Abstract
Background
Levofloxacin is used for the treatment of multidrug-resistant tuberculosis; however the optimal dose is unknown.
Methods
We used the hollow fiber system model of tuberculosis (HFS-TB) to identify 0–24 hour area under the concentration-time curve (AUC0-24) to minimum inhibitory concentration (MIC) ratios associated with maximal microbial kill and suppression of acquired drug resistance (ADR) of Mycobacterium tuberculosis (Mtb). Levofloxacin-resistant isolates underwent whole-genome sequencing. Ten thousands patient Monte Carlo experiments (MCEs) were used to identify doses best able to achieve the HFS-TB–derived target exposures in cavitary tuberculosis and tuberculous meningitis. Next, we used an ensemble of artificial intelligence (AI) algorithms to identify the most important predictors of sputum conversion, ADR, and death in Tanzanian patients with pulmonary multidrug-resistant tuberculosis treated with a levofloxacin-containing regimen. We also performed probit regression to identify optimal levofloxacin doses in Vietnamese tuberculous meningitis patients.
Results
In the HFS-TB, the AUC0-24/MIC associated with maximal Mtb kill was 146, while that associated with suppression of resistance was 360. The most common gyrA mutations in resistant Mtb were Asp94Gly, Asp94Asn, and Asp94Tyr. The minimum dose to achieve target exposures in MCEs was 1500 mg/day. AI algorithms identified an AUC0-24/MIC of 160 as predictive of microbiologic cure, followed by levofloxacin 2-hour peak concentration and body weight. Probit regression identified an optimal dose of 25 mg/kg as associated with >90% favorable response in adults with pulmonary tuberculosis.
Conclusions
The levofloxacin dose of 25 mg/kg or 1500 mg/day was adequate for replacement of high-dose moxifloxacin in treatment of multidrug-resistant tuberculosis.
Keywords: tuberculous meningitis, hollow fiber system model, artificial intelligence, Monte Carlo experiments, gyrA mutations
The treatment of multidrug-resistant (MDR) tuberculosis relies on latter generation quinolones, considered group A drugs by the World Health Organization. Moxifloxacin has been most commonly used, and doses of 800 mg/day have been proposed to minimize acquired drug resistance (ADR) [1, 2]. However, at this higher dose, moxifloxacin could be associated with higher rates of QT segment prolongation, which could progress to polymorphic ventricular tachycardia, an important concern especially when coadministered with other anti-tuberculous agents associated with this same adverse event [3]. Levofloxacin is felt to be safer in this regard; thus, it is important to determine if levofloxacin can replace high-dose moxifloxacin and, if so, to identify the optimal dose for MDR tuberculosis.
While pharmacokinetic-pharmacodynamic (PK/PD) studies of moxifloxacin and the earlier-generation ofloxacin and ciprofloxacin have been performed, levofloxacin as of yet has not been examined [1, 4, 5]. Clinical studies have identified the population PK parameters of levofloxacin in patients with tuberculosis and the penetration of levofloxacin into tuberculosis lesions [6–8]. Since moxifloxacin binds the Mycobacterium tuberculosis (Mtb) gyrase better than levofloxacin, in theory, it could be a better anti-tuberculosis agent [9]. However, moxifloxacin had lower early bactericidal activity in patients than levofloxacin [10]. On the other hand, murine studies demonstrated that standard-dose moxifloxacin was superior to high-dose (human equivalent 1000 mg) levofloxacin when used in combination with ethionamide, amikacin, and pyrazinamide after 4 and 5 months of therapy [10, 11]. Thus, it is as yet unclear if levofloxacin could be a sufficient replacement for moxifloxacin and, if so, what the dose equivalent to moxifloxacin 800 mg/day would be. Here, we performed a PK/PD study in the hollow fiber system model of tuberculosis (HFS-TB). We utilized the results and population PK parameters in Monte Carlo experiments (MCEs) to evaluate levofloxacin doses for patients with tuberculosis and identify the minimum inhibitory concentration (MIC) above which levofloxacin ceases to effectively kill Mtb. Next, we utilized machine learning (ML) methods to identify the magnitude of the PK/PD index associated with clinical outcomes in Tanzanian patients who were treated for pulmonary MDR tuberculosis [12]. We also compared the MCE dose-vs-outcome results in Vietnamese patients treated for tuberculous meningitis [13].
METHODS
Materials, Organisms, and Reagents
Mtb H37Ra (American Type Culture Collection 25177) was used for HFS-TB experiments, as described elsewhere [14]. Levofloxacin was purchased from the Baylor University Medical Center Pharmacy (Dallas, TX) and from Sigma (St. Louis, MO). Moxifloxacin-13Cd3 was purchased from Santa Cruz Biotechnology (Dallas, TX). We utilized the BACTEC MGIT 960 Mycobacterial Growth Indicator Tube system (MGIT; Franklin Lakes, NJ) to determine time-to-positivity (TTP) as a measure of bacterial burden.
MICs and Screening for Extracellular and Intracellular Effect
MICs were identified using the standard macrobroth dilution reference method, the MGIT assay, and Epsilometer test (E test) on Middlebrook 7H10 agar, as described previously [15, 16]. The concentrations tested in the MGIT assay were 0, 0.015, 0.03, 0.06, 0.125, 0.25, 0.5, 1, and 2 mg/L. The macrobroth dilution assay tested the same concentration series, but up to 16 mg/L. Next, we examined the microbial kill of levofloxacin co-incubated for 7 days with extracellular and intracellular Mtb in test tubes and 24-well plates, as described previously [15, 16].
Hollow Fiber System Model of Tuberculosis
HFS-TB construction, technical specifications, and inoculation with log-phase growth Mtb have been described in detail elsewhere as well as in this supplement [1, 14, 17–20]. Seven HFS-TB units were treated for 28 days via computerized syringe pumps that achieved the following levofloxacin 0–24 area under the concentration-time curve (AUC0-24): 0, 1.0, 1.25, 2.0, 10.5, 21, and 42 mg*h/L. We confirmed these exposures by sampling the central compartment at 0, 1, 4, 8, 12, 22, and 23.5 hours after the last dose. Since the levofloxacin half-life in epithelial lining fluid and alveolar macrophages is 8.1–14.3 hours [21], we mimicked the midway half-life of 11 hours. Levofloxacin concentrations were measured using the assay described in detail in the Supplementary Methods. The peripheral compartments were sampled on days 0, 3, 7, 10, 14, 21, and 28 for TTP and colony-forming unit (CFU) counts, as described previously [16, 22]. The levofloxacin-resistant isolates, which were captured by culturing on agar supplemented with 8 times the levofloxacin MIC, underwent whole-genome sequencing (WGS); identified mutations were confirmed by Sanger sequencing, as described elsewhere [16, 23].
PK modeling was performed in ADAPT 5, and both AUC0-24 and AUC0-24/MIC were calculated as described elsewhere [1, 16, 24]. The relationship between AUC0-24/MIC and total bacterial burden was examined using the inhibitory sigmoid maximal kill (Emax) model, and that for ADR using the quadratic function [1, 14–20, 22–25].
