Abstract
Background
Resistance to all first-line antibiotics necessitates the use of less effective or more toxic “reserve” agents. Gram-negative bloodstream infections (GNBSIs) harboring such difficult-to-treat resistance (DTR) may have higher mortality than phenotypes that allow for ≥1 active first-line antibiotic.
Methods
The Premier Database was analyzed for inpatients with select GNBSIs. DTR was defined as intermediate/resistant in vitro to all ß-lactam categories, including carbapenems and fluoroquinolones. Prevalence and aminoglycoside resistance of DTR episodes were compared with carbapenem-resistant, extended-spectrum cephalosporin-resistant, and fluoroquinolone-resistant episodes using CDC definitions. Predictors of DTR were identified. The adjusted relative risk (aRR) of mortality was examined for DTR, CDC-defined phenotypes susceptible to ≥1 first-line agent, and graded loss of active categories.
Results
Between 2009–2013, 471 (1%) of 45011 GNBSI episodes at 92 (53.2%) of 173 hospitals exhibited DTR, ranging from 0.04% for Escherichia coli to 18.4% for Acinetobacter baumannii. Among patients with DTR, 79% received parenteral aminoglycosides, tigecycline, or colistin/polymyxin-B; resistance to all aminoglycosides occurred in 33%. Predictors of DTR included urban healthcare and higher baseline illness. Crude mortality for GNBSIs with DTR was 43%; aRR was higher for DTR than for carbapenem-resistant (1.2; 95% confidence interval, 1.0–1.4; P = .02), extended-spectrum cephalosporin-resistant (1.2; 1.1–1.4; P = .001), or fluoroquinolone-resistant (1.2; 1.0–1.4; P = .008) infections. The mortality aRR increased 20% per graded loss of active first-line categories, from 3–5 to 1–2 to 0.
Conclusion
Nonsusceptibility to first-line antibiotics is associated with decreased survival in GNBSIs. DTR is a simple bedside prognostic measure of treatment-limiting coresistance.
Keywords: gram-negative bacteria, antimicrobial resistance, mortality
Resistance to all first-line agents or difficult-to-treat resistance (DTR) was observed in 1% of gram-negative bacteremias. DTR was identified at half the hospitals; nearly 80% of patients with DTR received “reserve” agents. Mortality risk increased with decreasing active first-line categories.
Antimicrobial resistance is estimated to cause 23000 deaths annually in the United States [1] and 700000 worldwide [2]. Plasmid-mediated transfer of resistance and nosocomial transmission of gram-negative (GN) bacteria, particularly Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter baumannii have led to widespread dissemination [3], outbreaks, and untreatable infections [4]. Existing surveillance systems track antimicrobial resistance within and across antibiotic categories [5]. However, estimating the burden of clinically meaningful coresistance and its relationship to outcome remains challenging.
In 2008, the US Centers for Disease Control and Prevention (CDC) and the European Centre for Disease Prevention and Control classified nonsusceptibility to ≥1 agent in ≥3 antimicrobial categories as multidrug resistant (MDR), and susceptibility limited to ≤2 categories as extensively drug-resistant (XDR) [6]. Although epidemiologically useful [7], assessment as XDR can entail cumbersome in vitro testing of up to 17 unique antibiotic categories [6]. Furthermore, requiring resistance to only 1 agent per category [8] and weighting all antibiotics equally regardless of efficacy and toxicity limits the bedside applicability of MDR and XDR as categories. Despite denoting escalating resistance, MDR and XDR infections have not consistently been associated with different patient outcomes [7, 9–11].
A practical approach to antimicrobial resistance in GN bacteria might focus on treatment-limiting resistance to all first-line agents, that is, all β-lactams, including carbapenems and β-lactamase inhibitor combinations, and fluoroquinolones, which can be described as difficult-to-treat resistance (DTR). Although strains susceptible to only 1 or 2 first-line agents also present challenges for choosing empiric therapy, DTR signifies no active first-line agents and an even higher level of resistance. Nonsusceptibility to all first-line, high-efficacy, low-toxicity agents very often leads to both discordant empirical regimens and subsequent reliance on less effective and/or more toxic “reserve” agents. For example, aminoglycosides and colistin/polymyxin-B are both nephrotoxic and poorly penetrate abdominal and pulmonary sites. Tigecycline blood levels are low relative to minimal inhibitory concentrations, and tigecycline use is associated with increased mortality rates [12–14].
GN bloodstream isolates are an important surveillance target for monitoring resistance, because cultures from nonsterile sites are less likely to represent true infections [15]. Using data from a well-distributed sample of US hospitals, we determined the 5-year prevalence of DTR among GN bloodstream infections (GNBSIs), associated risk factors and the effect of nonsusceptibility to first-line agents on in-hospital mortality rates.
METHODS
Data Source and Study Population
A retrospective cohort study was conducted using inpatient encounters (hospitalizations) from 2009to 2013 at 173 US hospitals reporting microbiology data with antimicrobial susceptibility results in the Premier Healthcare Database. Included patients had ≥1 blood culture isolate belonging to 1 of 5 GN bacterial taxa: Escherichia coli, Enterobacter spp., Klebsiella spp., P. aeruginosa, or A. baumannii complex. The Office of Human Subjects Research at the National Institutes of Health waived the need for institutional review board evaluation, because analyses were limited to existing deidentified data using a commercially available data source compliant with the Health Insurance Portability and Accountability Act.
DTR Definition and Relationship to CDC-defined Resistance Phenotypes
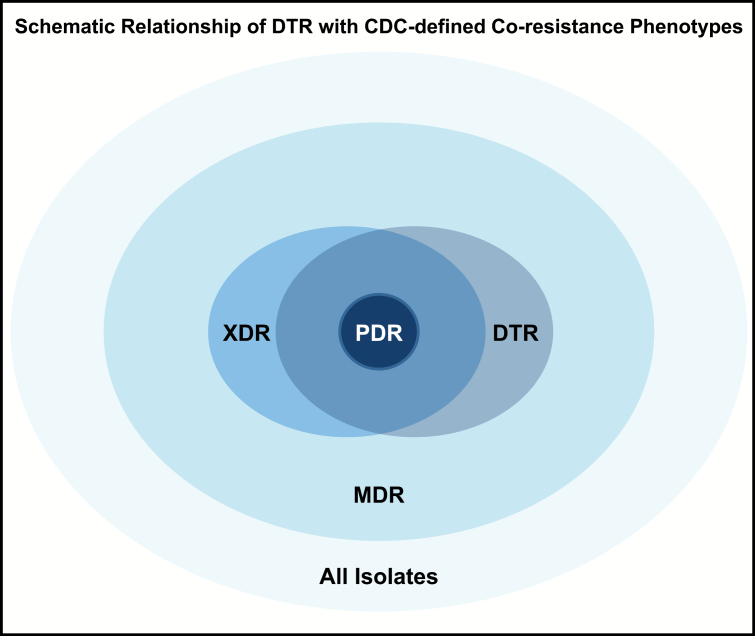
Experts in infectious diseases, critical care, microbiology, and epidemiology developed DTR consensus criteria for each bacterial taxon of interest (Table 1). A similar classification of resistance to the 4 major antibiotic classes (penicillins, cephalosporins, carbapenems, and fluoroquinolones) was previously published by the Robert Koch Institute as part of German infection control guidelines [16]. Susceptibility results for ≥1 carbapenem, ≥1 extended-spectrum cephalosporin, and ≥1 fluoroquinolone were required to determine DTR status. CDC surveillance definitions were also used to classify isolates into individual resistance phenotypes: carbapenem resistant (CR), extended-spectrum cephalosporin resistant (ECR) or fluoroquinolone resistant (FQR) [17]. The relationship between DTR and MDR, XDR, and pandrug-resistant categories is schematically represented in Figure 1 but not analyzed further. Susceptibility interpretations were as reported by each institution; individual institutional standards around susceptibility testing were not available (see Supplementary Methods).
