Abstract
Purpose
To explore the prognostic significance of mammary Paget’s disease (PD) in breast cancer (BC) patients and to investigate the association between clinical manifestation and outcome in invasive ductal carcinoma patients with PD (PD-IDC).
Patients and methods
Eighty-five patients diagnosed with mammary PD with underlying BC from 2006 to 2012 at Zhejiang Cancer Hospital were recruited. A matched group comprised 85 patients diagnosed with BC without PD. Patients were matched according to four variables: stage (0–IV), age at diagnosis (within 5 years), histologic subtype, and the year of surgery. The 74 patients diagnosed with PD-IDC were divided into three groups based on their clinical presentation.
Results
Compared with the matched group, the PD group had more HER2 positivity (P<0.01) and hormone receptor negativity (P<0.01), and a worse outcome (Kaplan–Meier analysis, P<0.001 for disease-free survival and P=0.002 for overall survival). Multivariate Cox regression analyses showed that PD was an independent prognostic predictor for BC patients with PD. In addition, the 22 PD-IDC patients who presented with skin lesions in the nipple/areola and a mass in the breast or axilla had a higher risk of disease relapse than patients who presented with a mass in the breast without skin lesions or patients who presented with skin changes without a palpable mass (adjusted hazards ratio, 0.24; 95% CI, 0.08–0.73; P=0.012 and adjusted hazard ratio, 0.30; 95% CI, 0.06–1.40; P=0.124, respectively).
Conclusion
PD is an independent prognostic indicator of outcome in BC patients with PD. Furthermore, the primary symptoms at presentation may be an available indicator of prognosis in PD-IDC.
Keywords: Paget’s disease, invasive ductal carcinoma, prognosis, clinical presentation
Introduction
Paget’s disease (PD) of the breast is a relatively rare clinical manifestation with a reported incidence of 1%–3% of all primary breast cancers (BCs).1–4 It is often present in ductal carcinoma in situ (DCIS) and/or invasive ductal carcinoma (IDC).5–7 It is characterized by eczematoid changes of the nipple–areola area. The epidermotropic theory and in situ malignant transformation theory have been raised to explain the pathogenesis of PD. The epidermotropic theory suggests that Paget’s cells arise from the underlying ductal tumor and migrate along the lactiferous ducts to reach the nipple epidermis. The in situ malignant transformation theory proposes that the Paget’s cells are malignant cells that appear in situ independently of any underlying precancerous or cancerous condition.7–10 PD of the breast is characterized by skin lesions of the nipple– areolar complex (NAC) with invasion of Paget’s cells, which are large, foamy cells that may contain mucin.3,11,12 Clinically, patients with PD of the breast typically present with nipple or areolar skin changes with or without an associated palpable mass in the breast. Skin changes in the NAC may include itching, erythema, scaly or flaky skin, bloody nipple discharge, nipple erosion or ulceration, and nipple retraction.13 Because the inchoate symptoms of PD are not standard and PD is commonly treated initially as a benign dermatologic condition, the diagnosis is often delayed for months or longer.14
Traditionally, the prognosis of PD patients with underlying cancer is primarily determined by the stage of malignancy, and the presence of PD was thought to have no effect on prognosis.11 Moreover, decisions regarding adjuvant treatment are based on the final histological analysis of the associated tumors. However, two small cohort case–control studies suggest that the presence of PD may be associated with poor outcomes in BC.15,16 Furthermore, Wong et al4 reported that patients who have PD with IDC have a greater risk of axillary lymph node (LN) metastasis than patients with IDC alone. Because of the uncommon nature of the disease, published research studies tend to have low numbers of patients treated over a long period of time. This study was conducted to further confirm whether PD is an adverse prognostic factor in patients with BC.
