Abstract
Objective
To assess the association of early morning serum cortisol with cognitive performance and brain structural integrity in community-dwelling young and middle-aged adults without dementia.
Methods
We evaluated dementia-free Framingham Heart Study (generation 3) participants (mean age 48.5 years, 46.8% men) who underwent cognitive testing for memory, abstract reasoning, visual perception, attention, and executive function (n = 2,231) and brain MRI (n = 2018) to assess total white matter, lobar gray matter, and white matter hyperintensity volumes and fractional anisotropy (FA) measures. We used linear and logistic regression to assess the relations of cortisol (categorized in tertiles, with the middle tertile as referent) to measures of cognition, MRI volumes, presence of covert brain infarcts and cerebral microbleeds, and voxel-based microstructural white matter integrity and gray matter density, adjusting for age, sex, APOE, and vascular risk factors.
Results
Higher cortisol (highest tertile vs middle tertile) was associated with worse memory and visual perception, as well as lower total cerebral brain and occipital and frontal lobar gray matter volumes. Higher cortisol was associated with multiple areas of microstructural changes (decreased regional FA), especially in the splenium of corpus callosum and the posterior corona radiata. The association of cortisol with total cerebral brain volume varied by sex (p for interaction = 0.048); higher cortisol was inversely associated with cerebral brain volume in women (p = 0.001) but not in men (p = 0.717). There was no effect modification by the APOE4 genotype of the relations of cortisol and cognition or imaging traits.
Conclusion
Higher serum cortisol was associated with lower brain volumes and impaired memory in asymptomatic younger to middle-aged adults, with the association being evident particularly in women.
Long-term elevation of cortisol levels negatively influences cardiometabolic changes.1 The effects of cortisol likely extend beyond cardiometabolic outcomes. Sustained cortisol elevation was shown to have deleterious effects on brain structure and function in animals.2 Similar alterations in brain structure and cognition were described in the Cushing syndrome.3–6 Adrenal insufficiency has also been linked to changes in the amygdala and hippocampus on MRI.7
Whether subtle alterations of the hypothalamic-pituitary-adrenal (HPA) axis among people without overt hormonal excess or deficiency relate to brain-related outcomes in the general population is unclear. A few studies have described an association between higher blood cortisol and decreased brain volumes,8–11 with some studies suggesting that changes in brain volumes correlate with cognitive decline associated with cortisol levels.10,12 Population-based studies have also seldom examined the association of circulating cortisol and early asymptomatic ischemic cerebrovascular disease.12,13 Extant studies of cortisol and brain-related outcomes have limitations. These studies are mainly clinical studies conducted in small and convenience samples. These have focused on older people, have not always excluded confounding conditions (e.g., dementia), and have seldom examined multiple brain regions focusing on the hippocampal and limbic areas, possibly because these are involved in regulating the HPA axis.8,9 It is important to examine other brain regions because corticosteroid receptors are expressed throughout the brain.14 Animal and small human studies suggest that the medial prefrontal cortex is affected by high cortisol levels.15–17
Using data from the Framingham Heart Study, a large community-based study, we assessed the cross-sectional associations of morning serum cortisol with cognitive performance and MRI-based brain structural measures. We hypothesized that higher blood cortisol is associated with worse cognitive functioning, reduced brain volumes, microvascular damage, and alterations in brain microstructure.
Methods
Study sample
The Framingham Heart Study is a single-site, longitudinal community-based cohort study that was initiated in 1948. Since the inception of the study, 3 generations of participants have been enrolled. The current study includes participants enrolled in the Third Generation Cohort of the Framingham Heart Study.18 The first examination of the generation 3 cohort conducted between 2002 and 2005 was attended by 4,095 participants (53.3% women, mean age 40 ± 9 years, age range 19–72 years) who had at least 1 parent recruited to the Framingham Offspring Cohort.19 Information on serum cortisol and covariates was ascertained from this baseline examination. The selection process to retain study participants for the present investigation is shown in figure e-1 (available from Dryad, doi.org/10.5061/dryad.gj21173). As part of an ancillary study, these participants were invited to undergo volumetric brain MRI and neuropsychological assessment. Of the participants with measured early morning serum cortisol (n = 4,036), 2,297 underwent a neuropsychological assessment. Of these, we excluded participants with a history of clinical dementia, stroke, or other neurologic conditions (brain tumor, major head trauma, multiple sclerosis) that could affect the cognitive or MRI assessments (n = 52). Other criteria of exclusion included use of exogenous glucocorticoids (n = 14). After exclusions, 2,231 participants were eligible for our investigation of cognitive measures, and 2,018 of those were eligible for investigation of MRI measures. Comparisons between the participants included in the cognitive performance (neuropsychological) and brain MRI analyses and those not included (table e-1, Dryad) showed that the included participants were more educated, less likely to be smokers or to have elevated blood glucose levels, and less likely to use diabetes medications.
Standard protocol approvals, registrations, and patient consents
The study protocol was approved by the institutional review board at the Boston University Medical Center, and all participants gave written informed consent.
