Abstract
Background
Global guidelines recommend preexposure prophylaxis (PrEP) for individuals with substantial human immunodeficiency virus (HIV) risk. Data on PrEP uptake in sub-Saharan Africa outside of clinical trials are limited. We report on “early adopters” of PrEP in the Sustainable East Africa Research in Community Health (SEARCH) study in rural Uganda and Kenya.
Methods
After community mobilization and PrEP education, population-based HIV testing was conducted. HIV-uninfected adults were offered PrEP based on an empirically derived HIV risk score or self-identified HIV risk (if not identified by score). Using logistic regression, we analyzed predictors of early PrEP adoption (starting PrEP within 30 days vs delayed/no start) among adults identified for PrEP.
Results
Of 21212 HIV-uninfected adults in 5 communities, 4064 were identified for PrEP (2991 by empiric risk score, 1073 by self-identified risk). Seven hundred and thirty nine individuals started PrEP within 30 days (11% of those identified by risk score; 39% of self-identified); 77% on the same day. Among adults identified by risk score, predictors of early adoption included male sex (adjusted odds ratio 1.53; 95% confidence interval, 1.09–2.15), polygamy (1.92; 1.27–2.90), serodiscordant spouse (3.89; 1.18–12.76), self-perceived HIV risk (1.66; 1.28–2.14), and testing at health campaign versus home (5.24; 3.33–8.26). Among individuals who self-identified for PrEP, predictors of early adoption included older age (2.30; 1.29–4.08) and serodiscordance (2.61; 1.01–6.76).
Conclusions
Implementation of PrEP incorporating a population-based empiric risk score, self-identified risk, and rapid initiation, is feasible in rural East Africa. Strategies are needed to overcome barriers to PrEP uptake, particularly among women and youth.
Clinical Trials Registration
Keywords: HIV prevention, preexposure prophylaxis, serodiscordant couple, HIV risk
In an East African population-based study, one-fifth of adults identified for preexposure prophylaxis (PrEP) started PrEP within 30 days. Early adoption was more likely among individuals with self-perceived human immunodeficiency virus risk and less likely among women and youth.
Oral tenofovir-based preexposure prophylaxis (PrEP) is highly effective in preventing human immunodeficiency virus (HIV) infection when taken consistently [1, 2]. PrEP is recommended in global guidelines for individuals at substantial risk of HIV acquisition [3] and rollout is beginning across sub-Saharan Africa. Despite the promise of PrEP, data on uptake in African settings outside of clinical efficacy trials are limited. Moreover, most studies to date have offered open-label PrEP to highly targeted populations, such as serodiscordant couples [4], young women [5, 6], or sex workers [7, 8]. Data on the feasibility of assessing PrEP eligibility based on population-level risk assessment, and subsequent uptake, are currently lacking.
“Early adopters” of PrEP (those who start PrEP within 30 days vs those who delay initiation or never start) can provide insights into the characteristics of individuals who elect to take PrEP. Early adopters are more likely to be opinion leaders and change agents [9]. As PrEP is newly introduced, they may become “PrEP champions” and promote further uptake in their communities. Identifying individuals less likely to start PrEP and barriers to uptake will also be critical for developing programs to support these populations.
We report on early adopters of PrEP in the Sustainable East Africa Research in Community Health (SEARCH) study, an ongoing population-based HIV test-and-treat and combination prevention trial in rural Kenya and Uganda. The study is implementing a PrEP intervention that includes targeted PrEP based on population-level and self-identification of HIV risk with rapid start offered. In this analysis, we describe predictors of early adoption of PrEP using data from 5 SEARCH communities that were among the earliest to implement the PrEP intervention.
METHODS
Study Design and Population
The SEARCH study (NCT01864603) is a cluster-randomized controlled trial that has enrolled >130000 adults in 32 communities in Kenya and Uganda since 2013 [10, 11]. In phase II of the trial (2016–2020), SEARCH is providing targeted PrEP as an intervention. In this descriptive study, we present data on HIV-uninfected adults (aged ≥18 years) in 5 SEARCH communities (2 in Homa Bay and Migori counties in Kenya and 3 in southwestern Uganda) in the phase II intervention arm.
Procedures
From June to September 2016, study staff conducted community-wide HIV and multidisease testing, using a hybrid approach combining mobile 2-week health campaigns followed by home-based testing for those who did not attend the campaign [10]. This approach resulted in testing of >95% of stable residents in the first 2 years of SEARCH [12]. Hybrid testing was preceded by 1 month of community mobilization and sensitization activities on PrEP, including meetings with community leaders and groups, such as health workers, religious leaders, youth, and workers in transportation and fishing industries. At health campaigns, PrEP education and discussions occurred on arrival at the campaign, with HIV counselors and clinicians, and at health discussion tents for women, men, and adolescents. During home-based testing, 1 staff member conducted HIV testing and counseling and provided information about PrEP.