Because the inchoate symptoms of PD are not standard and PD is commonly treated initially as a benign dermatologic condition, the diagnosis is often delayed for months or longer.16 Moreover, patients in our country are not proactive in seeking medical attention, and by the time they do see a physician, the symptoms tend to be severe. The accuracy and timeliness of preoperative assessment is therefore vital for PD-IDC patients. Zakaria et al’s1 study claimed that a palpable mass or suspicious mammogram portends a high likelihood of invasive cancer in patients with PD, and these factors were significantly associated with a worse outcome. Because of the limited number of patients with PD, studies on the relationship between clinical manifestations of PD-IDC and survival are rare. In the present study, we found that the clinical symptoms at presentation may be associated with outcome.
Materials and methods
Patients
Eighty-five patients diagnosed with nipple–areolar PD with underlying BC between January 1, 2006, and December 31, 2012, at Zhejiang Cancer Hospital in People’s Republic of China, were recruited as part of the PD group. Patients diagnosed with BC without PD were recruited into the matched group. Patients were matched at a 1:1 ratio according to four variables: stage (0–III), age at diagnosis (within 5 years), histologic subtype, and the year of surgery. No patients received preoperative chemotherapy or radiotherapy.
Clinicopathological data
The clinicopathological parameters evaluated included age at diagnosis, tumor size (mm), TNM stage (according to the American Joint Committee on Cancer, 7th ed.), the number of metastatic LNs, and the expression of estrogen receptor (ER), progesterone receptor (PR), and HER2. Tumors that were either ER or PR positive were considered hormone receptor-positive. HER2 scores of 0 and 1+ were considered negative, and a score of 3+ was defined as HER2-positive. Fluorescence in situ hybridization was used to confirm HER2 status when the HER2 score was 2+.
Follow-up
Most patients were followed regularly after surgery at Zhejiang Cancer Hospital. Follow-up via telephone call was performed if clinical records from follow-up examinations could not be obtained. The follow-up data included the time of follow-up, the time of recurrence and metastasis, site of metastases, and the time and cause of death. The length of overall survival (OS) and disease-free survival (DFS) was calculated as the time between the date of diagnosis and the last date for which vital status was available. All patients who remained alive on March 1, 2017, were censored.
Ethical approval
The study was conducted after an expedited review by the Medical Ethics Committee of Zhejiang Cancer Hospital on May 30, 2018. In this retrospective study, there is no potential risk to the subjects, and the subjects’ personal privacy is protected, therefore patient consent was not required. The submission of this study was approved by the board of ethics committee, reference number IRB-2018-162.
Statistical analysis
The significance of any correlation between clinicopathological variables was assessed by Pearson’s chi-squared or Fisher’s exact test. Survival curves were constructed using the Kaplan–Meier technique. Univariate and multivariate Cox regression analyses were performed to identify prognostic factors. Statistical analyses were performed using SPSS software, version 21 (IBM Corporation, Armonk, NY, USA). P<0.05 was considered statistically significant.
Results
Clinicopathological characteristics of the PD group
In total, 85 BC patients were diagnosed with mammary PD during the study period and were recruited into the study. Of these 85 patients with PD of the breast, 74 had received a diagnosis of IDC and 11 had received a diagnosis of DCIS. The median age at diagnosis was 51 years (range, 35–79 years). According to the staging criteria of the 7th American Joint Committee on Cancer, 10 (11.76%) patients were stage 0, 23 (27.1%) patients were stage I, 30 (35.3%) patients were stage II, and 22 (25.9%) patients were stage III. BC patients with PD were much more likely to have hormone receptor-negative (64 [75.3%] vs 31 [36.5%], P<0.01) and HER2-positive tumors (60 [70.6%] vs 28 [32.9%], P<0.01) than the matched patients with BC alone.