Assessment of cortisol
Fasting blood samples were drawn by venipuncture after ≈10 minutes of rest in a supine position in the morning, typically between 7:30 and 9 am. Participants were instructed to take all routine medications. Blood samples were centrifuged, and the serum/plasma fraction was stored at −70°C to −80°C until it was thawed for analysis. Serum cortisol concentration (micrograms per deciliter) was measured with a chemiluminescent immunoassay (Roche Diagnostics, Indianapolis, IN), with intra-assay coefficients of variation ranging from 3.3% to 10% for high concentrations and low concentrations. The limits of detection of the test were 0.036 to 63.4 µg/dL.
Cognitive evaluation
A comprehensive and standardized cognitive test battery administered by trained examiners was used to assess cognitive function. Initial screening evaluation was conducted between 2008 and 2011, with the Consortium to Establish a Registry for Alzheimer's Disease Word List (total, recall, and retention scores)20 and the Victoria Stroop test (interference score). Additional neuropsychological evaluation was performed just before or simultaneously with the MRI and included the Delayed Recall component of the Visual Reproductions Test, Similarities Test from the Wechsler Adult Intelligence Scale, Hooper Visual Organization Test (HVOT), Trails A (TrA), Trails B (TrB), Wechsler Memory Scale Logical Memory Test Immediate and Delayed Recall, and Delayed Recall of the Paired Associates Learning Test. These tests evaluate cognitive performance in verbal and visual memory, abstract reasoning, visual perception, attention, and executive function. We also assessed a global cognitive score. This variable was created previously with a principal component analysis and forcing a single score solution.21 The score combines weighted loadings for the Delayed Recall Component of the Visual Reproductions Test, Similarities Test, HVOT, Delayed Recall of the Paired Associates Learning Test, TrB, and Logical Memory. Higher scores across all cognitive endpoints indicate superior performance, except for Trail Making, for which higher scores indicate slower task completion.
Brain morphology assessment
The brain MRI was acquired with a 1.5T Siemens Avanto scanner (Malvern, PA) using 3 sequences: 3-dimensional T1-weighted coronal spoiled gradient recalled echo acquisition, fluid-attenuated inversion recovery (FLAIR) sequence, and diffusion tensor imaging (DTI). Segmentation and quantification of total intracranial volume, total cerebral brain volume, hippocampal volume, white matter hyperintensities (WMH), and gray matter were performed by automated procedures previously described.22 Total intracranial volume was determined by outlining intracranial vault lying above the tentorium.23 To distinguish CSF from brain matter, we used a semiautomated analysis of MRI pixel distributions for CSF, gray matter, and white matter.24 HPV was computed by a semiautomatic multiatlas hippocampal segmentation algorithm.25 We used a semiautomated procedure for the segmentation and quantification of WMH on FLAIR.26 The total cerebral brain and WMH volumes were corrected for differences in head size with the FLAIR-derived total cranial volume. Segmented gray matter maps were coregistered to a minimal deformation template27,28 for group statistical analyses. We used DTI measures of fractional anisotropy (FA), which is a sensitive indicator of white matter integrity.29 DTI data were preprocessed with the FMRIB Software Library Diffusion Toolbox (University of Oxford, UK)30,31 and included brain tissue extraction and correction for head motion and eddy currents. FA images were computed from DTI with FMRIB Software Library software tools31 and coregistered to a standard DTI template (FMRIB58_FA_1 mm.nii) using linear and nonlinear transformations. Each voxel corresponds to a similar brain location across all individuals. This enables the evaluation of the associations of both FA and gray matter density with various markers. Covert brain infarcts were defined as an area of abnormal signal intensity in a vascular distribution based on a size of ≥3 mm, as well as location and imaging characteristics of the lesion.32
Covariates
The covariates including sociodemographic and clinical characteristics were assessed by standardized questionnaires, physical examination, and laboratory tests.18 Educational achievement was defined as a binary variable (some college experience vs none). Current smokers were defined as those who reported having smoked ≥1 cigarette per day regularly during the year preceding the examination. Use of hormone replacement therapy (HRT) or oral contraceptive pills (OCPs) was assessed at the physician interview. Depressive symptoms were assessed with the 20-item Center for Epidemiologic Studies Depression Scale (CES-D).33 A cut point of ≥16 on the CES-D or use of antidepressants was used to define depression. Waist circumference, height, and weight were measured, and body mass index was calculated (kilograms per meter squared). Blood pressure (BP) was measured twice in the left arm of the seated participant with a mercury column sphygmomanometer. The average of the 2 readings was used as the examination BP, and hypertension was defined as a systolic BP ≥140 mm Hg, a diastolic BP ≥90 mm Hg, or self‐reported antihypertensive medication use. Diabetes mellitus was defined by a fasting glucose level ≥126 mg/dL or self‐reported use of glucose-lowering medications. Plasma total cholesterol, high‐density lipoprotein cholesterol, triglycerides, and fasting plasma glucose concentrations were measured with standard enzymatic methods, as previously described.18
Statistical analyses
The baseline characteristics of participants with cognitive performance measures were presented for the overall sample and by tertiles of cortisol. Cognitive scores were standardized to facilitate comparisons between performances on different cognitive tests and between brain structural outcomes.
