Abstract
Background
Previous studies suggest that human immunodeficiency virus (HIV)–infected cancer patients are less likely to receive cancer treatment. The extent to which this disparity affects the growing population of elderly individuals is unknown and factors that mediate these treatment differences have not been explored.
Methods
We studied 930359 Americans aged 66–99 years who were diagnosed with 10 common cancers. Surveillance, Epidemiology, and End Results–Medicare claims from 1991 to 2011 were used to determine HIV status and receipt of cancer treatment in 6 months following diagnosis. Mediation analysis was conducted to estimate the direct effect of HIV, and indirect effect through cancer stage at diagnosis and comorbidities, on cancer treatment.
Results
HIV-infected individuals (n = 687) were less likely to receive cancer treatment (70% vs 75% HIV uninfected; P < .01). This difference was larger in individuals aged 66–70 years, among whom only 65% were treated (vs 81% in HIV uninfected; P < .01), and time from cancer diagnosis to treatment was longer (median, 42.5 vs 36 days in HIV uninfected; P < .01). Accounting for potential confounders, HIV-infected individuals aged 66–70 years remained 20% less likely to receive cancer treatment (hazard ratio, 0.81 [95% confidence interval, .71–.92]). Seventy-five percent of this total effect was due to HIV itself, with a nonsignificant 24% mediated by cancer stage and comorbidities.
Conclusions
Lowest cancer treatment rates were seen in the younger subset of HIV-infected individuals, who would likely benefit most from treatment in terms of life expectancy.
Keywords: HIV/AIDS, cancer, SEER-Medicare, treatment, comorbidities
The larger differences seen in cancer treatment rates in younger individuals (≤70 years) with HIV, who may benefit the most from curative treatment in terms of added life expectancy, emphasize the need for improved guidance and clinical intervention.
Widespread and effective antiretroviral therapy (ART) has reduced the risk of developing AIDS and has greatly increased the life expectancy of human immunodeficiency virus (HIV)–infected individuals in the United States [1, 2]. As such, an increasing number of HIV-infected individuals are now at risk of cancers that typically occur with aging [3]. Given the increasing burden of cancer in the aging HIV-infected population, effective strategies to treat cancer in this population are needed. It is also important to ensure that patients are being properly referred for and are receiving these treatments. Unfortunately, recent studies suggest that HIV-infected individuals are significantly less likely to receive cancer treatment compared with uninfected individuals, but findings have differed by study population and cancer type [4, 5], including one study that found that HIV-infected individuals may even be overtreated for prostate cancer [6]. Although the incidence of many cancers increases with aging, studies that focus on treatment in elderly individuals with HIV are limited to only lung cancer [5, 7].
Understanding the effect of HIV on receipt of cancer treatment and identifying characteristics of HIV-infected individuals who do not receive timely and appropriate treatment are critical steps toward reducing the higher cancer-specific mortality reported for HIV-infected cancer patients [8, 9]. The extent to which time from cancer diagnosis to initiation of cancer treatment differs between HIV-infected and -uninfected individuals remains unclear, and research is needed to examine underlying drivers of HIV-related differences. For example, we hypothesize that HIV-infected individuals may be less likely to receive treatment because of competing medical conditions (comorbidities) or late-stage diagnosis, where harms of treatment may outweigh benefits. In this study, we focus on a growing subset of the HIV population in America—those who are aged 65 years or older and diagnosed with cancer—to examine factors associated with receipt of cancer treatment and delays in initiation of cancer therapy.
METHODS
We conducted a retrospective cohort study of 930359 older Americans in the Surveillance, Epidemiology, and End Results (SEER)–Medicare linkage database from 1991 to 2012, which includes individuals diagnosed with cancer in the SEER program who have been matched with their Medicare enrollment and billing claims records. The study goal was to focus on a diverse set of cancers, so we included the most common cancers in this elderly population (prostate, lung, breast, colorectal cancers) and also included both AIDS-defining (non-Hodgkin lymphoma [NHL]) and non-AIDS-defining cancers that are relatively common in HIV-infected individuals (cancer of the anus, bladder, kidney, and liver, and melanoma). This study was restricted to invasive cancers that were diagnosed (1) at ≥66 years of age to ensure at least 1 year of previous claims data; (2) on/after January 1992, when both the SEER and Medicare data were available; (3) before December 2011 to ensure 1 year of follow-up available after cancer diagnosis; and (4) among individuals with part A, part B, non–health maintenance organization coverage for at least 1 year before through 1 year after cancer diagnosis.
Cancer cases were defined as HIV-infected if an International Classification of Diseases, Ninth Revision code for HIV (042, 043, 044, or V08) was found in the Medicare Provider Analysis and Review (MEDPAR) file, or if ≥2 claims for those diagnosis codes were included in National Claims History (NCH) or the Outpatient files ≥30 days apart. If these criteria were not met up 365 days after the date of cancer diagnosis, cancer cases were considered HIV uninfected.
The study outcome, receipt of initial cancer treatment, was defined as one or any combination of surgery, radiotherapy, chemotherapy including oral prescriptions, hormone/biologic therapy, or transplant within 6 months of cancer diagnosis. Cancer treatment data were ascertained using the NCH, Outpatient, MEDPAR, and durable medical equipment (which contains data on oral chemotherapy) files of the SEER-Medicare linkage. For each cancer type, these files were searched for codes that indicate possible cancer treatments. Then, the final code list was compared against the National Comprehensive Cancer Network guidelines to ensure it included all standard treatments [10] and was further reviewed for completeness by an oncologist with expertise in using large databases to examine cancer treatment (G. S.).