Table 1.
Phenotypic Definitions of Difficult-to-Treat Resistance and Centers for Disease Control and Prevention-defined Individual Resistance Phenotype Among 5 Taxa of Gram-negative Bloodstream Infections
| Definitions | Agents Included | Defining Criteria |
|---|---|---|
| 2015 CDC definitions | ||
| Carbapenem resistanta | Imipenem, meropenem doripenem ertapenemb | Resistance to ≥1 carbapenem (Escherichia coli, Klebsiella spp, Enterobacter spp); intermediate or resistant to ≥1 carbapenem (Pseudomonas aeruginosa, Acinetobacter baumannii) |
| Extended-spectrum cephalosporin-resistantc | Ceftazidime, cefepime, ceftriaxone,c cefotaximec | Resistance to ≥1 extended-spectrum cephalosporin |
| Fluoroquinolone resistanta | Ciprofloxacin, levofloxacin, moxifloxacinc | Resistance to ≥1 fluoroquinolone |
| Proposed definition | ||
| Difficult-to-treat resistance | Intermediate or resistant to all reported agents in carbapenem, β-lactam, and fluoroquinolone categories (including additional agentse when results available) | |
Abbreviation: CDC, Centers for Disease Control and Prevention.
aBased on 2015 CDC definitions.
bApplicable for Enterobacteriaceae only.
cNot applicable for P. aeruginosa.
dDTR assessment requires in vitro testing against ≥1 carbapenem, ≥1 extended-spectrum cephalosporin, and ≥1 fluoroquinolone.
eIntermediate or resistant to piperacillin-tazobactam and ampicillin-sulbactam (A. baumannii only) and intermediate or resistant to aztreonam (not applicable for A. baumannii). These drugs were only included in the assessment of DTR when results were reported.
Figure 1.
Schematic relationship between difficult-to-treat resistance (DTR) and Centers for Disease Control and Prevention (CDC)–defined coresistance phenotypes. As represented in this figure, DTR isolates fall completely within the CDC-defined multidrug-resistant (MDR) phenotype and overlap with the extensively drug-resistant (XDR) phenotype. Although pandrug-resistant (PDR) organisms all fall within the DTR phenotype, the DTR definition introduced here offers more clinical utility, in that it requires resistance only to all first-line agents, instead of all antimicrobial agents available. (Note that proportions of overlap among coresistance phenotypes are not displayed to scale. Refer to Magiorakos et al [6] for CDC definitions.)
Study Design and Analysis
The prevalence of CR, ECR, and/or FQR was estimated from total GNBSIs with requisite susceptibility testing for that respective drug category. DTR prevalence estimates used several denominators: isolates with any susceptibility results, isolates from hospitals with continuous reporting, and isolates meeting minimum testing criteria. Isolates could contribute to estimates for >1 resistance category. A GNBSI episode encompassed all isolates of the same bacterial taxon within a 30-day period. Healthcare-associated DTR was defined as any GNBSI occurring >3 calendar days after hospital admission, or earlier in patients from a healthcare facility and/or receiving hemodialysis. Comorbid conditions and source of bacteremia were defined using International Classification of Diseases, Ninth Revision coding and the Elixhauser index [18] (Supplementary Table 1). The outcome was adjusted for the predicted mortality risk using the 3M All Patient Refined Diagnosis Related Group risk of mortality assignments [19]. Administration of reserve agent categories (aminoglycosides, colistin/polymyxin B, and tigecycline) and prevalence of aminoglycoside nonsusceptibility (intermediate/resistant) were compared across resistance phenotypes; the burdens of coresistance were compared across taxa.