Prognostic value of PD
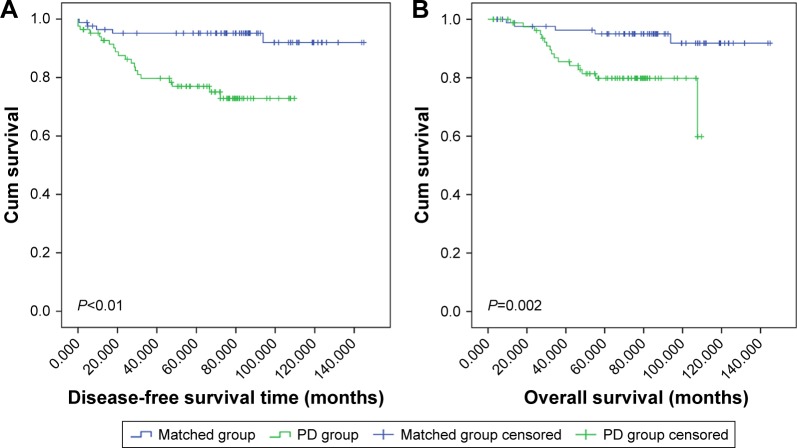
The median follow-up time was 66.5 months (range, 2.63– 109.70) in the PD group and 85.37 (range, 4.60–144.90) in the matched group. Univariate Cox regression analyses demonstrated that PD was related to poor outcomes in BC patients. Before adjustment for confounding variables, hazards ratio (HR) for DFS in PD patients was 4.96 (95% CI, 1.84–13.35; P=0.002), and the HR for OS of PD patients was 4.33 (95% CI, 1.55–12.11; P=0.005) (Tables 1 and 2). Kaplan–Meier analysis showed similar results (P<0.001 for DFS; P=0.002 for OS) (Figure 1). After adjustment for clinicopathological factors, PD was confirmed as an independent prognostic factor for DFS (adjusted HR, 2.96; 95% CI, 1.04–8.47; P=0.043).
Table 1.
Univariate and multivariate Cox regression analyses for DFS among patients diagnosed with BC with and without PD
| Variable | Crude HR | 95% CI | P-value | Adjusted HR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Age | ||||||
| <40 | 1 | |||||
| ≥40 | 2.25 | 0.30–16.64 | 0.427 | |||
| Diagnosis | ||||||
| DCIS | 1 | |||||
| IDC | 25.98 | 0.12–5,428.72 | 0.232 | |||
| TNM stage | ||||||
| 0 + I + II | 1 | 1 | ||||
| III | 4.965 | 2.22–11.10 | <0.01 | 7.38 | 3.24–16.86 | <0.01 |
| Hormone receptor | ||||||
| Negative | 1 | 1 | ||||
| Positive | 0.14 | 0.04–0.47 | 0.001 | 0.22 | 0.06–0.76 | 0.018 |
| HER2 | ||||||
| Negative | 1 | 1 | ||||
| Positive | 3.44 | 1.37–8.64 | 0.009 | 2.72 | 1.04–7.06 | 0.040 |
| PD | ||||||
| Without | 1 | 1 | ||||
| With | 4.96 | 1.84–13.35 | 0.002 | 2.96 | 1.04–8.47 | 0.043 |
Note: Bold-faced values are statistically significant (P<0.05).
Abbreviations: BC, breast cancer; DCIS, ductal carcinoma in situ; DFS, disease-free survival; HR, hazards ratio; IDC, invasive ductal carcinoma; PD, Paget’s disease.
Table 2.
Univariate and multivariate Cox regression analyses for OS time among patients diagnosed with BC with and without PD
| Variables | Crude HR | 95% CI | P-value | Adjusted HR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Age | ||||||
| <40 | 1 | |||||
| ≥40 | 23.15 | 0.03–19,086 | 0.360 | |||
| Diagnosis | ||||||
| DCIS | 1 | |||||
| IDC | 25.69 | 0.05–12,905.8 | 0.653 | |||
| Cancer stage | ||||||
| 0 + I + II | 1 | 1 | ||||
| III | 4.89 | 2.02–11.87 | <0.01 | 6.95 | 2.81–17.21 | <0.001 |
| Hormone receptor | ||||||
| Negative | 1 | 1 | ||||
| Positive | 0.16 | 0.05–0.56 | 0.004 | 0.23 | 0.06–0.83 | 0.025 |
| HER2 | ||||||
| Negative | 1 | 1 | ||||
| Positive | 2.98 | 1.13–7.81 | 0.027 | 2.29 | 0.82–6.37 | 0.114 |
| Paget’s disease | ||||||
| Without | 1 | 1 | ||||
| With | 4.33 | 1.55–12.11 | 0.005 | 2.63 | 0.88–7.88 | 0.083 |
Note: Bold-faced values are statistically significant (P<0.05).