Available covariates of interest in this study included age, sex, race, year of cancer diagnosis, metropolitan setting (big metropolitan/metropolitan vs urban/less urban/rural), cancer stage, comorbidities, and socioeconomic status (SES). To avoid collinearity and to best capture SES in regression models, a composite binary variable was created based on zip code–level median income, percentage of high-school graduates, and percentage of residents living below poverty. For prevalent comorbidities, the Medicare claims were searched for codes indicating the 15 other comorbidities (excluding cancer and HIV) included in the Charlson index in the year prior to cancer diagnosis [11].
Statistical Analysis
Demographic, clinical, and health characteristics of the study population, as well as the proportion of cancer cases receiving treatment within 6 months of cancer diagnosis, were described. Pearson χ2 tests and Wilcoxon rank-sum tests were used to compare these characteristics by HIV status for categorical and continuous variables, respectively; trend tests were used for ordered variables. Time from cancer diagnosis to treatment was compared by HIV status and age using Kaplan-Meier curves. Only the month and year of cancer diagnosis were available in SEER, so the date of diagnosis was assumed to be the 15th of the month. If the treatment date occurred within 15 days before the diagnosis date, we assumed they occurred at the same time.
Time-to-event analyses were used to formally compare receipt of cancer treatment by HIV status for each cancer type, and for all cancers combined with adjustment for cancer type. In the absence of treatment, participants were censored at the earliest of death or 183 days (6 months) after cancer diagnosis. Likelihood ratio tests were used to identify potential interactions between HIV and other covariates on time to treatment. A significant interaction was found for age (P < .05), so results are stratified by age 66–70 years (younger subset) and age >70 years (older subset).
Mediation analysis, using the inverse odds ratio weighting method [12], was employed to examine the extent to which cancer stage and comorbidities could explain the association between HIV and cancer treatment (Supplementary Data 1). Here, HRTOTAL = HRDIRECT × HRINDIRECT, where HRs correspond to hazard ratios for cancer treatment. HRTOTAL is the estimate of the overall association (all sources and pathways) between HIV and cancer treatment, after adjustment for measured confounding factors (race, sex, composite SES, year of diagnosis [Supplementary Data 2], and metropolitan setting). HRINDIRECT captures the “indirect” effect, which is the portion of the association between HIV and cancer treatment that is mediated, or accounted for, by differences in cancer stage and comorbidities. HRDIRECT estimates the direct effect of HIV itself, that is, the portion that does not work through cancer stage and comorbidities, although this direct effect might be mediated through other unmeasured pathways. Models were bootstrapped 500 times to estimate the standard errors. Finally, factors associated with receipt of cancer treatment among HIV-infected individuals were examined using standard Cox proportional hazards models restricted to the HIV subpopulation. Analyses were conducted using Stata version 14 and R version 3.3.1 software.
RESULTS
In this study of elderly Americans with cancer (n = 930359), 687 were HIV infected (0.07%), of whom the majority (n = 631) had their first HIV claim prior to cancer diagnosis (median, 1281 days [interquartile range {IQR}, 660–2144]; Table 1). Of the cancers examined, lung and prostate were the most common in both HIV-infected and uninfected individuals. HIV-infected individuals had lower proportions of bladder, breast, and colorectal cancers and higher proportions of anal cancer, liver cancer, and NHL, compared with HIV-uninfected individuals. HIV-infected cancer patients were younger (median, 71 years [IQR, 68–76 years]) than HIV-uninfected patients (median, 75 years [IQR, 71–81 years]), and a higher proportion of HIV-infected individuals were non-Hispanic black or Hispanic (31% and 11%, compared with 8% and 5% in HIV uninfected, respectively). Annual cancer screening, excluding the year before diagnosis, was higher in HIV-infected individuals (median, 0.47 screens per year vs 0.35 in HIV uninfected). Diagnosis of distant-stage cancers was equally common by HIV status (20%), whereas HIV-infected individuals were less likely to have localized/regional cancers (61% vs 65% in HIV uninfected) and more likely to have unknown stage. HIV-infected individuals were more likely to have a high number of comorbidities (26% had ≥4 comorbidities vs 11% in HIV uninfected) and to reside in metropolitan areas and areas of lower SES.
Table 1.