Monte Carlo Experiments Using HFS-TB Data
The EC80 (exposure associated with 80% of maximal kill) and the AUC0-24/MIC associated with ADR suppression, whichever was the higher value, was defined as the target exposure [20]. The PK parameter estimates and variability shown in Table 1, based on prior work by others, were entered in subroutine PRIOR of ADAPT 5 [6, 26]. We used a levofloxacin AUC cavitary penetration ratio of 1.33 for pulmonary tuberculosis and a cerebrospinal fluid (CSF)-to-serum penetration ratio of 0.7 for tuberculous meningitis, based on the work of others [7, 27, 28]. The following levofloxacin doses were examined in each 10000 subject MCE for pulmonary disease and tuberculous meningitis: 750, 1000, 1250, and 1500 mg. Target attainment probability (TAP) was calculated at each MIC, ranging from 0.0625 mg/L to 16 mg/L, based on the MIC distribution of 243 Mtb isolates by Rodriguez [29]. Since fluoroquinolone MIC susceptibility breakpoint and distribution in Mtb isolates are dependent on the media used and the MGIT is commonly used for MIC distribution, we performed a sensitivity analysis using the MGIT-derived MIC distribution in 30 wild-type isolates reported by Kambli et al [30]. The overall cumulative fraction of response (CFR) was then calculated for each dose weighted over this MIC distribution, as described elsewhere in this supplement [20].
Table 1.
Levofloxacin Pharmacokinetic Parameters and Variances
| Pharmacokinetic Parameters | Domain of Input (Mean ± SD) [6, 29] |
10000 Simulated Subjects (Mean ± SD) |
US Food and Drug Administration Package Insert (Mean ± SD) |
|---|---|---|---|
| Clearance, L/h | 7.63 ± 3.55 | 7.61 ± 1.88 | 8.58 ± 1.74 |
| Volume, L | 81.21 ± 41.66 | 81.33 ± 6.50 | 100 ± 16 |
| Absorption constant, per hour | 5.96 ± 2.38 | 5.95 ± 1.54 | … |
| 0–24 hour area under the concentration-time curve (mg*h/L) for 750 mg | … | 87.13 ± 15.6 | 90.7 ± 17.6 |
| Peak (mg/L) for 750 mg | … | 8.56 ± 2.55 | 8.6 ± 1.9 |
Abbreviation: SD, standard deviation.
Analyses of Clinical Data Using Machine-learning and Probit Regression
We used random forests and classification and regression tree (CART) analyses, which are described in full in the Supplemental Methods and elsewhere, to identify levofloxacin PK/PD parameters predictive of outcomes in MDR tuberculosis patients [20]. Outcomes examined were sputum conversion, ADR, and death in 41 Tanzanian patients with MDR tuberculosis treated with 750 mg levofloxacin, in combination with kanamycin, cycloserine, ethionamide, and pyrazinamide, who had levofloxacin concentrations measured 2 hours after dose 4 weeks after starting therapy [12]. First, we performed compartmental PK modeling, as previously described, and compared results with prior studies that used intensive sampling (Table 1) [31, 32]. We then implemented random forests and CART to identify and rank the most important predictors of sputum conversion [32, 33]. Next, we determined the probability of favorable outcomes, given a specific levofloxacin dose, in the Tanzanian patients with pulmonary MDR tuberculosis, as well as in Vietnamese patients with tuberculous meningitis, using separate probit regression models [12, 13].
RESULTS
Activity of Static Levofloxacin Concentrations
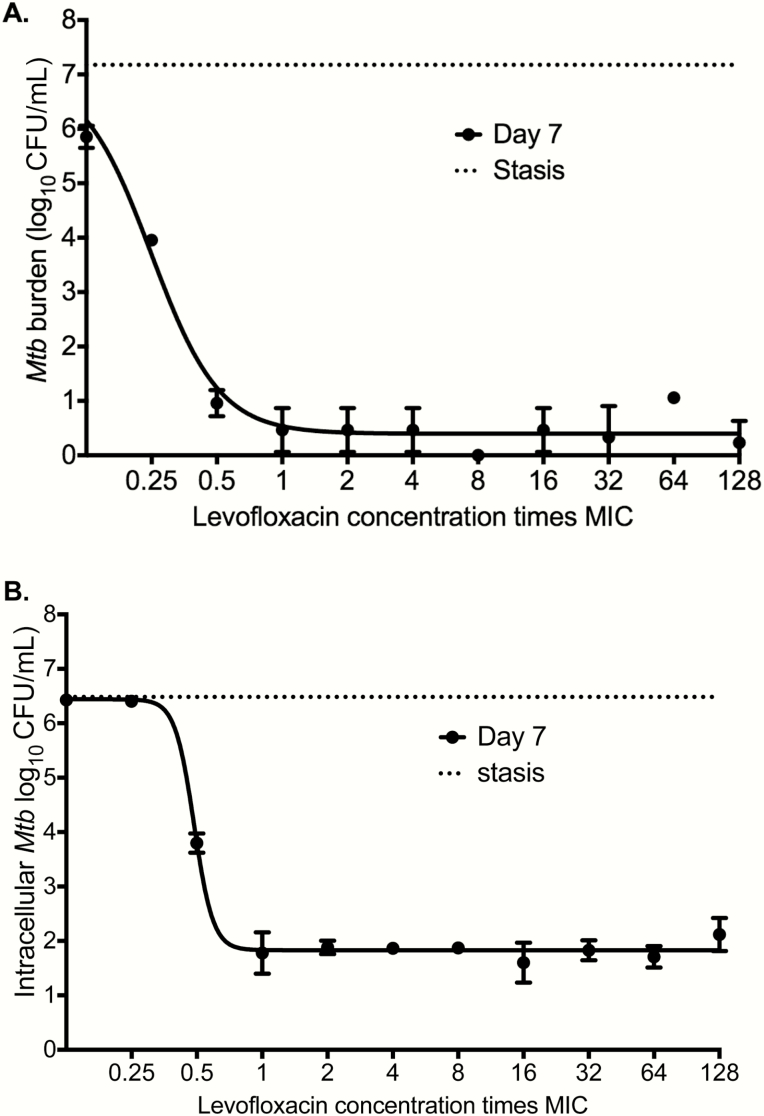
The levofloxacin MICs for Mtb H37Ra were 0.0625 and 0.125 mg/L in broth macrodilution on 2 separate occasions, 0.125 mg/L in the MGIT on 2 separate occasions, and 0.16 mg/L on E-test. We adopted an MIC of 0.125 mg/L. The inhibitory sigmoid Emax relationship between static concentration and extracellular log-phase growth Mtb burden in test tubes is shown in Figure 1A. The EC50 was 0.25 (95% confidence interval [CI], 0.22–0.28) times MIC and Emax was 6.62 (95% CI, 6.09–7.16) log10 CFU/mL. Figure 1B shows results for intracellular Mtb in 24-well plates. The EC50 was 0.44 (95% CI, 0.43–0.51) times MIC and Emax was 4.71 (95% CI, 4.38–5.08) log10 CFU/mL.
Figure 1.