Abbreviations: BC, breast cancer; DCIS, ductal carcinoma in situ; HR, hazards ratio; IDC, invasive ductal carcinoma; OS, overall survival; PD, Paget’s disease.
Figure 1.
Kaplan–Meier estimates of DFS and OS in BC patients with and without PD.
Note: The DFS for BC patients with and without PD (A) and the OS for BC patients with and without PD (B).
Abbreviations: BC, breast cancer; DFS, disease-free survival; OS, overall survival; PD, Paget’s disease.
Clinical presentation of PD-IDC patients
This study enrolled 74 PD patients diagnosed with PD-IDC that were divided into three groups based on their clinical presentation at diagnosis. For 15 patients (20.2%), the cardinal symptom was nipple discharge or skin lesions in the NAC (PD-IDC3). For 37 patients (50.0%), the chief complaint was a mass in the breast or axilla (PD-IDC2), and the remaining 22 patients (29.7%) presented with both nipple discharge or skin lesions in the NAC and a mass in the breast or axilla (PD-IDC1).
Prognostic value of cardinal clinical presentation in PD-IDC patients
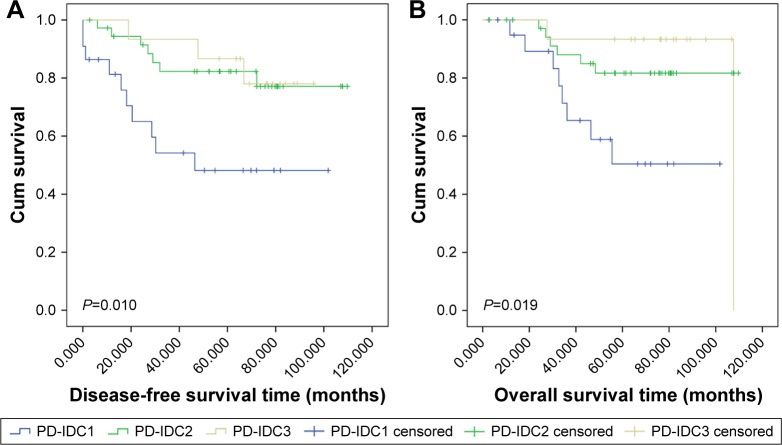
As shown in Figure 2, patients in the PD-IDC1 group had significantly worse OS (P=0.019) and DFS (P=0.010) than patients in the PD-IDC2 group or the PD-IDC3 group. Univariate Cox regression analyses gave the same results (crude HR, 0.28; 95% CI, 0.11–0.75; P=0.012 for DFS in PD-IDC2 patients; crude HR, 0.29; 95% CI, 0.10–0.86; P=0.026 for OS in PD-IDC2 patients; crude HR, 0.26; 95% CI, 0.07–0.95; P=0.041 for DFS in PD-IDC3 patients; and crude HR, 0.21; 95% CI, 0.04–0.98; P=0.047 for OS in PD-IDC3 patients) (Table 3). Data from the 74 PD-IDC patients were used in a multivariate analysis to test the predictive function of clinical presentation on OS and DFS, independent of other risk factors (age, sex, TNM stage, and hormone receptor and HER2 status). Based on this analysis, patients in the PD-IDC1 group would be predicted to have worse DFS than those in the PD-IDC2 or PD-IDC3 group (adjusted HR, 0.24; 95% CI, 0.08–0.73; P=0.012 for DFS in PD-IDC2 patients; adjusted HR, 0.30; 95% CI, 0.06–1.40; P=0.124 for DFS in PD-IDC3 patients).
Figure 2.
