylaxis (PrEP) population level intervention study in Victoria Australia: The PrEPX Study. Frontiers in public health 2018, 6:–151.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. STIs in Gay Men Action Group (STIGMA). Australian sexually transmitted infection & HIV testing guidelines 2014 for asymptomatic men who have sex with men Available at: http://stipu.nsw.gov.au/wp-content/uploads/STIGMA_Testing_Guidelines_Final_v5.pdf. Accessed 3 February 2015.
- 14. Cornelisse VJ, Chow EP, Huffam S, et al. Increased detection of pharyngeal and rectal gonorrhea in men who have sex with men after transition from culture to nucleic acid amplification testing. Sex Transm Dis 2017; 44:114–7. [DOI] [PubMed] [Google Scholar]
- 15. Wright E, Grulich A, Roy K, et al. Australasian society for HIV, viral hepatitis and sexual health medicine HIV pre-exposure prophylaxis: clinical guidelines. J Virus Erad 2017; 3:168–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lal L, Audsley J, Murphy DA, et al. ; VicPrEP Study Team Medication adherence, condom use and sexually transmitted infections in Australian preexposure prophylaxis users. AIDS 2017; 31:1709–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.