Characteristics of the Study Population of Elderly Americans With Cancer, by Human Immunodeficiency Virus Status
| Characteristic | HIV-Infected | HIV-Uninfected | P Valuea |
|---|---|---|---|
| No. (%) or Median (IQR) | No. (%) or Median (IQR) | ||
| Totalb | 687 (100) | 929672 (100) | |
| Type of cancer | |||
| Anus | 28 (4.1) | 2790 (0.3) | <.0001 |
| Bladder | 30 (4.4) | 60538 (6.5) | .02 |
| Breast | 45 (6.6) | 137333 (14.8) | <.0001 |
| Colorectum | 75 (10.9) | 145327 (15.6) | .0007 |
| Kidney | 22 (3.2) | 29024 (3.1) | .9 |
| Liver | 33 (4.8) | 14103 (1.5) | <.0001 |
| Lung | 163 (23.7) | 213630 (23.0) | .64 |
| Melanoma | 25 (3.6) | 29225 (3.1) | .46 |
| Non-Hodgkin lymphoma | 85 (12.4) | 67320 (7.2) | <.0001 |
| Prostate | 181 (26.3) | 230382 (24.8) | .34 |
| Sex | |||
| Female | 153 (22.3) | 406749 (43.8) | <.001 |
| Male | 534 (77.7) | 522923 (56.2) | |
| Age, y, median (IQR) | 71 (68–76) | 75 (71–81) | <.001 |
| Age category, y | |||
| 66–70 | 321 (46.7) | 228889 (24.6) | <.0001 |
| 71–75 | 184 (26.8) | 238222 (25.6) | |
| 76–80 | 105 (15.3) | 208559 (22.4) | |
| ≥81 | 77 (11.2) | 254002 (27.3) | |
| Race/ethnicity | |||
| Non-Hispanic white | 364 (53.0) | 766995 (82.5) | <.001 |
| Non-Hispanic black | 216 (31.4) | 74716 (8.0) | |
| Hispanic | 78 (11.4) | 43735 (4.7) | |
| Other/unknown | 29 (4.2) | 44226 (4.8) | |
| Year of cancer diagnosis | |||
| 1992–1995 | 47 (6.8) | 125947 (13.5) | <.001 |
| 1996–2000 | 80 (11.6) | 137318 (14.8) | |
| 2001–2005 | 223 (32.5) | 306217 (32.9) | |
| 2006–2011 | 337 (49.1) | 360190 (38.7) | |
| Cancer stage | |||
| Local/regional | 420 (61.1) | 608051 (65.4) | .006 |
| Distant | 141 (20.5) | 190013 (20.4) | |
| Unknown | 126 (18.3) | 131608 (14.2) | |
| Timing of first HIV claim relative to cancer, median (IQR) | |||
| Days before cancer (n = 631) | 1281 (660–2144) | NA | NA |
| Days after cancer (n = 56) | 74 (28–170) | ||
| Metropolitan area | |||
| No | 37 (5.4) | 158684 (17.1) | <.001 |
| Yes | 650 (94.6) | 770827 (82.9) | |
| No. of cancer screenings per year, median (IQR) | 0.47 (0.00–1.29) | 0.35 (0.00–1.07) | .001 |
| No. of comorbidities | |||
| 0 | 140 (20.4) | 337400 (36.3) | <.001 |
| 1–3 | 371 (54.0) | 492537 (53.0) | |
| ≥4 | 176 (25.6) | 99735 (10.7) | |
| Zip code–based measures of SES | |||
| Income, US dollars, median (IQR) | 41027 (31008–54977) | 45031 (34808–58401) | <.001 |
| Non–high school graduates, %, median (IQR) | 19.7 (10.8–31.9) | 15.9 (10.0–25.0) | <.001 |
| Residents below poverty level, %, median (IQR) | 13.3 (6.7–23.4) | 9.0 (5.2–15.5) | <.001 |
| Composite indictor of lower SESc | 331 (48.2) | 322272 (34.7) | <.001 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; NA, not applicable; SES, socioeconomic status.
aFor age, year, and comorbidities, P values are from trend tests.
bAmong all participants without HIV (n = 945916), 16244 (1.72%) were missing zip code–level median income information, and 15958 (1.69%) were missing zip code–level percentage of non–high school graduates/percentage of residents living below poverty level information. Among all participants with HIV (n = 715), 28 (3.92%) were missing zip code–level median income/percentage of non–high school graduates/percentage of residents living below poverty level.
cThis is a composite variable that equals 1 if a participant is living in an area where (1) the median income is below the median of median income of all available areas; (2) percentage of non–high school graduates is higher than the median of percentage of non–high school grads of all available areas; or (3) percentage of residents living below poverty is higher than the median of percentage of residents living below poverty level of all available areas. Otherwise this variable equals 0. If any of these 3 variables is missing, this variable is missing. Among all participants without/with HIV, 16244 and 28 participants were missing this variable, respectively.
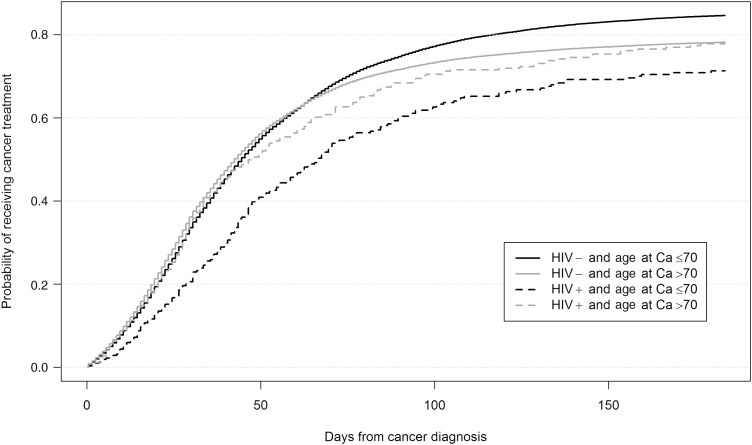
Overall, 68% of HIV-infected and 75% of HIV-uninfected individuals received cancer treatment within 6 months of cancer diagnosis (P < .01). However, the differences were actually restricted to individuals aged 66–70 years: only 65% of HIV-infected individuals received cancer treatment compared with 81% of HIV-uninfected individuals (P < .01; Table 2; Figure 1). Time from cancer diagnosis to treatment initiation was also longer in HIV-infected individuals overall, but particularly among the younger subpopulation, with a median of 43 days to first treatment compared with 36 days in HIV-uninfected persons (P < .01). The negative association between HIV and cancer treatment was also related to the type of cancer in the younger subpopulation: HIV-infected individuals were significantly less likely to be treated for colorectal, kidney, lung, and prostate cancers (P < .05). In the older subpopulation (>70 years), there were no differences between HIV-infected and -uninfected individuals in receipt (71% vs 73%, respectively) or time to treatment (median 31 vs 30 days, respectively) except for anal cancer, for which treatment was delayed in HIV (median, 61 days vs 35 in HIV uninfected; P = .02).