Univariate and multivariable mixed-effect models were developed used modified Poisson regression with log-link function and robust error variances, controlling for facility-level random effects, to estimate adjusted relative risk (aRR) and 95% confidence intervals (CIs) for factors significantly associated with GNBSIs with DTR (see Supplementary Data for full results) and in-hospital mortality. Separate models were developed to estimate the independent effects of resistance phenotype and the number of active first-line categories available on mortality risk. Multivariable models were adjusted for patient-, organism-, and hospital-level characteristics (covariates listed in the Supplementary Methods), for continuous reporting (see Supplementary Table 2 for hospital reporting patterns), and for previous episodes of bacteremia. Interactions between resistance phenotype and taxa or source of bacteremia were evaluated, as was possible multicollinearity. Among highly correlated variables (r > 0.8), those with the maximal effect on outcome were retained. Significance was assessed at the P < .05 level. Analyses were performed using SAS software (version 9.4).
RESULTS
Prevalence of DTR
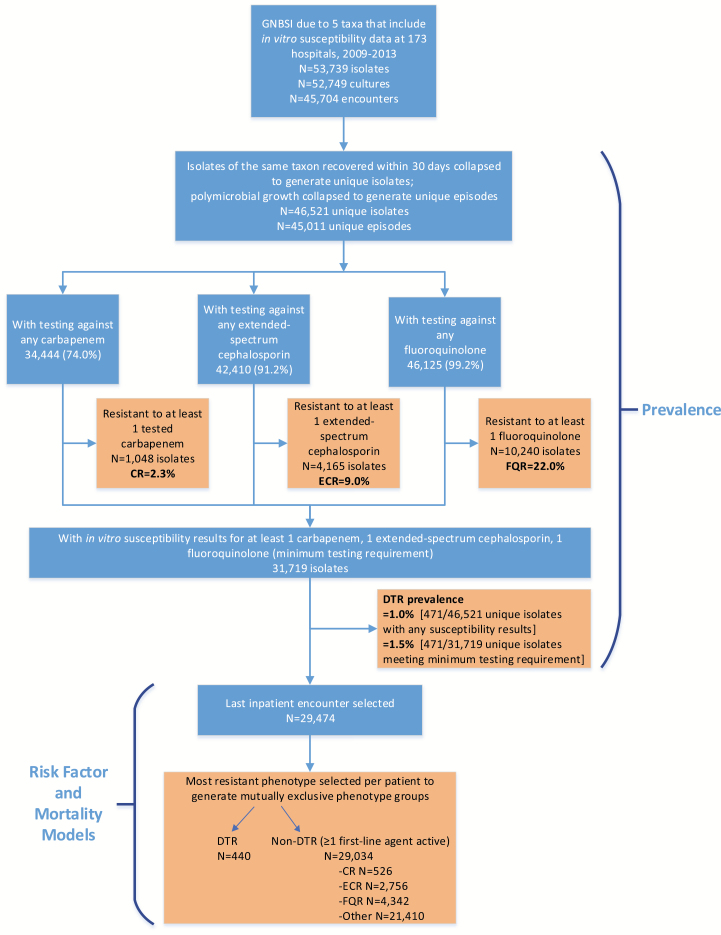
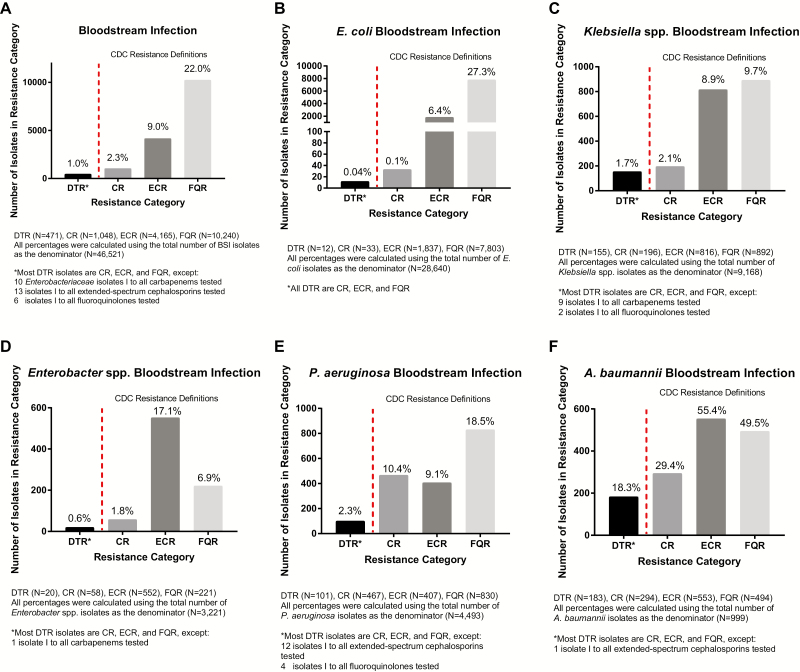
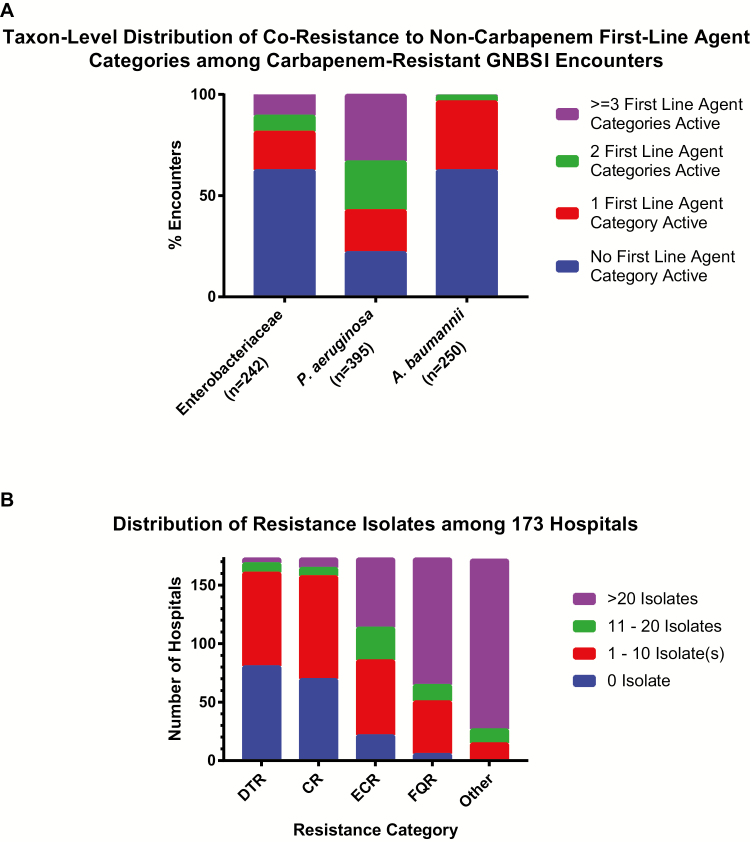
At 173 institutions from 2009 to 2013, a total of 46521 GNBSI isolates of interest were identified, spanning 45011 unique episodes and 29474 unique patients (Figure 2). Of these, 471 GNBSIs (1.0%) exhibited DTR, compared with 1048 (2.3%) for CR, 4165 (9.0%) for ECR, and 10240 (22%) for FQR (Figure 3). Characteristics of the 29474 unique patients with GNBSI, 21410 with nonresistant phenotypes, are presented in Table 2 and the Supplementary Data. The DTR prevalence was 1.1% when limited to hospitals with continuous reporting (n = 42). Among GNBSIs with minimum susceptibility testing, the prevalence was 1.5% (471 of 31719) overall and 2.5% (336 of 13445) among intensive care unit encounters. The prevalence of GNBSIs displaying DTR varied considerably across taxa, ranging from 0.04% for E. coli to 18.3% for A. baumannii (Figure 3). P. aeruginosa had a CR/DTR prevalence ratio of 4.5, reflecting the underlying susceptibility of many CR isolates to piperacillin-tazobactam (85.1% susceptible) and/or aztreonam (49.5% susceptible). Prevalence differences between CR and DTR were smaller but still significant for Klebsiella spp., Enterobacter spp., and Acinetobacter spp. (P < .005). CR P. aeruginosa was nearly twice as likely to be susceptible to ≥1 alternative first-line category as CR isolates in other taxa (Figure 4A).
Figure 2.
Flowchart showing selection process for inpatient encounters with gram-negative bloodstream infections (GNBSIs) exhibiting difficult-to-treat resistance (DTR) in the Premier Healthcare Database. To estimate DTR prevalence, all inpatient encounters reporting microbiology and in vitro antimicrobial susceptibility results were included if patients had ≥1 blood culture isolate belonging to 1 of 5 gram-negative bacterial taxa (see Methods). A GNBSI episode comprised all isolates of the same bacterial taxon within 30 days, and prevalence was calculated among episodes that met minimum testing requirements for DTR. For risk factor and outcome analysis, the most resistant phenotype from the last inpatient encounter was selected, given its proximity to the last known outcome for a given patient. Abbreviations: CR, carbapenem resistant; ECR, extended-spectrum cephalosporin resistant; FQR, fluoroquinolone resistant.
Figure 3.
Prevalence of difficult-to-treat resistance (DTR) compared with Centers for Disease Control and Prevention (CDC)–defined resistance phenotypes among gram-negative bloodstream isolates. The prevalences of DTR organisms and of CDC-defined phenotypes were compared across gram-negative bloodstream isolates for the 5 taxa of interest. Prevalence was calculated using all gram-negative bloodstream infection episodes, overall and for each respective organism. A, Overall prevalence of DTR compared with the CDC-defined phenotypes. B–F, Prevalence among Escherichia coli (B), Klebsiella spp. (C), Enterobacter spp. (D), Pseudomonas aeruginosa (E), and Acinetobacter baumannii (F) isolates. Abbreviations: CR, carbapenem resistant; ECR, extended-spectrum cephalosporin resistant; FQR, fluoroquinolone resistant; I, intermediate.
Table 2.