Levofloxacin concentration-effect against Mycobacterium tuberculosis (Mtb). Mtb cultures were co-incubated for 7 days, with 3 replicates for each drug concentration. Shown are mean values; error bars are for standard deviation. The stasis line indicates bacterial burden at the start of treatment. Each drug concentration is expressed as a multiple of minimum inhibitory concentration (MIC). (A) Levofloxacin effect against extracellular log-phase growth Mtb demonstrated a steep decline in bacterial burden below the MIC, with no increased killing at the concentration of 1 times MIC or above. Maximal kill (Emax) was >7 log10 colony-forming units (CFU)/mL below stasis. (B) We infected activated THP-1 cells and co-incubated them with levofloxacin for 7 days. The concentration-effect pattern was similar to that seen with extracellular Mtb co-incubation, with the difference that microbial kill below stasis was 2 log10 CFU/mL less than against extracellular bacilli, which means less efficacy against intracellular bacilli. A lower Emax of intracellular vs extracellular Mtb has also been seen with other quinolones such as moxifloxacin. Abbreviations: MIC, minimum inhibitory concentration; Mtb, Mycobacterium tuberculosis.
Exposure-Response in the HFS-TB
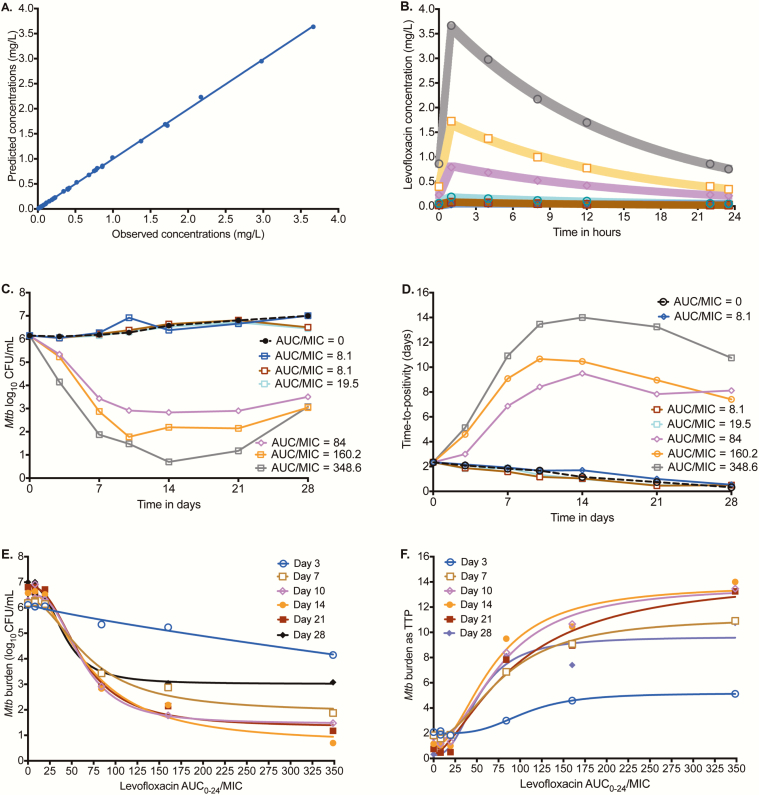
The PK model predicted vs observed concentrations in HFS-TB, shown in Figure 2A, had a slope of 0.995 ± 0.003 (r2 > 0.999), indicating minimal bias. The concentration-time profiles achieved are shown in Figure 2B.The levofloxacin clearance was 0.040 ± 0.002 L/h, indicating a 5% (minimal) technical variation between the HFS-TB units; the half-life was 10.84 hours, which is 98.55% accurate for the intended 11 hours. The time-kill curves, based on CFU/mL readout, are shown in Figure 2C, which demonstrates 2 clusters. Those with an AUC0-24/MIC <20 had effect similar to the nontreated controls, while higher exposures demonstrated considerable microbial kill. Figure 2D shows the same pattern when TTP was used as the bacterial burden readout. Inhibitory sigmoid Emax modeling of AUC0-24/MIC vs log10 CFU/mL for each day of therapy is shown in Figure 2E. Supplementary Table 1 demonstrates a remarkably consistent EC50 and Hill slope on all sampling days. The TTP-based inhibitory sigmoid Emax fits are shown in Figure 2F and Supplementary Table 1. We calculated the EC80 averaged across all sampling days, which was an AUC0-24/MIC of 146.40 (95% CI, 112.1–180.8).
Figure 2.
Levofloxacin pharmacokinetics (PKs) and time-kill curves in the hollow fiber system model of tuberculosis (HFS-TB). (A) Model-predicted vs observed levofloxacin concentrations in the hollow fiber system. (B) Levofloxacin concentrations achieved in each HFS-TB at each time point were used for PK modeling. The shaded area is the 95% confidence interval for the PK model-derived concentrations for each dose; the observed concentrations show that the model described the data well. (C) Time change in colony-forming units per milliliter (CFU/mL) with duration of therapy shows that the exposures could be separated into 2 groups. The highest exposure achieved near sterilization on day 14, followed by rebound on day 21. (D) Time-to-positivity (TTP) shows the same pattern as with CFU/mL. (E) The inhibitory sigmoid maximal kill (Emax) model curves are shown based on the CFU/mL readout. (F) Inhibitory sigmoid Emax modeling using TTP as a measure of bacterial burden demonstrated a higher exposure associated with 50% of maximal kill than was observed for CFU/mL. Abbreviations: AUC0-24, 0–24 hour area under the concentration-time curve; MIC, minimum inhibitory concentration; Mtb, Mycobacterium tuberculosis.
Evolution of Resistance in the HFS-TB
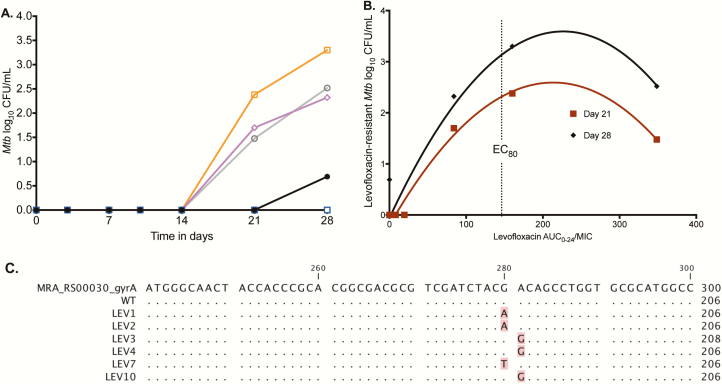
The evolution of the levofloxacin-resistant Mtb subpopulation is shown in Figure 3A; ADR arose on day 21. The relationship between AUC0-24/MIC and the levofloxacin-resistant subpopulation is shown in Figure 3B. The figure shows that the EC80 AUC0-24/MIC of 146 also amplified the proportion of levofloxacin-resistant Mtb to near maximal, similar to what was noted with moxifloxacin and ciprofloxacin [1, 5]. Suppression of ADR was at an AUC0-24/MIC of 360. Levofloxacin-resistant isolates from the HFS-TB underwent WGS that identified the following gyrA mutations: Asp94Gly, Asp94Asn, and Asp94Tyr. The results were confirmed by Sanger sequencing, shown in Figure 3C. Indeed, results show that all mutations were in the quinolone resistance determining region [34].