Table 2.
Cancer Treatment by Age, Human Immunodeficiency Virus Infection Status, and Cancer Typea
| Age 66–70 y | Age >70 y | |||||
|---|---|---|---|---|---|---|
| Cancer Type | HIV-Infected | HIV-Uninfected | P Valueb | HIV-Infected | HIV-Uninfected | P Valueb |
| Total | ||||||
| Total No. (% treated) | 321 (64.8) | 228889 (81.2) | <.01 | 366 (70.8) | 700783 (72.7) | .41 |
| Days to treatment | 42.5 (24–68.5) | 36 (20–61) | <.01 | 31 (18–58) | 30 (17–50) | .20 |
| Anus | ||||||
| Total No. (% treated) | 17 (82.4) | 658 (90.3) | .28 | 11 (90.9) | 2132 (80.5) | .39 |
| Days to treatment | 33 (16–62) | 36 (24–50) | .83 | 61 (40–102) | 35 (23–50) | .02 |
| Bladder | ||||||
| Total No. (% treated) | …c | 11560 (92.5) | .76 | 20 (90.0) | 48978 (92.0) | .75 |
| Days to treatment | 18 (12–20) | 17 (8–25) | .96 | 15.5 (11–25) | 16 (8–25) | .98 |
| Breast | ||||||
| Total No. (% treated) | 12 (91.7) | 34143 (95.8) | .47 | 33 (93.9) | 103190 (90.7) | .52 |
| Days to treatment | 24 (6–44) | 29 (17–44) | .38 | 26 (8–35) | 28 (16–44) | .09 |
| Colorectum | ||||||
| Total No. (% treated) | 23 (73.9) | 26863 (90.0) | .01 | 52 (80.8) | 118464 (84.1) | .51 |
| Days to treatment | 29 (15–41) | 20 (11–30) | .06 | 20.5 (13–29) | 20 (11–30) | .79 |
| Kidney | ||||||
| Total No. (% treated) | 11 (50.0) | 7632 (83.6) | <.01 | 12 (66.7) | 21392 (67.7) | .94 |
| Days to treatment | 48 (27–53) | 25 (13–46) | .21 | 32.5 (18–45) | 25 (14–46) | .60 |
| Liver | ||||||
| Total No. (% treated) | 18 (44.4) | 3560 (46.4) | .87 | 15 (26.7) | 10543 (32.7) | .62 |
| Days to treatment | 40 (22–83.5) | 50 (29–79) | .57 | 87.5 (27.5–160) | 48 (28–76) | .45 |
| Lung | ||||||
| Total No. (% treated) | 86 (65.1) | 52288 (75.4) | .03 | 77 (58.4) | 161342 (58.7) | .97 |
| Days to treatment | 43 (32–66.5) | 35 (22–52) | .01 | 30 (19–54) | 36 (23–55) | .24 |
| Melanoma | ||||||
| Total No. (% treated) | …c | 7093 (88.6) | .81 | 18 (88.9) | 22132 (86.9) | .80 |
| Days to treatment | 18 (14–23) | 29 (17–44) | .22 | 39 (18–54) | 29 (17–45) | .30 |
| Non-Hodgkin lymphoma | ||||||
| Total No. (% treated) | 46 (54.4) | 13483 (60.3) | .41 | 39 (56.4) | 53837 (52.1) | .59 |
| Days to treatment | 38 (22–57) | 40 (26–61) | .58 | 34 (27–60) | 40 (26–59) | .90 |
| Prostate | ||||||
| Total No. (% treated) | 92 (62.0) | 71609 (78.0) | <.01 | 89 (70.8) | 158773 (68.9) | .70 |
| Days to treatment | 66 (43–89) | 63 (41–90) | .60 | 56 (32–82) | 49 (32–74) | .29 |
Abbreviation: HIV, human immunodeficiency virus.
aPercentage of participants receiving any treatment refers to the 6-month period after diagnosis. Days to treatment refers to the median number of days (and the interquartile range) from cancer diagnosis to first treatment among those receiving any treatment.
b P values were calculated using rank-sum test.
cDue to Surveillance, Epidemiology, and End Results–Medicare data use restrictions, entries with <11 individuals have been suppressed.
Figure 1.
Probability of receiving cancer (Ca) treatment within 6 months of diagnosis, by age and human immunodeficiency virus (HIV) status.
There remained no difference in receipt of treatment by HIV status in the older subpopulation, even after adjustment for confounding variables (HRTOTAL, 1.05 [95% confidence interval {CI}, .93–1.18]; Table 3). In the younger subpopulation, HIV-infected individuals had a significantly lower rate of cancer treatment compared with HIV-uninfected individuals for all cancer combined even after adjustment (HRTOTAL, .81 [95% CI, .71–.92] vs unadjusted HR, 0.65 [95% CI, .57–.75]). This 19% lower rate of cancer treatment can be apportioned into an approximately 15% “direct” reduction due to HIV (HRDIRECT, 0.85 [95% CI, .72–.99]) plus a nonsignificant 5% reduction mediated by cancer stage and comorbidities (HRINDIRECT, 0.95 [95% CI, .87–1.04]). Thus, comorbidities and stage at cancer diagnosis together mediated only 24% of the total effect of HIV on delayed cancer treatment (ie, log[HRINDIRECT]/log[HRTOTAL]), whereas the direct effect of HIV infection itself accounts for the remaining 76% of the total effect.
Table 3.