Before HIV testing, study staff collected sociodemographic information from community members. These characteristics (eg, age, sex, marital status, polygamy, educational attainment, circumcision, occupation, and alcohol use) were used to identify persons at higher risk of HIV acquisition, based on an empirically derived risk score. The risk score was based on applying ensemble supervised machine learning methods to seroconversion data from the first 2 years of the SEARCH phase I intervention arm, with the goal of minimizing the number of PrEP starts while ensuring coverage of 50% of seroconversions [13]. The score provided a region-specific algorithm to identify PrEP candidates (classified as identified by score vs not).
HIV antibody testing was conducted, followed by counseling on test results, risk factors for HIV acquisition, and strategies for HIV prevention. Information was provided on PrEP, including how it works and is taken, adverse effects, and who might benefit from PrEP. Counselors engaged participants in discussion of their potential risks for HIV based on personal or partner factors (eg, knowledge of partner HIV status, concurrent partnerships, condom use, and circumcision) and the HIV risk score described above to facilitate self-assessment of risk.
PrEP was offered to HIV-uninfected individuals based on (1) risk score or (2) self-identified risk of HIV among individuals not identified by the score. In addition, HIV-uninfected individuals in serodiscordant partnerships were encouraged to start PrEP during HIV counseling or based on referral from HIV clinics by their partners. For participants identified for PrEP by risk score, counselors explained “from what we have learned from your community, and what you shared at the beginning of the campaign, we think that you would benefit from taking PrEP.” After counseling, study staff documented whether individuals were interested in PrEP and whether they were identified by risk score or self-identified risk.
Individuals identified for PrEP were offered transportation to clinics to start PrEP the same day or within several days. In 4 communities, on-site PrEP initiation was offered at the health campaign but not at home. PrEP eligibility included negative HIV testing performed by SEARCH within the past 4 weeks (based on country-standard antibody testing algorithm), no history of hepatitis B infection (by self-report), and no symptoms of acute HIV infection. Phlebotomy was performed for baseline creatinine testing. After providing written informed consent, participants were provided tenofovir disoproxil fumarate (300 mg) with emtricitabine (200 mg) or lamivudine (150 mg), free of charge. Follow-up was scheduled at 4 weeks, 12 weeks, and then quarterly for up to 144 weeks, after which the study will facilitate the transition of care to local health facilities.
Before HIV testing and counseling, individuals were asked about their current self-perceived risk of HIV acquisition (“Do you think you are at risk for HIV infection?”). The response to this question (yes, no, or “I don’t know”) was used to analyze “self-perceived risk” but was not incorporated into counseling discussions on HIV, PrEP, and self-assessment of risk.
Statistical Analyses
Descriptive statistics were used to characterize individuals identified for PrEP by risk score or who self-identified for PrEP. Serodiscordant spouses were identified by linking HIV testing data on the head of household to their spouse, and were also classified as identified by risk score or self-identified for PrEP. “Early adopters” were defined as those who started PrEP within 30 days of testing (based on enrollment in the PrEP study and receipt of medication). Univariate and multivariate predictors of being an early adopter were analyzed using logistic regression with cluster-robust standard errors (to account for household-level clustering) with community as a fixed effect. Analyses were conducted using SAS software, version 9.4.
Ethics Approvals
The SEARCH study was approved by the institutional review boards of Makerere University, the Uganda National Council of Science and Technology, the Kenya Medical Research Institute, and the University of California, San Francisco. All SEARCH participants provided verbal informed consent; PrEP participants provided written consent in their preferred language.
RESULTS
PrEP Uptake and Timing of PrEP Initiation
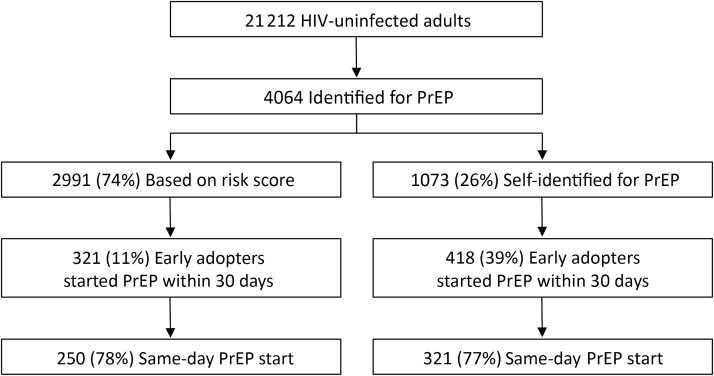
Of 21212 HIV-uninfected adults in 5 SEARCH communities, 4064 were identified as eligible for PrEP, of whom 2991 (74%) were identified based on empiric risk score and 1073 (26%) who were not identified based on the risk score self-identified for PrEP (Figure 1). Of individuals identified by risk score, 321 (11%) were early adopters and initiated PrEP within 30 days; of these 321 early adopters, 250 (78%) started PrEP on the day of HIV testing and PrEP counseling. Of individuals who self-identified for PrEP, 418 (39%) were early adopters, of whom 321 (77%) started PrEP same-day. Overall, 631 adults (275 identified by risk score, 356 self-identified) initiated PrEP within 1–2 days, 717 (309 identified by risk score, 408 self-identified) within 3–7 days, and 739 (321 identified by risk score, 418 self-identified) within 8–30 days (Figure 2).