Characteristics of Unique Inpatient Gram-negative Bloodstream Infection Encounters Classified by Resistance Phenotype at 173 US Hospitals From 2009 to 2013 (N = 29474)
|
Characteristic |
Mutually Exclusive Categories of Unique Encounters With GNBSI Isolates, No. (%)a | ||||
|---|---|---|---|---|---|
| No First-line Agent Active | ≥1 First-line Agent Active | ||||
| DTR (n = 440) | CR (n = 526) |
ECR (n = 2756) |
FQR (n = 4342) |
Other (Nonresistant) (n = 21410) |
|
| Patient-level characteristics | |||||
| Patient demographics | |||||
| Age group, y | |||||
| <18 | <5 (NR)b | 9 (1.7) | 50 (1.8) | 30 (0.7) | 634 (3.0) |
| 18–44 | 59 (13.4) | 62 (11.8) | 251 (9.1) | 328 (7.6) | 2284 (10.7) |
| 45–64 | 166 (37.7) | 194 (36.9) | 838 (30.4) | 1259 (29) | 5976 (27.9) |
| >64 | 214 (48.6) | 261 (49.6) | 1617 (58.7) | 2725 (62.8) | 12516 (58.5) |
| Sex | |||||
| Male | 222 (50.5) | 292 (55.5) | 1443 (52.4) | 2253 (51.9) | 9238 (43.2) |
| Female | 218 (49.6) | 234 (44.5) | 1313 (47.6) | 2089 (48.1) | 12172 (56.9) |
| Race | |||||
| White | 280 (63.6) | 352 (66.9) | 1717 (62.3) | 2985 (68.8) | 15240 (71.2) |
| Black | 101 (23.0) | 119 (22.6) | 537 (19.5) | 815 (18.8) | 3418 (16.0) |
| Hispanic/other | 59 (13.4) | 55 (10.5) | 502 (18.2) | 542 (12.5) | 2752 (12.9) |
| Encounter characteristics | |||||
| Admission year | |||||
| 2009 | 83 (18.9) | 105 (20.0) | 427 (15.5) | 813 (18.7) | 3529 (16.5) |
| 2010 | 101 (23.0) | 110 (20.9) | 564 (20.5) | 984 (22.7) | 4726 (22.1) |
| 2011 | 104 (23.6) | 105 (20.0) | 537 (19.5) | 963 (22.2) | 4773 (22.3) |
| 2012 | 84 (19.1) | 117 (22.2) | 558 (20.3) | 822 (18.9) | 4518 (21.1) |
| 2013 | 68 (15.5) | 89 (16.9) | 670 (24.3) | 760 (17.5) | 3864 (18.1) |
| Elixhauser comorbidity index, median (IQR) | 6 (4, 8) | 5, (3, 7) | 5 (3, 7) | 4 (3, 6) | 4 (3, 6) |
| APR DRG risk of mortalityc | |||||
| Minor | 5 (1.1) | 16 (3.0) | 169 (6.1) | 357 (8.2) | 2541 (11.9) |
| Moderate | 23 (5.2) | 43 (8.2) | 360 (13.1) | 711 (16.4) | 4315 (20.2) |
| Major | 99 (22.5) | 127 (24.1) | 845 (30.7) | 1423 (32.8) | 6926 (32.4) |
| Extreme | 313 (71.1) | 340 (64.6) | 1382 (50.2) | 1851 (42.6) | 7625 (35.6) |
| ICU stay | 321 (73.0) | 328 (62.4) | 1401 (50.8) | 1845 (42.5) | 8547 (39.9) |
| Ventilator use | 226 (51.4) | 220 (41.8) | 606 (22.0) | 651 (15.0) | 2919 (13.6) |
| Neutropenia | 12 (2.7) | 19 (3.6) | 60 (2.2) | 122 (2.8) | 663 (3.1) |
| Isolated taxon | |||||
| Escherichia coli | 10 (2.3) | 18 (3.4) | 1542 (56.0) | 3777 (87) | 12595 (58.8) |
| Klebsiella spp. | 127 (28.9) | 36 (6.8) | 469 (17.0) | 129 (3.0) | 4280 (20.0) |
| Enterobacter spp. | 15 (3.4) | 36 (6.8) | 349 (12.7) | 41 (0.9) | 1535 (7.2) |
| Pseudomonas aeruginosa | 88 (20.0) | 307 (58.4) | 154 (5.6) | 272 (6.3) | 2219 (10.4) |
| Acinetobacter baumannii | 157 (35.7) | 93 (17.7) | 114 (4.1) | 11 (0.3) | 226 (1.1) |
| Multiorganism | 43 (9.8) | 36 (6.8) | 128 (4.6) | 112 (2.6) | 555 (2.6) |
| Bacteremia sourced | |||||
| Urinary only | 98 (22.3) | 114 (21.7) | 1135 (41.2) | 2128 (49.0) | 9599 (44.8) |
| Respiratory only | 70 (15.9) | 93 (17.7) | 232 (8.4) | 300 (6.9) | 1710 (8.0) |
| Abdominal only | 27 (6.1) | 32 (6.1) | 170 (6.2) | 267 (6.2) | 1433 (6.7) |
| Skin and soft tissue only | 13 (3.0) | 29 (5.5) | 84 (3.1) | 85 (2.0) | 456 (2.1) |
| Other site | 89 (20.2) | 128 (24.3) | 575 (20.9) | 825 (19.0) | 5318 (24.8) |
| Multisite | 143 (32.5) | 130 (24.7) | 560 (20.3) | 737 (17.0) | 2894 (13.5) |
| Healthcare associatede | 285 (64.8) | 325 (61.8) | 1118 (40.6) | 1141 (26.3) | 4835 (22.6) |
| Discharge status | |||||
| Deathf | 190 (43.2) | 183 (34.8) | 609 (22.1) | 795 (18.3) | 3161 (14.8) |
| Discharge to an institution | 230 (52.3) | 276 (52.5) | 1594 (57.8) | 2162 (49.8) | 8731 (40.8) |
| Otherg | 20 (4.6) | 67 (12.7) | 553 (20.1) | 1385 (31.9) | 9518 (44.5) |
| Hospital characteristics | |||||
| Bed capacity, No. | |||||
| ≤299 | 141 (32.1) | 172 (32.7) | 859 (31.2) | 1596 (36.8) | 8533 (39.9) |
| 300–499 | 158 (35.9) | 159 (30.2) | 1022 (37.1) | 1421 (32.7) | 6284 (29.4) |
| ≥500 | 141 (32.1) | 195 (37.1) | 875 (31.8) | 1325 (30.5) | 6593 (30.8) |
| Urban hospital | 426 (96.8) | 487 (92.6) | 2599 (94.3) | 3924 (90.4) | 19249 (89.9) |
| Teaching hospital | 258 (58.6) | 317 (60.3) | 1267 (46.0) | 2049 (47.2) | 10166 (47.5) |
| Region | |||||
| East North Central | 88 (20.0) | 88 (16.7) | 353 (12.8) | 681 (15.7) | 3540 (16.5) |
| East South Central | 31 (7.1) | 19 (3.6) | 74 (2.7) | 219 (5.0) | 797 (3.7) |
| Middle Atlantic | 91 (20.7) | 135 (25.7) | 502 (18.2) | 545 (12.6) | 2501 (11.7) |
| Mountain | 26 (5.9) | 30 (5.7) | 104 (3.8) | 126 (2.9) | 839 (3.9) |
| New England | 8 (1.8) | 21 (4.0) | 119 (4.3) | 175 (4.0) | 998 (4.7) |
| Pacific | 36 (8.2) | 40 (7.6) | 480 (17.4) | 591 (13.6) | 3249 (15.2) |
| South Atlantic | 112 (25.5) | 133 (25.3) | 735 (26.7) | 1160 (26.7) | 5275 (24.6) |
| West North Central | 13 (3.0) | 25 (4.8) | 128 (4.6) | 301 (6.9) | 1799 (8.4) |
| West South Central | 35 (8.0) | 35 (6.7) | 261 (9.5) | 544 (12.5) | 2412 (11.3) |
Abbreviations: APR DRG, All Patient Refined Diagnosis Related Group; CR, carbapenem resistant; DTR, difficult-to-treat resistance; ECR, extended-spectrum cephalosporin resistant; FQR, fluoroquinolone resistant; GNBSI, gram-negative bloodstream infection; ICU, intensive care unit; IQR, interquartile range; NR, not reported.
aData represent No. (column %) unless otherwise specified.
bSpecific counts <5 are not reported (NR) to maintain deidentification.
cProvided by 3M.
dDefined using International Classification of Diseases, Ninth Revision codes, listed in Supplementary Table 1.
eDefined based on infection detection day, admission source, and International Classification of Diseases, Ninth Revision codes, listed in Supplementary Table 1.
f”Death” as discharge status includes discharge to hospice.
g”Other” includes discharge to home/self-care, left against medical advice, discharged/transferred to court/law enforcement, and still a patient/expected to return.
