Abstract
Background and aim
Despite several studies being conducted to examine the associations between the NUDT15 R139C polymorphism and thiopurine-induced leukopenia in the Asian population, the results remain inconsistent. This meta-analysis determined the risk of thiopurine-induced leukopenia conferred by the NUDT15 R139C polymorphism.
Materials and methods
All eligible studies published in English up to May 2018 were identified by searching PubMed, Web of Science, Embase, and the Cochrane Library. Pooled OR and 95% CI were calculated using fixed- or random-effect model.
Results
In all, total of 14 studies containing 918 patients and 2,341 controls were included; of these, 8 studies concerned inflammatory bowel disease (IBD) and 4 concerned acute lymphoblastic leukemia (ALL). Overall, the results indicated that the NUDT15 R139C polymorphism was associated with leukopenia induced by thiopurines (OR =9.04, 95% CI 6.05–13.50, P<0.001 for the dominant model; OR =24.26, 95% CI 11.38–51.71, P<0.001 for the recessive model; OR =7.60, 95% CI 4.97–11.61, P<0.001 for the CT vs TT model; OR =38.47, 95% CI 17.78–83.24, P<0.001 for the CC vs TT model). In subgroup analyses, significant associations were found among patients with IBD (OR =7.57, 95% CI 5.16–11.12, P<0.001 for the dominant model), ALL (OR =13.13, 95% CI 3.43–50.23 P<0.001 for the dominant model), and other diseases (OR =31.22, 95% CI 1.20–814.07, P=0.04 for the dominant model). In addition, the R139C variant was strongly associated with early (<8 weeks) (OR =15.53, 95% CI 7.91–30.50, P<0.001 for the dominant model) and late leukopenia (≥8 weeks) (OR =2.92, 95% CI 2.01–4.24, P<0.001 for the dominant model). Moreover, these findings were sufficiently robust when studies without Hardy–Weinberg equilibrium test were excluded.
Conclusion
This meta-analysis verified the strong association between the NUDT15 R139C polymorphism and thiopurine-induced leukopenia (both early and late leukopenia) in an Asian population with IBD, ALL, and other diseases. NUDT15 R139C genotyping should be prioritized to predict leukopenia among Asians.
Keywords: NUDT15 R139C, leukopenia, thiopurine, polymorphism, IBD, ALL, meta-analysis
Introduction
Thiopurines (mercaptopurine, thioguanine, and azathioprine) are commonly used immunosuppressive and anticancer agents. In inflammatory bowel disease (IBD), including ulcerative colitis and Crohn’s disease, thiopurines are the first-line regimens in patients with corticosteroid refractory or corticosteroid dependant.1–3 Thiopurines are also commonly prescribed for patients with acute lymphoblastic leukemia (ALL), as prolonged daily exposure to mercaptopurine is an important component of contemporary treatment regimens.4,5 Unfortunately, up to 40% of patients discontinue thiopurine therapy owing to intolerable adverse effects, especially leukopenia, which is the most common and life-threatening toxicity in Asian patients.5,6 Therefore, predictors that can forecast the risk of leukopenia are strongly needed to accurately and efficiently use thiopurines.
The metabolism of thiopurines is complex and involves several primary active intermediates, such as nucleoside triphosphates, 6-thio-GTP, and 6-thio-dGTP.7 Nucleoside diphosphate-linked moiety X-type motif 15 (NUDT15) is a 164-amino-acid protein that belongs to the nudix hydrolase enzyme family, which is hypothesized to catalyze the hydrolysis of 6-thio-GTP and 6-thio-dGTP, thus preventing their incorporation into DNA and playing a negative role with respect to the adverse effects of thiopurines.8 Studies revealed that the novel gene NUDT15 R139C variant (rs116855232; C415T; encoding p.Arg139Cys) could decrease thermal stability of NUDT15.9 Moreover, in 2014, Yang et al10 first reported that the NUDT15 R139C variant confers susceptibility to thiopurine-induced leukopenia. Since then, there have been numerous publications, mostly in patients of Asian origin, that further supported Yang et al’s findings.11–15 Furthermore, this variant is most common in East Asians, rare in Europeans, and not observed in Africans. Hence, we aimed to perform a systematic review and meta-analysis to gather and analyze the data regarding the association between NUDT15 R139C variant and thiopurine-induced leukopenia in Asians.