Figure 1.
Preexposure prophylaxis uptake among human immunodeficiency virus–uninfected adults in 5 Sustainable East Africa Research in Community Health communities in rural Kenya and Uganda from June to October 2016. Abbreviations: HIV, human immunodeficiency virus; PrEP, preexposure prophylaxis.
Figure 2.
Cumulative number of participants initiating preexposure prophylaxis (PrEP) by days since offer of PrEP during community-wide human immunodeficiency virus testing from June to October 2016 in 5 Sustainable East Africa Research in Community Health communities in rural Kenya and Uganda.
Characteristics of Individuals Identified for PrEP
Among adults identified for PrEP, 1934 (48%) were female, 1929 (47%) were 18–25 years, and 2012 (50%) were from Kenya (Table 1). More than half (56%; n = 2290) were married; 1490 (37%) were single. Three-quarters (76%; n = 3103) tested at health campaigns, and one-quarter (24%; n = 961) through home-based testing.
Table 1.
Characteristics of Human Immunodeficiency Virus-uninfected Adults Identified for Preexposure Prophylaxis (PrEP) and Early Adopters of PrEP From June to October 2016 in 5 Sustainable East Africa Research in Community Health Communities in Rural Kenya and Uganda
| Characteristic | Identified for PrEP, No. (%) | Early Adopters, No. (%) (n = 739) | ||
|---|---|---|---|---|
| Total (n = 4064) | Risk Scorea (n = 2991) |
Self-identifieda (n = 1073) |
||
| Female sex | 1934 (48) | 1384 (46) | 550 (51) | 365 (49) |
| Age group, y | ||||
| 18–25 | 1929 (47) | 1666 (56) | 263 (25) | 237 (32) |
| 26–35 | 1400 (34) | 1039 (35) | 361 (34) | 249 (34) |
| 36–45 | 455 (11) | 198 (7) | 257 (24) | 138 (19) |
| 46–55 | 198 (5) | 70 (2) | 128 (12) | 83 (11) |
| >55 | 82 (2) | 18 (1) | 64 (6) | 32 (4) |
| Educational attainment | ||||
| Primary school | 2687 (66) | 1961 (66) | 726 (68) | 509 (69) |
| Any secondary school or above | 1146 (28) | 895 (30) | 251 (23) | 155 (21) |
| Occupationb | ||||
| Formal sector | 477 (12) | 352 (12) | 125 (12) | 61 (8) |
| High-risk informal sector | 933 (23) | 792 (26) | 141 (13) | 158 (21) |
| Low-risk informal sector | 2122 (52) | 1441 (48) | 681 (63) | 444 (60) |
| Unemployed or disabled | 255 (6) | 217 (7) | 60 (6) | 53 (7) |
| Marital status | ||||
| Single | 1490 (37) | 1299 (43) | 191 (18) | 185 (25) |
| Married | 2290 (56) | 1501 (50) | 789 (74) | 484 (65) |
| Polygamous (among married) | 520 (23) | 331 (22) | 189 (24) | 134 (28) |
| Divorced | 60 (1) | 45 (2) | 15 (1) | 10 (1) |
| Separated | 131 (3) | 100 (3) | 31 (3) | 26 (4) |
| Widowed | 90 (2) | 44 (1) | 46 (4) | 33 (4) |
| Serodiscordant spouse | 36 (2) | 18 (1) | 18 (2) | 18 (2) |
| Circumcised (among men) | 780 (37) | 557 (35) | 223 (43) | 156 (42) |
| Alcohol use | ||||
| 1–7 d/mo | 565 (14) | 470 (16) | 95 (9) | 83 (11) |
| >7 d/mo | 445 (11) | 295 (10) | 150 (14) | 87 (12) |
| Migration out of the community in past 12 mo | ||||
| 1–6 mo | 252 (6) | 220 (7) | 32 (3) | 26 (4) |
| >6 mo | 242 (6) | 211 (7) | 31 (3) | 20 (3) |
| Self-perceived HIV riskc | 1430 (35) | 899 (30) | 531 (49) | 367 (50) |
| Region | ||||
| Kenya | 2012 (50) | 1585 (53) | 427 (40) | 368 (50) |
| Southwestern Uganda | 2052 (50) | 1406 (47) | 646 (60) | 371 (50) |
| Point of contact | ||||
| Community health campaign | 3103 (76) | 2045 (68) | 1058 (99) | 701 (95) |
| Home-based testing | 961 (24) | 946 (32) | 15 (1) | 38 (5) |
Abbreviations: HIV, human immunodeficiency virus; PrEP, preexposure prophylaxis.
aMutually exclusive categories: individuals who had not been identified by the risk score could self-identify for PrEP.
bFormal sector occupations included teacher, student, government worker, military worker, health worker, and factory worker; high-risk informal sector occupations included fishmonger, fisherman, bar owner, bar worker, transport, and tourism; low-risk informal sector occupations included farmer, shopkeeper, market vendor, hotel worker, homemaker, household worker, miner, and construction worker.
cIndividuals were asked about their current self-perceived risk of HIV before counseling on HIV test results and PrEP.
