Abstract
Phospholipids can interact strongly with ions at physiological concentrations, and these interactions can alter membrane properties. Here, we describe the effects of calcium ions on the dynamics in phospholipid membranes. We used a combination of time-resolved ultrafast two-dimensional infrared spectroscopy and molecular dynamics simulations. We found that millimolar Ca2+ concentrations lead to slower fluctuations in the local environment at the lipid-water interface of membranes with phosphatidylserine. The effect was only observed in bilayers containing anionic phosphatidylserine; membranes composed of only zwitterionic phosphatidylcholine did not experience a slowdown. Local water dynamics were measured using the ester groups as label-free probes and were found to be up to 50% slower with 2.5 mM Ca2+. Molecular dynamics simulations show that Ca2+ primarily binds to the carboxylate group of phosphatidylserines. These findings have implications for apoptotic and diseased cells in which phosphatidylserine is exposed to extracellular calcium and for the biophysical effects of divalent cations on lipid bilayers.
Introduction
Composed of tens of thousands of lipid species and host to thousands of different proteins, membranes are much more than barriers. Indeed, membranes mediate a wide array of biological processes, from sensing and signaling to transport and adhesion (1, 2, 3, 4, 5). Lipid species are distributed asymmetrically across the leaflets, but the role of heterogeneity is not well understood because investigating membrane structure remains a challenging task. For example, zwitterionic phosphatidylcholines (PC) and negatively charged phosphatidylserines (PS) make up a large portion of the plasma membrane, but PS lipids are encountered almost exclusively on the cytoplasmic leaflets of membranes (6). Cations such as calcium (Ca2+) interact with PS headgroups, altering membrane properties (7, 8). In healthy cells, PS is only exposed to low (∼100 nM) Ca2+ concentrations (9). However, extracellular environments, such as blood, contain free calcium concentrations as high as 2 mM (9), and PS is exposed to these environments during apoptosis and blood coagulation (4, 10).
Exposure of PS lipids to millimolar concentrations of Ca2+ causes model membranes to rigidify and phase separate (6, 7, 8, 9). Ca2+ can also alter bilayer curvature and even rupture PS-containing membranes (11, 12, 13, 14). Many of these studies found that the effects observed were absent for PC lipids and became more pronounced as the abundance of PS increased (13, 14, 15, 16, 17, 18, 19, 20, 21), implying that the negatively charged PS lipids condense Ca2+ onto the bilayer interface. Recent studies using molecular dynamics simulations have shown that Ca2+ cations become localized near PS lipid headgroups, leading to changes in area per lipid, partial phase separation, and membrane bending propensity (18, 19, 22, 23). The physiological implications of PS-Ca2+ complexation are not fully understood, but binding may play a role in cytoplasmic Ca2+ concentration regulation (19), membrane fusion (5), PS-rich domain formation (18), and recruitment of signaling proteins that jointly bind PS lipids and calcium ions (6, 18, 19). In this work, we directly address the effect of Ca2+ on the dynamics of PS-containing mixed membranes at extracellular concentrations to better understand the origins of the effects calcium ions have on lipid membranes.
Structural and dynamic effects of calcium ions
Lipid bilayer interactions with Ca2+ have been studied using multiple techniques. For example, Fourier-transform infrared (FTIR) spectroscopy has shown that Ca2+ binding to PS lipids changes the electrostatic environment around the phosphate and ester groups (7). The changes to the phosphate peaks are similar to those induced by membrane dehydration, whereas Ca2+ splits the ester peak, producing sharp features (7, 8, 24, 25, 26). NMR measurements indicate that Ca2+ induces two separate rigid conformations in the PS lipid headgroups while only minimally influencing the acyl tails (18, 27, 28). Molecular dynamics (MD) simulations suggest that Ca2+ decreases the area per lipid (23, 29) and slows down molecular motion (18, 29, 30). In many of these MD studies, Ca2+ primarily binds to the carboxylate region, with some secondary binding to the phosphate (19, 20, 22).
Despite extensive studies, a comprehensive molecular picture of ion effects on local interactions and interfacial dynamics is lacking (18, 30, 31). Different studies produce contradictory interpretations regarding the binding site of Ca2+. In FTIR studies, for example, Ca2+ binding seems to mostly impact the ester and phosphate groups (7, 24, 25) of PS, whereas MD indicates phosphates and carboxylates are involved (19, 22, 32, 33), and some studies show binding modes involving the esters (19, 23, 30). The location of bound Ca2+ likely depends on the concentration of Ca2+, which explains some of this discrepancy (19, 21, 23). Moreover, recent comparison of MD simulations with NMR data shows that binding affinity to esters is overestimated, which could be another source of discrepancy between simulations and experiments (34, 35). Physiological Ca2+ concentrations are ∼100 nM in the cytosol and ∼2 mM in extracellular fluid and blood plasma (5, 9); some of the previous studies were carried out with higher concentrations (17, 27, 36).
Carbonyl IR absorption line shapes are sensitive to the local environment. Frequencies shift as a result of electrostatic interactions such as hydrogen bonding. Measured peaks correspond to an ensemble distribution of frequencies and environments and as a result contain information about the range of noncovalent interactions experienced by the carbonyls. However, although IR bands provide an ensemble-averaged structural view, a more detailed picture is obtained by examining the dynamics of these vibrational modes using time-resolved spectroscopy.
IR absorption and relaxation in condensed phases occur within picoseconds. The dynamics probed on these timescales involve small motions, such as water reorientation and hydrogen-bond formation, rather than the larger structural changes observed via EPR (electron paramagnetic resonance) and NMR. These techniques have been used fruitfully to study bilayer dynamics and interactions with ions. Lipids, however, undergo dynamics on a wide range of timescales. Ultrafast IR spectroscopy provides access to the picosecond timescales and as such is complementary to more common experimental techniques (37, 38). In protein biophysics, ultrafast IR studies have shed light on the fluctuating movement of ions and water around proteins, which are almost static on picosecond timescales (38). Ultrafast IR spectroscopy has recently been applied to lipid membranes by Righini and others (37, 39, 40, 41, 42), but to our knowledge, the effects of cations on anionic lipids have not been investigated experimentally on ps timescales.
PC and PS have a pair of ester carbonyls, which can be used as intrinsic, nonperturbative probes of the interfacial environment. Ester carbonyls have been used previously to measure membrane electrostatics and are sensitive to ion binding (7, 8, 24, 26) and water penetration into the hydrophobic region of the membrane (37, 39, 43). In addition, PS lipids contain a fully hydrated carboxylate group (Fig. 1). Simulations have suggested that Ca2+ interacts strongly with these groups; however, as we show below, binding to carboxylates produces only small shifts to the asymmetric COO− absorption band.
Figure 1.

Calcium ion interactions with the headgroups of phosphatidylcholine (PC, left) and phosphatidylserine (PS, right) lipids. To see this figure in color, go online.
Here, we investigate Ca2+ binding and its effects on hydrogen-bond dynamics at the lipid-water interface in membranes containing mixed PC and PS lipids using ultrafast vibrational spectroscopy and MD simulations. We found that calcium ions bind to the PS carboxylates and slow down fluctuations in the electric field near the lipid-water interface without significantly affecting water penetration. These effects were consistent for PC/PS membranes with differing PS ratios, including membranes containing 10 mol% PS designed to mimic the plasma membrane. Overall, our results are interpreted as evidence that extracellular calcium concentrations can rigidify membranes after PS exposure toward the outer leaflet.