Association Between Human Immunodeficiency Virus Status and Receipt of Cancer Treatment, Stratified by Age and Cancer Type
| Cancer Type | Age 66–70 y (n = 229210) |
Age >70 y (n = 701149) |
|||
|---|---|---|---|---|---|
| Unadjusted HR | HRTOTALa | HRDIRECT | HRINDIRECT | HRTOTALa | |
| Total | 0.65 (.57–.75) | 0.81 (.71–.92) | 0.85 (.72–.99) | 0.95 (.87–1.04) | 1.05 (.93–1.18) |
| Anus | 0.77 (.45–1.30) | 1.08 (.52–2.23) | 1.19 (.57–2.47) | 0.91 (.59–1.40) | 0.88 (.47–1.65) |
| Bladder | 1.31 (.68–2.53) | 1.32 (.82–2.14) | 1.34 (.74–2.44) | 0.98 (.69–1.41) | 1.05 (.66–1.66) |
| Breast | 0.99 (.55–1.79) | 1.08 (.50–2.33) | 0.75 (.22–2.56) | 1.44 (.67–3.08) | 1.37 (.97–1.95) |
| Colorectum | 0.55 (.34–.88) | 0.63 (.40–.98) | 0.58 (.32–1.05) | 1.09 (.71–1.68) | 0.92 (.68–1.25) |
| Kidney | 0.39 (.16–.93) | 0.41 (.15–1.12) | 0.64 (.25–1.65) | 0.64 (.28–1.47) | 0.95 (.47–1.90) |
| Liver | 1.06 (.53–2.12) | 1.17 (.51–2.67) | 1.18 (.50–2.79) | 0.99 (.59–1.67) | 0.71 (.27–1.90) |
| Lung | 0.65 (.50–.85) | 0.73 (.57–.93) | 0.73 (.55–.97) | 0.99 (.85–1.16) | 1.07 (.80–1.43) |
| Melanoma | 1.25 (.56–2.79) | 1.49 (.50–4.45) | 2.66 (.75–9.45) | 0.56 (.15–2.11) | 0.98 (.60–1.60) |
| NHL | 1.13 (.76–1.67) | 1.20 (.79–1.83) | 1.16 (.63–2.12) | 1.03 (.69–1.54) | 1.22 (.80–1.86) |
| Prostate | 0.65 (.50–.85) | 0.71 (.54–.94) | 0.77 (.58–1.02) | 0.93 (.80–1.08) | 1.05 (.82–1.35) |
Data are presented as HR (95% confidence interval). Significant HRs are highlighted in bold.
Abbreviations: HR, hazard ratio; NHL, non-Hodgkin lymphoma.
aInverse odds ratio weighting method was used to calculate HRs for the total (HRTOTAL), direct (HRDIRECT), and indirect (HRINDIRECT) effects of human immunodeficiency virus on time to cancer treatment. Cancer diagnosis year, sex, race/ethnicity, age (categorical), zip code–level socioeconomic status (composite variable), metropolitan area/not metropolitan area were modeled as confounding variables for the direct effect; stage at cancer diagnosis and number of comorbidities were modeled as mediating variables.
Among the specific cancer types, HIV-infected individuals with colorectal, lung, and prostate cancers had lower rates of treatment compared with HIV-uninfected individuals (Table 3; HRTOTAL < 1.0; P < .05), and these differences were not mediated by cancer stage and comorbidities. For example, HIV-infected individuals with colorectal cancer had a 37% lower rate of cancer treatment compared with HIV-uninfected individuals (HRTOTAL, 0.63 [95% CI, .40–.98]), and HRINDIRECT was not significantly <1.0. HIV-infected individuals with prostate cancer had a 29% lower rate (HRTOTAL, 0.71 [95% CI, .54–.94]), and those with lung cancer had a 27% lower rate of cancer treatment (HRTOTAL, 0.73 [95% CI, .57–.93]). Moreover, results for lung cancer indicated a significant direct contribution from HIV (HRDIRECT, 0.73 [95% CI, .55–.97]). There were no differences in treatment of anal, bladder, breast, or liver cancers, melanoma, or NHL between HIV-infected and uninfected individuals.
Among those with HIV, treatment varied considerably by cancer type, from as high as 93% for breast cancer to only 36% for liver cancer. Men, non-Hispanic blacks, younger individuals, and those diagnosed at distant or unknown stage had significantly lower rates of cancer treatment (Table 4). However, after mutually adjusting for all other variables, only age, prior cancer screening rate, and cancer stage were associated with cancer treatment rates. HIV-infected individuals with distant (adjusted HR [aHR], 0.71 [95% CI, .54–.94]) and unknown stage (aHR, 0.43 [95% CI, .28–.66]) had at least a 30% lower rate of cancer treatment compared with individuals with local/regional cancers. Trends in cancer treatment across age indicate an increase in treatment up to age 80 years (aHR for age 71–75 years: 1.15 [95% CI, .92–1.44]; aHR for age 76–80 years: 1.24 [95% CI, .94–1.63]) compared with individuals 66–70 years old; the oldest individuals (≥81 years) had lower treatment rates (aHR, 0.69 [95% CI, .49–.98]). More than 80% of HIV-infected cancer cases also had at least 1 other comorbidity, but there was no clear association with cancer treatment.
Table 4.