We used multivariable linear (for continuous measures) or logistic (for binary variables) regression models to relate serum cortisol to each measure of cognitive performance and brain structure. We categorized serum cortisol into tertiles because the middle tertile of the distribution of cortisol in our sample roughly corresponded to the commonly accepted range of normality in clinical practice. Using the categorization by tertiles, we then assessed the relations between serum cortisol and cognitive or brain structural outcomes, with the middle cortisol tertile as the reference category. In the first model (model 1), we adjusted for age, sex, and use of OCPs or HRT, with the addition of age squared for the MRI outcomes and education for the cognitive outcomes. In a subsequent model (model 2), we additionally adjusted for cardiovascular risk factors, including smoking status, systolic BP, use of antihypertensives, impaired fasting plasma glucose (>100 mg/dL), and body mass index. We tested for interactions of serum cortisol with sex and APOE4 genotype (known to be associated with the risk of dementia) by including multiplicative interaction terms in the models, and we stratified analyses presented when necessary on the basis of the significance of the interaction term. We used logistic regression for several outcomes: presence of any covert brain infarct (≥1 covert brain infarcts vs none) and cerebral microbleeds, with adjustments performed similarly to those done for previous models. We also used linear regressions to investigate associations of serum cortisol with white matter and gray matter integrity at the voxel-based level. Models included serum cortisol, transformed into a categorical variable using tertile groups, as the independent variable and FA and gray matter density as dependent variables. All models were adjusted for age, current smoking, hyperglycemia, systolic BP, antihypertensive treatment, body mass index, hormone (OCP/HRT) use, and time interval between serum cortisol assay and MRI assessment. In secondary analyses, we repeated the regression analyses after excluding participants with depression (CES-D score ≥16 or on antidepressants).
The significance of obtained T maps on brain MRI was assessed using threshold-free cluster enhancement at the <0.05 significance level and correcting for multiple comparison with permutation-based correction (n = 1,000).34 To provide a description of white matter tracts or gray matter regions to which the significant voxels likely belonged, we overlaid the corrected T maps with the Johns Hopkins University probabilistic fiber map and the Brodmann area atlases, warped to the minimum deformation template space.35
All analyses were performed with SAS version 9.4 (SAS Institute Inc, Cary, NC) and R version 2.10.0 (R Development Core Team, 2009, Vienna, Austria). Results were considered statistically significant if p < 0.10 for tests of interactions and p < 0.05 for all other tests.
Data availability
Anonymized data will be shared by request from any qualified investigator for purposes of replicating procedures and results.
Results
Baseline characteristics
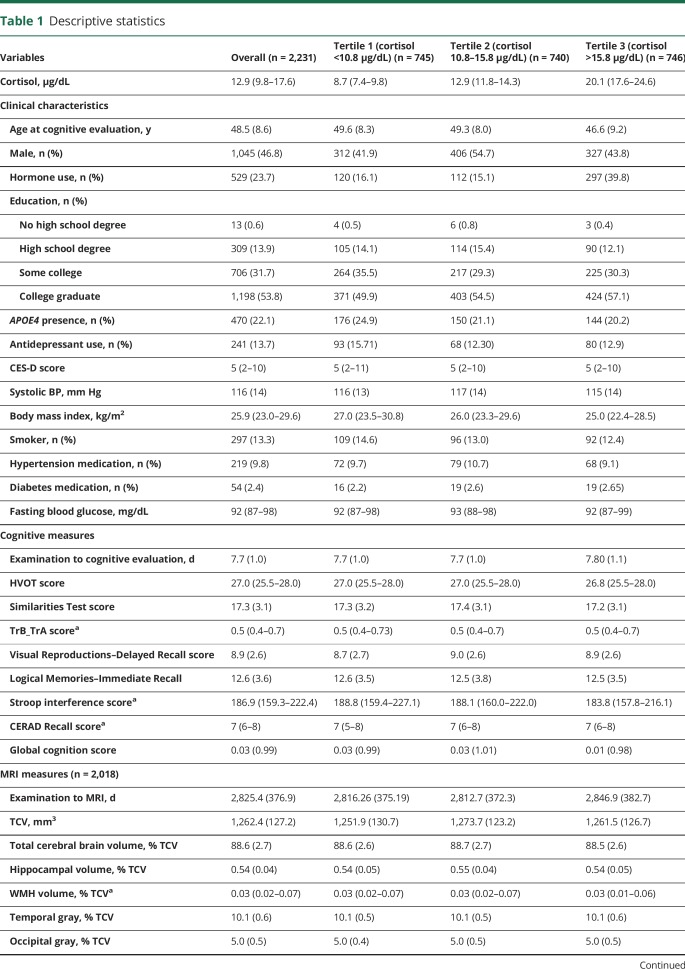
The selection process of study participants is presented in figure e-1 (available from Dryad, doi.org/10.5061/dryad.gj21173). The study sample consisted of 2,231 participants; 46.8% were men, and their mean age was 48.52 years (SD 8.64 years). The median (25th–75th percentile) serum cortisol level was 12.92 (9.82–17.58) µg/dL. The characteristics of the overall study sample and by tertiles of cortisol are shown in table 1. The mean time durations between the baseline examination (examination 1) and cognitive and brain MRI assessments were 0.02 and 7.74 years, respectively.
Table 1.