Materials and Methods
Detailed descriptions of the experimental methods, data analysis, and MD trajectories are included in the Supporting Materials and Methods. Here, we provide a summary of the methods.
Sample preparation
Vesicles were prepared with 100, 50, 10, and 0 mol% 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (POPS), with the remaining lipid being 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) as shown in Table 1. The final lipid and ion concentrations were 51 and 2.5 mM, respectively, producing a 20:1 lipid/ion ratio. To ensure lipids were not in a dehydrated cochleate phase, we examined an IR absorbance marker of cochleate formation (see Supporting Materials and Methods, Sections 2 and 3) (44).
Table 1.
List of Membrane Compositions Simulated and Analyzed Using FTIR and 2D IR Spectroscopy
| Lipid Composition | Lipid/Ca2+ Ratio |
|---|---|
| POPS | No Ca2+ |
| POPS | 20:1 |
| POPS/DOPC (1:1) | No Ca2+ |
| POPS/DOPC (1:1) | 20:1 |
FTIR spectroscopy
Samples were held between two CaF2 windows with a 50 μm spacer. Spectra were measured at room temperature with a Bruker Vertex 70 spectrometer (Bruker, Billerica, MA) purged with dry air. All spectra were collected at 1 cm−1 resolution, and 64 scans were averaged per sample.
Ultrafast 2D IR spectroscopy
Our two-dimensional infrared (2D IR) spectrometer has been described in detail previously (45). Briefly, ∼100 fs mid-IR pulses were split into pump and probe pulses. A Ge-based pulse shaper was used to generate excitation pulse pairs. The time delay between pump pulses was Fourier transformed to generate the excitation frequency axis. The probe pulse arrived after a waiting time, t2, and was dispersed onto a 128-pixel-wide MCT (mercury cadmium telluride) array to generate the detection axis. The probe pulse was polarized perpendicular to the pump pulses to enhance cross peaks and minimize scatter. Spectra were measured at a series of waiting times to extract dynamical information from the 2D line shapes through center line slope analysis.
MD simulations
Simulations were performed using the same POPS/DOPC and lipid/Ca2+ ratios as in experiments. Each system was comprised of 200 lipids, with 100 in each leaflet. The ratio of water/lipid molecules was larger than 38 to completely hydrate the bilayer systems. We used the CHARMM36 force field and the CHARMM implementation of TIP3P (transferrable intermolecular potential 3p) water (46, 47, 48). To more accurately model interactions between cations and anionic lipids, we used the NBFIX (non-bonded fix) parameters developed by Venable et al. (49, 50) that changed the van der Waals parameters for Na+ and Ca2+ to better fit experimental data. We use sodium as the counterion for POPS. In the systems containing calcium ions, we removed 10 sodium ions and replaced another 10 with calcium to reach a lipid/Ca2+ ratio of 20:1. The simulation temperature was 298 K, above the lipid-crystalline phase transition temperatures for the two lipids, and the pressure was kept constant at 1 bar. The starting configurations of the hydrated bilayers were prepared by the CHARMM-GUI membrane builder (51, 52), which places the lipids randomly within the bilayer. Each system was first minimized using a conjugate gradient algorithm and then equilibrated for 375 ps using the CHARMM-GUI six-step equilibration protocol (53). Finally, 400-ns production trajectories were carried out for each system.
Results
Absorption spectra of the ester and carboxylate groups (see the structures in Fig. 1) probe the electrostatics in the headgroup region. Ultrafast 2D IR provides two key pieces of information: 1) the diagonal peaks are narrower than absorption peaks and allow the different hydrogen-bond populations to be resolved, and 2) the growth of off-diagonal features with waiting time provides a direct measure of the subpicosecond changes to the environment around the carbonyls (41). Note that here we probe the PS and PC lipids together because the carbonyl vibrations are highly overlapped, and as such, the experiments measure an average of the two components. To ensure that MD simulations and experiments sample similar environments, simulations are carried out with the same lipid and ion compositions in our experimental samples.
IR absorption spectroscopy
The presence of calcium in the lipid systems leads to a variety of changes in peak positions and shapes in the IR spectra of systems with PS, as shown in Fig. 2. The carboxylate peak shifts to lower frequencies by ∼1 cm−1. This slight shift is shown in the difference spectrum in Fig. 2 b. A shift in this peak to lower frequencies generally indicates that both oxygens participate in calcium binding, whereas a shift to higher frequencies indicates contact only with one oxygen (54, 55, 56).
Figure 2.
Ester carbonyl (∼1740 cm−1), and carboxylate (1620 cm−1) absorption spectra in 1:1 PS/PC lipid vesicles with (green) and without (red) CaCl2. (a) Normalized FTIR absorbance spectra of the carboxylate and ester are shown shifted to ease visualization. (b) The difference between the normalized spectra is shown. (c) The second-derivative spectrum showing the number of features in each peak is given. To see this figure in color, go online.
Calcium changes ester-peak splitting in systems with 1:1 PS/PC composition, as shown by the second derivative spectrum in Fig. 2 c. This change is accompanied by an increase in intensity at lower frequencies. Without calcium, the ester peak consists of two features, which have been previously assigned to 0 and 1 hydrogen-bond populations (25). Upon adding calcium, four separate features are observed. These features are visible in the difference spectrum (Fig. 2 b) and in the second derivatives (Fig. 2 c).
Ultrafast 2D IR spectroscopy
2D IR spectroscopy enables us to quantify the subpicosecond hydrogen-bond dynamics at the ester group positions. We monitor the dynamics by analyzing the line shapes at different pump-probe delays or waiting times.
A 2D IR spectrum can be interpreted as an excitation-to-detection frequency correlation map analogous to homonuclear COSY NMR spectroscopy, as shown in Fig. 3 (38, 57, 58). A pair of pump pulses generates the excitation frequency ω1 (horizontal axis), followed by a probe pulse to generate the detection axis ω3 (vertical axis). When the probe pulse passes through the sample, a positive signal is generated because of stimulated emission from the first excited state down to the ground state. This positive signal contours are colored red. A negative signal, colored blue, is generated because of absorption from the first excited state to the second excited state. The negative and positive signals appear in the same region on the pump axis but are offset on the probe axis because of vibrational anharmonicity (59, 60, 61, 62).
Figure 3.
2D IR spectra of 1:1 POPS/DOPC with 2.5 mM CaCl2 with a t2 of 0.30 ps (left) and 1.5 ps (right). Below is a difference spectrum from these two 2D IR spectra. Black and white rectangles highlight the regions with maximum growth and decay, respectively. To see this figure in color, go online.
We varied the waiting time between the pump and the probe pulses to investigate dynamics. Changes in peak shapes with waiting time report on the fluctuations in IR absorption frequency. The signal at short waiting times is highly correlated; it is strongest when the pump and probe frequencies are equal. The signal is less correlated at longer time delays, as the environment evolves with time. Changes in the spectra also result from vibrational relaxation, which leads to decreases in intensity. If vibrational relaxation is faster at some frequencies than others, then vibrational relaxation can also alter the line shapes measured at different waiting times.