Characteristics Associated With Receipt of Cancer Treatment Among Human Immunodeficiency Virus–infected Cancer Patients
| Characteristic | Unadjusted HR (95% CI) |
Fully Adjusted HR (95% CI) |
|---|---|---|
| No. of cancer cases | 687 | 687 |
| Type of cancer | ||
| Anus | 1.35 (.86–2.10) | 1.10 (.69–1.77) |
| Bladder | 4.41 (2.87–6.77) | 3.72 (2.34–5.91) |
| Breast | 3.05 (2.12–4.39) | 2.05 (1.28–3.27) |
| Colorectal | 1.86 (1.35–2.56) | 1.69 (1.20–2.39) |
| Kidney | 0.84 (.47–1.49) | 0.68 (.37–1.25) |
| Liver | 0.52 (.29–.94) | 0.50 (.27–.92) |
| Lung | 1.00 (ref) | 1.00 (ref) |
| Melanoma | 1.90 (1.20–3.01) | 1.49 (.91–2.45) |
| Non-Hodgkin lymphoma | 0.88 (.63–1.25) | 1.42 (.88–2.28) |
| Prostate | 0.76 (.58–.99) | 0.63 (.47–.86) |
| Sex | ||
| Female | 1.00 (ref) | 1.00 (ref) |
| Male | 0.64 (.52–.79) | 0.82 (.62–1.09) |
| Age category, y | ||
| 66–70 | 1.00 (ref) | 1.00 (ref) |
| 71–75 | 1.37 (1.10–1.69) | 1.15 (.92–1.44) |
| 76–80 | 1.41 (1.09–1.83) | 1.24 (.94–1.63) |
| ≥81 | 1.04 (.75–1.43) | 0.69 (.49–.98) |
| Race/ethnicity | ||
| Non-Hispanic white | 1.00 (ref) | 1.00 (ref) |
| Non-Hispanic black | 0.75 (.61–.93) | 0.83 (.65–1.05) |
| Hispanic | 1.01 (.75–1.34) | 1.03 (.75–1.40) |
| Other/unknown | 0.94 (.58–1.54) | 1.22 (.74–2.03) |
| Year of cancer diagnosis | ||
| 1992–1995 | 1.00 (ref) | 1.00 (ref) |
| 1996–2000 | 1.55 (1.00–2.41) | 1.06 (.67–1.69) |
| 2001–2005 | 1.04 (.70–1.55) | 0.78 (.51–1.20) |
| 2006–2011 | 1.01 (.69–1.48) | 0.76 (.50–1.15) |
| Timing of HIV claim relative to cancer | ||
| HIV diagnosed after cancer | 1.05 (.74–1.49) | 1.17 (.81–1.68) |
| 0–1000 days before cancer | 1.00 (ref) | 1.00 (ref) |
| 1001–2000 days before cancer | 1.11 (.88–1.39) | 1.07 (.85–1.35) |
| >2000 days before cancer | 1.10 (.87–1.39) | 1.04 (.81–1.34) |
| No. of cancer screenings per year | 1.01 (.95–1.08) | 1.08 (1.00–1.15) |
| Cancer stage | ||
| Local/regional | 1.00 (ref) | 1.00 (ref) |
| Distant | 0.77 (.60–.98) | 0.71 (.54–.94) |
| Unknown | 0.54 (.41–.72) | 0.43 (.28–.66) |
| Metropolitan area | ||
| No | 1.00 (ref) | 1.00 (ref) |
| Yes | 1.16 (.77–1.73) | 0.98 (.65–1.49) |
| No. of comorbidities | ||
| 0 | 1.00 (ref) | 1.00 (ref) |
| 1–3 | 1.11 (.88–1.40) | 1.25 (.98–1.59) |
| ≥4 | 0.87 (.66–1.15) | 1.01 (.75–1.35) |
| Indictor of lower SES | ||
| No | 1.00 (ref) | 1.00 (ref) |
| Yes | 0.87 (.73–1.05) | 0.88 (.71–1.08) |
Data are presented as HR (95% confidence interval). Significant HRs are highlighted in bold.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; SES, socioeconomic status.
DISCUSSION
Based on the limited inclusion of elderly individuals with HIV in previous research, this study explored treatment for a variety of cancer types in a cohort of nearly 1 million elderly Americans. We found that individuals with HIV were less likely to receive timely cancer treatment compared to those without HIV. In fact, 35% of HIV-infected individuals aged 66–70 years received no cancer treatment within 6 months of cancer diagnosis, and there were modest delays in time to treatment compared to HIV-uninfected individuals. After taking into account potential confounding variables, HIV-infected individuals still had 20% lower treatment rates overall, and treatment rates were lower specifically for HIV-infected individuals with colorectal, lung, and prostate cancer. Importantly, these less-treated cancers represent 3 of the 4 most common cancers studied in this HIV population. Two important clinical factors, cancer stage at diagnosis and burden of comorbidities, may explain 24% of the difference in cancer treatment rates. A recent study estimated that over a 5-year period, 10% of HIV-infected individuals in the United States developed cancer at age 65 years or older [13], and cancer is the leading cause of non-AIDS-associated death [8, 9]. Thus, with the current aging of the HIV population, understanding the complexities in provision of cancer care and treatment will be essential to reduce disparities, prevent premature death, and improve quality of life.