Kaplan–Meier estimates of DFS and OS in the PD-IDC1, PD-IDC2, and PD-IDC3 group.
Notes: The DFS for PD-IDC1, PD-IDC2, and PD-IDC3 group (A) and the OS for PD-IDC1, PD-IDC2, and PD-IDC3 group (B) PD-IDC: infiltrating ductal carcinoma with PD. PD-IDC1: patients presenting with skin changes in the nipple–areola area or nipple discharge and a mass in the nipple or axilla (22 cases). PD-IDC2: patients presenting with a mass in the nipple or axilla (37 cases). PD-IDC3: patients presenting with skin changes in the nipple–areola area or nipple discharge (15 cases).
Abbreviations: DFS, disease-free survival; OS, overall survival; PD, Paget’s disease; PD-IDC, invasive ductal carcinoma with PD.
Table 3.
Univariate and multivariate Cox regression analyses for OS and DFS among PD-IDC patients
| Clinical presentation | Crude HR | 95% CI | P-value | Adjusted HR | 95% CI | P-value | |
|---|---|---|---|---|---|---|---|
| DFS | PD-IDC1 | 1 | 0.018 | 1 | 0.026 | ||
| PD-IDC2 | 0.28 | 0.11–0.75 | 0.012 | 0.24 | 0.08–0.73 | 0.012 | |
| PD-IDC3 | 0.26 | 0.07–0.95 | 0.041 | 0.30 | 0.06–1.40 | 0.124 | |
| OS | PD-IDC1 | 1 | 0.034 | 1 | 0.148 | ||
| PD-IDC2 | 0.29 | 0.10–0.86 | 0.026 | 0.34 | 0.10–1.11 | 0.074 | |
| PD-IDC3 | 0.21 | 0.04–0.98 | 0.047 | 0.34 | 0.07–1.83 | 0.211 |
Notes: Bold-faced values are statistically significant (P<0.05); PD-IDC1: patients presenting with skin changes in the nipple–areola area or nipple discharge and a mass in the nipple or axilla (22 cases). PD-IDC2: patients presenting with a mass in the nipple or axilla (37 cases). PD-IDC3: patients presenting with skin changes in the nipple–areola area or nipple discharge (15 cases).
Abbreviations: DFS, disease-free survival; OS, overall survival; PD, Paget’s disease; PD-IDC, invasive ductal carcinoma with PD.
Discussion
Mammary PD is a rare pathological subtype of BC that occurs along with eczematoid lesions of the nipple–areola area. There are differences in clinical presentation, method of diagnosis, and treatment between BC patients with PD and BC patients with other pathologic subtypes. Postoperative adjuvant treatment for BC patients with PD was selected according to the current National Comprehensive Cancer Network guidelines and the experience of the treating physicians. Based on version 2.2016 of the National Comprehensive Cancer Network guidelines, there are no category 1 data that specifically address the local management of PD. Systemic treatment is based on the stage and biological characteristics of any underlying cancer. However, PD is not specifically considered. Estimating the prognosis of mammary PD accurately and objectively was the focus of this study.
PD of the breast is a relatively rare clinical condition, and so research regarding its role in patients’ outcomes is scant, particularly within populations of Chinese women. Retrospective studies, albeit with small cohorts, do exist in the literature to guide therapeutic decisions. Several previous series demonstrated that PD negatively influences BC survival. For example, in a study by Ordz-Pagan, the PD group had an overall 5-year survival of 81.2%, vs 93.8% in the non-PD group (Kaplan–Meier log-rank test, P=0.03). However, there was no difference in DFS (Kaplan–Meier log-rank test, P=0.30). In addition, the adjusted HR for the overall 5-year survival (2.26) suggested that the presence of PD may independently confer a worse survival.13 Kanitakis12 reported that patients with PD and IDC had worse outcomes than patients with IDC of a similar stage and similar clinical characteristics but without PD. However, that study did not consider DCIS. Wong et al’s4 study demonstrated that patients with PD and IDC are more likely to present with axillary LN metastases than women with IDC alone. We performed a matched case–control study between 85 BC patients with PD and a matched group of 85 BC patients without PD. The OS and DFS of the PD group were lower than those of the matched group (Kaplan–Meier analysis, P<0.001 for DFS and P=0.002 for OS).