Figure 4.
A, Distribution of active first-line agent categories by taxon among carbapenem-resistant (CR) with gram-negative bloodstream infection (GNBSI) encounters, comparing the availability of active noncarbapenem first-line agent categories among CR GNBSI episodes as a measure of the degree of coresistance across the various taxa of CR isolates. The population used for this analysis includes episodes of difficult-to-treat resistance (DTR) in addition to Centers for Disease Control and Prevention–defined CR episodes and excludes multiorganism bacteremia. B, Distribution of isolates by resistance category among 173 hospitals in the Premier Healthcare Database, showing the absolute number of isolates displaying each resistance phenotype across hospitals. More than half of all hospitals in this data set had ≥1 DTR isolate from 2009 to 2013. Abbreviations: ECR, extended-spectrum cephalosporin resistant; FQR, fluoroquinolone resistant.
The 173-hospital sample resembled geographic and teaching status distributions in the American Hospital Association annual survey but was weighted toward larger hospitals (Supplementary Table 3). Ninety-two (53%) hospitals distributed across all 9 US census divisions had ≥1 GNBSI episode displaying DTR (Figure 4B and Supplementary Table 2). Likewise, 103 similarly distributed hospitals had ≥1 case of CR. Few hospitals had ≥10 GNBSIs classified as either DTR or CR over 5 years (Figure 4B). Model results for DTR risk factors, adjusted mortality risk, and proportional susceptibility of isolates to individual agents are reported by taxa for 2009 and 2013 in the Supplementary Data (Supplementary Tables 4–7).
Impact of DTR
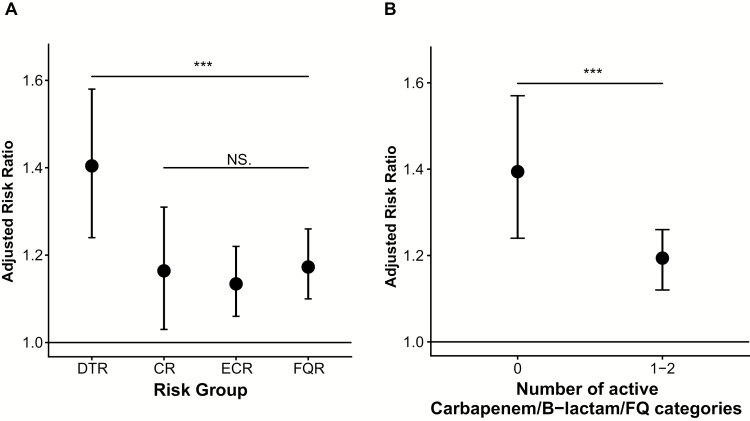
Of 29474 patients with GNBSI, 3161 (15%) died in the hospital or were discharged to hospice. There were 190 deaths (or discharges to hospice) among the 440 patients with GNBSIs exhibiting the DTR phenotype (unadjusted mortality rate in patients with DTR, 43%; see Supplementary Data for predictors of DTR). In comparison, the unadjusted mortality rate was 35% for CR (183 of 526 patients), 22% for ECR (609 of 2756), and 18% for FQR (795 of 4342). This hierarchical pattern of mortality (DTR > CR > ECR > FQR) was observed for each GN taxa evaluated (Table 2). After adjustment for confounders, all resistant phenotypes remained individually associated with an increased mortality risk compared with nonresistant GNBSIs. Patients with GNBSIs and DTR had a 40% higher adjusted mortality risk than those with nonresistant GNBSI (aRR, 1.4; 95% CI, 1.2–1.6; P < .001). Adjusted risk did not differ significantly among CR, ECR, or FQR phenotypes (P ≥ .45 for all; Supplementary Table 5 and Figure 5A), whereas DTR had a 20% higher adjusted mortality risk relative to CR (aRR, 1.2; 95% CI, 1.0–1.4; P = .02), ECR (1.2; 1.1–1.4; P = .001), and FQR (1.2; 1.0–1.4; P = .008) (Figure 5B). Taxon-level distributions of DTR encounters and mortality rates, as well as taxon-specific crude and aRR of mortality are displayed in Figure 6A–6D.
Figure 5.
Adjusted relative mortality risk among inpatients with gram-negative bloodstream infections across resistance phenotypes (A) and diminished active first-line agent categories (B) at 173 US hospitals from 2009 to 2013. Variables adjusted for in both models included age, sex, Elixhauser index, 3M All Patient Refined Diagnosis Related Group risk of mortality assignment, infection site, intensive care unit stay, neutropenia, and ventilator use at the patient level; taxa/species, healthcare-associated status, culture day relative to hospital admission, and year at the organism level; and region, bed capacity, urban location, teaching status, and hospital indicator at the hospital level. A, The reference category was “all other isolates.” The significance of differences between difficult-to-treat resistance (DTR) and the other phenotypes was also tested: DTR versus carbapenem resistant (CR), P = .02; DTR versus extended-spectrum cephalosporin resistant (ECR), P = .001; and DTR versus fluoroquinolone resistant (FQR), P = .008. *Not significant (NS) for all pairwise comparisons among CR, ECR, and FQR categories. B, The reference category was 3–5 active categories of first-line agents (and 3–4 active categories for Acinetobacter baumannii isolates). Categories included carbapenems, extended-spectrum cephalosporins, quinolones, β-lactam/β-lactamase inhibitors (piperacillin-tazobactam), and monobactam (aztreonam); for A. baumannii cases, monobactams were not included, and the β-lactam/β-lactamase inhibitor category also included ampicillin-sulbactam. P values for comparison of categories are as follows: 0 versus 3–5 categories, P < .001; 1–2 versus 3–5 categories, P = .001. *P = .01 for the comparison of 0 versus 1–2 categories. Abbreviation: FQ, fluoroquinolone.
Figure 6.