The majority of individuals identified by risk score were male (54%), young (56% aged 18–25 years), and uncircumcised (65%). Half were married (22% polygamous), 43% were single, and 14% had migrated outside the community in the past year. One-quarter (26%) were employed in high-risk informal sector occupations, such as fishing, bar work, transportation, or tourism [14, 15].
Most individuals who self-identified for PrEP were older (42% aged ≥36 years), employed in the low-risk informal sector (63% employed as farmers, shopkeepers, market vendors, hotel workers, homemakers, miners, or construction workers), and had lower educational attainment (9% had no formal education). The majority (74%) were married (24% polygamous). Nearly all (99%) who self-identified for PrEP were tested at health campaigns rather than home.
Self-perceived Risk of HIV Acquisition
Before HIV testing and counseling, community members were asked about their current self-perceived risk of HIV. Of adults identified for PrEP, 35% initially reported self-perceived risk of HIV before HIV testing (30% of those identified by risk score and 49% of those who self-identified for PrEP) and 5% reported that they did not know if they were at risk.
Predictors of Initiating PrEP Within 30 Days
Among adults identified for PrEP by risk score, in a multivariate model adjusted for community, occupation, alcohol intake, and circumcision, being male (adjusted odds ratio [aOR], 1.53; 95% confidence interval [CI], 1.09–2.15), in a polygamous marriage (1.92; 1.27–2.90), having a serodiscordant spouse (3.89; 1.18–12.76), self-perceived current risk of HIV acquisition (1.66; 1.28–2.14), and testing at the health campaign versus during home-based testing (5.24; 3.33–8.26) were associated with greater odds of early PrEP adoption (Table 2). Having a secondary level of education or higher was negatively associated with PrEP uptake (aOR, 0.53; 95% CI, .20–.98) compared with no formal education.
Table 2.
Predictors of Early Preexposure Prophylaxis Uptake Among Individuals Identified by Risk Score From June to October 2016 in 5 Sustainable East Africa Research in Community Health Communities in Rural Kenya and Ugandaa
| Characteristic | OR (95% CI) | P Value | aOR (95% CI) | P Value |
|---|---|---|---|---|
| Sex | ||||
| Female | Reference | … | Reference | … |
| Male | 1.25 (.99–1.57) | .06 | 1.53 (1.09–2.15) | .01 |
| Age group, y | ||||
| 18–25 | Reference | … | Reference | … |
| 26–35 | 1.17 (.91–1.51) | .21 | 0.97 (.72–1.29) | .81 |
| 36–45 | 1.61 (1.05–2.46) | .03 | 0.96 (.58–1.59) | .88 |
| 46–55 | 2.13 (1.15–3.95) | .02 | 1.22 (.59–2.52) | .60 |
| >55 | 1.17 (.27–5.14) | .84 | 0.62 (.11–3.59) | .59 |
| Educational attainment | ||||
| No formal education | Reference | … | Reference | … |
| Primary school | 0.69 (.41–1.14) | .14 | 0.68 (.39–1.18) | .17 |
| Any secondary school or above | 0.45 (.26–.77) | .004 | 0.53 (.20–.98) | .04 |
| Marital status | ||||
| Single | Reference | … | Reference | … |
| Married | 1.15 (.90–1.48) | .25 | 0.73 (.52–1.03) | .07 |
| Divorced | 1.15 (.45–2.98) | .77 | 1.32 (.48–3.67) | .59 |
| Separated | 1.74 (.99–3.06) | .055 | 1.67 (.84–3.30) | .14 |
| Widowed | 1.46 (.60–3.52) | .40 | 1.17 (.41–3.32) | .77 |
| Polygamous marriage | 1.88 (1.32–2.69) | .001 | 1.92 (1.27–2.90) | .002 |
| Serodiscordant spouse | 4.26 (1.59–11.45) | .004 | 3.89 (1.18–12.76) | .03 |
| Migration out of the community in past 12 mo | ||||
| 0 mo | Reference | … | Reference | … |
| 1–6 mo | 0.60 (.35–1.01) | .053 | 1.14 (.65–2.01) | .65 |
| >6 mo | 0.34 (.17–0.67) | .002 | 0.81 (.39–1.69) | .58 |
| Self-perceived HIV risk | ||||
| No | Reference | … | Reference | … |
| Yes | 2.24 (1.77–2.84) | <.001 | 1.66 (1.28–2.14) | <.001 |
| Do not know | 1.56 (.93–2.63) | .09 | 1.29 (.76–2.21) | .35 |
| Point of contact | ||||
| Home-based testing | Reference | … | Reference | … |
| Community health campaign | 5.95 (3.96–9.94) | <.001 | 5.24 (3.33–8.26) | <.001 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio.
aMultivariate models were adjusted for community, occupation, alcohol intake, and circumcision.