In previous studies, PD patients with underlying invasive cancer had larger tumors, lower ER and PR expression, and higher HER2 expression than BC patients without PD.16–19 In this study, age, sex, tumor size, and the number of involved axillary LNs were matched between BC patients with and without PD. In this study as well, patients with PD had lower ER and PR expression and higher HER2 expression (Table 4). In clinical studies, overexpression of HER2, absence of hormone receptor expression, grade 3 lesions, a young age, and the number of axillary LN metastases are adverse prognostic factors.20 We were therefore interested in determining whether the poor outcomes of patients in the PD group were due to overexpression of HER2, the absence of hormone receptors, and other unfavorable prognostic factors. As shown in Tables 1 and 2, multivariate Cox regression analyses identified PD as an independent prognostic factor for DFS after adjustment for age, histological subtype, TNM stage, hormone receptor status, and HER2 expression. In 2013, Durkan et al21 reported that patients with PD and DCIS had a worse outcome than those with PD alone. For patients with isolated PD, survival at both 5 and 10 years was 100%. For the 22 patients with PD and DCIS, survival at 5 and 10 years was 94.7%±5.1% and 75.85%±17.4%, respectively.21 However, the present study indicated no significant difference in survival between DCIS patients with or without PD. This may be due to the shorter follow-up period or the small sample size.
Table 4.
Patient clinicopathological characteristics
| Variable | PD group | Matched group | χ2 | P-value |
|---|---|---|---|---|
| Age | ||||
| ≥40 | 80 (94.1%) | 77 (90.6%) | 0.75 | 0.387 |
| <40 | 5 (5.9%) | 8 (9.4%) | ||
| Histology | ||||
| IDC | 74 | 74 | χ2=0 | 1 |
| DCIS | 11 | 11 | ||
| TNM stage | ||||
| 0 + I + II | 63 | 63 | χ2=0 | 1 |
| III | 22 | 22 | ||
| Tumor size | ||||
| ≤2 cm | 35 | 37 | χ2=0.096 | 0.76 |
| >2 cm | 50 | 48 | ||
| ALN | ||||
| Positive | 38 | 40 | χ2=1.151 | 0.283 |
| Negative | 47 | 45 | ||
| Hormone receptor | ||||
| Positive | 21 (24.7%) | 54 (63.5%) | χ2=25.98 | 0.001 |
| Negative | 64 (75.3%) | 31 (36.5%) | ||
| HER2 | ||||
| Positive | 60 (70.6%) | 28 (32.9%) | χ2=24.12 | 0.001 |
| Negative | 25 (29.4%) | 57 (67.1%) |
Note: Bold-faced values are statistically significant (P<0.05).
Abbreviations: ALN, axillary lymph node; DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma; PD, Paget’s disease; PD-IDC, invasive ductal carcinoma with PD.
The typical clinical presentation of PD of the breast is skin alterations in the nipple–areolar area. PD patients with BC may present with lesions in the nipple or areola, a palpable mass in the breast or axilla, or no clinical manifestations. Skin changes in the nipple–areolar region may include itching, erythema, scaly or flaky skin, bloody nipple discharge, nipple erosion or ulceration, and nipple retraction.15 It is vital to understand the cardinal symptoms of PD because they can be the only signs of BC. Physical examinations should be performed in the clinic to detect palpable masses or thickening of the parenchyma.6 Tissue biopsies should be performed in all patients presenting with skin lesions of the nipple.3 However, mammography and physical exam may significantly underestimate the presence and extent of the underlying disease. Morrogh et al22 concluded that MRI is a more sensitive method to detect occult breast malignancies.