A study using linked HIV and cancer registries in Texas found that HIV-infected individuals with lung cancer were less likely to receive cancer treatment compared with HIV-uninfected individuals [4], and HIV-infected individuals were at least 20% less likely to receive cancer treatment for a variety of other cancers as well [14]. In a prior study using Medicare data, no differences in treatment for non-small-cell lung cancer were seen by HIV status [5]. This is consistent with the present study, as no differences were seen in treatment rates considering the Medicare population as a whole, including for lung cancer. However, when we stratified our Medicare population by age, treatment disparities in the younger subset were observed, a finding consistent with the majority of prior studies that focused on relatively younger populations [4–6, 14, 15]. Notably, age-related treatment patterns differed by HIV status: Treatment rates in HIV-infected individuals increased with age, whereas treatment decreased with age in HIV-uninfected individuals. The decline in cancer treatment with age in HIV-uninfected individuals is consistent with prior studies of breast, colon, prostate, and other types of cancer treatment in the general population [16–18]. However, the observed increase in treatment with age up to age 80 years in the HIV population is a novel finding with no clear explanation. Together, these patterns highlight that age modifies the association between HIV and cancer treatment in the aging population, and may help to explain inconsistent results across other study populations.
As our study highlights, the growing problem of multimorbidity and thus need for polypharmacy is greatly amplified in the elderly HIV population with cancer [19, 20]—80% of HIV-infected cancer patients in our study had at least 1 of 15 other comorbidities. We hypothesized that cancer treatment rates may be lower because of medical indication against treatment, perceived low benefit-to-harm ratio, or competing health risks. Therefore, unlike prior research, we considered factors such as comorbidities and stage at diagnosis as mediators, rather than confounders, of the association between HIV status and receipt of cancer treatment. Although cancer stage at diagnosis was an important predictor of treatment among those with HIV, there was no consistent association between comorbidity score and cancer treatment. As such, cancer stage and medical comorbidities combined accounted for only a nonsignificant 24% of the difference in cancer treatment rates between HIV-infected and HIV-uninfected cases in the 66–70 year-old age group.
Thus, our findings suggest that HIV infection itself, which accounted for 76% of the total effect in younger cancer patients (HRDIRECT in our mediation analyses), is the predominant comorbidity adversely associated with cancer treatment in individuals aged 66–70 years. It is also likely that the total effect attributable to HIV remains affected by unmeasured confounding such as education or health behavior, or additional mediating variables that are yet to be identified. We conducted a sensitivity analysis where we added the annual number of cancer screenings to the mediation analysis of the younger subpopulation, as a marker of access to care or differences in engagement in routine care that might affect receipt of treatment. Despite observed differences in cancer screening by HIV status, this did not change the estimates of the direct (HR, 0.84 [95% CI, .71–.99]) or indirect (HR, 0.96 [95% CI, .88–1.05]) effects of HIV on cancer treatment.
Our findings of differences in treatment by HIV are consistent with findings from a recent survey of oncologists, in which approximately 20% said they would modify prescribing behavior based on HIV status [21]. In addition, the majority of providers felt that sufficient clinical management guidelines were not available to aid in treatment decision making. These findings and the extensive literature focusing on interactions between ART and chemotherapeutics [22–24] point to a need for guidelines that support individualized cancer treatment plans, in close coordination between HIV physicians and oncologists. Prior research has shown that having multidisciplinary care teams can result in treatment rates and outcomes in HIV-infected individuals that are comparable to those without HIV [25].
Prior research in the general population suggests that longer time from cancer diagnosis to treatment could be associated with worse outcomes in breast, colorectal, and melanoma skin cancer [26]. Factors related to delayed treatment include older age, low SES, multiple comorbidities, nonwhite race/ethnicity, nonprivate health insurance, and diagnosis at a referring hospital [27–29]. The present study adds to the literature by providing evidence that HIV is also associated with treatment delays, and presents the first data on time to cancer treatment in older Americans with HIV. This is an important absolute measure to consider in addition to the overall treatment rates. Prior studies have focused on treatment delays of 15–30 days [27, 28, 30], so it is unclear whether our observed delay of 7 days would negatively impact patient outcomes, or simply reflects the increased time needed for treatment planning in these potentially more complicated patients with HIV/AIDS.
Unfortunately, indicators of HIV progression such as CD4 cell count and HIV RNA load are not available in SEER-Medicare, and data on antiretroviral claims only started in 2007 with 50%–70% Medicare part D coverage, so we could not explore the extent to which severity of HIV or ART use was associated with cancer treatment. However, in the analysis of the HIV subpopulation, we included timing of first HIV diagnostic claim relative to cancer diagnosis, which was not associated with cancer treatment. In addition, analyzing the details of complex treatment algorithms was beyond the scope of this study, so future research should address whether the types or completeness of treatment, and subsequent survival, differ by HIV status and effective ART use. A strength of this study is the population-based design, which allowed us to examine several common cancers in a representative population. Although the number of cases is small for some individual cancers, this study represents one of the largest possible samples of older HIV-infected adults with cancer, since SEER-Medicare captures >25% of elderly Americans [31].
The elderly HIV population is at high risk for cancer, yet there are limited data specific to cancer treatment in aging HIV populations. This study begins to fill the gaps in our knowledge with regard to cancer care disparities across a variety of cancer types and subgroups less likely to receive treatment. HIV-infected individuals differ in many ways compared to HIV-uninfected individuals with regard to the development and management of cancer that may ultimately affect survival. Even after accounting for many of these factors, HIV-infected individuals still had lower treatment rates for common cancers, particularly the younger subset that might benefit the most from improved cancer survival in terms of additional life expectancy. The results of this study go beyond simply quantifying these disparities and highlight the need for a multilevel evaluation of barriers to care and for multidisciplinary teams to manage these complex cases, particularly younger individuals with common cancers, who currently experience low treatment rates.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. This study used the linked Surveillance, Epidemiology, and End Results (SEER)-Medicare database. The authors acknowledge the efforts of the National Cancer Institute; Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services, Inc (IMS); and the SEER Program tumor registries in the creation of the SEER-Medicare database. The authors thank Winnie Ricker at IMS for programming support.