Descriptive statistics
Associations between serum cortisol and MRI measures
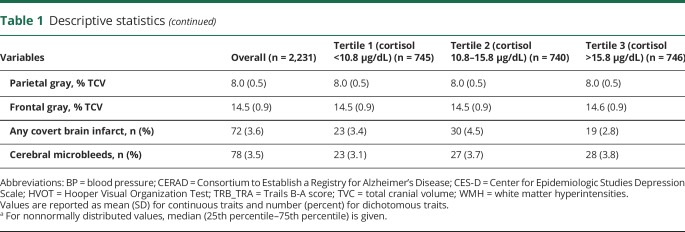
The relation of serum cortisol and brain volumes is shown in table 2 and tables e-2 and e-5 through e-7 (available from Dryad, doi.org/10.5061/dryad.gj21173). After adjustment for potential confounders, compared to the middle tertile, the highest tertile of cortisol was associated with lower total cerebral brain (β [standard error (SE)] = −0.38 [0.14], p = 0.008), parietal gray matter volume (β [SE] = −0.06 [0.03], p = 0.046), and frontal gray matter volumes matter volume (β [SE] = −0.12 [0.04], p = 0.006) (table 2). The lowest tertile of cortisol was not associated with any brain volume measures.
Table 2.
Association of serum cortisol with cognitive and brain outcomes in the Framingham Heart Study
There was no statistically interaction between cortisol and APOE4 with regard to brain structural measures. However, there was an effect modification of sex on the association between cortisol and total cerebral brain volume (p = 0.048). Analyses stratified by sex showed that, compared to the middle tertile, the highest tertile of cortisol was associated with lower total cerebral brain volume among women (β [SE]: −0.73 [0.21], p = 0.001) but not among men (β [SE]: −0.07 [0.19], p = 0.717). The lowest tertile of cortisol was not associated with total cerebral brain volume in either sex.
Voxel-based associations of serum cortisol with white matter and gray matter integrity
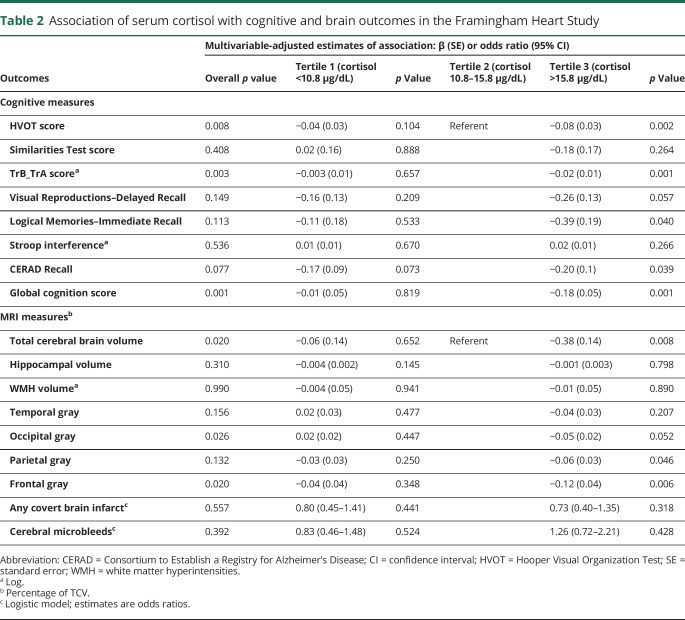
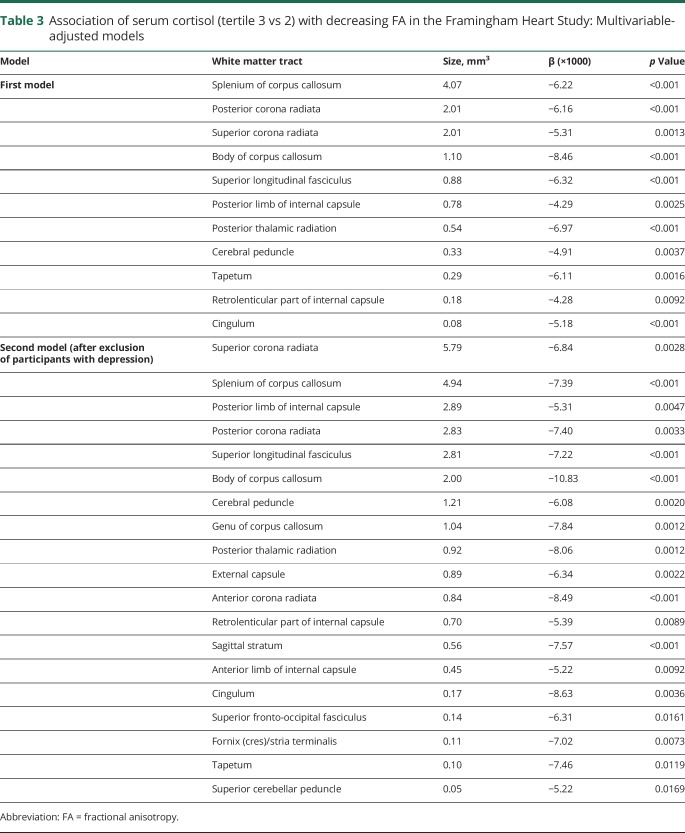
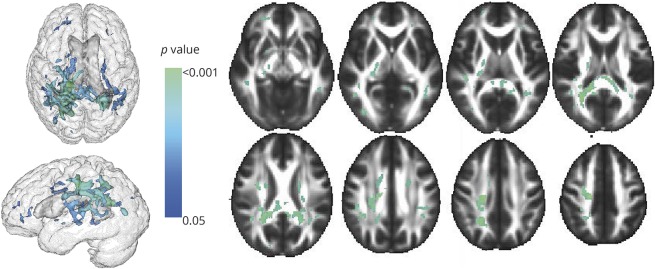
Compared to the middle cortisol tertile, the highest cortisol tertile was independently associated with lower FA within tracts covering 13.3 mm3 of the white matter (table 3). The white matter tracts most strongly associated with serum cortisol (figure 1) included the splenium and body of corpus callosum region (4.07 and 1.08 mm3, respectively) and the superior and posterior part of the corona radiata (2.01 and 2.01 mm3, respectively).