Among individuals who self-identified for PrEP, the unadjusted odds of initiating PrEP increased with increasing age, although this trend did not remain significant in multivariate analysis (Table 3). In a multivariate model, older age (46–55 vs 18–25 years; aOR, 2.30; 95% CI, 1.29–4.08) and having a serodiscordant spouse (2.61; 1.01–6.76) were associated with greater odds of being an early adopter, whereas having primary (0.53; .33–.87) or secondary education or above (vs no formal education; 0.53; .33–.93) and testing at the health campaign (vs at home; 0.14; .004–.50) were associated with lower odds of early PrEP initiation.
Table 3.
Predictors of Early Preexposure Prophylaxis (PrEP) Uptake Among Individuals Self-identified for PrEP From June to October 2016 in 5 Sustainable East Africa Research in Community Health Communities in Rural Kenya and Ugandaa
| Characteristic | OR (95% CI) | P Value | aOR (95% CI) | P Value |
|---|---|---|---|---|
| Sex | ||||
| Female | Reference | … | Reference | … |
| Male | 0.76 (.59–.97) | .03 | 0.76 (.53–1.9) | .13 |
| Age group, y | ||||
| 18–25 | Reference | … | Reference | … |
| 26–35 | 1.44 (1.02–2.02) | .04 | 1.35 (.88–12.06) | .17 |
| 36–45 | 1.81 (1.25–2.62) | .002 | 1.33 (.82–2.14) | .24 |
| 46–55 | 2.97 (1.92–4.60) | <.001 | 2.30 (1.29–4.08) | .004 |
| >55 | 2.17 (1.24–3.80) | .007 | 1.54 (.77–3.08) | .22 |
| Educational attainment | ||||
| No formal education | Reference | … | Reference | … |
| Primary school | 0.50 (.32–.76) | .001 | 0.53 (.33–.87) | .01 |
| Any secondary school or above | 0.40 (.25–.64) | <.001 | 0.53 (.33–.93) | .03 |
| Marital status | ||||
| Single | Reference | … | Reference | … |
| Married | 1.54 (1.09–2.18) | .01 | 0.81 (.50–1.30) | .38 |
| Divorced | 1.15 (.34–3.88) | .83 | 1.04 (.27–3.97) | .96 |
| Separated | 1.09 (.48–2.49) | .83 | 0.60 (.23–1.59) | .30 |
| Widowed | 3.25 (1.67–6.34) | .001 | 1.14 (.48–2.68) | .77 |
| Polygamous marriage | 1.09 (.78–1.52) | .60 | 0.81 (.56–1.17) | .26 |
| Serodiscordant spouse | 2.92 (1.09–7.85) | .03 | 2.61 (1.01–6.76) | .048 |
| Migration out of the community in past 12 mo | ||||
| 0 mo | Reference | … | Reference | … |
| 1–6 mo | 0.67 (.30–1.49) | .32 | 1.10 (.47–2.57) | .83 |
| >6 mo | 0.80 (.40–1.63) | .55 | 1.04 (.49–2.23) | .91 |
| Self-perceived HIV risk | ||||
| No | Reference | … | Reference | … |
| Yes | 1.16 (.89–1.50) | .27 | 1.29 (.97–1.72) | .08 |
| Do not know | 0.54 (.29–1.04) | .07 | 0.64 (.32–1.29) | .21 |
| Point of contact | ||||
| Home-based testing | Reference | … | Reference | … |
| Community health campaign | 0.16 (.04–.56) | .004 | 0.14 (.004–.50) | .002 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio.
aMultivariate models were adjusted for community, occupation, alcohol intake, and circumcision.
DISCUSSION
In the context of the rapid introduction of PrEP via community-wide HIV testing and population-based and self-identification of risk, nearly one-fifth of >4000 adults identified as eligible for PrEP started PrEP within 30 days. In 5 SEARCH communities that were among the first to implement our PrEP intervention, the majority of these early adopters started PrEP on the same day it was offered. However, only 11% of community members identified as being at higher risk of HIV acquisition based on an empiric risk score started PrEP within 30 days and were less likely to do so than those who self-identified for PrEP. Moreover, fewer than half of individuals identified for PrEP initially reported self-perceived risk of HIV before receiving counseling on their HIV test results and education about PrEP.