The changes in the ester peak with waiting time can be illustrated by plotting a difference spectrum made by subtracting a spectrum with a short waiting time from a spectrum with a longer waiting time, as shown in Fig. 3. Loss of signal for peaks that are initially positive (red) appears as a negative feature (blue) and vice versa. In general, the signal increases in off-diagonal regions and decays along the diagonal, especially near the low-frequency side of the peak below 1730 cm−1. The decay is greatest along the diagonal between 1710 and 1720 cm−1. The off-diagonal growth is broader and smaller than the decay. We observe the same pattern for all samples regardless of lipid composition or presence of Ca2+ (see Supporting Materials and Methods, Section 4).
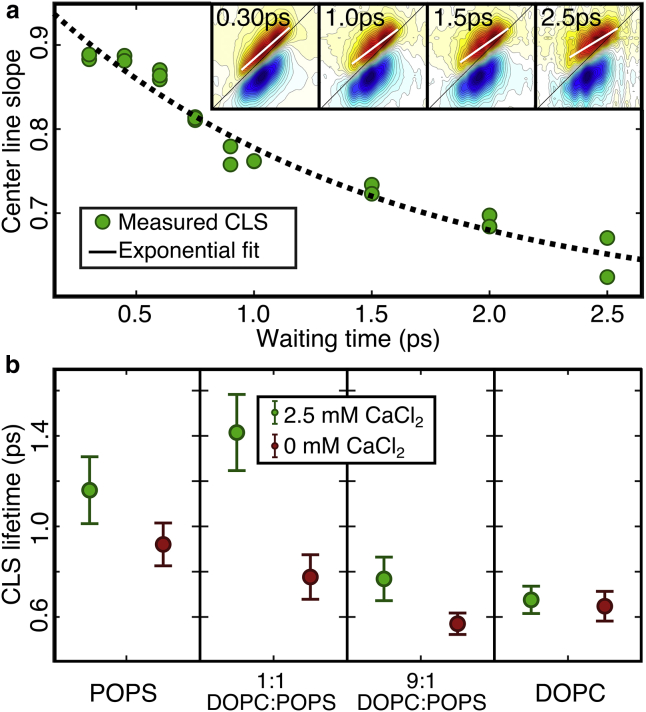
Because spectra can be interpreted as an excitation-to-detection frequency correlation map, diagonal elongation implies that the two frequencies are correlated, whereas rounder peaks imply loss of correlation at longer waiting times. Loss of correlation can be quantified by center line slope (CLS) analysis (40, 41, 58, 63, 64). The CLS is the line along the ridge of maximal intensity in the spectrum. The CLS decay with waiting time is equivalent to the normalized frequency-frequency correlation function (FFCF), which decays as the oscillator loses “memory” of its initial frequency. The FFCF has been described in detail elsewhere (63, 65). Frequency fluctuations are due to the environment, and thus the FFCF is a direct measure of fluctuations in local electrostatics. Fig. 4 a shows examples of spectra at various waiting times with a CLS fit to the ester peaks. Fitting the time-dependent changes of the CLS to a monoexponential decay yields FFCF lifetimes. These lifetimes are plotted in Fig. 4 b for all samples.
Figure 4.
(a) CLS decay and an exponential fit for esters in 1:1 POPS/DOPC vesicles with 2.5 mM CaCl2. The inset shows some of the corresponding 2D IR spectra with their center line in white. (b) CLS lifetimes for all systems studied with (green) and without (red) CaCl2 are shown. To see this figure in color, go online.
We observe two significant patterns in the correlation lifetimes. 1) In systems with PS lipids, Ca2+ leads to longer relaxation lifetimes or slower ester carbonyl dynamics. Ca2+ leads to slower dynamics for all samples containing PS lipids, but the effect is greatest in the 1:1 PS/PC membranes. In the 1:9 PS/PC sample, the difference is only about ∼30%. No change was observed in the membranes composed of just PC lipids. 2) Dynamics are slower in systems with large amounts of POPS, indicating that POPS induces slower interfacial dynamics compared to DOPC. Changes related to the lipid composition are smaller than the Ca2+-dependent changes. For example, in the 1:1 PS/PC mixtures, Ca2+ slows the dynamics by ∼40% (with an increase relation time from 0.7 to 1.4 ps).
Diagonal slices of the 2D IR spectra are comparable to IR absorption spectra. The comparison is useful for interpreting absorption spectra because both are related to the distribution of environments within the samples. However, IR absorption peak intensities are proportional to the C=O transition dipole moment squared (μ2), whereas 2D IR peak intensities are proportional to μ4 (59). Consequently, 2D IR spectroscopy enhances the intensity of sharp spectral features. Comparison between diagonal slices and similar IR absorption spectra has previously been used by Dunkelberger et al. to study protein secondary structure (57).
Fig. 5 shows IR peaks and 2D IR diagonal slices for our systems with and without Ca2+. The diagonal slices of the spectra taken with calcium ions exhibit three main peaks centered at 1745, 1730, and 1715 cm−1. The sharp features observed in the absorption spectra are not present in 2D IR. We observe a general broadening and a shift to lower frequencies after adding calcium to systems with PS. The individual peaks that make up the overall band do not shift, however. The apparent broadening and red shift are instead due to changes in their relative intensities.
Figure 5.
(a) FTIR detail of the ester peak for 1:1 POPS:DOPC vesicles with (green) and without (red) CaCl2. (b) Diagonal slices from 2D IR spectra of all systems studied with (green) and without (red) CaCl2 are shown. Vertical gray lines highlight the three overlapping peaks observed in the diagonal slices. To see this figure in color, go online.
MD simulations
MD trajectory analysis informs our interpretation of the FTIR and 2D IR spectra. These spectra probe the local environment of the lipid, and therefore the simulations should reproduce local features and not necessarily long-range correlations; see Fig. S1 for a convergence test. Qualitatively, we observe that calcium ions, which were placed in bulk water at the beginning of each trajectory, diffused toward the carboxylate region and remained within the lipid-water interface for the remainder of the trajectory. More quantitative analyses were performed to pinpoint the Ca2+ location within the interface. Plots of the location of calcium relative to lipid carbonyls are shown in Figs. S5 and S7. Table 2 shows the number of oxygen atoms from various functional groups within 3 Å of the calcium ions. Ca2+ shows strong preferential binding to the carboxylates, as the number of oxygens from these groups is the largest among the lipid groups. Furthermore, this number is greater than two, which shows that on average, more than one carboxylate coordinates each calcium ion. This suggests that, by binding two or more carboxylates, strong electrostatic interactions effectively cross-link lipids. This assignment is also in agreement with the experimental carboxylate asymmetric stretch FTIR spectra, in which the small red shift can be attributed to binding of Ca2+ in a bidentate-type configuration involving both oxygen atoms. A 2:1 lipid/ion complex is in agreement with previous Ca2+-binding measurements involving PS (17, 18, 21, 28). Furthermore, Table 2 shows that ions interact weakly with the ester carbonyls, and direct complexation by the phosphate groups is negligible.