Figure 3.
Acquired resistance in the hollow fiber system and whole genome sequencing. (A) The acquired levofloxacin-resistant bacterial burden (log10 colony-forming units per milliliter [CFU/mL]) arose in 3 of the levofloxacin-treated systems on day 21, under the 3 highest 0–24 hour area under the concentration-time curve/minimum inhibitory concentration (AUC0-24/MIC) exposures of 84 (magenta diamonds), 160.2 (orange squares), and 348.6 (gray open circles), which were higher than nontreated controls (black solid circles), as shown. On day 28, the proportion of the levofloxacin-resistant subpopulation to the total hollow fiber system model of tuberculosis (HFS-TB) CFU/mL was 6.56% in the HFS-TB exposed to an AUC0-24/MIC = 84, 100% for AUC0-24/MIC = 160.2, and 27.5% for AUC0-24/MIC = 348.6. (B) Based on the size of the levofloxacin-resistant subpopulation, it can be seen that the exposure associated with 80% of maximal kill was associated with near maximal resistance amplification. The suppression of resistance can be read off the graph as an AUC0-24/MIC = 360. (C) The Sanger sequence alignment compares wild-type to levofloxacin-resistant strains from the HFS-TB and shows nucleotide changes at positions 280 and 281. Abbreviations: AUC0-24, 0–24 hour area under the concentration-time curve; EC80, exposure associated with 80% of maximal kill; MIC, minimum inhibitory concentration; Mtb, Mycobacterium tuberculosis.
MCE Dose Selection for Pulmonary and Meningeal Tuberculosis
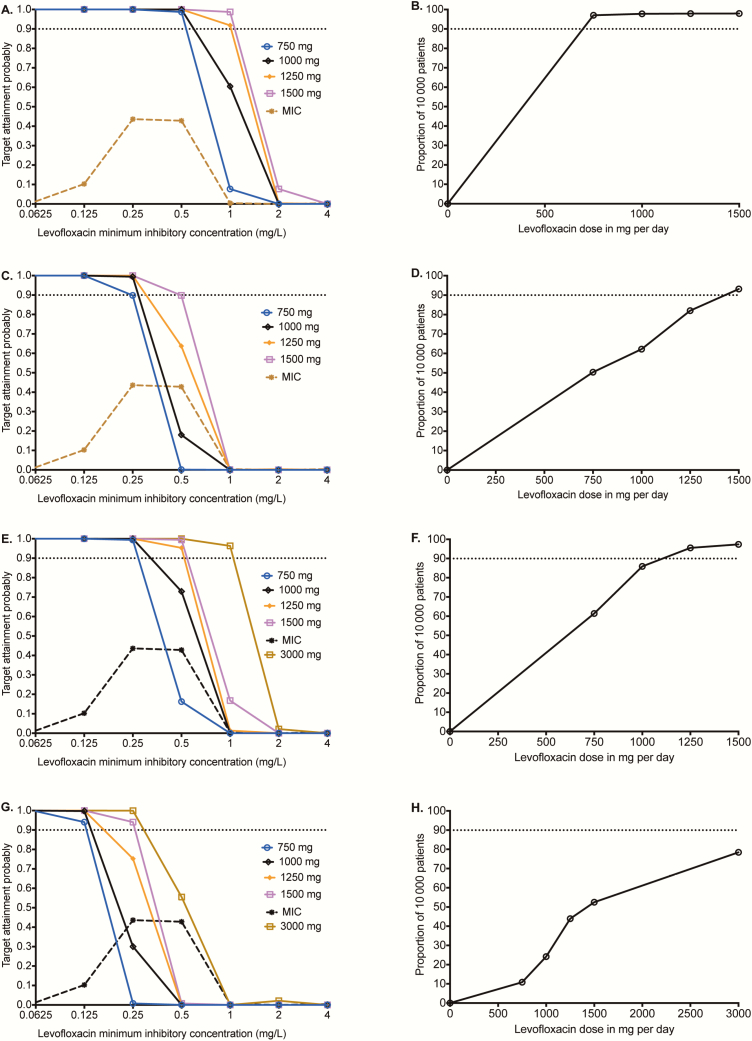
The TAPs of different doses in pulmonary tuberculosis for the EC80 target exposure are shown in Figure 4A at each MIC. The CFR, which is the proportion of patients with pulmonary tuberculosis who achieved target exposures with each dose, were as shown in Figure 4B. However, the EC80 target maximally amplifies ADR. Therefore, we performed MCEs using the target exposure associated with ADR suppression, with results shown in Figure 4C and 4D, which revealed an optimal dose of 1500 mg/day, based on CFR. At that dose, the proposed susceptibility breakpoint was 0.5 mg/L. Next, we performed a sensitivity analysis utilizing MGIT-derived levofloxacin MIC distribution in which 83.33% of Mtb isolates had an MIC ≤0.38 mg/L and 16.77% had an MIC of 0.75 mg/L [30]. The 1500-mg/day TAP for resistance suppression was 99.29% at an MIC of 0.38 mg/L and 15.67% at an MIC of 0.75 mg/L; thus, the proposed susceptibility breakpoint would remain the same at 0.5 mg/L even in MGIT assays.
Figure 4.
Monte Carlo experiments of different levofloxacin doses for pulmonary and meningeal tuberculosis. (A) Target attainment probability (TAP) for the exposure associated with 80% of maximal kill (EC80) exposure in pulmonary cavities. At a dose of 1000 mg, TAP falls below 90% above a minimum inhibitory concentration (MIC) of 0.5 mg/L. (B) Proportion of 10000 patients treated with different doses who achieved the EC80 in pulmonary cavities reveals an optimal dose of 1250 mg. (C) TAP of different doses for achieving 0–24 hour area under the concentration-time curve (AUC0-24)/MIC = 360 target exposure associated with resistance suppression in pulmonary cavities. At a dose of 1500 mg, the TAP falls below 90% above an MIC of 0.5 mg/L. (D) The levofloxacin dose that would achieve the exposure associated with resistance suppression in >90% of patients with pulmonary cavities was 1500 mg per day. (E and F) In tuberculous meningitis, the TAP for the EC80 target fell below 90% above an MIC of 0.5 mg/L for all doses, except the 3000 mg a day. (G and H) TAP of different doses in achieving exposure associated with resistance suppression in the cerebrospinal fluid precipitously drops above an MIC of 0.25 mg/L, demonstrating the impact of drug penetration on site of effect. Abbreviation: MIC, minimum inhibitory concentration.
In tuberculous meningitis, the performance of doses in achieving the EC80 target were as shown in Figure 4E. The CFRs for each dose are shown in Figure 4F. A dose of 1250 mg/day was required to achieve the EC80 in >90% of patients with tuberculous meningitis. For completeness, we also examined the higher target for suppression of ADR. The results shown in Figure 4G and 4H indicate that even at a dose of 3000 mg/day (ie, 4 times the current standard dose), only 78% of patients would achieve that target. However, since tuberculous meningitis is a paucibacillary disease with low risks of ADR, the EC80 target will likely be clinically sufficient.