Table 3.
Association of serum cortisol (tertile 3 vs 2) with decreasing FA in the Framingham Heart Study: Multivariable-adjusted models
Figure 1. Regions of the cerebral white matter in which cortisol (highest tertile vs middle tertile) is associated with decreased fractional anisotropy.
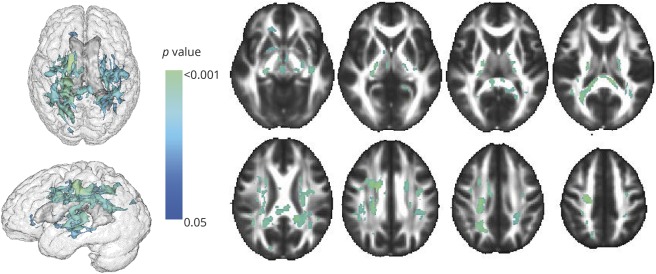
Among participants without depression (CES-D score <16 and no use of antidepressants), compared to the middle cortisol tertile, the highest cortisol tertile was associated with decreased FA within white matter tracts covering 28.4 mm3. The white matter tracts most associated with blood cortisol concentrations were the superior and posterior corona radiata (5.79 and 2.83 mm3), the splenium of the corpus callosum region (4.94 mm3), and the posterior limb of the internal capsule (2.89 mm3) (figure 2).
Figure 2. Regions of the cerebral white matter in which cortisol (highest tertile vs middle tertile) is associated with decreased fractional anisotropy, people with depression (Center for Epidemiologic Studies Depression Scale score <16 and no depression medications) excluded.
.
There were no associations between serum cortisol and gray matter density, overall and in subgroup analyses.
Associations between serum cortisol and cognition
The relation of cortisol and cognitive performance measures is displayed in table 3 and tables e-s and e-5 through e-7 (available from Dryad, doi.org/10.5061/dryad.gj21173). After multivariable adjustment for potential confounders, compared to the middle tertile, the highest tertile of cortisol was associated with poor global cognition (β [SE] = −0.18 [0.05], p = 0.001) and with poorer performance on HVOT (β [SE] = −0.08 [0.05], p = 0.002) and TrB_TrA (β [SE] = −0.02 [0.01], p = 0.001) (table 2). The lowest tertile of cortisol was not associated with any cognitive measure (table 2).
There was an interaction between the presence of APOE4 allele and cortisol on the TrA score (p = 0.062). When stratified by the presence or absence of the APOE4 allele, the highest tertile of cortisol was associated with poor performance on TrA compared to the middle tertile group among those with the APOE4 allele (p = 0.021) but not among those without the APOE4 allele (p = 0.430). The lower tertile of cortisol was not associated with TrA score in either APOE4 group. There was no effect modification of sex on the association between serum cortisol and cognitive outcomes.
The results of the association of cortisol with cognitive or brain structural outcomes did not change when persons with a diagnosis of major depressive disorder (CES-D score ≥16 or use of depression medications) were excluded from the analysis ( tables e-3 and e-4, available from Dryad, doi.org/10.5061/dryad.gj21173).
Discussion
Our findings suggest that middle-aged and young–to–middle-aged adults in their 40s with higher serum cortisol concentrations (highest tertile) perform worse on tasks of visual perception, executive function, and attention and have less gray matter (total and regional) compared to persons with moderate cortisol levels (middle tertile), with women being potentially more susceptible to the influence of glucocorticoids. Blood cortisol concentration was also associated with structural changes in white matter integrity. The association of cortisol and cognitive functioning was in the same direction as for the brain volumes. Our findings are consistent with the concept that increasing levels of circulating glucocorticoids are associated with worse cognitive functioning.
To date, most prior studies that have investigated the relation between the HPA axis and brain volumes have focused on the hippocampal volume as the imaging measure of importance. Some studies reported more hippocampal atrophy with higher serum cortisol concentrations,36–38 while others reported no association.10,39,40 Some of the observed differences may be related to a difference in medium of measurement of cortisol (salivary or urine vs blood) or the use of evening instead of morning cortisol. Indeed, some studies showed that serum cortisol levels (assessed in the evening) were associated with poorer performance across all cognitive domains and reduced volume of the hippocampus.41 Similar findings were described with evening salivary cortisol, while morning cortisol was more selectively associated with speed and executive functioning.11
Our study is a novel exploration of the associations of both high and low morning levels of serum cortisol levels with imaging measures of brain integrity and cognition in mid-adulthood. We expanded the findings from extant studies showing an association between serum cortisol and gray matter in regions other than the hippocampus. Although prior data suggest that hippocampal atrophy is evident in hypercortisolism, our study did not find a robust association between serum cortisol and hippocampal volume. It is possible that subtle changes such as subregional hippocampal atrophy or early neuronal or synaptic loss occur independently of total hippocampal volume changes and could not be detected with the current methods.