In the present study, 74 PD patients were diagnosed with IDC. Of these, 37 patients had a palpable breast mass, 15 patients presented with skin alterations in the nipple– areolar area, and 22 patients presented with skin alterations and a mass in the breast. Zakaria et al’s1 study found that a palpable mass or suspicious mammogram presages a high probability of infiltrating cancer in patients with PD, and these factors were significantly associated with a worse outcome. Furthermore, our research indicates that the presenting symptom of PD-IDC patients correlates with tumor progression and prognosis. The PD-IDC patients with NAC lesions and a mass in the breast or axilla had lower OS and DFS (Kaplan–Meier log-rank, P=0.019 for OS and P=0.01 for DFS). In this study, the extensive nature of most patients’ symptoms at the time of diagnosis suggested that they had been experiencing these clinical symptoms for a long time before seeking treatment. Not seeking timely medical attention may lead to the appearance of additional clinical symptoms, and the appearance of additional symptoms predicts worse survival. Therefore, early detection and consultation is very important.
To our knowledge, although this is not the first case– control study to report PD as an independent prognostic indicator in BC, this study included the largest number of patients. In addition, this is the first study to evaluate the association between clinical manifestations of PD-IDC and outcome. Clinical manifestation was identified as an independent prognostic indicator of OS and DFS in PD-IDC. This study may provide a stronger understanding of BC in PD. However, larger-sample, prospective clinical studies are needed to support these findings.
Conclusion
The outcomes of the PD group were worse than those of the matched group. PD is an independent prognostic indicator of outcome in BC patients with PD. Furthermore, the primary symptoms at presentation may be an available indicator of prognosis in PD-IDC.
Acknowledgments
This work was funded by National Natural Science Foundation of China (Grant numbers 81672597), Ministry of Health and Department of Health in Zhejiang (Grant numbers 2015PYA001), and Zhejiang TCM research grant (Grant numbers 2014ZB069).
Footnotes
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zakaria S, Pantvaidya G, Ghosh K, Degnim AC. Paget’s disease of the breast: accuracy of preoperative assessment. Breast Cancer Res Treat. 2007;102(2):137–142. doi: 10.1007/s10549-006-9329-2. [DOI] [PubMed] [Google Scholar]
- 2.Dalberg K, Hellborg H, Wärnberg F. Paget’s disease of the nipple in a population based cohort. Breast Cancer Res Treat. 2008;111(2):313–319. doi: 10.1007/s10549-007-9783-5. [DOI] [PubMed] [Google Scholar]
- 3.Trebska-Mcgowan K, Terracina KP, Takabe K. Update on the surgical management of Paget’s disease. Gland Surg. 2013;2(3) doi: 10.3978/j.issn.2227-684X.2013.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong SM, Freedman RA, Sagara Y, et al. The effect of Paget disease on axillary lymph node metastases and survival in invasive ductal carcinoma. Cancer. 2015;121(24):4333–4340. doi: 10.1002/cncr.29687. [DOI] [PubMed] [Google Scholar]
- 5.Helme S, Harvey K, Agrawal A. Breast-conserving surgery in patients with Paget’s disease. Br J Surg. 2015;102(10):1167–1174. doi: 10.1002/bjs.9863. [DOI] [PubMed] [Google Scholar]
- 6.Lim HS, Jeong SJ, Lee JS, et al. Paget disease of the breast: mammographic, US, and MR imaging findings with pathologic correlation. Radiographics. 2011;31(7):1973–1987. doi: 10.1148/rg.317115070. [DOI] [PubMed] [Google Scholar]