Table 2.
Number of Oxygen Atoms from Different Functional Groups Surrounding Ca2+ Ions in Simulations Computed from the Last 200 ns of the 400 ns Trajectories
| Group | POPS | 1:1 POPS/DOPC |
|---|---|---|
| Phosphate | 0.018 | 0.020 |
| Carboxylate | 2.84 | 2.59 |
| Ester | 0.13 | 0.072 |
| Water | 4.65 | 4.89 |
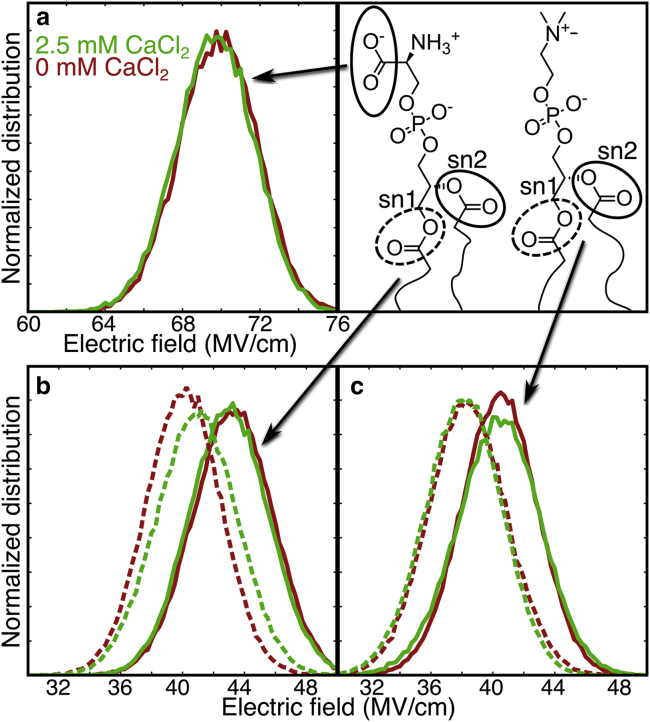
Because IR frequencies are sensitive to electrostatic environments (66, 67, 68), we compute the distribution of electric fields along the C=O bonds from the trajectories. Fig. 6 shows the distribution of electric fields at the carboxylate positions. These do not change significantly with Ca2+, which explains the underlying reason why carboxylate peak frequencies shift by only ∼1 cm−1 with Ca2+ (Fig. 2 a). The average electric field around the ester groups (Fig. 6, b and c), on the other hand, is shifted in the presence of Ca2+. In the 100% POPS system (Fig. S8), the Ca2+ affected the electric field for both the sn1 and sn2 ester carbonyls, although the effect was greater for the sn1 esters. In the system with 1:1 POPS/DOPC (Fig. 6), an effect was only observed for the sn1 ester of POPS.
Figure 6.
Electric field projected onto carbonyl bonds in simulations. (a) The electric field distribution for the carboxylates in a 100% POPS system with and without Ca2+ is shown. (b) Electric field distributions for POPS esters and (c) DOPC esters with and without Ca2+ in a 1:1 mixture of POPS and DOPC are shown. Distributions for the pure POPS bilayer are shown in the Supporting Materials and Methods. These plots are obtained by first averaging the electric fields along all carbonyl bonds in each time frame and then binning those averages. To see this figure in color, go online.
Local water dynamics at the ester carbonyls are quantified through hydrogen-bond correlation functions between water OH groups and the carbonyl oxygen atom. Hydrogen-bond lifetimes are typically in the picosecond range; thus, we use configurations from five short (2 ns) trajectories saved every 10 fs. The results of this analysis are shown in Fig. S10. In general, we observe that Ca2+ slows down hydrogen-bond dynamics at the ester carbonyl positions. The effect only appears to be significant for DOPC esters in the 1:1 POPS/DOPC system in the simulations.
Together, these results point toward calcium perturbing the lipid-water interface through direct effects such as binding to carboxylates, as well as indirect effects such as altering the electrostatic environment and hydrogen-bond dynamics at the ester carbonyl positions. In the next section, we combine the results from absorption spectra, ultrafast 2D IR spectra, and simulations to extract a comprehensive molecular picture of the changes that Ca2+ binding induces on the lipid bilayer.
Discussion
Ca2+ primarily binds carboxylates
MD trajectories indicate that Ca2+ primarily binds to the carboxylates. These results are consistent with previous literature (19, 22, 32, 33) but are surprising in the context of the IR results. Previous FTIR results show minimal changes to the carboxylate peaks and large changes to the phosphate and ester peaks (25). Even though the calcium ions primarily bind with the carboxylates in our MD trajectories, their effect on the electric field around the carboxylates is minimal because of screening by water molecules and averaging of orientations. In contrast, the less polar domain of the carbonyl experiences more significant electric field (Fig. 6). This is consistent with recent 2D IR and ab initio MD simulations by Kuroda and co-workers in which no changes in the IR spectra or relaxation dynamics of the acetate carboxylate were observed in solutions up to 6 M NaCl (69). Recent results from our own group also point toward larger changes in carboxylate frequencies with a monodentate binding configuration (70). Likewise, in our study, carboxylates are fully hydrated and primarily experience electric field fluctuations from the surrounding water, and the ion does not appear to modify the local dynamics. Calcium ions exert a larger effect on the partially hydrated ester groups at the lipid-water interface, even though the esters rarely bind Ca2+. Further investigation is required to experimentally determine the location of Ca2+ ions.
Ca2+ changes the dynamics and structure of the membrane-water interface
The ester region remains hydrated
Previous experiments show that Ca2+ impacts the overall hydration of bilayers. Binder et al. found that dried lipid films hydrate differently in the presence of Ca2+ (71), calorimetric data suggests that PS-Ca2+ binding is driven entropically by both Ca2+ and lipid dehydration (21), and PS-Ca2+ binding changes the antisymmetric phosphate group’s IR absorption the same way that lipid dehydration does (7, 26). PS-rich membranes can even form a separate dehydrated phase, forming cylindrical aggregates, with excessive calcium ions (28, 44). The small changes in our measured ester line shapes, both FTIR and 2D IR, indicate that the carboxylate region remains hydrated. In fact, hydrogen-bond populations increase slightly with Ca2+, which points toward a slight increase in hydration.
Calcium-free ester FTIR peak shapes for PS-containing membranes (Figs. 2 and 5) show the characteristic two-band structure observed in similar bilayers (41, 42, 43, 72, 73). The overall peak is the sum of two Gaussians, each centered where one of the second derivative’s minima appears: 1730 and 1745 cm−1. These peaks, referred to as peak A and peak B, respectively (41, 73), indicate that the lipid esters experience two distinct electrostatic environments. The origins of these peaks are attributed to hydrogen bonding (41, 43). Peak A corresponds to esters with a single hydrogen bond to water (C=O-HOH), whereas peak B corresponds to esters without a hydrogen bond. The center frequency of Peak A is ∼15 cm−1 lower than the center frequency of peak B, consistent with the measured shifts for hydrogen-bond formation (74). In FTIR spectra of lipids with one ester isotope labeled such that each of the two tails absorbs in a different region, peak A and peak B were observed in each of the individual esters, showing that the two groups experience similar environments (25, 37). Recent experiments have demonstrated that peak A experiences faster dynamics than peak B and has a shorter vibrational lifetime (41), which is consistent with our difference spectra (Figs. 3 and S4). Also visible in the difference spectra are off-diagonal crosspeaks, which grow as a function of the pump-probe delay. We attribute these to hydrogen-bond switching between peak A and peak B. Esters that experience the breaking or forming of a hydrogen bond between the pump and probe pulses exhibit peaks at peak A in one frequency axis and peak B in the other frequency axis. Our simulations show the fast decay of hydrogen bonding between water and the carbonyl esters (Fig. S10).