Relationships Among Exposure, Concentration, Dose, and Outcome in Patients
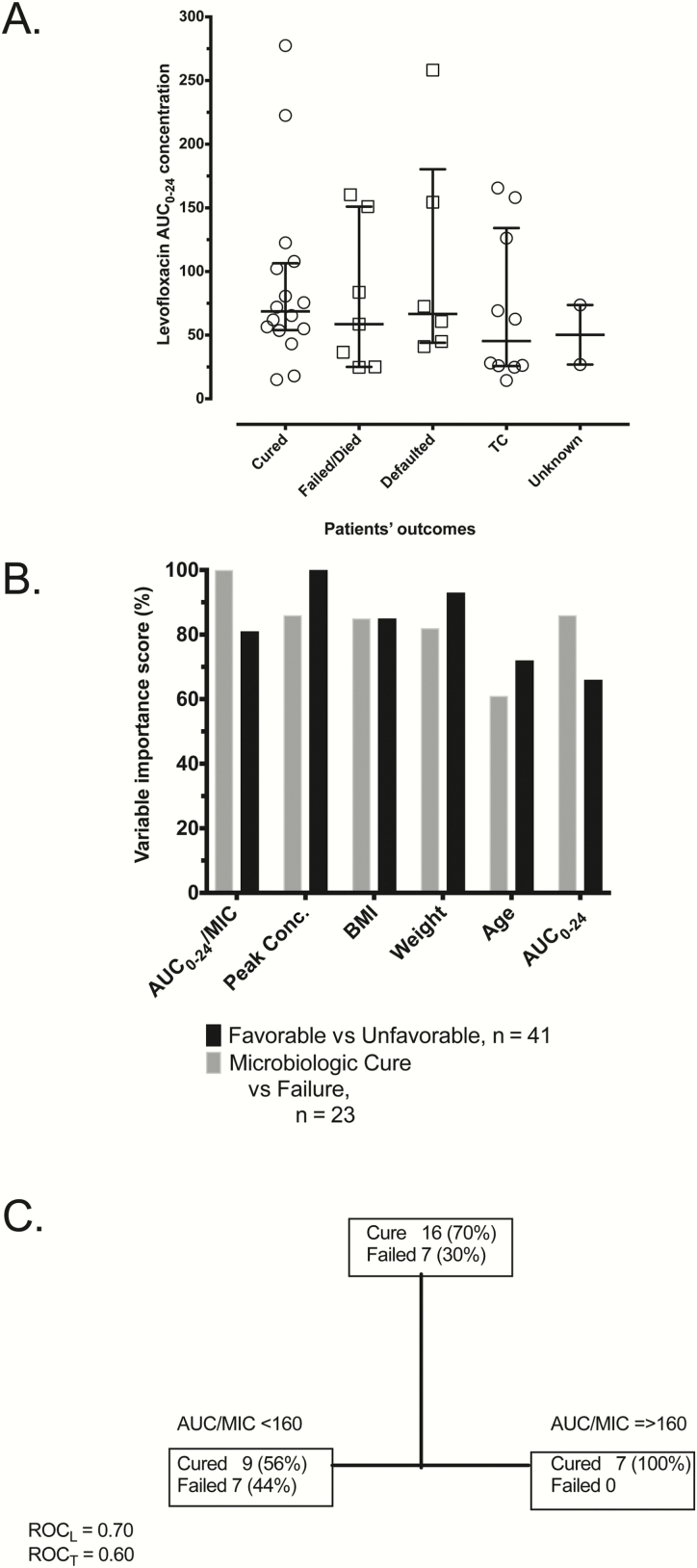
Clinical details of the 41 Tanzanian patients with pulmonary MDR tuberculosis have been published [12]. MICs were performed for ofloxacin in 31 of the 41 patients, but not for levofloxacin, using Sensititre; the MIC distribution is shown in Figure 5A. The mean ± standard deviation PK model–derived levofloxacin AUC0-24 was 83.02 ± 65.00 mg*h/L in the Tanzanian patients, which is virtually the same as those identified with more intensive PK sampling in Table 1 and elsewhere. The following unfavorable outcomes were ascertained: sputum conversion status unknown 2 (5%), defaulted 6 (15%), death 6 (15%), and development of ADR 1 (2%). There was no significant difference in the distribution of the median levofloxacin peak concentration, AUC0-24, or AUC0-24/MIC by treatment outcome based on the Kruskal-Wallis test (Figure 5B). However, Figure 5C shows the ranking of important variables from the 2 ML algorithms: comparing microbiologic cure vs failure and favorable vs unfavorable outcomes. For microbiologic cure vs failure, the levofloxacin AUC0-24/MIC ratio was the most important predictor at 100% importance, followed by levofloxacin 2-hour peak concentration at 86% and body mass index (BMI) at 85%. For favorable vs unfavorable outcomes in the entire dataset, peak concentration was the primary node (100%), followed by weight at 93%, BMI at 85%, and AUC0-24/MIC ratio at 81%. Figure 5D shows a representative CART tree, which revealed that the AUC0-24/MIC threshold predictive of microbiologic failure was 160. However, the MIC used was for ofloxacin, which is often 1 tube dilution higher than levofloxacin, so that the threshold value would translate to a putative AUC0-24/MIC of 320.
Figure 5.
Artificial intelligence–derived predictors of outcomes in patients. (A) There were no statistically significant differences in the distribution of the median levofloxacin 0–24 hour area under the concentration-time curve (AUC0-24) by treatment outcome using standard statistical inferences. (B) Ranking of important variables from 2 random forest models based on 2 definitions of outcome revealed the effect of drug concentration on outcomes. (C) Representative classification and regression tree used to generate the random forest model output shows a threshold AUC0-24/minimum inhibitory concentration of 160. Once the threshold exposure has been identified using machine learning, it can be used in standard statistics to show an odds of failure below this threshold, which in this case shows a higher odds ratio of failure with a 95% confidence interval of 1.15 to infinity (given the 0% failure rate above the threshold value). Abbreviations: AUC0-24, 0–24 hour area under the concentration-time curve; BMI, body mass index; MIC, minimum inhibitory concentration; ROCL, receiver operating characteristic Learn set; ROCT, receiver operating characteristic Test set; TC, treatment complete.