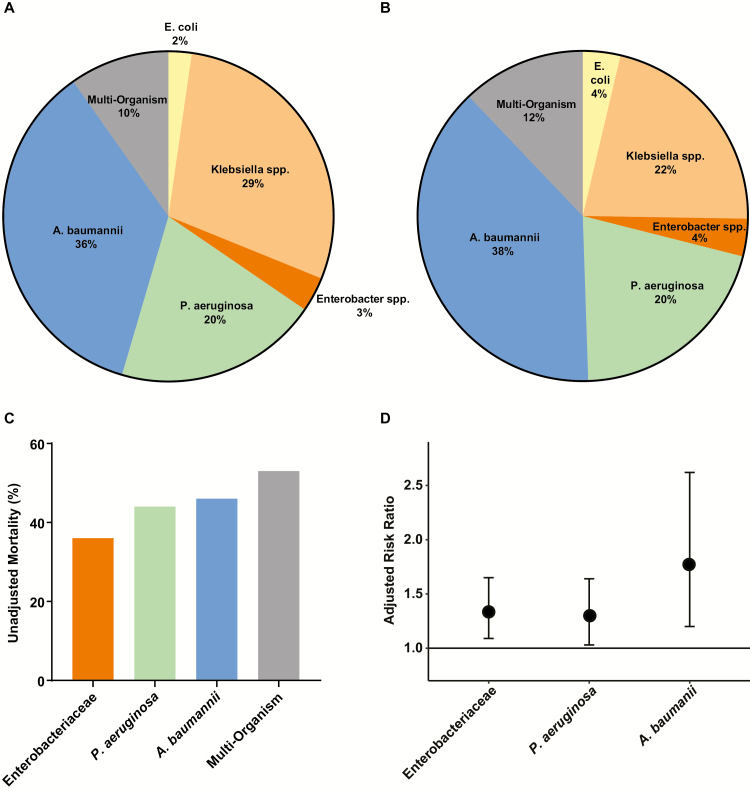
Difficult-to-treat resistance (DTR)–associated mortality by taxon of bloodstream isolate. A, B, Taxon-specific distributions of DTR encounters (n = 440) (A) and deaths (n = 190) (B), respectively. C, Unadjusted mortality rates (in-hospital death or discharge to hospice). D, Estimates of adjusted relative risk of death from DTR obtained from taxon-specific models (reference category, encounters with susceptible gram-negative bloodstream isolates). The differences in adjusted relative risk of death among DTR Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter baumannii were not statistically significant (P ≥ .05 for all three 2-way comparisons). Note that there were only 10 encounters with DTR Escherichia coli, so the 3 taxa of Enterobacteriaceae are presented as 1 group to ensure better model fit.
In sensitivity analyses exploring whether observed taxon-level resistance differences affected outcome, adjusted mortality risk was higher for DTR than for CR among patients with P. aeruginosa (P = .04). Enterobacteriaceae and A. baumannii had much fewer cases of CR not classified as DTR for this comparison and combined was not significant (P = .7; Supplementary Figure 3A and 3B). The adjusted risk of mortality for CR, ECR, and FQR was similar to nonresistant cases for P. aeruginosa (all not significant), but higher for Enterobacteriaceae and A. baumannii (P < .001 for all). As empiric validation of the DTR concept, the aRR of mortality increased in a graded fashion with the stepwise loss of active first-line agent categories. Compared with patients with 3–5 active antibiotic categories (3–4 for A. baumannii; Supplementary Table 6 and Figure 5B), those with only 1–2 categories had a 20% higher adjusted mortality risk (aRR, 1.2; 95% CI, 1.1–1.3 and P = .001) and those with none (ie, DTR) had a 40% higher adjusted mortality risk (1.4; 1.2–1.6; P < .0001). Multicollinearity was not observed between DTR and other variables included in the model; interactions among variables did not significantly influence the effect of DTR on mortality risk.
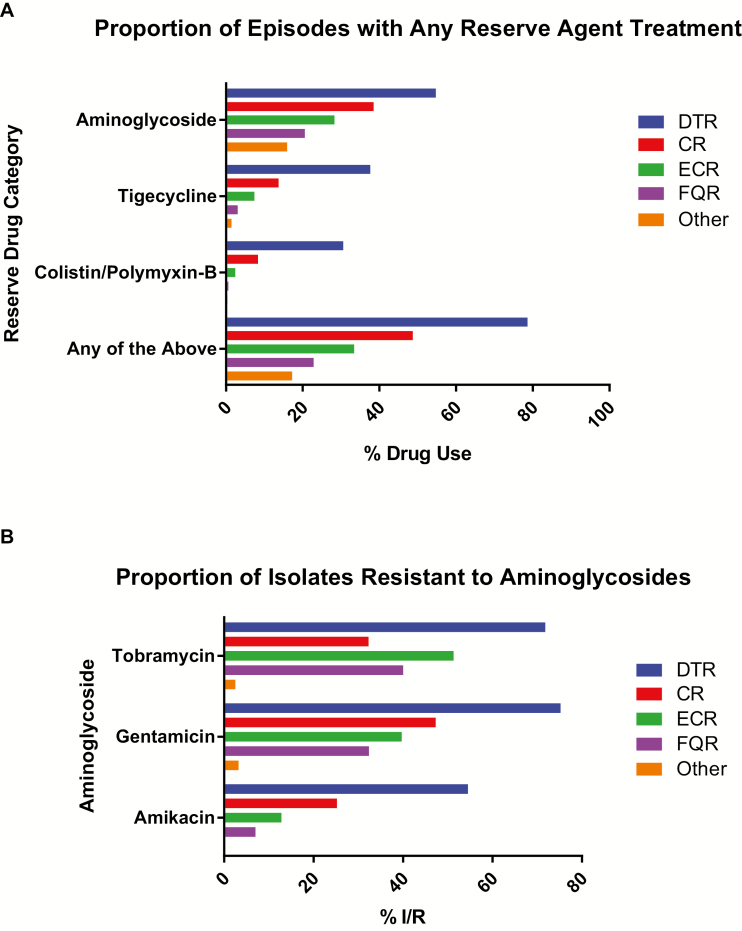
Patients with DTR had longer hospital stays than those with nonresistant GNBSIs (median [interquartile range], 14 [7–29] vs 6 [4–11] days; P < .001). Aminoglycoside, tigecycline, and/or colistin/polymyxin-B use correlated with the degree of resistance (Figure 7A); reserve agents were administered to 79% of patients with DTR, 47% with CR, 31% with ECR, 21% with FQR, and 15% with nonresistant phenotypes. Tigecycline was nearly 3-fold and colistin/polymyxin-B nearly 4-fold more likely to be administered in DTR than in CR GNBSIs. Of DTR isolates, 74% were nonsusceptible to gentamicin, 71% to tobramycin, and 54% to amikacin, all higher than among the CDC-defined phenotypes (Figure 7B). Of GNBSIs with susceptibility results for these 3 aminoglycosides (n = 28259), 1% overall versus 33% of DTR isolates displayed class resistance. Only 14% of CR, 4% of ECR, and 0.5% of FQR episodes were resistant to all aminoglycosides. Adjusted mortality risk did not differ significantly between patients with DTR and those who simultaneously met criteria for all 3 CDC-defined resistance phenotypes (CR, ECR, and FQR) but otherwise had ≥1 active first-line agent (n = 176; P = .3). However, these patients still exhibited greater use of aminoglycosides (P = .04), tigecycline (P < .001), and colistin/polymyxin-B (P < .001).
Figure 7.
A, Proportion of episodes treated with intravenous aminoglycoside, tigecycline, or colistin/polymixin-B, shown as the proportion of episodes treated with ≥1 dose of intravenous reserve agents: aminoglycoside (gentamycin, tobramycin, and/or amikacin), tigecycline, or colistin/polymixin-B, by resistance phenotype. B, Proportion of isolates resistant to aminoglycosides among those from patients with gram-negative bloodstream infections exhibiting resistant phenotype(s) at 173 US hospitals from 2009 to 2013. The display shows the proportion of isolates in each resistance phenotype that are intermediate or resistant in vitro to ≥1 of the 3 aminoglycosides. The “Other” category represents isolates that could not be classified into one of the listed resistance categories. Abbreviations: CR, carbapenem resistant; ECR, extended-spectrum cephalosporin resistant; DTR, difficult-to-treat resistance; FQR, fluoroquinolone resistant.