Ester peaks in the presence of both calcium and PS contain at least four different spectral features as clearly shown in the second-derivative spectrum (Fig. 2 c). These sharp peaks have been observed before for PS lipids in the presence of calcium (7, 24) and have been explained either as being due to increased rigidity in the motions of the esters and lipid tails (7) or as being due to interfacial water trapped near the ester by a calcium-lipid complex (24). Our simulations do not show enough large differences in interfacial water trapping or ester rigidity between the systems with and without calcium to be able to draw a clear picture about their relevance to explain the spectral features.
The diagonal slices from 2D spectra at high PS concentrations and 2 mM Ca2+ do not exhibit the same four peaks as FTIR spectra of the same systems. These differences can be attributed to how the transition dipole moments, μ, of oscillators contribute to the spectra. In FTIR, the signal is proportional to μ2, whereas in 2D IR, the signal is proportional to μ4. For this reason, small-amplitude peaks can be buried within the background. Furthermore, our 2D IR spectral resolution may contribute to the suppression of these sharp peaks.
Diagonal slices, with or without calcium, exhibit peak A (or A1) and peak B at 1728 and 1743 cm−1, respectively (Fig. 5). Additionally, a shoulder is observed between 1710 and 1715 cm−1, shifted ∼15 cm−1 from peak A. This is the shift expected to result from an ester participating in two hydrogen bonds. For convenience, we refer to the single-hydrogen-bond feature as peak A1 and the double-hydrogen-bond feature as peak A2 in the 2D IR slices. Cation-carbonyl interactions do not produce a sufficiently large shift to explain this feature (75). This feature near 1715 cm−1 is observed in all the samples, including the system with only DOPC and no Ca2+, and decays faster than any other peak as a function of waiting time (Figs. 3 and S4). We interpret the feature between 1710 and 1715 cm−1 as a small population of esters with two hydrogen bonds to water. In the absorbance spectra, both features appear under peak A because of their broadness and the small amplitude of peak A2 relative to peak A1. The relative amplitudes of the diagonal 2D IR peaks changed with calcium.
The amplitude of peak A2 increasing in POPS and the 1:1 mixture provides evidence that Ca2+ increases ester hydration. In general, Ca2+ increases the interfacial polarity, and as a result, the overall peak shifts toward lower frequencies. This shift can also be seen in the FTIR of PS-containing systems, particularly the difference spectrum in Fig. 2 b.
Calcium ions slow down interfacial dynamics
The experimental CLS and MD trajectories (Figs. 4 and S10) show that calcium in POPS-containing bilayers slows down the picosecond local water dynamics at the ester positions. The measurements show that in the 1:1 POPS/DOPC mixture, these fluctuations can be slower by ∼50%, which is significant given the 20:1 lipid/ion ratio used in these experiments. We postulate that ion-mediated electrostatic cross-linking between PS lipids contributes significantly to the slowdown in dynamics. Further, a single lipid-ion-lipid cluster may affect the dynamics of its surrounding neighbors (four to six lipids), which would explain the considerable slowdown observed even at physiological ion concentrations. Furthermore, these local interactions may explain the more global changes in membrane properties such as rigidification. Karathanou and Bondar pointed out that increased rigidity and slower diffusion may be connected to changes in the hydrogen-bond network at the lipid-water interface in membranes with anionic lipids (31). Our simulations similarly suggest that the interfacial hydrogen bond between water and ester lipids slows down slightly when Ca2+ binds anionic lipids (Fig. S10). The simulations also indicate that the slowdown is not restricted to the lipids directly binding Ca2+, as hydrogen-bond dynamics slowed around DOPC despite calcium binding to POPS. Melcrová et al. found slower fluorescence relaxation times for a fluorophore near the lipid esters with Ca2+, further suggesting that Ca2+ induces slower dynamics at the lipid-water interface (22). Here, we have directly measured the predicted changes in the dynamics with a label-free method and have found the degree to which calcium ions can slow down molecular dynamics at the lipid-water interface. The degree of change induced by Ca2+ binding appears to be composition dependent, with the greatest change occurring in a bilayer that is 1:1 PS/PC, but changes at 1:9 PS/PC are still significant. The pure PC bilayers were not significantly affected, which may be explained by the absence of a net negative charge in a bilayer composed of zwitterionic lipids.
To summarize, calcium binding to PS lipids slows the dynamics at the ester position and introduces more heterogeneous molecular environments. The slowdown cannot be attributed to complete ester dehydration or decreased hydrogen bonding to water, so we interpret these results as evidence that the lipid headgroups adopt more rigid but disordered conformations on the molecular level. The decrease in dynamics observed experimentally indicates that the network of hydrogen bonds between lipids and water at the polar-nonpolar interface becomes slower, as it was shown by Karathanou and Bondar (34). This is bolstered by recent NMR work showing that binding to Ca2+ induces two different rigid conformations in the glycerol backbone of lipid headgroups (18).
Ca2+-lipid interactions with physiologically relevant amounts of PS
Approximately 10–20% of the lipids on the inner leaflet of the plasma membrane are PS lipids (6), which is approximated by our 1:9 PS/PC sample. The inner leaflet is never exposed to millimolar Ca2+ concentrations. However, there are situations in which a membrane leaflet with ∼10% PS may experience such concentrations. During apoptosis and blood coagulation, proteins scramble the lipid distribution between leaflets (6). PS scrambling also occurs in several pathological cell types, including sickle cells and some cancer cells (8). Together, these results point toward membrane rigidification as one of the biological roles of Ca2+ and a feature of apoptotic cells.
In the absorption spectra of 1:9 PS/PC systems with and without calcium, we did not observe significant Ca2+-induced changes to either the ester or carboxylate stretches (Fig. S9). In our 2D IR measurements, however, differences became clear. In the diagonal slices of the 2D spectra, shown in Fig. 5, the intensity at low frequencies increases with calcium. Changes in dynamics also occur in the 1:9 PS/PC system with Ca2+ (Fig. 4). Because the same patterns occur when the bilayer is composed primarily of PC, these findings may be generalized to model systems primarily containing zwitterionic lipids.
Conclusions
We have demonstrated, both through experiment and simulation, how calcium binding to PS lipids influences the interfacial dynamics and intermolecular interactions. The lipid-water interface near the esters in membranes bound to Ca2+ experiences strong electrostatic interactions and distinctly slowed dynamics. This occurred even in membranes with 90% zwitterionic PC lipids.
