ABSTRACT
Long non-coding RNAs (lncRNAs) have been shown to play a significant role in the progression of many cancers, including pancreatic cancer (PC). However, the biological function and regulatory mechanisms of lncRNAs in PC remains largely unclear. The aim of this study was to identify and evaluate the potential functions of lncRNAs in PC and reveal the underlying mechanisms of their effects. Screening of published microarray data (GEO accession Nos. GSE16515 and GSE32688), revealed lncRNA AFAP1-AS1 to be one of the most upregulated lncRNAs in PC tissues. High expression of AFAP1-AS1 was correlated with advanced stages, tumor size and lymph node metastasis, as well as with poorer overall survival in patients with PC. Functionally, knockdown of AFAP1-AS1 by transfection with siRNA inhibited the proliferative and invasive capacities of PaCa-2 and SW1990 PC cells, promoted apoptosis of PC cells in vitro, and impaired in-vivo tumorigenicity. In particular, it was hypothesized that AFAP1-AS1 may act as a competitive endogenous RNA (ceRNA), effectively becoming a sink for miR-133a whose expression was found to be downregulated in PC tissues and cell lines, and which was negatively correlated with the expression of AFAP1-AS1. We also found that the IGF1R oncogene which is an important regulator of MEK/ERK signaling pathway, was positively regulated by AFAP1-AS1 through ameliorating miR-133a-mediated IGF1R repression in PC tissues. Moreover, we demonstrated that knockdown of IGF1R by transfection with si-IGF1R suppressed cell proliferation, invasion and migration of PaCa-2 and SW1990 PC cells, suggesting that IGF1R may function as an oncogene in PC cells. Further investigations revealed that miR-133a reversed the biological effects of AFAP1-AS1 on PC cells. Collectively, the findings provide new evidence that AFAP1-AS1 could regulate the progression of pancreatic cancer by acting as a ceRNA, and suggest it has potential for use as both a biomarker for the early detection PC and for the development of individualized therapies for PC.
Keywords: Pancreatic cancer, LncRNA AFAP1-AS1/miR-133a/IGF1R axies, competitive endogenous RNA (ceRNA)
Introduction
Pancreatic cancer (PC) is one of the leading causes of cancer-related mortality worldwide. In 2017, there were an estimated 43,090 deaths due to pancreatic cancer in the United States [1]. Despite considerable efforts in recent years to improve clinical treatment of PC, there has been little improvement in disease prognosis, largely because it is a highly invasive and metastatic cancer [2]. Therefore, there is an urgent need to enhance current understanding of the underlying mechanisms associated with PC, in order to inform and improve current therapeutic approaches.
Long non-coding RNAs (lncRNAs) are a diverse class of RNA transcripts with a length of more than 200 nucleotides that lack a significant protein-coding capacity. Increasing evidence has demonstrated that lncRNAs play important roles in multiple biological processes including cell proliferation, invasion, metastasis and apoptosis [3–5]. Dysregulated lncRNA expression has been reported in a variety of human cancers, including nasopharyngeal carcinoma (NPC), lung, breast and colorectal cancers. In particular, a number of specific lncRNAs, such as HOTAIR, MALAT1 and MEG3 are emerging as critical regulators of tumor progression and metastasis [6–10]. However, to date, only a few studies have focused on the roles of lncRNAs in PC.
Among the thousands of lncRNAs that have been identified in human cells, only a relatively small number of lncRNAs have been shown to play crucial roles in a range of biological processes by modulating gene expression at the epigenetic, transcriptional and posttranscriptional levels [11,12]. Recently, certain lncRNAs have also been reported to serve as competing endogenous RNAs (ceRNAs; also known as micro RNA (miRNA) sponges) for shared to sponge miRNAs, thereby regulating the expression of target gene [13]. Li et al. demonstrated that lncRNA NORAD effectively acted as an effective sink for miR-125a-3p, thereby increasing the expression of RhoA, and promoting the migration and invasion of PC cells [14]. Cao et al. found that lncRNA growth arrest-specific transcript 5 (GAS5) antagonized the chemoresistance of PC cells through the down-regulation of miR-181c-5p [15]. These studies collectively suggest that lncRNAs function as ceRNAs and are involved in PC.