Materials and methods
Search strategy
A literature research was conducted using PubMed, Web of Science, Embase, and Cochrane Library up to May 2018 with English-language restriction. Relevant studies were searched using the terms [NUDT15 or rs116855232 or NUDT15 R139C or NUDT15 c.415C>T] AND [variant or genetic polymorphism or polymorphisms or mutation]. Additional studies were identified by screening references in the retrieved articles and preceding reviews on the topic.
Inclusion and exclusion criteria
Studies were included if they met the following criteria: 1) they described the association between NUDT15 R139C polymorphism and risks of thiopurine-induced leukopenia in Asians; and 2) they reported the genotype frequencies of cases and controls or the data could be calculated from the paper. Accordingly, the exclusion criteria were 1) reviews or letters, or case report; 2) duplicate data; 3) reports only concerned with leukopenia; and 4) studies with sample size <30.
Date extraction and quality assessment
Two of the authors independently selected the articles and extracted data with consensus regarding all search terms. If the data were not identical, both investigators would check the data again to arrive at an agreement. If they could not reach an agreement, an expert (W.D.) would intervene and help make a decision. The following items were collected from the eligible articles: first author’s name, year of publication, country of origin, ethnicity, year of publication, type of disease, leukopenia-onset time, number of cases and controls, age of onset, thiopurine regimens, leukopenia criteria, number of early leukopenia cases induced by thiopurines, number of late leukopenia cases induced by thiopurines, and the method of single-nucleotide polymorphism detection.
The quality of the selected studies was independently evaluated on the basis of the Newcastle–Ottawa Scale (NOS).16 Studies with six or more stars were considered as high quality.
Statistical analysis
Meta-analysis was performed using the Cochrane Collaboration Revman 5.3 (Copenhagen, 2014) and STATA package version 12.0 (StataCorp LP, College Station, TX). The pooled ORs ad 95% CIs were calculated to evaluate the association between the NUDT15 R139C polymorphism and thiopurine-induced leukopenia risk. In addition, subgroup analyses were performed based on disease type and leukopenia-onset time when adequate data were available. We used the chi-squared-based Q statistic to assess the heterogeneity between the studies.17,18 When I2>50% and P≤0.05, the heterogeneity was considered significant, and the random-effects model was used to analyze the data; otherwise, the fixed-effects model was used.19 Hardy–Weinberg equilibrium (HWE) among the control subjects was examined with the chi-squared test. We evaluated the association of NUDT15 R139C polymorphism with susceptibility to thiopurine-induced leukopenia in the dominant, recessive, codominant, and heterozygous models. Then, we calculated the sensitivity to evaluate the stability of the results after eliminating the studies without HWE. Publication bias was diagnosed using Begg’s funnel plot20 and Egger’s linear regression.21 P<0.05 was regarded as a state of disequilibrium.
Results
Study characteristics
In total, 97 abstracts were identified and assessed from the initial literature search. According to the inclusion and exclusion criteria, 14 studies10–14,22–30 with full text were eligible for this meta-analysis. Figure 1 shows the flow chart of included studies. All studies were conducted in Asia and all study participants were Asian. There were 14 eligible studies including 918 cases (leukopenia) and 2,341 controls (without leukopenia) regarding the NUDT15 R139C variant. Disease type included IBD (Crohn’s disease or ulcerative colitis), ALL, autoimmune hepatitis, and various neurological disease, including myasthenia gravis, chronic inflammatory demyelinating polyneuropathy, neuromyelitis optica, vasculitis, and others. Leukopenia with onset within 8 weeks was defined as early leukopenia; otherwise, it was defined as late leukopenia.31 Blood samples were obtained from enrolled patients and used to determine genetic polymorphisms in all of the included studies. The distribution of genotypes in controls was consistent with HWE for all, except two studies.11,12 According to the NOS, the quality of all enrolled studies was high. Table 1 showed the characteristics of the included studies.
Figure 1.

Flow chart showing study selection procedure.
Table 1.