These results are relevant in the context of PS lipids on plasma membranes. PS on the extracellular leaflet is one of the hallmarks of both apoptosis and blood coagulation, although PS is also seen on the outer leaflet in several types of abnormal cells, including red blood cells affected by sickle cell anemia (8). These interactions have been shown to dramatically alter the properties of model membranes through rigidification, changes in membrane curvature, domain formation, and decreased lateral diffusion. We have investigated the molecular origin of the rigidification and decreased lateral diffusion, finding that calcium binding does not dehydrate the ester region, but even with only 10% PS lipids, the local dynamics slow around the esters. The slower local dynamics provide a molecular explanation for the Ca2+-dependent decreases in fluidity observed on longer length and timescales.
There is considerable room for further improvement in the force fields for ion-membrane interactions. Particularly, small differences in short-range interactions may determine the local geometries (34). Most of our conclusions are, however, based on long-range effects (i.e., electric field generated by the ion at significant distances from the carbonyl groups), which are less sensitive to the details of the force field. This work demonstrates the power of direct comparison between simulations and experiments. Indeed, our 2D IR measurements provide a direct, nonperturbative probe of the interfacial dynamics and demonstrate the capabilities of 2D IR spectroscopy as a biophysical tool to study molecular interactions in heterogeneous systems. Ultrafast data may serve as a useful benchmark for future refinement of MD models.
Other divalent cations have been shown to associate strongly with PS lipids, such as Mg2+ (22, 76) and Cd2+ (77), and are relevant either because of their physiological ubiquity or toxic effects. Interactions between PS lipids and many of these cations have been studied with FTIR and have been shown to be ion dependent. Further exploration with ultrafast 2D IR and simulations will provide insights into the interactions between these other cations and PS lipids.
Acknowledgments
We gratefully acknowledge financial support from the Welch Foundation (F-1891 and F-1896), the National Science Foundation under grant BIO-1815354, the College of Natural Sciences at the University of Texas at Austin, and the National Institutes of Health under grant GM111364. Some of the molecular dynamics simulations were performed at the Texas Advanced Computing Center.
Editor: D. Peter Tieleman.
Footnotes
Supporting Materials and Methods and ten figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(18)31022-1.
Author Contributions
All authors contributed to conceiving the study. M.L.V. performed the experimental measurements, and A.E.C. performed the simulations. M.L.V. and A.E.C. analyzed the data. M.L.V. wrote the manuscript. All authors contributed to editing the manuscript.
Supporting Material
References
- 1.Phillips R., Ursell T., Sens P. Emerging roles for lipids in shaping membrane-protein function. Nature. 2009;459:379–385. doi: 10.1038/nature08147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lizardo D.Y., Parisi L.R., Atilla-Gokcumen G.E. Noncanonical roles of lipids in different cellular fates. Biochemistry. 2018;57:22–29. doi: 10.1021/acs.biochem.7b00862. [DOI] [PubMed] [Google Scholar]
- 3.Engelman D.M. Membranes are more mosaic than fluid. Nature. 2005;438:578–580. doi: 10.1038/nature04394. [DOI] [PubMed] [Google Scholar]
- 4.Zwaal R.F., Comfurius P., Bevers E.M. Surface exposure of phosphatidylserine in pathological cells. Cell. Mol. Life Sci. 2005;62:971–988. doi: 10.1007/s00018-005-4527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mokkila S., Postila P.A., Róg T. Calcium assists dopamine release by preventing aggregation on the inner leaflet of presynaptic vesicles. ACS Chem. Neurosci. 2017;8:1242–1250. doi: 10.1021/acschemneuro.6b00395. [DOI] [PubMed] [Google Scholar]
- 6.Leventis P.A., Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu. Rev. Biophys. 2010;39:407–427. doi: 10.1146/annurev.biophys.093008.131234. [DOI] [PubMed] [Google Scholar]
- 7.Dluhy R., Cameron D.G., Mendelsohn R. Fourier transform infrared spectroscopic studies of the effect of calcium ions on phosphatidylserine. Biochemistry. 1983;22:6318–6325. [Google Scholar]
- 8.Casal H.L., Martin A., Hauser H. Infrared studies of fully hydrated unsaturated phosphatidylserine bilayers. Effect of Li+ and Ca2+ Biochemistry. 1987;26:7395–7401. doi: 10.1021/bi00397a030. [DOI] [PubMed] [Google Scholar]
- 9.Xu N., Francis M., Stevens T. Studies on the resolution of subcellular free calcium concentrations: a technological advance. Focus on “detection of differentially regulated subsarcolemmal calcium signals activated by vasoactive agonists in rat pulmonary artery smooth muscle cells”. Am. J. Physiol. Cell Physiol. 2014;306:C636–C638. doi: 10.1152/ajpcell.00046.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fadok V.A., Voelker D.R., Henson P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 11.Düzgünes N., Nir S., Papahadjopoulos D. Calcium- and magnesium-induced fusion of mixed phosphatidylserine/phosphatidylcholine vesicles: effect of ion binding. J. Membr. Biol. 1981;59:115–125. doi: 10.1007/BF01875709. [DOI] [PubMed] [Google Scholar]
- 12.Tsai H.H., Juang W.F., Lee J.B. Molecular mechanism of Ca(2+)-catalyzed fusion of phospholipid micelles. Biochim. Biophys. Acta. 2013;1828:2729–2738. doi: 10.1016/j.bbamem.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 13.Marr J.M., Li F., Schultz Z.D. The role of lateral tension in calcium-induced DPPS vesicle rupture. Langmuir. 2012;28:11874–11880. doi: 10.1021/la301976s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali Doosti B., Pezeshkian W., Lobovkina T. Membrane tubulation in lipid vesicles triggered by the local application of calcium ions. Langmuir. 2017;33:11010–11017. doi: 10.1021/acs.langmuir.7b01461. [DOI] [PubMed] [Google Scholar]
- 15.Onishi S., Ito T. Calcium-induced phase separations in phosphatidylserine--phosphatidylcholine membranes. Biochemistry. 1974;13:881–887. doi: 10.1021/bi00702a008. [DOI] [PubMed] [Google Scholar]
- 16.Ross M., Steinem C., Janshoff A. Visualization of chemical and physical properties of calcium-induced domains in DPPC/DPPS Langmuir−Blodgett layers. Langmuir. 2001;17:2437–2445. [Google Scholar]
- 17.Hui S.W., Boni L.T., Isac T. Identification of phosphatidylserine and phosphatidylcholine in calcium-induced phase separated domains. Biochemistry. 1983;22:3511–3516. [Google Scholar]