We observed that high levels of cortisol are associated with microstructural white matter injury in several tracts, particularly the corpus callosum, which has also been reported to be associated with cortisol in other studies.13 Our findings also extend observations from prior studies on the association of cortisol and white matter tracts,13 which are scarce and limited by their small size and, for some, restricted to clinical settings with the inclusion of individuals with extreme phenotypes of cortisol42,43 or with depression.44 White matter integrity is significantly associated with processing speed, which in turn is strongly associated with higher general cognitive ability.45 Thus, disruption of information transfer by white matter damage could partially explain the impairments to cognitive ability associated with higher cortisol concentrations observed in our study. We did not observe any association of blood cortisol levels and WMH. This may simply be related to the fact that WMH could be the final stage of progressive white matter degeneration,29 a process that may not be sufficiently advanced in middle-aged individuals.
Although several endocrine, metabolic, and vascular abnormalities have been inferred to underlie the structural changes in the brain related to cortisol, the causal pathway remains unknown. These brain-related effects are mediated through glucocorticoid receptors, which abound in the brain.14,46–48 In addition to the direct effects of corticosteroids, hypertension and hyperglycemia (frequently present in the Cushing syndrome) may also contribute to brain damage. Evidence suggests that corticosteroid receptors in the brain inhibit the HPA axis and that depletion of these receptors results in hypersecretion of glucocorticoids.49 With aging, brain volume reduction will lead to a reduction in the concentrations of corticosteroid receptors.50 Although the hippocampal glucocorticoid receptors are most vulnerable to this downregulation,49 glucocorticoid receptors are expressed not only in the hippocampus but also throughout the brain,14 and our data suggest that the associations of cortisol with brain structure are not specific to the hippocampus but are more diffuse throughout the gray matter. The stronger relationship with gray matter than with white matter may be consistent with our observations because gray matter volume loss starts at an earlier age and progresses more rapidly than white matter volume loss.51 Alternatively, HPA axis dysregulation may have come first. Indirect evidence for this originates from studies that found that cumulative physiologic wear and tear or allostatic load increases the risk of cognitive decline.52,53 It is also possible that HPA axis dysregulation resulted from repeated depressive episodes earlier in life,54 which, in turn, led to brain volume reduction and poorer cognitive performance. However, in secondary analyses, we excluded participants with depressive disorder, making this explanation less likely.
The strengths of this study include its community-based design, the large sample size, the exclusion of participants with dementia, and the adjustment for potential confounders, including accounting for the presence of the APOE4 allele. Second, the extensive measures of several brain regions, including the microstructure (FA and white matter intensity), allowed us to examine differential associations between HPA axis activity and the various brain volumes, as well as traits indicative of alterations along several white matter tracts. Third, the inclusion of measures of cognitive function allowed us to examine whether the associations with MRI measures were also recapitulated in the associations of blood cortisol with cognitive measures.
Our study has limitations. It was a cross-sectional investigation; we cannot comment on the temporal sequence between HPA axis dysregulation and changes in cognition or brain volumes. We used early morning cortisol assessed only once in each participant, which may not represent long-term exposure to cortisol and may exhibit a higher level of within-individual variation than other measures of exposure to cortisol such as urinary or salivary cortisol. The constraints of a large, longitudinal observational cohort precluded the collection of either salivary cortisol or 24-hour urine cortisol or of several dynamic measures of cortisol levels during the day. Indeed, the 24-hour urinary cortisol excretion is more representative of longer-term exposure to cortisol and thus would have provided with a better picture of the associations. While a single assessment of cortisol is a limitation, cortisol concentrations have been found to have a high degree of within-individual stability over a 2-year period in prior large epidemiologic studies of the stability (correlation coefficient 0.70).55
It is important to point out that our study reflects a community-based sample and is not directly comparable to the examination of participants in a research setting to diagnose hypercortisolism or hypocortisolism. Our community-based study does not characterize people with extreme phenotypes related to blood cortisol levels because we did not carry out further screening or diagnostic testing beyond the assessment of serum cortisol. Lastly, the Framingham Heart Study Third Generation Cohort includes relatively young participants of European ancestry and consequently is not representative of all age groups in the general population of the United States.
Glossary
- BP
blood pressure
- CES-D
Center for Epidemiologic Studies Depression Scale
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- FLAIR
fluid-attenuated inversion recovery
- HPA
hypothalamic-pituitary-adrenal
- HRT
hormone replacement therapy
- HVOT
Hooper Visual Organization Test
- OCP
oral contraceptive pill
- SE
standard error
- TrA
Trails A
- TrB
Trails B
- WMH
white matter hyperintensities
Author contributions
Drs. Echouffo-Tcheugui and Seshadri had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Echouffo-Tcheugui and Seshadri. Acquisition of data: Vasan, Beiser, DeCarli, and Seshadri. Analysis and interpretation of data: Conner, Maillard, Himali, Echouffo-Tcheugui, Beiser, and Seshadri; Drafting of the manuscript: Echouffo-Tcheugui. Critical revision of the manuscript for important intellectual content: Conner, Himali, Maillard, DeCarli, Beiser, Vasan, and Seshadri; Statistical analysis: Conner, Himali, and Maillard. Obtained funding: DeCarli, Vasan, and Seshadri. Study supervision: Seshadri.