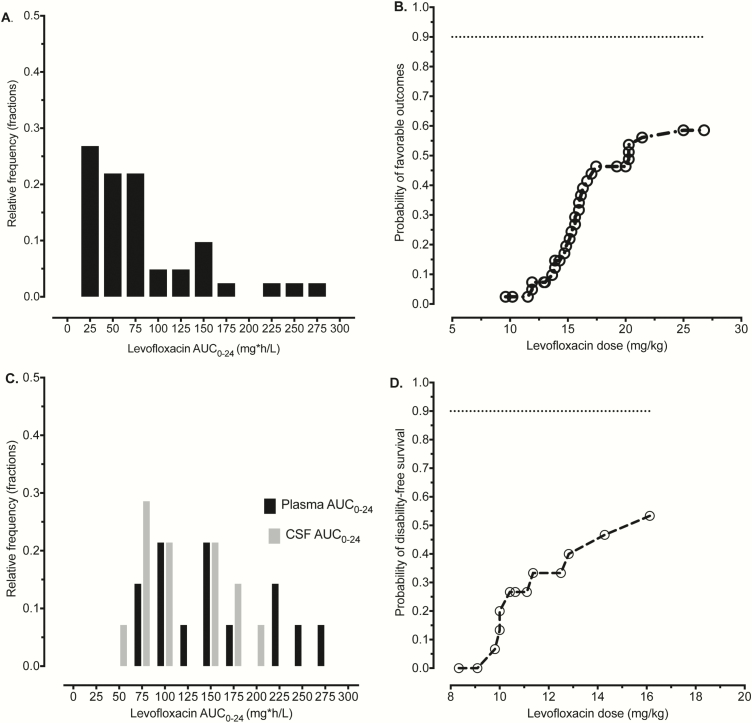
The levofloxacin serum AUC distribution in Tanzanian patients with pulmonary MDR tuberculosis is shown in Figure 6A. We utilized a probit regression model of levofloxacin dose (milligrams per kilogram) vs outcome in the same patients, with results shown in Figure 6B. Interestingly, the maximum probability of favorable outcomes was 60% in pulmonary disease, which is suboptimal. The dose on the flat portion of the probit curve was about 25 mg/kg or a total 1250 mg, given the weight of patients in Tanzania. Next, we analyzed data from Vietnamese patients with tuberculous meningitis on treatment with a levofloxacin-based regimen whose clinical characteristics have been published elsewhere [13]. The levofloxacin serum and CSF AUCs in these patients were as shown in Figure 6C. The levofloxacin penetration ratio into CSF observed was 0.71, which is close to the 0.70 used in our MCEs. Probit analyses revealed the relationship between dose and probability of disability-free survival shown in Figure 6D. The disability-free survival rates were also poor; however, they were in line with the current outcomes with first-line anti-tuberculosis therapy.
Figure 6.
Probit regression in patients treated with levofloxacin-based regimens. (A) Area under the concentration-time curves (AUCs) achieved in the blood of patients with pulmonary tuberculosis in Tanzania were in the range identified with intensive pharmacokinetic sampling schemes. (B) The probit model of levofloxacin dose (milligrams per kilogram) administered to patients vs probability of good outcomes in pulmonary multidrug-resistant (MDR) tuberculosis in Tanzanian patients. The curve flattened out at 60% probability of good outcome for pulmonary MDR tuberculosis, which was a suboptimal response but in the range seen for MDR tuberculosis outcomes. (C) Distributions of AUCs in the blood and cerebrospinal fluid of Vietnamese patients with meningitis shows a distribution similar to that of Tanzanian patients for the blood. (D) Probit model in patients with drug-susceptible tuberculosis who also received rifampin for tuberculous meningitis showed a curve that was still on a steep rise and had not reached maximum, suggesting room for improvement with increased doses. Abbreviations: AUC0-24, 0–24 hour area under the concentration-time curve; CSF, cerebrospinal fluid.
DISCUSSION
Aubry et al have shown that the ability of a quinolone to inhibit Mtb gyrase DNA supercoiling activity by 50% (IC50) was 3 mg/L for gatifloxacin, 4.5 mg/L for moxifloxacin, and 5 mg/L for levofloxacin. The concentration producing 50% of the maximum DNA cleavage (CC50) was 4 mg/L for gatifloxacin, 4 mg/L for moxifloxacin, but 12 mg/L for levofloxacin [35]. In Table 2 we compare levofloxacin PK/PD parameters, MCE-derived dose, to those of moxifloxacin and gatifloxacin, derived from our separate but similarly designed studies [1, 14]. Based on speed of kill and time to ADR, the ranking would be gatifloxacin > levofloxacin > moxifloxacin. ADR arose faster with moxifloxacin than with levofloxacin, while gatifloxacin was the most effective at suppressing ADR. Indeed, the gatifloxacin-resistant subpopulation was only 5% of the total on day 28 in the systems that amplified resistance the most, as opposed to 100% for both moxifloxacin and levofloxacin (Table 2). Thus, based on PK/PD considerations, levofloxacin can indeed replace moxifloxacin in pulmonary tuberculosis, while gatifloxacin could be the best drug to start off with. However, in elderly patients gatifloxacin increased the rate of dysglycemia 4.3-fold compared to other antibiotics, while levofloxacin increased it 1.5-fold, and moxifloxacin did not [36]. Dysglycemia has been encountered in 2%–9% of MDR tuberculosis patients on gatifloxacin, and the incidence of this adverse event could be higher at the advocated dose of 800 mg [14, 37].
Table 2.
Effect of Levofloxacin, Moxifloxacin, and Gatifloxacin in Hollow Fiber System Model of Tuberculosis and Monte Carlo Experiments from Different Studies With Similar Experimental Design
| Variables | Moxifloxacin | Gatifloxacin | Levofloxacin |
|---|---|---|---|
| Maximal kill (log10 CFU/mL/day) | 0.57 | 0.68 | 0.61 |
| Time to 1% acquired drug resistance (days) | 10 | 21 | 21 |
| % population resistant at end of experiment | 100 | 5 | 100 |
| Area under the concentration-time curve/minimum inhibitory concentration resistance suppression | 53 | 184 | 360 |
| Exposure associated with 80% of maximal kill | 56 | 184 | 146 |
| Monte Carlo experiment–derived dose (mg/day): resistance suppression | |||
| Pulmonary tuberculosis | 800 | 800 | 1500 |
| Meningeal tuberculosis | - | 1200 | >3000 |
We identified the levofloxacin dose that gives equivalent effect to moxifloxacin, 800 mg/day, using HFS-TB in tandem with MCE, AI-based analyses, and probit models of clinical data. This is consistent with prior findings that show that the EC80 and exposure suppressing ADR in the HFS-TB correspond closely to the exposures associated with optimal response in patients, as do susceptibility breakpoints based on these exposures [20, 25, 38–42]. The equivalent dose should be able to minimize ADR, which is encountered for quinolones and aminoglycosides in 9%–14% of patients being treated for MDR tuberculosis at standard doses [43, 44]. We have utilized amplicon-based next-generation and confirmatory Sanger sequencing in 158 MDR tuberculosis isolates from 4 countries and determined that 6.3% of 1811 loci examined exhibited at least some mutant population (≥1% of reads) in at least 1 isolate, with the largest being gyrA. Thus, subpopulations of fluoroquinolone resistance are often encountered in MDR tuberculosis [45]. Mutations were within the same resistance determining codon (gyrA 94) identified in the HFS-TB with suboptimal levofloxacin exposures, consistent with our antibiotic resistance arrow-of-time model [46, 47]. Since resistance to quinolones reduces cure rates considerably, attaining ADR suppression targets is crucial [48, 49]. For pulmonary tuberculosis, the levofloxacin dose that is able to achieve suppression of ADR was 1500 mg/day or around 20 mg/kg/day, as shown in Figure 4. Thus, the inferiority of levofloxacin to moxifloxacin when added to amikacin, ethionamide, and pyrazinamide, as demonstrated in mice, could be due to a differential dose issue. At high enough doses, levofloxacin could be equivalent to moxifloxacin [10, 11]. In the case of tuberculous meningitis, the dose that would suppress ADR was higher than what is currently known to be tolerated by patients. However, given the lower bacterial burden in CSF compared to pulmonary cavities, the PK/PD goal of therapy in tuberculous meningitis may not be resistance suppression but rather the EC80. In that case, the levofloxacin dose of 1500 mg/day could still be used for tuberculous meningitis.