- 18.Boettcher J.M., Davis-Harrison R.L., Rienstra C.M. Atomic view of calcium-induced clustering of phosphatidylserine in mixed lipid bilayers. Biochemistry. 2011;50:2264–2273. doi: 10.1021/bi1013694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melcrová A., Pokorna S., Cwiklik L. The complex nature of calcium cation interactions with phospholipid bilayers. Sci. Rep. 2016;6:38035. doi: 10.1038/srep38035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine Z.A., Vernier P.T. Calcium and phosphatidylserine inhibit lipid electropore formation and reduce pore lifetime. J. Membr. Biol. 2012;245:599–610. doi: 10.1007/s00232-012-9471-1. [DOI] [PubMed] [Google Scholar]
- 21.Sinn C.G., Antonietti M., Dimova R. Binding of calcium to phosphatidylcholine–phosphatidylserine membranes. Colloids Surf. Physicochem. Eng. Asp. 2006;282–283:410–419. [Google Scholar]
- 22.Martín-Molina A., Rodríguez-Beas C., Faraudo J. Effect of calcium and magnesium on phosphatidylserine membranes: experiments and all-atomic simulations. Biophys. J. 2012;102:2095–2103. doi: 10.1016/j.bpj.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vernier P.T., Ziegler M.J., Dimova R. Calcium binding and head group dipole angle in phosphatidylserine-phosphatidylcholine bilayers. Langmuir. 2009;25:1020–1027. doi: 10.1021/la8025057. [DOI] [PubMed] [Google Scholar]
- 24.Hübner W., Mantsch H.H., Hauser H. Conformation of phosphatidylserine in bilayers as studied by Fourier transform infrared spectroscopy. Biochemistry. 1994;33:320–326. doi: 10.1021/bi00167a042. [DOI] [PubMed] [Google Scholar]
- 25.Hübner W., Blume A. Interactions at the lipid–water interface. Chem. Phys. Lipids. 1998;96:99–123. [Google Scholar]
- 26.Choi S., Ware W., Jr., Phillips W.M. Infrared spectroscopic studies on the phosphatidylserine bilayer interacting with calcium ion: effect of cholesterol. Biochemistry. 1991;30:8563–8568. doi: 10.1021/bi00099a011. [DOI] [PubMed] [Google Scholar]
- 27.Roux M., Bloom M. Ca2+, Mg2+, Li+, Na+, and K+ distributions in the headgroup region of binary membranes of phosphatidylcholine and phosphatidylserine as seen by deuterium NMR. Biochemistry. 1990;29:7077–7089. doi: 10.1021/bi00482a019. [DOI] [PubMed] [Google Scholar]
- 28.Roux M., Bloom M. Calcium binding by phosphatidylserine headgroups. Deuterium NMR study. Biophys. J. 1991;60:38–44. doi: 10.1016/S0006-3495(91)82028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porasso R.D., López Cascales J.J. Study of the effect of Na+ and Ca2+ ion concentration on the structure of an asymmetric DPPC/DPPC + DPPS lipid bilayer by molecular dynamics simulation. Colloids Surf. B Biointerfaces. 2009;73:42–50. doi: 10.1016/j.colsurfb.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 30.Böckmann R.A., Grubmüller H. Multistep binding of divalent cations to phospholipid bilayers: a molecular dynamics study. Angew. Chem. Int. Ed. Engl. 2004;43:1021–1024. doi: 10.1002/anie.200352784. [DOI] [PubMed] [Google Scholar]
- 31.Karathanou K., Bondar A.N. Dynamic water hydrogen-bond networks at the interface of a lipid membrane containing palmitoyl-oleoyl phosphatidylglycerol. J. Membr. Biol. 2018;251:461–473. doi: 10.1007/s00232-018-0023-1. [DOI] [PubMed] [Google Scholar]
- 32.Pedersen U.R., Leidy C., Peters G.H. The effect of calcium on the properties of charged phospholipid bilayers. Biochim. Biophys. Acta. 2006;1758:573–582. doi: 10.1016/j.bbamem.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 33.Ishiyama T., Shirai S., Morita A. Molecular dynamics study of structure and vibrational spectra at zwitterionoic lipid/aqueous KCl, NaCl, and CaCl2 solution interfaces. J. Chem. Phys. 2018;148:222801. doi: 10.1063/1.5006543. [DOI] [PubMed] [Google Scholar]
- 34.Catte A., Girych M., Vilov S. Molecular electrometer and binding of cations to phospholipid bilayers. Phys. Chem. Chem. Phys. 2016;18:32560–32569. doi: 10.1039/c6cp04883h. [DOI] [PubMed] [Google Scholar]
- 35.Melcr J., Martinez-Seara H., Ollila O.H.S. Accurate binding of sodium and calcium to a POPC bilayer by effective inclusion of electronic polarization. J. Phys. Chem. B. 2018;122:4546–4557. doi: 10.1021/acs.jpcb.7b12510. [DOI] [PubMed] [Google Scholar]
- 36.Pabst G., Hodzic A., Laggner P. Rigidification of neutral lipid bilayers in the presence of salts. Biophys. J. 2007;93:2688–2696. doi: 10.1529/biophysj.107.112615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volkov V.V., Nuti F., Righini R. Hydration and hydrogen bonding of carbonyls in dimyristoyl-phosphatidylcholine bilayer. J. Am. Chem. Soc. 2006;128:9466–9471. doi: 10.1021/ja0614621. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh A., Ostrander J.S., Zanni M.T. Watching proteins wiggle: mapping structures with two-dimensional infrared spectroscopy. Chem. Rev. 2017;117:10726–10759. doi: 10.1021/acs.chemrev.6b00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volkov V.V., Chelli R., Righini R. Electrostatic interactions in phospholipid membranes revealed by coherent 2D IR spectroscopy. Proc. Natl. Acad. Sci. USA. 2007;104:15323–15327. doi: 10.1073/pnas.0706426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kel O., Tamimi A., Fayer M.D. Ultrafast structural dynamics inside planar phospholipid multibilayer model cell membranes measured with 2D IR spectroscopy. J. Am. Chem. Soc. 2013;135:11063–11074. doi: 10.1021/ja403675x. [DOI] [PubMed] [Google Scholar]
- 41.Stevenson P., Tokmakoff A. Ultrafast fluctuations of high amplitude electric fields in lipid membranes. J. Am. Chem. Soc. 2017;139:4743–4752. doi: 10.1021/jacs.6b12412. [DOI] [PubMed] [Google Scholar]
- 42.Volkov V.V., Chelli R., Righini R. Domain formation in lipid bilayers probed by two-dimensional infrared spectroscopy. J. Phys. Chem. B. 2006;110:1499–1501. doi: 10.1021/jp056396o. [DOI] [PubMed] [Google Scholar]
- 43.Blume A., Hübner W., Messner G. Fourier transform infrared spectroscopy of 13C = O-labeled phospholipids hydrogen bonding to carbonyl groups. Biochemistry. 1988;27:8239–8249. doi: 10.1021/bi00421a038. [DOI] [PubMed] [Google Scholar]
- 44.Flach C.R., Mendelsohn R. A new infrared spectroscopoic marker for cochleate phases in phosphatidylserine-containing model membranes. Biophys. J. 1993;64:1113–1121. doi: 10.1016/S0006-3495(93)81477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edington S.C., Gonzalez A., Baiz C.R. Coordination to lanthanide ions distorts binding site conformation in calmodulin. Proc. Natl. Acad. Sci. USA. 2018;115:E3126–E3134. doi: 10.1073/pnas.1722042115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klauda J.B., Venable R.M., Pastor R.W. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B. 2010;114:7830–7843. doi: 10.1021/jp101759q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacKerell A.D., Bashford D., Karplus M. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 48.Durell S.R., Brooks B.R., Ben-Naim A. Solvent-induced forces between two hydrophilic groups. J. Phys. Chem. 1994;98:2198–2202. [Google Scholar]