Characteristics of studies included in the meta-analysis
| Study | Year | Ethnicity | Diseases | Number | Leukopenia diagnostic criteria | Genotype (case/control) | PHWE | NOS | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Case/Control | WT Ho | Ht | VR Ho | |||||||
| Asada et al22 | 2016 | Japanese | IBD | 45/116 | WBC <3,000/μL | 25/102 | 18/14 | 2/0 | 0.489 | 9 |
| Chao et al11 | 2017 | Chinese | IBD | 177/555 | WBC <3,500/μL | 90/467 | 76/88 | 11/0 | 0.043 | 9 |
| Kakuta et al13 | 2016 | Japanese | IBD | 34/101 | WBC <3,000/μL | 19/88 | 10/13 | 5/0 | 0.489 | 9 |
| Sato et al23 | 2017 | Japanese | IBD | 59/90 | WBC <3,000/μL | 29/80 | 24/9 | 6/1 | 0.223 | 8 |
| Sutiman et al25 | 2018 | Asian | IBD | 10/119 | WBC <3,000/μL | 3/108 | 5/11 | 2/0 | 0.597 | 8 |
| Wang et al27 | 2018 | Chinese | IBD | 19/80 | WBC <3,000/μL or ANC <1,500/μL | 11/64 | 8/16 | 0/0 | 0.320 | 8 |
| Yang et al10 | 2014 | Korean | IBD | 346/632 | WBC <3,000/μL | 199/589 | 133/43 | 14/0 | 0.376 | 7 |
| Zhu et al29 | 2016 | Chinese | IBD | 65/188 | WBC <3,500/μL | 25/171 | 36/17 | 4/0 | 0.516 | 6 |
| Chiengthong et al12 | 2016 | Thai | ALL | 28/54 | ANC <500/μL | 18/52 | 9/1 | 1/1 | 0.000 | 6 |
| Tanaka et al26 | 2018 | Japanese | ALL | 38/57 | WBC <2,000/μL or ANC <1,000/μL | 19/51 | 13/5 | 6/1 | 0.071 | 9 |
| Zhou et al28 | 2018 | Chinese | ALL | 23/82 | WBC <2,000/μL | 11/63 | 10/19 | 2/0 | 0.235 | 9 |
| Zhu et al30 | 2018 | Chinese | ALL | 48/140 | WBC <2,000/μL | 15/136 | 27/4 | 6/0 | 0.864 | 6 |
| Kim et al14 | 2017 | Korean | Neurological diseases | 20/64 | WBC <3,500/μL | 12/59 | 3/5 | 5/0 | 0.745 | 7 |
| Shah et al24 | 2017 | Indian | IBD + AIH | 6/63 | WBC <3,000/μL | 0/60 | 5/3 | 1/0 | 0.846 | 9 |
Notes: PHWE was calculated by goodness-of fit chi-squared test, PHWE <0.05 was considered statistically significant.
Abbreviations: AIH, autoimmune hepatitis; ALL, acute lymphoblastic leukemia; Ht, heterozygote; HWE, Hardy–Weinberg equilibrium; IBD, inflammatory bowel disease; NOS, Newcastle–Ottawa Scale; VR Ho, variant homozygote; WBC, white blood cell; WT Ho, wild-type homozygote.
Quantitative data synthesis
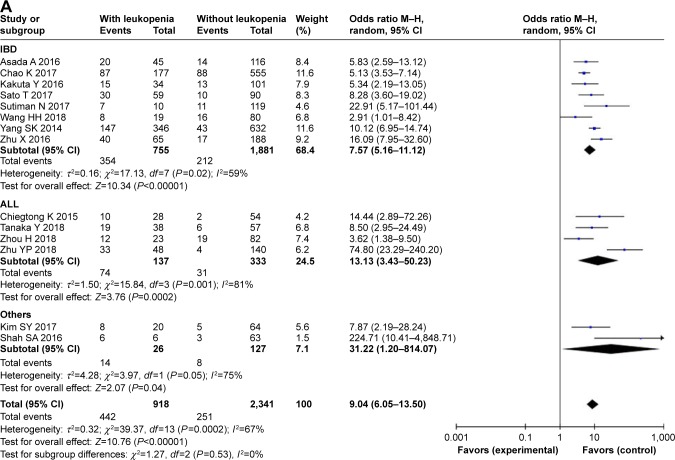
In this meta-analysis, the allelic frequency of NUDT15 R139C was 11.2% (732/6,518), and there were 28.2% (918/3,259) patients with thiopurine-induced leukopenia. Overall, NUDT15 R139C polymorphism significantly increased susceptibility to thiopurine-induced leukopenia. This association was observed under all four models: dominant model (OR =9.04, 95% CI 6.05–13.50, P<0.001); recessive model (OR =24.26, 95% CI 11.38–51.71, P<0.0010); CT vs TT model (OR =7.60, 95% CI 4.97–11.61, P<0.001); and CC vs TT model (OR =38.47, 95% CI 17.78–83.24, P<0.001) (Figure 2, Table 2).
Figure 2.
Meta-analysis of the association between NUDT15 c.415C.T polymorphism and susceptibility to thiopurine-induced leukopenia under dominant model.