Disclaimer. The funder played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. The interpretation and reporting of these data are the sole responsibility of the authors.
Financial support. This study was supported, in part, by the Johns Hopkins University Center for AIDS Research (grant number P30AI094189) and the Intramural Research Program of the National Cancer Institute.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Greene M, Justice AC, Lampiris HW, Valcour V. Management of human immunodeficiency virus infection in advanced age. JAMA 2013; 309:1397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. May MT, Ingle SM. Life expectancy of HIV-positive adults: a review. Sex Health 2011; 8:526–33. [DOI] [PubMed] [Google Scholar]
- 3. Grulich AE. Living longer with HIV: what does it mean for cancer risk?Curr Opin HIV AIDS 2009; 4:1–2. [DOI] [PubMed] [Google Scholar]
- 4. Suneja G, Shiels MS, Melville SK, Williams MA, Rengan R, Engels EA. Disparities in the treatment and outcomes of lung cancer among HIV-infected individuals. AIDS 2013; 27:459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee JY, Moore PC, Steliga MA. Do HIV-infected non-small cell lung cancer patients receive guidance-concordant care?Med Care 2013; 51:1063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murphy AB, Bhatia R, Martin IK et al. Are HIV-infected men vulnerable to prostate cancer treatment disparities?Cancer Epidemiol Biomarkers Prev 2014; 23:2009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sigel K, Crothers K, Dubrow R et al. Prognosis in HIV-infected patients with non-small cell lung cancer. Br J Cancer 2013; 109:1974–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coghill AE, Shiels MS, Suneja G, Engels EA. Elevated cancer-specific mortality among HIV-infected patients in the United States. J Clin Oncol 2015; 33:2376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marcus JL, Chao C, Leyden WA et al. Survival among HIV-infected and HIV-uninfected individuals with common non-AIDS-defining cancers. Cancer Epidemiol Biomarkers Prev 2015; 24:1167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Comprehensive Cancer Network NCCN guidelines for treatment of cancer by site. Available at: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. Accessed 8 September 2016.
- 11. Quan H, Sundararajan V, Halfon P et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–9. [DOI] [PubMed] [Google Scholar]
- 12. Tchetgen Tchetgen EJ. Inverse odds ratio-weighted estimation for causal mediation analysis. Stat Med 2013; 32:4567–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yanik EL, Katki HA, Engels EA. Cancer risk among the HIV-infected elderly in the United States. AIDS 2016; 30:1663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suneja G, Lin CC, Simard EP, Han X, Engels EA, Jemal A. Disparities in cancer treatment among patients infected with the human immunodeficiency virus. Cancer 2016; 122:2399–407. [DOI] [PubMed] [Google Scholar]
- 15. Suneja G, Shiels MS, Angulo R et al. Cancer treatment disparities in HIV-infected individuals in the United States. J Clin Oncol 2014; 32:2344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schonberg MA, Marcantonio ER, Li D, Silliman RA, Ngo L, McCarthy EP. Breast cancer among the oldest old: tumor characteristics, treatment choices, and survival. J Clin Oncol 2010; 28:2038–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol 2011; 29:235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schrag D, Cramer LD, Bach PB, Begg CB. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst 2001; 93:850–7. [DOI] [PubMed] [Google Scholar]
- 19. Althoff KN, Smit M, Reiss P, Justice AC. HIV and ageing: improving quantity and quality of life. Curr Opin HIV AIDS 2016; 11:527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smit M, Brinkman K, Geerlings S et al. ATHENA Observational Cohort Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015; 15:810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suneja G, Boyer M, Yehia BR et al. Cancer treatment in patients with HIV infection and non-AIDS-defining cancers: a survey of US oncologists. J Oncol Pract 2015; 11:e380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berretta M, Caraglia M, Martellotta F et al. Drug-drug interactions based on pharmacogenetic profile between highly active antiretroviral therapy and antiblastic chemotherapy in cancer patients with HIV infection. Front Pharmacol 2016; 7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rudek MA, Flexner C, Ambinder RF. Use of antineoplastic agents in patients with cancer who have HIV/AIDS. Lancet Oncol 2011; 12:905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mounier N, Katlama C, Costagliola D, Chichmanian RM, Spano JP. Drug interactions between antineoplastic and antiretroviral therapies: implications and management for clinical practice. Crit Rev Oncol Hematol 2009; 72:10–20. [DOI] [PubMed] [Google Scholar]
- 25. Lim C, Goutte N, Gervais A et al. Standardized care management ensures similar survival rates in HIV-positive and HIV-negative patients with hepatocellular carcinoma. J Acquir Immune Defic Syndr 2012; 61:581–7. [DOI] [PubMed] [Google Scholar]
- 26. Neal RD, Tharmanathan P, France B et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer 2015; 112(Suppl 1):S92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bleicher RJ, Ruth K, Sigurdson ER et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol 2016; 2:330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol 2016; 2:322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lott JP, Narayan D, Soulos PR, Aminawung J, Gross CP. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol 2015; 151:731–41. [DOI] [PubMed] [Google Scholar]
- 30. Amri R, Bordeianou LG, Sylla P, Berger DL. Treatment delay in surgically-treated colon cancer: does it affect outcomes?Ann Surg Oncol 2014; 21:3909–16. [DOI] [PubMed] [Google Scholar]
- 31. Engels EA, Pfeiffer RM, Ricker W, Wheeler W, Parsons R, Warren JL. Use of Surveillance, Epidemiology, and End Results–Medicare data to conduct case-control studies of cancer among the US elderly. Am J Epidemiol 2011; 174:860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.