- 49.Venable R.M., Luo Y., Pastor R.W. Simulations of anionic lipid membranes: development of interaction-specific ion parameters and validation using NMR data. J. Phys. Chem. B. 2013;117:10183–10192. doi: 10.1021/jp401512z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim S., Patel D.S., Im W. Bilayer properties of lipid a from various gram-negative bacteria. Biophys. J. 2016;111:1750–1760. doi: 10.1016/j.bpj.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee J., Cheng X., Im W. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J. Chem. Theory Comput. 2016;12:405–413. doi: 10.1021/acs.jctc.5b00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu E.L., Cheng X., Im W. CHARMM-GUI membrane builder toward realistic biological membrane simulations. J. Comput. Chem. 2014;35:1997–2004. doi: 10.1002/jcc.23702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jo S., Lim J.B., Im W. CHARMM-GUI membrane builder for mixed bilayers and its application to yeast membranes. Biophys. J. 2009;97:50–58. doi: 10.1016/j.bpj.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DePalma J.W., Kelleher P.J., Johnson M.A. Coordination-dependent spectroscopic signatures of divalent metal ion binding to carboxylate head groups: H2- and He-tagged vibrational spectra of M2+·RCO2¯ (M = Mg and Ca, R = -CD3, -CD2CD3) complexes. J. Phys. Chem. Lett. 2017;8:484–488. doi: 10.1021/acs.jpclett.6b02964. [DOI] [PubMed] [Google Scholar]
- 55.Sutton C.C., da Silva G., Franks G.V. Modeling the IR spectra of aqueous metal carboxylate complexes: correlation between bonding geometry and stretching mode wavenumber shifts. Chemistry. 2015;21:6801–6805. doi: 10.1002/chem.201406516. [DOI] [PubMed] [Google Scholar]
- 56.Deacon G.B., Phillips R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980;33:227–250. [Google Scholar]
- 57.Dunkelberger E.B., Grechko M., Zanni M.T. Transition dipoles from 1D and 2D infrared spectroscopy help reveal the secondary structures of proteins: application to amyloids. J. Phys. Chem. B. 2015;119:14065–14075. doi: 10.1021/acs.jpcb.5b07706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kwak K., Park S., Fayer M.D. Frequency-frequency correlation functions and apodization in two-dimensional infrared vibrational echo spectroscopy: a new approach. J. Chem. Phys. 2007;127:124503. doi: 10.1063/1.2772269. [DOI] [PubMed] [Google Scholar]
- 59.Hamm P., Zanni M.T. Cambridge University Press; Cambridge, UK: 2011. Concepts and Methods of 2D Infrared Spectroscopy. [Google Scholar]
- 60.Ganim Z., Tokmakoff A. Spectral signatures of heterogeneous protein ensembles revealed by MD Simulations of 2DIR spectra. Biophys. J. 2006;91:2636–2646. doi: 10.1529/biophysj.106.088070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng J., Kwak K., Fayer M.D. Ultrafast 2D IR vibrational echo spectroscopy. Acc. Chem. Res. 2007;40:75–83. doi: 10.1021/ar068010d. [DOI] [PubMed] [Google Scholar]
- 62.Hochstrasser R.M. Two-dimensional spectroscopy at infrared and optical frequencies. Proc. Natl. Acad. Sci. USA. 2007;104:14190–14196. doi: 10.1073/pnas.0704079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo Q., Pagano P., Cheatum C.M. Line shape analysis of two-dimensional infrared spectra. J. Chem. Phys. 2015;142:212427. doi: 10.1063/1.4918350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fenn E.E., Fayer M.D. Extracting 2D IR frequency-frequency correlation functions from two component systems. J. Chem. Phys. 2011;135:074502. doi: 10.1063/1.3625278. [DOI] [PubMed] [Google Scholar]
- 65.Mukamel S. Multidimensional femtosecond correlation spectroscopies of electronic and vibrational excitations. Annu. Rev. Phys. Chem. 2000;51:691–729. doi: 10.1146/annurev.physchem.51.1.691. [DOI] [PubMed] [Google Scholar]
- 66.Bagchi S., Falvo C., Hochstrasser R.M. 2D-IR experiments and simulations of the coupling between amide-I and ionizable side chains in proteins: application to the Villin headpiece. J. Phys. Chem. B. 2009;113:11260–11273. doi: 10.1021/jp900245s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.la Cour Jansen T., Knoester J. A transferable electrostatic map for solvation effects on amide I vibrations and its application to linear and two-dimensional spectroscopy. J. Chem. Phys. 2006;124:044502. doi: 10.1063/1.2148409. [DOI] [PubMed] [Google Scholar]
- 68.Fried S.D., Bagchi S., Boxer S.G. Measuring electrostatic fields in both hydrogen-bonding and non-hydrogen-bonding environments using carbonyl vibrational probes. J. Am. Chem. Soc. 2013;135:11181–11192. doi: 10.1021/ja403917z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang X., Kumar R., Kuroda D.G. Acetate ion and its interesting solvation shell structure and dynamics. J. Chem. Phys. 2018;148:094506. [Google Scholar]
- 70.Edington S.C., Baiz C.R. Vibrational relaxation in EDTA is ion-dependent. J. Phys. Chem. A. 2018;122:6585–6592. doi: 10.1021/acs.jpca.8b06075. [DOI] [PubMed] [Google Scholar]
- 71.Binder H., Zschörnig O. The effect of metal cations on the phase behavior and hydration characteristics of phospholipid membranes. Chem. Phys. Lipids. 2002;115:39–61. doi: 10.1016/s0009-3084(02)00005-1. [DOI] [PubMed] [Google Scholar]
- 72.Hübner W., Mantsch H.H. Orientation of specifically 13C=O labeled phosphatidylcholine multilayers from polarized attenuated total reflection FT-IR spectroscopy. Biophys. J. 1991;59:1261–1272. doi: 10.1016/S0006-3495(91)82341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stevenson P., Tokmakoff A. Infrared insights into the effect of cholesterol on lipid membranes. Chem. Phys. 2018;512:146–153. [Google Scholar]
- 74.Reppert M., Tokmakoff A. Computational amide I 2D IR spectroscopy as a probe of protein structure and dynamics. Annu. Rev. Phys. Chem. 2016;67:359–386. doi: 10.1146/annurev-physchem-040215-112055. [DOI] [PubMed] [Google Scholar]
- 75.Okur H.I., Kherb J., Cremer P.S. Cations bind only weakly to amides in aqueous solutions. J. Am. Chem. Soc. 2013;135:5062–5067. doi: 10.1021/ja3119256. [DOI] [PubMed] [Google Scholar]
- 76.Schultz Z.D., Pazos I.M., Levin I.W. Magnesium-induced lipid bilayer microdomain reorganizations: implications for membrane fusion. J. Phys. Chem. B. 2009;113:9932–9941. doi: 10.1021/jp9011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kerek E., Hassanin M., Prenner E.J. Inorganic mercury and cadmium induce rigidity in eukaryotic lipid extracts while mercury also ruptures red blood cells. Biochim. Biophys. Acta. 2018;1860:710–717. doi: 10.1016/j.bbamem.2017.12.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.