DISCUSSION
DTR, nonsusceptibility to all first-line agents, is a clinically relevant resistance paradigm that emphasizes the loss of high-efficacy, low-toxicity treatment options. Whereas traditional definitions of coresistance are essential epidemiologic tools calibrated for surveillance, the DTR concept was developed for select GN taxa to reflect how resistance impacts antibiotic decisions at the bedside. Our analysis demonstrates how shared electronic health record-based information systems integrating microbiology and susceptibility results with patient characteristics can offer a meaningful avenue to understand real-world infections and the impact of antimicrobial resistance on a large scale. In this well-distributed cohort of US hospitals, 1% of GN bacteremia episodes were caused by DTR strains.
Nearly 4 of 5 patients with DTR received reserve agents that are ordinarily avoided when first-line agents (ie, carbapenems, other β-lactams, and fluoroquinolones) are active. Aminoglycosides were the most frequently prescribed second-line agent for GNBSIs with DTR, yet one-third of DTR isolates were resistant to all 3 commonly available drugs in this class. Tigecycline and colistin/polymyxin-B were also used more frequently for DTR than non-DTR bloodstream isolates. When susceptible to other first-line agents, isolates classified as CR, ECR, or FQR all had a surprisingly similar impact on mortality. In contrast, the risk of death significantly increased in a stepwise fashion as the number of active first-line agent categories fell to 0, supporting the clinical relevance of the DTR concept.
The 2015 White House National Action Plan for Combating Antibiotic-resistant Bacteria calls for reliable metrics to estimate the clinical impact of interventions to contain and decrease antibiotic resistance [20]. Seemingly specific traits, such as extended-spectrum β-lactamase and carbapenemase production, are heterogeneous entities that are difficult and costly to detect and characterize [21]. These labels may help track transmission and guide therapy but do not directly address the high frequency of coresistance [22], which severely constrains treatment options. Global designations, such as MDR and XDR [6], fill this gap somewhat, but, unlike DTR, they do not account for the efficacy and safety of available antibiotic options. For example, some XDR isolates of P. aeruginosa might be susceptible to levofloxacin or piperacillin-tazobactam.
Likewise, highly resistant A. baumannii might be effectively treated with ampicillin-sulbactam. As such, XDR infections do not consistently result in worse outcomes than those caused by MDR isolates [7, 9–11]. More readily measurable than XDR, the DTR metric reflects how treatment-limiting resistance is understood and managed at the bedside, particularly with regard to the use of reserve agents. Although DTR is familiar to infection specialists, explicitly defining DTR will ideally lead to wider recognition among all providers, easier-to-use and more relevant resistance tracking, and improvements in both empiric and targeted treatment regimens.
GNBSIs can culminate in septic shock and death when antimicrobial therapy is delayed or inappropriate [23]. It is common practice to administer an empiric aminoglycoside when clinical decline is observed during treatment with first-line antibiotics. The high proportion of aminoglycoside resistance among DTR bloodstream isolates emphasizes the difficulty of choosing salvage antibiotics for clinical decompensation due to suspected resistance. DTR was associated with GNBSIs that were recurrent, polymicrobial, due to A. baumannii, of urinary or multiple potential sources, and/or in urban hospitals. Notably, the antibiotics received were not included in our models; the DTR profile implicitly represents scenarios with severe treatment constraints, and we intended to isolate the effect of such profiles on mortality risk. However, we did adjust for previous bloodstream infections to mitigate the impact of prior treatment for bacteremia on mortality.
The 2013 CDC national estimate of deaths attributable to antimicrobial resistance (6.1%) was derived from a single center and was an average for all antimicrobial-resistant infections [24]. However, efforts to determine the impact of resistance on bloodstream infection outcomes have been hampered by access to linked microbiology and patient-level data, as well as variable definitions and methods for underlying diseases, pathogens, and treatment-related factors [25, 26]. Falagas et al [27] pooled multiple small studies on CR Enterobacteriaceae and found an association with mortality.
In Enterobacteriaceae, CR is generally associated with resistance to other high-efficacy, low-toxicity agents and therefore indirectly overlaps with our concept of DTR. Compared with DTR episodes, however, CR episodes not exhibiting DTR (ie, with ≥1 alternative active first-line agent) were associated with lower reserve agent use, lower aminoglycoside resistance, and lower aRR of mortality. We observed a greater preponderance of active first-line alternatives to treat CR P. aeruginosa, compared with other taxa. Retention of more first-line agents for CR P. aeruginosa compared with other taxa may explain its relatively large survival advantage over DTR.
Similarly, GNBSIs due to MDR isolates empirically treated with appropriate antibiotics have not been associated with higher mortality rates [28]. A multicenter study of GNBSIs in European intensive care units using tracer phenotypes (ceftazidime resistance for A. baumannii and P. aeruginosa; third-generation cephalosporin resistance for E. coli) also did not show a significant effect of resistance on survival, possibly owing to retained susceptibility to other β-lactams and/or fluoroquinolones. Furthermore, first-line antibiotic options have been shown to mitigate the adverse impact of resistance on survival in gram-positive infections [29, 30].
Owing to its simplicity and ease of recognition, our analysis of DTR should alert all providers to its serious implications, and ideally it will encourage infection specialists and clinical pharmacists to help direct and monitor the use of agents that less frequently used and more toxic and/or less effective. In addition to its patient management challenges, DTR is also a public health threat that contributes to antibiotic overuse, the international spread of plasmids, and the need for costly infection control measures in healthcare facilities.
A similar threat from XDR tuberculosis was foreseen by global leadership, and, despite its relatively low prevalence, XDR tuberculosis was incorporated into regional and global surveillance operations [31]. Although their overall burden is also low, GNBSIs displaying DTR was found in half of the hospitals in our sample across all 9 US census regions. For these reasons, the prevalence of DTR has important public health implications and might serve as a useful surrogate marker for the success or failure of local and national measures aimed at controlling the spread of antimicrobial resistance.
Our study has several limitations. First, our cohort was derived from a convenience sample of hospitals, which may not be nationally representative. However, the included hospitals generally resemble US hospitals across a broad variety of characteristics. Second, we were unable to assess strain virulence or adequacy of source control and used encounter-level diagnostic codes for source of bacteremia, which may not necessarily represent the true source. Third, despite controlling for a wide range of related variables, residual confounding for severity of illness may still exist; as expected from other studies, more resistant strains in our study occurred in patients who were sicker at baseline.
Fourth, we are unable to comment on long-term outcomes or the generalizability of our estimates to non-US regions. Fifth, we were unable to report on trends in DTR due to break-point changes and their piecemeal implementation at different hospitals. Testing practices varied across hospitals, potentially biasing the results of our classification system. Sixth, though DTR can be readily applied to other pathogens and infection sites, the classification scheme requires periodic revision as new antibiotics become available and patterns of resistance change. For example, future studies will need to incorporate recently approved agents (ceftazidime-avibactam, ceftolozane-tazobactam, and meropenem-vaborbactam) into the matrix used here for defining DTR. As such, DTR is not a fixed phenotype but rather a flexible framework that will evolve with pathogens and our armamentarium for combating them.