Notes: (A) All leukopenia. (B) Early leukopenia. (C) Late leukopenia.
Abbreviations: ALL, acute lymphoblastic leukemia; IBD, inflammatory bowel disease.
Table 2.
Summary of ORs of the NUDT15 R139C polymorphism and thiopurine-induced leukopenia
| Disease | n | Dominant model | Recessive model | Ht vs WT Ho | VR Ho vs WT Ho | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-valuea | OR (95% CI) | P-valuea | OR (95% CI) | P-valuea | OR (95% CI) | P-valuea | ||
| Total | |||||||||
| All studies | 14 | 9.04 (6.05–13.50) | 0.0002 | 24.26 (11.38–51.71)b | 0.90 | 7.60 (4.97–11.61) | 0.0001 | 38.47 (17.78–83.24)b | 0.78 |
| Studies with HWE | 12 | 9.60 (6.09–15.12) | 0.0009 | 25.13 (10.71–58.99)b | 0.99 | 7.85 (4.87–12.65) | 0.0008 | 40.79 (17.00–97.86)b | 0.94 |
| IBD | 8 | 7.57 (5.16–11.12) | 0.02 | 32.06 (11.10–92.56)b | 0.90 | 6.55 (4.42–9.70) | 0.02 | 51.22 (17.44–150.48)b | 0.89 |
| ALL | 4 | 13.13 (3.43–50.23) | 0.001 | 12.43 (3.62–42.69)b | 0.48 | 12.62 (2.91–54.78) | 0.001 | 19.88 (5.82–67.94)b | 0.35 |
| Others | 2 | 31.22 (1.20–814.07)b | 0.05 | 42.43 (4.23–425.17)b | 0.89 | 19.07 (0.31–1160.45) | 0.007 | 67.02 (4.62–972.29)b | 0.43 |
Notes:
Test for heterogeneity.
Fixed-effect model was used when the P for heterogeneity test was ≥0.05, otherwise the random-effect model was used.
Abbreviations: ALL, acute lymphoblastic leukemia; IBD, inflammatory bowel disease; n, number of studies; Ht + VR Ho vs WT Ho, dominant model; HWE, Hardy–Weinberg equilibrium; VR Ho, variant homozygote; SNP, single-nucleotide polymorphism; vs Ht + WT Ho, recessive model.
In IBD patients, eight studies with 755 cases and 1,881 controls were identified. Overall, a significantly increased risk was found under all four models: dominant model (OR =7.57, 95% CI 5.16–11.12, P<0.001); recessive model (OR =32.06, 95% CI 11.10–92.56, P<0.001); CT vs TT model (OR =6.55, 95% CI 4.42–9.70, P<0.001); and CC vs TT model (OR =51.22, 95% CI 17.44–150.48, P<0.001). Similar results were observed in the subgroup of ALL and other diseases (Table 2).
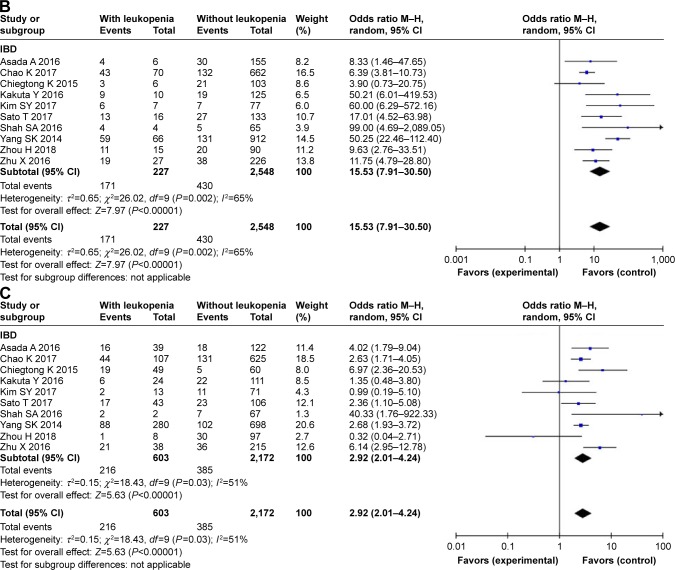
For early leukopenia, 10 studies with 227 cases and 2,548 controls were identified. Overall, we found a significantly increased risk under the dominant model (OR =15.53, 95% CI 7.91–30.50, P<0.001). For late leukopenia, 10 studies with 603 cases and 2,172 controls were included. Similar results were obtained in the development of late leukopenia (OR =2.92, 95% CI 2.01–4.24, P<0.001) (Figure 2).