Study funding
This work was supported by the Framingham Heart Study’s National Heart, Lung, and Blood Institute Study (contract N01-HC-25195) and HHSN268201500001I (R.S.V.) and by grants from the NIH, National Institute of Neurologic Disorders and Stroke (R01-NS017950 and UH2 NS100605 [S.S.]) and the National Institute on Aging (R01 AG054076, AG008122, AG033193, AG033040, U01 AG049505, and AG052409 [all S.S.]), as well as the following grants: T32 HL125232 (J.B.E-T.), R01HL093328 (R.S.V.), R01HL107385 (R.S.V.), and R01HL126136 (R.S.V.).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
Publication history
Received by Neurology March 6, 2018. Accepted in final form August 10, 2018.
References
- 1.Walker BR. Glucocorticoids and cardiovascular disease. Eur J Endocrinol 2007;157:545–559. [DOI] [PubMed] [Google Scholar]
- 2.Mcewen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann NY Acad Sci 2010;1186:190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patil CG, Lad SP, Katznelson L, Laws ER. Brain atrophy and cognitive deficits in Cushing's disease. Neurosurg Focus 2007;23:E11. [DOI] [PubMed] [Google Scholar]
- 4.Bourdeau I, Bard C, Noël B, et al. Loss of brain volume in endogenous Cushing's syndrome and its reversibility after correction of hypercortisolism. J Clin Endocrinol Metab 2002;87:1949–1954. [DOI] [PubMed] [Google Scholar]
- 5.Forget H, Lacroix A, Cohen H. Persistent cognitive impairment following surgical treatment of Cushing's syndrome. Psychoneuroendocrinology 2002;27:367–383. [DOI] [PubMed] [Google Scholar]
- 6.Forget H, Lacroix A, Somma M, Cohen H. Cognitive decline in patients with Cushing's syndrome. J Int Neuropsychol Soc 2000;6:20–29. [DOI] [PubMed] [Google Scholar]
- 7.Herbert J, Goodyer IM, Grossman AB, et al. Do corticosteroids damage the brain? J Neuroendocrinol 2006;18:393–411. [DOI] [PubMed] [Google Scholar]
- 8.Lee BK, Glass Ta, McAtee MJ, et al. Associations of salivary cortisol with cognitive function in the Baltimore Memory Study. Arch Gen Psychiatry 2007;64:810–818. [DOI] [PubMed] [Google Scholar]
- 9.Pulopulos MM, Hidalgo V, Almela M, Puig-Perez S, Villada C, Salvador A. Hair cortisol and cognitive performance in healthy older people. Psychoneuroendocrinology 2014;44:100–111. [DOI] [PubMed] [Google Scholar]
- 10.MacLullich AMJ, Deary IJ, Starr JM, Ferguson KJ, Wardlaw JM, Seckl JR. Plasma cortisol levels, brain volumes and cognition in healthy elderly men. Psychoneuroendocrinology 2005;30:505–515. [DOI] [PubMed] [Google Scholar]
- 11.Geerlings MI, Sigurdsson S, Eiriksdottir G, et al. Salivary cortisol, brain volumes, and cognition in community-dwelling elderly without dementia. Neurology 2015;85:976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox SR, MacPherson SE, Ferguson KJ, et al. Does white matter structure or hippocampal volume mediate associations between cortisol and cognitive ageing? Psychoneuroendocrinology 2015;62:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox SR, Bastin ME, Ferguson KJ, et al. Brain white matter integrity and cortisol in older men: the Lothian Birth Cohort 1936. Neurobiol Aging 2015;36:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci 2005;6:463–475. [DOI] [PubMed] [Google Scholar]
- 15.Lyons DM, Lopez JM, Yang C, Schatzberg AF. Stress-level cortisol treatment impairs inhibitory control of behavior in monkeys. J Neurosci 2000;20:7816–7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol 2004;60:236–248. [DOI] [PubMed] [Google Scholar]
- 17.Stomby A, Boraxbekk C-J, Lundquist A, et al. Higher diurnal salivary cortisol levels are related to smaller prefrontal cortex surface area in elderly men and women. Eur J Endocrinol 2016;175:117–126. [DOI] [PubMed] [Google Scholar]
- 18.Splansky GL, Corey D, Yang Q, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol 2007;165:1328–1335. [DOI] [PubMed] [Google Scholar]
- 19.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: the Framingham offspring study. Am J Epidemiol 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 20.Moms JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD), part I: clinical and neuropsychological assessment of Alzheimer's disease. Neurology 1989;39:1159. [DOI] [PubMed] [Google Scholar]
- 21.Pase MP, Beiser A, Enserro D, et al. Association of ideal cardiovascular health with vascular brain injury and incident dementia. Stroke 2016;47:1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- 23.DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke 2005;36:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeCarli C, Miller BL, Swan GE, et al. Predictors of brain morphology for the men of the NHLBI twin study. Stroke 1999;30:529–536. [DOI] [PubMed] [Google Scholar]
- 25.Aljabar P, Heckemann RA, Hammers A, Hajnal JV, Rueckert D. Multi-atlas based segmentation of brain images: atlas selection and its effect on accuracy. Neuroimage 2009;46:726–738. [DOI] [PubMed] [Google Scholar]