We identified the MIC above which levofloxacin therapy is expected to fail in MCE. We identified this as an MIC of 0.5 mg/L for both MGIT and agar dilution using Middlebrook 7H11. At 1 dilution higher (1 mg/L), one would need to double the dose to achieve the same TAP. Since, unlike gatifloxacin, exposures associated with the EC50 and EC80 actually amplify ADR, we did not examine the effect of higher doses, such as 3000 mg/day, at higher MICs. The doses would be several times greater than the standard, and the safety is unclear, as is the effect given resistance amplification. Thus, we did not propose a susceptible dose-dependent zone for levofloxacin, as was the case with gatifloxacin and other drugs [14, 50].
Our study has a few limitations. First, the levofloxacin concentrations we utilized as an external check on PK modeling were from a retrospective study. Moreover, except for levofloxacin, concentrations of other drugs that comprised the MDR tuberculosis regimen were not measured. However, the observations of low levofloxacin concentrations associated with unfavorable outcomes in patients are similar to those derived from the modeling exercise and thus likely accurate. Second, the clinical dataset utilized for external validation of modeling for tuberculous meningitis was relatively small. However, the high rate of failure and use of the index of disability-free survival made it possible to model, despite the small sample size. On the other hand, disability-free survival cannot be solely attributable to tuberculous meningitis. The effect of other factors such as penetration of companion antituberculosis compounds into the CSF will be important to consider. Yet, both model-predicted and clinically observed unfavorable outcomes were associated with low CSF exposures and identified similar doses for success. Therefore, despite these limitations, these findings are likely to be true.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. T. G. and D. D. designed the study; T. G., D. D., and P. B. performed the hollow fiber studies; D. D. wrote the first draft of the manuscript; T. K. performed DNA extraction; T. G. and S. S. performed the WGS analysis; T. G. performed PK/PD modeling and MCE; J. P. and T. G. performed Probit and CART analysis of clinical data; S. G. M., C. P., S. B., P. A., G. T., and S. K. H. performed the clinical studies and/or analyses; D. D., J. P., S. S., and T.G. wrote the manuscript; all authors edited and contributed to the final version of the manuscript.
Financial support. This work was supported by the Baylor Research Institute (T. G.) and the Wellcome Trust, UK (G. T.). Portions of the Tanzania study were funded by the National Institutes of Health and the National Institute of Allergy and Infectious Diseases (U01AI119954 to S. K. H.). This supplement was sponsored by Baylor Research Institute, Dallas, TX.
Supplement sponsorship. This supplement is sponsored by the Baylor Institute of Immunology Research of the Baylor Research Institute.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Gumbo T, Louie A, Deziel MR, Parsons LM, Salfinger M, Drusano GL. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J Infect Dis 2004; 190:1642–51. [DOI] [PubMed] [Google Scholar]
- 2. Drusano GL, Louie A, Deziel M, Gumbo T. The crisis of resistance: identifying drug exposures to suppress amplification of resistant mutant subpopulations. Clin Infect Dis 2006; 42:525–32. [DOI] [PubMed] [Google Scholar]
- 3. Komatsu R, Honda M, Holzgrefe HH, et al. . Sensitivity of common marmosets to detect drug-induced QT interval prolongation: moxifloxacin case study. J Pharmacol Toxicol Methods 2010; 61:271–6. [DOI] [PubMed] [Google Scholar]
- 4. Shandil RK, Jayaram R, Kaur P, et al. . Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob Agents Chemother 2007; 51:576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gumbo T, Louie A, Deziel MR, Drusano GL. Pharmacodynamic evidence that ciprofloxacin failure against tuberculosis is not due to poor microbial kill but to rapid emergence of resistance. Antimicrob Agents Chemother 2005; 49:3178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peloquin CA, Hadad DJ, Molino LP, et al. . Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob Agents Chemother 2008; 52:852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kempker RR, Barth AB, Vashakidze S, et al. . Cavitary penetration of levofloxacin among patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother 2015; 59:3149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mase SR, Jereb JA, Gonzalez D, et al. . Pharmacokinetics and dosing of levofloxacin in children treated for active or latent multidrug-resistant tuberculosis, Federated States of Micronesia and Republic of the Marshall Islands. Pediatr Infect Dis J 2016; 35:414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ginsburg AS, Grosset JH, Bishai WR. Fluoroquinolones, tuberculosis, and resistance. Lancet Infect Dis 2003; 3:432–42. [DOI] [PubMed] [Google Scholar]
- 10. Johnson JL, Hadad DJ, Boom WH, et al. . Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis 2006; 10:605–12. [PubMed] [Google Scholar]
- 11. Ahmad Z, Tyagi S, Minkowski A, Peloquin CA, Grosset JH, Nuermberger EL. Contribution of moxifloxacin or levofloxacin in second-line regimens with or without continuation of pyrazinamide in murine tuberculosis. Am J Respir Crit Care Med 2013; 188:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ebers A, Stroup S, Mpagama S, et al. . Determination of plasma concentrations of levofloxacin by high performance liquid chromatography for use at a multidrug-resistant tuberculosis hospital in Tanzania. PLoS One 2017; 12:e0170663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thwaites GE, Bhavnani SM, Chau TT, et al. . Randomized pharmacokinetic and pharmacodynamic comparison of fluoroquinolones for tuberculous meningitis. Antimicrob Agents Chemother 2011; 55:3244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deshpande D, Pasipanodya JG, Srivastava S, et al. . Gatifloxacin pharmacokinetics/pharmacodynamics-based optimal dosing for pulmonary and meningeal multidrug-resistant tuberculosis. Clin Infect Dis 2018; 67(Suppl 3):S293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deshpande D, Srivastava S, Pasipanodya JG, et al. . Linezolid for infants and toddlers with disseminated tuberculosis: first steps. Clin Infect Dis 2016; 63: 80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deshpande D, Srivastava S, Chapagain M, et al. . Ceftazidime-avibactam has potent sterilizing activity against highly drug-resistant tuberculosis. Sci Adv 2017; 3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gumbo T, Lenaerts AJ, Hanna D, Romero K, Nuermberger E. Nonclinical models for antituberculosis drug development: a landscape analysis. J Infect Dis 2015; 211(Suppl 3):S83–95. [DOI] [PubMed] [Google Scholar]
- 18. Gumbo T, Louie A, Liu W, et al. . Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob Agents Chemother 2007; 51:2329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Srivastava S, Pasipanodya JG, Meek C, Leff R, Gumbo T. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J Infect Dis 2011; 204:1951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gumbo T, Alffenaar JWC. An introduction to pharmacokinetics/pharmacodynamics methods and scientific evidence base for dosing of second line tuberculosis drugs. Clin Infect Dis 2018; 67(Suppl 3):S267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Conte JE Jr, Golden JA, McIver M, Zurlinden E. Intrapulmonary pharmacokinetics and pharmacodynamics of high-dose levofloxacin in healthy volunteer subjects. Int J Antimicrob Agents 2006; 28:114–21. [DOI] [PubMed] [Google Scholar]