Test of heterogeneity
There was significant heterogeneity for overall comparisons in the dominant (CT + TT vs TT: P=0.0002) and heterozygote model (CT vs CC: P=0.0001), but not in the recessive (TT vs CC + CT: P=0.90) and homozygote comparisons (TT vs CC: P=0.78). In the subgroup analysis by disease type, results were similar in IBD, ALL, and other diseases subgroup. For early and late leukopenia, there was significant heterogeneity (Pearly =0.002, Plate =0.03) (Table 2).
Publication bias
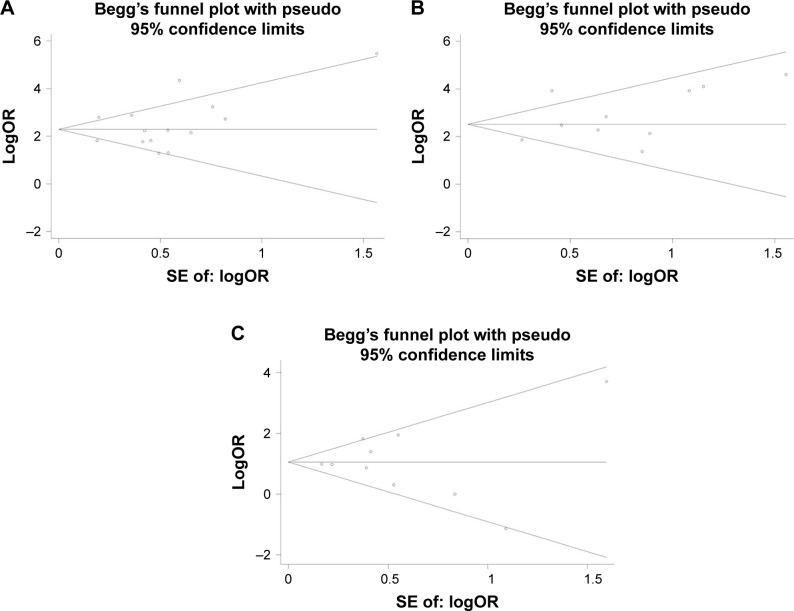
Begg’s funnel plot and Egger’s test were performed to address potential publication bias in the available literature. The shape of funnel plots did not indicate any evidence of funnel plot asymmetry (Figure 3). Egger’s test also revealed that there was no statistical significance of evaluation of publication bias under the dominant model (415C>T: P=0.497, early leukopenia: P=0.245, late leukopenia: P=0.952).
Figure 3.
Begg’s funnel plot for publication bias under dominant model.
Notes: (A) All leukopenia. (B) Early leukopenia. (C) Late leukopenia.
Discussion
As prodrugs, thiopurines undergo a series of enzymatic reactions that results in several active species, consisting of 6-thioguanine-monophosphate (6-TGMP), 6-thioguanine-diphosphate (6-TGDP), and 6-thioguanine-triphosphate (6-TGTP).32 6-TGTP is further reduced to 6-deoxythioguanosine-triphosphate (6-TdGTP). Then, 6-TGTP and 6-TdGTP are incorporated into the RNA or DNA to trigger a futile mismatch repair and, eventually, apoptosis.33,34 Studies from comprehensive in vitro and vivo studies strongly indicated that NUDT15 could hydrolyze 6-TdGTP and 6-TGTP and then prevent the incorporation of these thiopurine metabolites into DNA (DNA-TG), thereby acting as a barrier to the efficacy of thiopurines in cells, and consequently, cytotoxicity.8,9,35,36 Moreover, the R139C mutation of NUDT15 can decrease the thermal stability of NUDT15 that results in NUDT15 degradation9 and on average, only about 10% of thiopurine dose routinely tolerated is by patients with wild-type alleles.8
Routine examination of (thiopurine methyltransferase) TPMT genotype status prior to initiation of thiopurine therapy has been recommended in several clinical practice guidelines to reduce the risks of myelosuppression.37,38 However, series studies have confirmed that in Asian patients the variations in TPMT gene were rare and might not be clinically relevant in predicting toxicity.15,39,40 NUDT15 c.415C>T is a missense variant in the NUDT15 gene (rs116855232, encoding p.Arg139Cys) and the allele frequency of the mutation is ~10%–20% in the Asian population.10,22,41 By contrast, it is much lower in Caucasians with an occurrence of only 0.4%. Recently, researchers have reported that the NUDT15 R139C variant is strongly associated with thiopurine-related myelosuppression in patients with IBD10,25 and in children with ALL.29,42 Studies reported that NUDT15 c.415C>T may be a better predictor for thiopurine-reduced leukopenia than TPMT polymorphism in the Asian population.