To our knowledge, this study is among the first and largest to evaluate PrEP uptake in sub-Saharan Africa when offered via a population-based approach. Our findings demonstrate the feasibility of evaluation for PrEP and rapid initiation in community-based settings and highlight the need for ongoing sensitization and education about PrEP, as well as strategies to facilitate discussions of self-assessment of risk and uptake among those at risk.
Our PrEP intervention includes a combined approach to assessing PrEP eligibility through population-based risk assessment and self-identification of HIV risk. Our approach is novel in that it aims to target those at highest risk on a population level to maximize impact rather than offering PrEP based on membership in specific risk groups [13]. Other risk scoring tools have been developed for serodiscordant couples, pregnant women, and younger women in southern and eastern Africa [16–18], but not for the general population, and none were derived using machine learning.
Our score is based on demographic characteristics that are asked of all community members and requires neither asking sensitive sexual behavior questions (including the sex of partners) during screening nor testing for non-HIV sexually transmitted infections. Our approach also provides an opportunity for counseling and self-assessment of risk. This is in line with World Health Organization guidance, which recommends offering PrEP to those who request it in the context of a generalized epidemic [19], because persons who request PrEP are likely to be at higher risk of HIV [20]. As PrEP rollout expands, further assessment of PrEP uptake after the application of our risk score and others is needed, as are data on the predictive performance of these scores [21]. Future studies should examine the whether adding targeted behavioral data to population-level risk assessment improves score performance.
We found that only 35% of community members who were identified for PrEP initially reported self-perceived risk of HIV. Because community members in our study were asked about perceived risk before counseling on HIV test results and PrEP, self-perception of risk may have increased during counseling, leading individuals to self-identify for PrEP. In addition, our study findings and those of others suggest that brief questions with discrete answer choices may not fully capture risk perception. In the placebo-controlled FEM-PrEP trial that enrolled Kenyan, Tanzanian, and South African women, half of seroconverters reported no perceived risk at the visit preceding seroconversion [22]. In HPTN 082 (an ongoing open-label PrEP study among young women in South Africa and Zimbabwe), at baseline, 47% of participants reported no risk of HIV in the next year [5].
As PrEP implementation expands, further strategies are needed to rapidly and sensitively facilitate self-assessment and reporting of risk and recognition of risk based on empiric scoring tools. Future research should include quantitative and qualitative assessments of HIV risk perception, as well as the perceived severity of HIV infection, which can drive demand for PrEP [23]. Work is also needed on methods to communicate the results of risk scoring tools and triangulate these results with individuals’ self-perceived risk.
Sex was an important factor in the early adoption of PrEP. Among community members identified for PrEP by risk score, women were less likely than men to be early adopters. Placebo-controlled PrEP trials that exclusively enrolled African women demonstrated limited use of the study product (pills or gel) among participants [24, 25]. Although data are limited on open-label PrEP uptake among women, recent studies have found higher levels of PrEP initiation [6, 26]. Our qualitative work in SEARCH communities suggests that many women recognize their risk of HIV acquisition but feel the need to seek consent from their male partners before starting PrEP [23, 27]. This may pose a substantial barrier to uptake for women who have partners who are HIV uninfected or of unknown status but desire PrEP owing to their own risk or perceived risk from their partners.
Age was also a significant factor in the early adoption of PrEP. Younger adults (aged 18–25 years) were less likely to initiate PrEP, in multivariate analysis among individuals who self-identified for PrEP and in univariate analysis among those identified by risk score. Given the rapid increase in HIV prevalence by the time youth (particularly young women) reach their mid-20s in SEARCH communities [28] and in much of southern and eastern Africa [29, 30], this is a priority population for HIV prevention efforts. A recent study found that oral PrEP was acceptable to young men and women in Cape Town [31]. However, studies demonstrate gaps along all steps of the HIV treatment cascade for young persons [11, 32] suggesting that engagement with PrEP may also present a challenge. Ongoing studies offering PrEP to youth will provide valuable insights into drivers of and barriers to PrEP uptake in this population.
More early adopters started PrEP after testing at health campaigns, compared with home-based testing. This finding may have been related to differences in how PrEP was offered in these testing venues (with more opportunities for PrEP education/discussion and on-site start at campaigns) or in the healthcare-seeking behaviors of these groups (because persons who did not present to campaigns were subsequently offered testing at home). Our results suggest that additional strategies are needed to provide PrEP education and engage individuals who are less likely to seek HIV testing, and to reduce barriers to PrEP initiation after testing at home.
This study is subject to several limitations. Fewer individuals may have been early PrEP adopters owing to the short time frame over which PrEP was introduced in communities before being offered. In other settings (eg, the United States), uptake was slow when PrEP was first introduced [33, 34]. PrEP uptake may also have been lower because we offered PrEP within a study before it was available through public sector clinics. Although PrEP was provided free of charge and at several clinics within each community, PrEP initiators were required to provide written consent.