- 22. Deshpande D, Srivastava S, Nuermberger E, Pasipanodya JG, Swaminathan S, Gumbo T. A faropenem, linezolid, and moxifloxacin regimen for both drug-susceptible and multidrug-resistant tuberculosis in children: FLAME path on the milky way. Clin Infect Dis 2016; 63:S95–S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Srivastava S, Magombedze G, Koeuth T, et al. . Linezolid dose that maximizes sterilizing effect while minimizing toxicity and resistance emergence for tuberculosis. Antimicrob Agents Chemother 2017; 61:e00751–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. D’Argenio DZ, Schumitzky A, Wang X.. ADAPT 5 user’s guide: pharmacokinetic/pharmacodynamic systems analysis software. Los Angeles: Biomedical Simulations Resource, 2009. [Google Scholar]
- 25. Pasipanodya J, Gumbo T. An oracle: antituberculosis pharmacokinetics-pharmacodynamics, clinical correlation, and clinical trial simulations to predict the future. Antimicrob Agents Chemother 2011; 55:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Preston SL, Drusano GL, Berman AL, et al. . Levofloxacin population pharmacokinetics and creation of a demographic model for prediction of individual drug clearance in patients with serious community-acquired infection. Antimicrob Agents Chemother 1998; 42:1098–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Drusano GL, Preston SL, Gotfried MH, Danziger LH, Rodvold KA. Levofloxacin penetration into epithelial lining fluid as determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob Agents Chemother 2002; 46:586–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pea F, Pavan F, Nascimben E, et al. . Levofloxacin disposition in cerebrospinal fluid in patients with external ventriculostomy. Antimicrob Agents Chemother 2003; 47:3104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rodríguez JC, Ruiz M, López M, Royo G. In vitro activity of moxifloxacin, levofloxacin, gatifloxacin and linezolid against Mycobacterium tuberculosis. Int J Antimicrob Agents 2002; 20:464–7. [DOI] [PubMed] [Google Scholar]
- 30. Kambli P, Ajbani K, Nikam C, et al. . Determination of MICs of levofloxacin for Mycobacterium tuberculosis with gyrA mutations. Int J Tuberc Lung Dis 2015; 19:1227–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 2013; 208:1464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Swaminathan S, Pasipanodya JG, Ramachandran G, et al. . Drug concentration thresholds predictive of therapy failure and death in children with tuberculosis: bread crumb trails in random forests. Clin Infect Dis 2016; 63:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Breiman L. Random forests. Machine Learning 2001; 45:5–32. [Google Scholar]
- 34. Maruri F, Sterling TR, Kaiga AW, et al. . A systematic review of gyrase mutations associated with fluoroquinolone-resistant Mycobacterium tuberculosis and a proposed gyrase numbering system. J Antimicrob Chemother 2012; 67:819–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aubry A, Pan XS, Fisher LM, Jarlier V, Cambau E. Mycobacterium tuberculosis DNA gyrase: interaction with quinolones and correlation with antimycobacterial drug activity. Antimicrob Agents Chemother 2004; 48:1281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park-Wyllie LY, Juurlink DN, Kopp A, et al. . Outpatient gatifloxacin therapy and dysglycemia in older adults. N Engl J Med 2006; 354:1352–61. [DOI] [PubMed] [Google Scholar]
- 37. Chiang CY, Van Deun A, Rieder HL. Gatifloxacin for short, effective treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2016; 20:1143–7. [DOI] [PubMed] [Google Scholar]
- 38. Gumbo T, Pasipanodya JG, Romero K, Hanna D, Nuermberger E. Forecasting accuracy of the hollow fiber model of tuberculosis for clinical therapeutic outcomes. Clin Infect Dis 2015; 61(Suppl 1):S25–31. [DOI] [PubMed] [Google Scholar]
- 39. Gumbo T, Pasipanodya JG, Nuermberger E, Romero K, Hanna D. Correlations between the hollow fiber model of tuberculosis and therapeutic events in tuberculosis patients: learn and confirm. Clin Infect Dis 2015; 61(Suppl 1):S18–24. [DOI] [PubMed] [Google Scholar]
- 40. Pasipanodya JG, Nuermberger E, Romero K, Hanna D, Gumbo T. Systematic analysis of hollow fiber model of tuberculosis experiments. Clin Infect Dis 2015; 61(Suppl 1):S10–7. [DOI] [PubMed] [Google Scholar]
- 41. Gumbo T, Angulo-Barturen I, Ferrer-Bazaga S. Pharmacokinetic-pharmacodynamic and dose-response relationships of antituberculosis drugs: recommendations and standards for industry and academia. J Infect Dis 2015; 211(Suppl 3):S96–S106. [DOI] [PubMed] [Google Scholar]
- 42. Gumbo T, Pasipanodya JG, Wash P, Burger A, McIlleron H. Redefining multidrug-resistant tuberculosis based on clinical response to combination therapy. Antimicrob Agents Chemother 2014; 58:6111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cegielski JP, Dalton T, Yagui M, et al. ; Global Preserving Effective TB Treatment Study (PETTS) Investigators. Extensive drug resistance acquired during treatment of multidrug-resistant tuberculosis. Clin Infect Dis 2014; 59:1049–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kempker RR, Kipiani M, Mirtskhulava V, Tukvadze N, Magee MJ, Blumberg HM. Acquired drug resistance in Mycobacterium tuberculosis and poor outcomes among patients with multidrug-resistant tuberculosis. Emerg Infect Dis 2015; 21:992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Operario DJ, Koeppel AF, Turner SD, et al. . Prevalence and extent of heteroresistance by next generation sequencing of multidrug-resistant tuberculosis. PLoS One 2017; 12:e0176522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schmalstieg AM, Srivastava S, Belkaya S, et al. . The antibiotic resistance arrow of time: efflux pump induction is a general first step in the evolution of mycobacterial drug resistance. Antimicrob Agents Chemother 2012; 56:4806–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gumbo T. Biological variability and the emergence of multidrug-resistant tuberculosis. Nat Genet 2013; 45:720–1. [DOI] [PubMed] [Google Scholar]
- 48. Ahuja SD, Ashkin D, Avendano M, et al. ; Collaborative Group for Meta-Analysis of Individual Patient Data in MDR-TB. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med 2012; 9:e1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cegielski JP, Kurbatova E, van der Walt M, et al. ; Global PETTS Investigators. Multidrug-resistant tuberculosis treatment outcomes in relation to treatment and initial versus acquired second-line drug resistance. Clin Infect Dis 2016; 62:418–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zuur MA, Pasipanodya JG, van Soolingen D, van der Werf TS, Gumbo T, Alffenaar JC. Intermediate susceptibility dose-dependent breakpoints for high dose rifampicin, isoniazid and pyrazinamide treatment in multidrug-resistant tuberculosis programmes. Clin Infect Dis 2018; 67:1743–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.