The current meta-analysis found that the allele frequency of NUDT15 c.415C>T was 11.2% (732/6518) and the risk of developing leukopenia was significantly increased by 3.02-fold compared with patients without the T-allele mutation, which was similar to the range observed on other studies in East Asians.27,29 We calculated that the NUDT15 c.415C>T allele was strongly associated with an increased risk of developing thiopurine-related leukopenia and this result was confirmed among studies with HWE. In the subgroup of IBD, ALL, and other diseases, similar results were observed. Among these, thiopurine-induced leukopenia among Asian populations have been robustly linked to the NUDT15 R139C genetic variant. Therefore, the utility of routine genotyping for the NUDT15 R139C variant before initiating thiopurine therapy should be considered to reduce the risk of thiopurine-related leukopenia.
Meanwhile, there have been different observations in recent studies of the NUDT15 R139C variant in East Asians. Yang et al showed that NUDT15 R139C was associated with both early and late leukopenia in the Korean population.10 In addition, Asada et al found that a decrease in the white blood cell count was rapidly induced (within 2 weeks) after thiopurine initiation in patients carrying NUDT15 R139C. However, Kakuta et al13 reported that the presence of NUDT15 R139C was associated with only early leukopenia, but not late leukopenia, in the Japanese population. This meta-analysis showed that the Asian population NUDT15 R139C was significantly associated with the development of late leukopenia (OR =2.92, 95% CI 2.01–4.24) and a much stronger association with early leukopenia (OR =15.53, 95% CI 7.91–30.50). We also found that more patients develop late leukopenia (21.7%, 603/2,775) than early leukopenia (8.2%, 227/2,775). Several studies22,41 also found that patients with T mutation were more likely to develop severe leukopenia. The reason for the discrepancy might be owing to different azathiopurine dosage and different diagnostic criteria of leukopenia. Further studies are necessary to clarify this association.
Some of the highlights of our meta-analysis should be noted. First, this research sheds light on the relationship between the NUDT15 R139C genetic polymorphism and the increased susceptibility to thiopurine-induced leukopenia in the Asian population. Second, the exhaustive inclusion criteria and articles on different types of disease enhanced the power and validity of our conclusion. Furthermore, all literature included had acceptable quality scores (scored at least 6). We also acknowledge that our study has some limitations. First, we only investigated the NUDT15 p.Arg139Cys mutation, but not other coding variants of NUDT15, such as NUDT15 p.Val18_Val19insGlyVal and p.Val18Ile. Second, the search terms were chosen only from the genetic aspect, and did not include leukopenia and toxicity to thiopurines. Third, the number of the studies, especially for ALL patients, was not sufficiently large.
Conclusion
In summary, we verified the strong association between NUDT15 c.415C>T polymorphism and thiopurine-induced leukopenia (both early and late leukopenia) in an Asian population with IBD, ALL, and other diseases. Thus, NUDT15 c.415C>T genotyping should currently be prioritized for the prediction of leukopenia among the Asian population, and its application in precision medicine should be considered in the future. Further research is necessary to verify this relationship and determine the precise mechanism.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lichtenstein GR, Abreu MT, Cohen R, Tremaine W, American Gastro-enterological Association American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130(3):940–987. doi: 10.1053/j.gastro.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 2.Timmer A, McDonald JW, Tsoulis DJ, Macdonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;9:CD000478. doi: 10.1002/14651858.CD000478.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Timmer A, Patton PH, Chande N, McDonald JW, MacDonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2016;5:CD000478. doi: 10.1002/14651858.CD000478.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Childhood ALL Collaborative Group Duration and intensity of maintenance chemotherapy in acute lymphoblastic leukaemia: overview of 42 trials involving 12000 randomised children. Lancet. 1996;347(9018):1783–1788. doi: 10.1016/s0140-6736(96)91615-3. [DOI] [PubMed] [Google Scholar]