Of note, although transportation to the clinic for same-day PrEP initiation was offered, no additional incentives or transport reimbursement were provided, thus enhancing applicability to real-life settings. Another limitation is that serodiscordant spouses were identified by linking HIV test results of the head of household to their self-reported spouse; the study survey did not ask about discordant or secondary partnerships. This approach does not identify serodiscordant partnerships among individuals who are not married, not living together, or not married to the head of household.
In conclusion, our study demonstrates that a PrEP implementation approach combining a population-based risk score and self-identification of risk, as well as rapid initiation of PrEP, is feasible in rural East Africa. However, most individuals who were identified by risk score neither reported self-perceived risk of HIV nor started PrEP within 30 days. The SEARCH study is offering PrEP initiation on an ongoing basis for persons in serodiscordant partnerships or newly self-identifying as at risk. Qualitative work is being conducted in SEARCH communities to further clarify reasons for declining PrEP, and the study is implementing efforts to address barriers to and optimize PrEP uptake. As PrEP is rolled out across sub-Saharan Africa, scalable strategies are needed to facilitate recognition of HIV risk and overcome barriers to PrEP uptake, maximizing the impact of this HIV prevention modality.
Notes
Acknowledgments. The Sustainable East Africa Research in Community Health (SEARCH) project gratefully acknowledges the Ministries of Health of Uganda and Kenya, our research team, collaborators and advisory boards, and especially all communities and participants involved. We thank Maneet Kaur and Jeanna Wallenta from the SEARCH data team for their contributions to the statistical analyses for this study.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH), the Gates Foundation, or Gilead.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, NIH (grants U01AI099959 and R01AI074345), the Bill & Melinda Gates Foundation (grant OPP1159068), Gilead Sciences, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant K12HD052163).
Potential conflicts of interest. Gilead Sciences donated tenofovir disoproxil fumarate/emtricitabine as a study drug, but had no other role in the design or conduct of the study or the analysis or interpretation of data. C. A. K. has received grant support paid to her institution from the Gilead Research Scholars Program in Human Immunodeficiency Virus. F. M. is an employee of the Infectious Diseases Research Collaboration. C. R. C. has received personal fees for expert testimony in a legal case, consultancy fees from Symbiomix, and a grant and travel accommodations from the NIH. D. V. H. has received grant funding paid to her institution from the NIH. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Baeten JM, Donnell D, Ndase P et al. ; Partners PrEP Study Team Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thigpen MC, Kebaabetswe PM, Paxton LA et al. ; TDF2 Study Group Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2nd ed Geneva, Switzerland: World Health Organization, 2016. [Google Scholar]
- 4. Baeten JM, Heffron R, Kidoguchi L et al. ; Partners Demonstration Project Team Integrated delivery of antiretroviral treatment and pre-exposure prophylaxis to HIV-1-serodiscordant couples: a prospective implementation study in Kenya and Uganda. PLoS Med 2016; 13:e1002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Celum C, Delany-Moretlwe S, Hosek S et al. . Risk behavior, perception, and reasons for PrEP among young African women in HPTN 082. Presented at: Conference on Retroviruses and Opportunistic Infections; 2018;Boston, Massachusetts. [Google Scholar]
- 6. Bekker LG, Roux S, Sebastien E et al. ; HPTN 067 (ADAPT) study team Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomised, open-label, phase 2 trial. Lancet HIV 2018; 5:e68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eakle R, Gomez GB, Naicker N et al. ; TAPS Demonstration Project Team HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: results from a prospective observational demonstration project. PLoS Med 2017; 14:e1002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cowan F, Davey C, Mushati P et al. . Results of the SAPPH-IRe trial: a cluster randomised trial of a combination intervention to empower female sex workers in Zimbabwe to link and adhere to antiretrovirals for treatment and prevention. Presented at: International AIDS Conference; 2016;Durban, South Africa. [Google Scholar]
- 9. Rogers EM. Diffusion of innovations. 5th ed New York, NY: Free Press, 2003. [Google Scholar]
- 10. Chamie G, Clark TD, Kabami J et al. . A hybrid mobile approach for population-wide HIV testing in rural east Africa: an observational study. Lancet HIV 2016; 3:e111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petersen M, Balzer L, Kwarsiima D et al. . Association of implementation of a universal testing and treatment intervention with HIV diagnosis, receipt of antiretroviral therapy, and viral suppression in East Africa. JAMA 2017; 317:2196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kwarisiima D, Kabami J, Sang N et al. . Who remains untested following near-universal (>95%) population HIV testing? Presented at: Conference on Retroviruses and Opportunistic Infections; 2018;Boston, Massachusetts. [Google Scholar]