- 7.Lopes Filho LL, Lopes IM, Lopes LR, Enokihara MM, Michalany AO, Matsunaga N. Mammary and extramammary Paget’s disease. Anais brasileiros de dermatologia. Mar. 2015;90(2):225–231. doi: 10.1590/abd1806-4841.20153189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandoval-Leon AC, Drews-Elger K, Gomez-Fernandez CR, Yepes MM, Lippman ME. Paget’s disease of the nipple. Breast Cancer Res Treat. 2013;141(1):1–12. doi: 10.1007/s10549-013-2661-4. [DOI] [PubMed] [Google Scholar]
- 9.Yim JH, Wick MR, Philpott GW, Norton JA, Doherty GM. Underlying pathology in mammary Paget’s disease. Ann Surg Oncol. 1997;4(4):287–292. doi: 10.1007/BF02303576. [DOI] [PubMed] [Google Scholar]
- 10.Paone JF, Baker RR. Pathogenesis and treatment of Paget’s disease of the breast. Cancer. 1981;48(3):825–829. doi: 10.1002/1097-0142(19810801)48:3<825::aid-cncr2820480326>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Adams SJ, Kanthan R. Paget’s disease of the male breast in the 21st century: A systematic review. Breast. 2016;29:14–23. doi: 10.1016/j.breast.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Kanitakis J. Mammary and extramammary Paget’s disease. J Eur Acad Dermatol Venereol. 2007;21(5):581–590. doi: 10.1111/j.1468-3083.2007.02154.x. [DOI] [PubMed] [Google Scholar]
- 13.Muttarak M, Siriya B, Kongmebhol P, Chaiwun B, Sukhamwang N. Paget’s disease of the breast: clinical, imaging and pathologic findings: a review of 16 patients. Biomed Imaging Interv J. 2011;7(2):e16. doi: 10.2349/biij.7.2.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng S, Song QK, Zhao L, et al. Characteristics of mammary Paget’s disease in China: a national-wide multicenter retrospective study during 1999–2008. Asian Pac J Cancer Prev. 2012;13(5):1887–1893. doi: 10.7314/apjcp.2012.13.5.1887. [DOI] [PubMed] [Google Scholar]
- 15.Ling H, Hu X, Xu XL, Liu ZB, Shao ZM. Patients with nipple-areola Paget’s disease and underlying invasive breast carcinoma have very poor survival: a matched cohort study. PLoS One. 2013;8(4):e61455. doi: 10.1371/journal.pone.0061455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ortiz-Pagan S, Cunto-Amesty G, Narayan S, et al. Effect of Paget’s disease on survival in breast cancer: an exploratory study. Arch Surg. 2011;146(11):1267–1270. doi: 10.1001/archsurg.2011.278. [DOI] [PubMed] [Google Scholar]
- 17.Kothari AS, Beechey-Newman N, Hamed H, et al. Paget disease of the nipple: a multifocal manifestation of higher-risk disease. Cancer. 2002;95(1):1–7. doi: 10.1002/cncr.10638. [DOI] [PubMed] [Google Scholar]
- 18.Wolber RA, Dupuis BA, Wick MR. Expression of c-erbB-2 oncoprotein in mammary and extramammary Paget’s disease. Am J Clin Pathol. 1991;96(2):243–247. doi: 10.1093/ajcp/96.2.243. [DOI] [PubMed] [Google Scholar]
- 19.Fu W, Lobocki CA, Silberberg BK, Chelladurai M, Young SC. Molecular markers in Paget disease of the breast. J Surg Oncol. 2001;77(3):171–178. doi: 10.1002/jso.1090. [DOI] [PubMed] [Google Scholar]
- 20.Taucher S, Rudas M, Mader RM, et al. Do we need HER-2/neu testing for all patients with primary breast carcinoma? Cancer. 2003;98(12):2547–2553. doi: 10.1002/cncr.11828. [DOI] [PubMed] [Google Scholar]
- 21.Durkan B, Bresee C, Bose S, Phillips EH, Dang CM. Paget’s disease of the nipple with parenchymal ductal carcinoma in situ is associated with worse prognosis than Paget’s disease alone. Am Surg. 2013;79(10):1009–1012. [PubMed] [Google Scholar]
- 22.Morrogh M, Morris EA, Liberman L, van Zee K, Cody HS, King TA. MRI identifies otherwise occult disease in select patients with Paget disease of the nipple. J Am Coll Surg. 2008;206(2):316–321. doi: 10.1016/j.jamcollsurg.2007.07.046. [DOI] [PubMed] [Google Scholar]