- 5.Schmiegelow K, Nielsen SN, Frandsen TL, Nersting J. Mercaptopurine/methotrexate maintenance therapy of childhood acute lymphoblastic leukemia: clinical facts and fiction. J Pediatr Hematol Oncol. 2014;36(7):503–517. doi: 10.1097/MPH.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002;50(4):485–489. doi: 10.1136/gut.50.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karran P. Thiopurines, DNA damage, DNA repair and therapy-related cancer. Br Med Bull. 2006:79–80. 153–170. doi: 10.1093/bmb/ldl020. [DOI] [PubMed] [Google Scholar]
- 8.Moriyama T, Nishii R, Perez-Andreu V, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016;48(4):367–373. doi: 10.1038/ng.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valerie NC, Hagenkort A, Page BD, et al. NUDT15 Hydrolyzes 6-Thio-DeoxyGTP to mediate the anticancer efficacy of 6-thioguanine. Cancer Res. 2016;76(18):5501–5511. doi: 10.1158/0008-5472.CAN-16-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang SK, Hong M, Baek J, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014;46(9):1017–1020. doi: 10.1038/ng.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao K, Wang X, Cao Q, et al. Combined detection of NUDT15 variants could highly predict thiopurine-induced leukopenia in Chinese patients with inflammatory bowel disease: a multicenter analysis. Inflamm Bowel Dis. 2017;23(9):1592–1599. doi: 10.1097/MIB.0000000000001148. [DOI] [PubMed] [Google Scholar]
- 12.Chiengthong K, Ittiwut C, Muensri S, et al. NUDT15 c.415C.T increases risk of 6-mercaptopurine induced myelosuppression during maintenance therapy in children with acute lymphoblastic leukemia. Haematologica. 2016;101(1):e24–e26. doi: 10.3324/haematol.2015.134775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakuta Y, Naito T, Onodera M, et al. NUDT15 R139C causes thiopurine-induced early severe hair loss and leukopenia in Japanese patients with IBD. Pharmacogenomics J. 2016;16(3):280–285. doi: 10.1038/tpj.2015.43. [DOI] [PubMed] [Google Scholar]
- 14.Kim SY, Shin JH, Park JS, et al. NUDT15 p.R139C variant is common and strongly associated with azathioprine-induced early leukopenia and severe alopecia in Korean patients with various neurological diseases. J Neurol Sci. 2017;378:64–68. doi: 10.1016/j.jns.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 15.Liang DC, Yang CP, Liu HC, et al. NUDT15 gene polymorphism related to mercaptopurine intolerance in Taiwan Chinese children with acute lymphoblastic leukemia. Pharmacogenomics J. 2016;16(6):536–539. doi: 10.1038/tpj.2015.75. [DOI] [PubMed] [Google Scholar]
- 16.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 20.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asada A, Nishida A, Shioya M, et al. NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2016;51(1):22–29. doi: 10.1007/s00535-015-1142-4. [DOI] [PubMed] [Google Scholar]
- 23.Sato T, Takagawa T, Kakuta Y, et al. NUDT15, FTO, and RUNX1 genetic variants and thiopurine intolerance among Japanese patients with inflammatory bowel diseases. Intest Res. 2017;15(3):328–337. doi: 10.5217/ir.2017.15.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah SA, Paradkar M, Desai D, Ashavaid TF. Nucleoside diphosphate-linked moiety X-type motif 15 C415T variant as a predictor for thiopurine-induced toxicity in Indian patients. J Gastroenterol Hepatol. 2017;32(3):620–624. doi: 10.1111/jgh.13494. [DOI] [PubMed] [Google Scholar]
- 25.Sutiman N, Chen S, Ling KL, et al. Predictive role of NUDT15 variants on thiopurine-induced myelotoxicity in Asian inflammatory bowel disease patients. Pharmacogenomics. 2018;19(1):31–43. doi: 10.2217/pgs-2017-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka Y, Nakadate H, Kondoh K, Nakamura K, Koh K, Manabe A. Interaction between NUDT15 and ABCC4 variants enhances intolerability of 6-mercaptopurine in Japanese patients with childhood acute lymphoblastic leukemia. Pharmacogenomics J. 2018;18(2):275–280. doi: 10.1038/tpj.2017.12. [DOI] [PubMed] [Google Scholar]