In conclusion, DTR is a novel classifier of antimicrobial coresistance that integrates the impact of resistance on antibiotic choices and the effect of these choices on clinical outcome. This epidemiologic tool reflects the bedside management challenge of treating GN bacterial infections resistant to all first-line antibiotics. One percent of GNBSIs exhibit DTR, that is, resistance to all carbapenems, other β-lactams, and fluoroquinolones. These highly resistant strains are encountered in hospitals across the United States. As a category that considers the count, efficacy, and toxicity of available antibiotics, DTR more fully encompasses key aspects of antimicrobial resistance that affect mortality risk.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Study concept: S. S. K. and R. L. D. Study design: S. S. K., J. A., Y. L. L., A. B. S., E. R., D. P. R., C. R., M. K., J. P. D., J. H. P., D. C. H., S. F., and R. L. D. Acquisition, analysis, or interpretation of data: S. S. K., J. A., Y. L. L., A. B. S., E. R., D. P. R., J. P. D., and R. L. D. Drafting of the manuscript: S. S. K., J. A., and A. B. S. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: J. A., A. B. S., and Y. L. L. Obtaining of funding: S. S. K. and D. P. R. Study supervision: S. S. K., J. A., A. B. S., D. P. R., S. F., and R. L. D. Full access to all study data: S. S. K., J. A., Y. L. L., and A. B. S. Final responsibility for the decision to submit for publication: S. S. K. Members of DTR consensus classification panel: S. S. K., T. N. P., C. R., M. K., J. P. D., D. C. H., S. F., R. L. D., and 1 nonauthor participant, Alexander Kallen.
Acknowledgments. We thank Henry Masur, MD for reviewing the manuscript draft and Alexander Kallen, MD and Dean Follmann, PhD for offering critical feedback on study design. We also thank Kelly Byrne for formatting the manuscript text, tables, figures, and supplementary material.
Disclaimer. The findings and conclusions in this study are those of the authors and do not necessarily represent the official position of the National Institutes of Health, the US CDC, or the Agency for Healthcare Research and Quality.
Financial support. This study was funded in part by the National Institutes of Health Intramural Research Program of the Clinical Center and the National Institute of Allergy and Infectious Diseases respectively and in part by the Agency for Healthcare Research and Quality (grant K08HS025008 to C. R.) and the National Cancer Institute (contract HHSN261200800001E).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: American Thoracic Society meeting, San Francisco, California, 13–18 May 2016; Infectious Diseases Society of America meeting, New Orleans, Louisiana, 26–30 October 2016.
References
- 1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013–508.pdf. Accessed 20 October 2017. [Google Scholar]
- 2. O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. In: Review on antimicrobial resistance. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. 2014. Available at: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf. Accessed 20 June 2018. [Google Scholar]
- 3. Nordmann P, Naas T, Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 2011; 17:1791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGann P, Snesrud E, Maybank R, et al. Escherichia coli harboring mcr-1 and blaCTX-M on a novel IncF plasmid: first report of mcr-1 in the United States. Antimicrob Agents Chemother 2016; 60:4420–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fridkin SK, Cleveland AA, See I, Lynfield R. Emerging infections program as surveillance for antimicrobial drug resistance. Emerg Infect Dis 2015; 21:1578–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–81. [DOI] [PubMed] [Google Scholar]
- 7. Zilberberg MD, Nathanson BH, Sulham K, Fan W, Shorr AF. Multidrug resistance, inappropriate empiric therapy, and hospital mortality in Acinetobacter baumannii pneumonia and sepsis. Crit Care 2016; 20:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zeng ZR, Wang WP, Huang M, Shi LN, Wang Y, Shao HF. Mechanisms of carbapenem resistance in cephalosporin-susceptible Pseudomonas aeruginosa in China. Diagn Microbiol Infect Dis 2014; 78:268–70. [DOI] [PubMed] [Google Scholar]
- 9. Dimopoulos G, Koulenti D, Tabah A, et al. Bloodstream infections in ICU with increased resistance: epidemiology and outcomes. Minerva Anestesiol 2015; 81:405–18. [PubMed] [Google Scholar]
- 10. Burnham JP, Lane MA, Kollef MH. Impact of sepsis classification and multidrug-resistance status on outcome among patients treated with appropriate therapy. Crit Care Med 2015; 43:1580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tabah A, Koulenti D, Laupland K, et al. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med 2012; 38:1930–45. [DOI] [PubMed] [Google Scholar]
- 12. Satlin MJ, Jenkins SG, Walsh TJ. The global challenge of carbapenem-resistant Enterobacteriaceae in transplant recipients and patients with hematologic malignancies. Clin Infect Dis 2014; 58:1274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vicari G, Bauer SR, Neuner EA, Lam SW. Association between colistin dose and microbiologic outcomes in patients with multidrug-resistant gram-negative bacteremia. Clin Infect Dis 2013; 56:398–404. [DOI] [PubMed] [Google Scholar]
- 14. Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012; 54:1699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen AL, Calfee D, Fridkin SK, et al. ; Society for Healthcare Epidemiology of America and the Healthcare Infection Control Practices Advisory Committee Recommendations for metrics for multidrug-resistant organisms in healthcare settings: SHEA/HICPAC position paper. Infect Control Hosp Epidemiol 2008; 29:901–13. [DOI] [PubMed] [Google Scholar]
- 16. Robert Koch Institute. Hygiene measures for infection or colonization with multidrug-resistant gram-negative bacilli. Commission recommendation for hospital hygiene and infection prevention (KRINKO) at the Robert Koch Institute (RKI) [in German]. Bundesgesundheitsblatt 2012; 55:1311–54. [DOI] [PubMed] [Google Scholar]
- 17. National Healthcare Safety Network, Centers for Disease Control and Prevention. Antibiotic resistance patient safety atlas: phenotypic definitions. 2016. Available at: https://www.cdc.gov/hai/pdfs/patient-safety-atlas/AR-Patient-Safety-Atlas-Phenotype-Definitions.pdf. Accessed 20 June 2018. [Google Scholar]
- 18. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–9. [DOI] [PubMed] [Google Scholar]
- 19. Baram D, Daroowalla F, Garcia R, et al. Use of the All Patient Refined-Diagnosis Related Group (APR-DRG) risk of mortality score as a severity adjustor in the medical ICU. Clin Med Circ Respirat Pulm Med 2008; 2:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. The White House. National Action Plan for Combating Antibiotic-resistant Bacteria. Available at: https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed 20 June 2018. [Google Scholar]
- 21. Guh AY, Bulens SN, Mu Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012–2013. JAMA 2015; 314:1479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wong PH, von Krosigk M, Roscoe DL, Lau TT, Yousefi M, Bowie WR. Antimicrobial co-resistance patterns of gram-negative bacilli isolated from bloodstream infections: a longitudinal epidemiological study from 2002–2011. BMC Infect Dis 2014; 14:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leibovici L, Shraga I, Drucker M, Konigsberger H, Samra Z, Pitlik SD. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J Intern Med 1998; 244:379–86. [DOI] [PubMed] [Google Scholar]
- 24. Roberts RR, Hota B, Ahmad I, et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis 2009; 49:1175–84. [DOI] [PubMed] [Google Scholar]
- 25. Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis 2006; 42:S82–9. [DOI] [PubMed] [Google Scholar]
- 26. Lambert ML, Suetens C, Savey A, et al. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect Dis 2011; 11:30–8. [DOI] [PubMed] [Google Scholar]
- 27. Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis 2014; 20:1170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lye DC, Earnest A, Ling ML, et al. The impact of multidrug resistance in healthcare-associated and nosocomial gram-negative bacteraemia on mortality and length of stay: cohort study. Clin Microbiol Infect 2012; 18:502–8. [DOI] [PubMed] [Google Scholar]
- 29. Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol 2005; 26:166–74. [DOI] [PubMed] [Google Scholar]
- 30. Blot S, Depuydt P, Vandewoude K, De Bacquer D. Measuring the impact of multidrug resistance in nosocomial infection. Curr Opin Infect Dis 2007; 20:391–6. [DOI] [PubMed] [Google Scholar]
- 31. World Health Organization. Global tuberculosis report, 2017. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed 20 June 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.