- 26.Carmichael O, Mungas D, Beckett L, et al. MRI predictors of cognitive change in a diverse and carefully characterized elderly population. Neurobiol Aging 2012;33:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee DY, Fletcher E, Martinez O, et al. Regional pattern of white matter microstructural changes in normal aging, MCI, and AD. Neurology 2009;73:1722–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kochunov P, Lancaster JL, Thompson P, et al. Regional spatial normalization: toward an optimal target. J Comput Assist Tomogr 2001;25:805–816. [DOI] [PubMed] [Google Scholar]
- 29.Maillard P, Fletcher E, Harvey D, et al. White matter hyperintensity penumbra. Stroke 2011;42:1917–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23(suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 31.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage 2012;62:782–790. [DOI] [PubMed] [Google Scholar]
- 32.Das RR, Seshadri S, Beiser AS, et al. Prevalence and correlates of silent cerebral infarcts in the Framingham Offspring Study. Stroke 2008;39:2929–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 34.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009;44:83–98. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Zhang J, Oishi K, et al. Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. Neuroimage 2010;52:1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lupien SJ, de Leon M, de Santi S, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci 1998;1:69–73. [DOI] [PubMed] [Google Scholar]
- 37.Wolf OT, Convit A, De Leon MJ, Caraos C, Qadri SF. Basal hypothalamo-pituitary-adrenal axis activity and corticotropin feedback in young and older men: relationships to magnetic resonance imaging-derived hippocampus and cingulate gyrus volumes. Neuroendocrinology 2002;75:241–249. [DOI] [PubMed] [Google Scholar]
- 38.Vythilingam M, Vermetten E, Anderson GM, et al. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol Psychiatry 2004;56:101–112. [DOI] [PubMed] [Google Scholar]
- 39.O'Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry 2004;161:2081–2090. [DOI] [PubMed] [Google Scholar]
- 40.Colla M, Kronenberg G, Deuschle M, et al. Hippocampal volume reduction and HPA-system activity in major depression. J Psychiatr Res 2007;41:553–560. [DOI] [PubMed] [Google Scholar]
- 41.Knoops AJG, Gerritsen L, van der Graaf Y, Mali WPTM, Geerlings MI. Basal hypothalamic pituitary adrenal axis activity and hippocampal volumes: the SMART-Medea study. Biol Psychiatry 2010;67:1191–1198. [DOI] [PubMed] [Google Scholar]
- 42.Pires P, Santos A, Vives-Gilabert Y, et al. White matter alterations in the brains of patients with active, remitted, and cured Cushing syndrome: a DTI study. Am J Neuroradiol 2015;36:1043–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pires P, Santos A, Vives-Gilabert Y, et al. White matter involvement on DTI-MRI in Cushing's syndrome relates to mood disturbances and processing speed: a case-control study. Pituitary 2017;20:340–348. [DOI] [PubMed] [Google Scholar]
- 44.Liu X, Watanabe K, Kakeda S, et al. Relationship between white matter integrity and serum cortisol levels in drug-naive patients with major depressive disorder: diffusion tensor imaging study using tract-based spatial statistics. Br J Psychiatry 2016;208:585–590. [DOI] [PubMed] [Google Scholar]
- 45.Penke L, Maniega SM, Bastin ME, et al. Brain white matter tract integrity as a neural foundation for general intelligence. Mol Psychiatry 2012;17:1026–1030. [DOI] [PubMed] [Google Scholar]
- 46.Patel PD, Lopez JF, Lyons DM, Burke S, Wallace M, Schatzberg AF. Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. J Psychiatr Res 2001;34:383–392. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the Rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci 2000;20:4657–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr Rev 1998;19:269–301. [DOI] [PubMed] [Google Scholar]
- 49.Sapolsky RM, Plotsky PM. Hypercortisolism and its possible neural bases. Biol Psychiatry 1990;27:937–952. [DOI] [PubMed] [Google Scholar]
- 50.Kalimi M. Glucocorticoid receptors: from development to aging: a review. Mech Ageing Dev 1984;24:129–138. [DOI] [PubMed] [Google Scholar]
- 51.Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology 2005;64:1032–1039. [DOI] [PubMed] [Google Scholar]
- 52.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci USA 2001;98:4770–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE. Allostatic load as a predictor of functional decline: MacArthur Studies of Successful Aging. J Clin Epidemiol 2002;55:696–710. [DOI] [PubMed] [Google Scholar]
- 54.Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 2011;73:114–126. [DOI] [PubMed] [Google Scholar]
- 55.Rosmalen JGM, Kema IP, Wüst S, et al. 24 H urinary free cortisol in large-scale epidemiological studies: short-term and long-term stability and sources of variability. Psychoneuroendocrinology 2014;47:10–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator for purposes of replicating procedures and results.