- 27.Wang HH, He Y, Wang HX, et al. Comparison of TPMT and NUDT15 polymorphisms in Chinese patients with inflammatory bowel disease. World J Gastroenterol. 2018;24(8):941–948. doi: 10.3748/wjg.v24.i8.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou H, Li L, Yang P, et al. Optimal predictor for 6-mercaptopurine intolerance in Chinese children with acute lymphoblastic leukemia: NUDT15, TPMT, or ITPA genetic variants? BMC Cancer. 2018;18(1):516. doi: 10.1186/s12885-018-4398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu X, Wang XD, Chao K, et al. NUDT15 polymorphisms are better than thiopurine S-methyltransferase as predictor of risk for thiopurine-induced leukopenia in Chinese patients with Crohn’s disease. Aliment Pharmacol Ther. 2016;44(9):967–975. doi: 10.1111/apt.13796. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y, Yin D, Su Y, et al. Combination of common and novel rare NUDT15 variants improves predictive sensitivity of thiopurine-induced leukopenia in children with acute lymphoblastic leukemia. Haematologica. 2018;103(7):e293–e295. doi: 10.3324/haematol.2018.187658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis JD, Abramson O, Pascua M, et al. Timing of myelosuppression during thiopurine therapy for inflammatory bowel disease: implications for monitoring recommendations. Clin Gastroenterol Hepatol. 2009;7(11):1195–1201. doi: 10.1016/j.cgh.2009.07.019. quiz 1141–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meijer B, Mulder CJ, de Boer NK. NUDT15: a novel player in thiopurine metabolism. J Gastrointestin Liver Dis. 2016;25(2):261–262. doi: 10.15403/jgld.2014.1121.252.nud. [DOI] [PubMed] [Google Scholar]
- 33.Ebbesen MS, Nersting J, Jacobsen JH, et al. Incorporation of 6-thioguanine nucleotides into DNA during maintenance therapy of childhood acute lymphoblastic leukemia-the influence of thiopurine methyltransferase genotypes. J Clin Pharmacol. 2013;53(6):670–674. doi: 10.1002/jcph.81. [DOI] [PubMed] [Google Scholar]
- 34.Hedeland RL, Hvidt K, Nersting J, et al. DNA incorporation of 6-thioguanine nucleotides during maintenance therapy of childhood acute lymphoblastic leukaemia and non-Hodgkin lymphoma. Cancer Chemother Pharmacol. 2010;66(3):485–491. doi: 10.1007/s00280-009-1184-5. [DOI] [PubMed] [Google Scholar]
- 35.Takagi Y, Setoyama D, Ito R, Kamiya H, Yamagata Y, Sekiguchi M. Human MTH3 (NUDT18) protein hydrolyzes oxidized forms of guanosine and deoxyguanosine diphosphates: comparison with MTH1 and MTH2. J Biol Chem. 2012;287(25):21541–21549. doi: 10.1074/jbc.M112.363010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang JJ, Landier W, Yang W, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. 2015;33(11):1235–1242. doi: 10.1200/JCO.2014.59.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burnett HF, Tanoshima R, Chandranipapongse W, Madadi P, Ito S, Ungar WJ. Testing for thiopurine methyltransferase status for safe and effective thiopurine administration: a systematic review of clinical guidance documents. Pharmacogenomics J. 2014;14(6):493–502. doi: 10.1038/tpj.2014.47. [DOI] [PubMed] [Google Scholar]
- 38.Relling MV, Gardner EE, Sandborn WJ, et al. Clinical Pharmacogenetics Implementation Consortium Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin Pharmacol Ther. 2013;93(4):324–325. doi: 10.1038/clpt.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gazouli M, Pachoula I, Panayotou I, et al. Thiopurine S-methyltransferase genotype and the use of thiopurines in paediatric inflammatory bowel disease Greek patients. J Clin Pharm Ther. 2010;35(1):93–97. doi: 10.1111/j.1365-2710.2009.01041.x. [DOI] [PubMed] [Google Scholar]
- 40.Takatsu N, Matsui T, Murakami Y, et al. Adverse reactions to azathioprine cannot be predicted by thiopurine S-methyltransferase genotype in Japanese patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2009;24(7):1258–1264. doi: 10.1111/j.1440-1746.2009.05917.x. [DOI] [PubMed] [Google Scholar]
- 41.Lee JH, Kim TJ, Kim ER, et al. Measurements of 6-thioguanine nucleotide levels with TPMT and NUDT15 genotyping in patients with Crohn’s disease. PLoS One. 2017;12(12):e0188925. doi: 10.1371/journal.pone.0188925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka Y, Kato M, Hasegawa D, et al. Susceptibility to 6-MP toxicity conferred by a NUDT15 variant in Japanese children with acute lymphoblastic leukaemia. Br J Haematol. 2015;171(1):109–115. doi: 10.1111/bjh.13518. [DOI] [PubMed] [Google Scholar]