- 13. Zheng W, Balzer L, van der Laan M, Petersen M; SEARCH Collaboration Constrained binary classification using ensemble learning: an application to cost-efficient targeted PrEP strategies. Stat Med 2018; 37:261–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kissling E, Allison EH, Seeley JA et al. . Fisherfolk are among groups most at risk of HIV: cross-country analysis of prevalence and numbers infected. AIDS 2005; 19:1939–46. [DOI] [PubMed] [Google Scholar]
- 15. Lindan CP, Anglemyer A, Hladik W et al. ; Crane Survey Group High-risk motorcycle taxi drivers in the HIV/AIDS era: a respondent-driven sampling survey in Kampala, Uganda. Int J STD AIDS 2015; 26:336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Balkus JE, Brown E, Palanee T et al. . An empiric HIV risk scoring tool to predict HIV-1 acquisition in African women. J Acquir Immune Defic Syndr 2016; 72:333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pintye J, Drake AL, Kinuthia J et al. . A risk assessment tool for identifying pregnant and postpartum women who may benefit from preexposure prophylaxis. Clin Infect Dis 2017; 64:751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kahle EM, Hughes JP, Lingappa JR et al. ; Partners in Prevention HSVHIV Transmission Study and the Partners PrEP Study Teams An empiric risk scoring tool for identifying high-risk heterosexual HIV-1-serodiscordant couples for targeted HIV-1 prevention. J Acquir Immune Defic Syndr 2013; 62:339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization. WHO implementation tool for pre-exposure prophylaxis (PrEP) of HIV infection. Module 1: clinical. Geneva, Switzerland: World Health Organization, 2017. [Google Scholar]
- 20. Grant RM, Glidden DV. HIV moments and pre-exposure prophylaxis. Lancet 2016; 387:1507–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balkus JE, Brown ER, Palanee-Phillips T et al. . Performance of a validated risk score to predict HIV-1 acquisition among African women participating in a trial of the dapivirine vaginal ring. J Acquir Immune Defic Syndr 2018; 77:e8–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Corneli AL, McKenna K, Headley J et al. ; FEM-PrEP Study Group A descriptive analysis of perceptions of HIV risk and worry about acquiring HIV among FEM-PrEP participants who seroconverted in Bondo, Kenya, and Pretoria, South Africa. J Int AIDS Soc 2014; 17:19152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Camlin C, Getahun M, Ayieko J et al. . Understanding PrEP demand among adolescents/young adults and HIV discordant couples in SEARCH: qualitative findings. Presented at: 9th IAS Conference on HIV Science; 2017;Paris, France. [Google Scholar]
- 24. Marrazzo JM, Ramjee G, Richardson BA et al. ; VOICE Study Team Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Damme L, Corneli A, Ahmed K et al. ; FEM-PrEP Study Group Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lahuerta M, Zerbe A, Baggaley R et al. . Feasibility, acceptability, and adherence with short-term HIV preexposure prophylaxis in female sexual partners of migrant miners in Mozambique. J Acquir Immune Defic Syndr 2017; 76:343–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koss C, Ayieko J, Owaraganise A et al. . Survey of early barriers to PrEP uptake among clients and community members in the SEARCH study in rural Kenya and Uganda. Presented at: 9th IAS Conference on HIV Science; 2017;Paris, France. [Google Scholar]
- 28. Kadede K, Ruel T, Kabami J et al. ; SEARCH team Increased adolescent HIV testing with a hybrid mobile strategy in Uganda and Kenya. AIDS 2016; 30:2121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. UNAIDS. Global AIDS update Available at: http://www.unaids.org/en/resources/documents/2016/Global-AIDS-update-2016. Accessed 3 June 2016.
- 30. Abdool Karim Q, Kharsany AB, Leask K et al. . Prevalence of HIV, HSV-2 and pregnancy among high school students in rural KwaZulu-Natal, South Africa: a bio-behavioural cross-sectional survey. Sex Transm Infect 2014; 90:620–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gill K, Pidwell T, Dietrich J et al. . A demonstration open label study to assess the acceptability, safety and use of Truvada pre-exposure prophylaxis in healthy, HIV-uninfected adolescents, 15–19 years of age. Presented at: 9th IAS Conference on HIV Science; 2017; Paris, France. [Google Scholar]
- 32. Justman J, Hoos D, Kalton G et al. . Real progress in the HIV epidemic: PHIA findings from Zimbabwe, Malawi, and Zambia. Presented at: Conference on Retroviruses and Opportunistic Infections; 2017; Seattle, Washington. [Google Scholar]
- 33. Krakower DS, Mimiaga MJ, Rosenberger JG et al. . Limited awareness and low immediate uptake of pre-exposure prophylaxis among men who have sex with men using an internet social networking site. PLoS One 2012; 7:e33119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Flash C, Landovitz R, Giler RM et al. . Two years of Truvada for pre-exposure prophylaxis utilization in the US. J Int AIDS Soc 2014; 17:19730. [DOI] [PMC free article] [PubMed] [Google Scholar]