In the present study, we found that the AFAP1-AS1 RNA gene was significantly upregulated in PC tissues and cell lines. Upregulation of AFAP1-AS1 was also found to be associated with poor prognosis in PC patients. Further, knockdown of AFAP1-AS1 suppressed cell proliferation and invasion, and induced cell apoptosis in vitro as well as inducing tumor growth in vivo. Further investigation revealed that AFAP1-AS1 could act as a ceRNA for miR-133a and thereby modulate the expression of the IGF1R oncogene. Taken together, these results implicate AFAP1-AS1 as being involved in the regulation of pancreatic carcinogenesis by acting as a ceRNA and therefore suggest that it may have potential as a therapeutic target for the treatment of PC.
Materials and methods
Patients and samples
Pancreatic cancer and matched adjacent non-tumor tissues from 63 patients were obtained during surgery at the Department of Hepatology and the Department of Hepatopancreatobiliary Surgery, Shanghai East Hospital, Tongji University School of Medicine between January 2015 and December 2016. All patient characteristics are presented in Supplementary Table 1. The patients provided signed, informed consent for their tissues to be used for scientific research. Ethical approval for the study was obtained from the Shanghai East Hospital, Tongji University School of Medicine. Diagnosis was based on pathological evidence, and the specimens were immediately snap-frozen and stored at −80°C prior to microarray and real-time PCR analyses.
Choice of differentially expressed lncRNAs list using heat map analysis
We obtained the microarray date from Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/), and the GEO accession number are GSE16515 and GSE32688. The date was generated using the genechip Affymetrix Human Genome U133 Plus 2.0 Array GPL570 (HG-U133¬_Plus_2), which completely coverage Human Genome U133 Set plus 6500 additional genes for analysis of over 47,000 transcripts. Observations with adjusted p-values ≥ 0.05 were removed, and thus excluded from further analysis. The heat map of the 48 lncRNAs most obvious differences was created using a method of hierarchical clustering by GeneSpring GX, version 7.3 (Agilent Technologies, California, United States).
Quantitative real-time PCR analysis
Total RNA from pancreatic cancer and matched adjacent non-tumor tissues was isolated using TRIzol (Invitrogen, CA) according to manufacturer’s instructions. After reverse transcription, cDNA was amplified by using SYBR-Green Premix (Takara, Otsu, Japan). The qRT-PCR assays were carried out using SYBR Green Master Mixture (Roche) reagent on a 7500 Fast Real-Time PCR System (Applied Biosystems, USA). The data were analyzed by delta Ct method. The sequences of primers were purchased from Guangzhou RiboBio Co.Ltd: lncRNA AFAP1-AS1, forward 5′-AATGGTGGTAGGAGGGAGGA-3' and reverse 5'-CACACAGGGGAATGAAGAGG-3'; IGF1R, forward 5' CCGCTGCCAGAAAATGTGCCCA-3' and reverse 5'-TGTCGTTGTCAGGCG CGCTG-3'; GAPDH forward, 5'-GAAGATGGTGATGGGATTTC-3', and reverse, ‘-GAAGGTGAAGGTCGGAGT-3'; miR-133a forward, 5'-TTTGGTCCCCTTCAACC-3' and reverse, 5'-GTGCAGGGTCCGAGGT-3'; U6 forward, 5'-AAAGACCTGTACGCCAACAC-3' and reverse, 5'-GTCATACTCCTGCTTGCTGAT-3'. The expression of miR-133a and lncRNA AFAP1-AS1 in tissue was normalized to the expression of U6 and GAPDH, respectively. The data were analyzed by delta Ct method.
Cell lines and cell culture
The human pancreatic cancer cell lines AsPC-1, BxPC-3, PANC-1, PaCa-2 and SW1990 were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and were cultured as ATCC protocol described. Primary cultures of normal human pancreatic duct epithelial cells (HPDE6c7) were obtained from fresh specimens of the adjacent non-tumor pancreatic tissue following the procedures previously reported [16,17], and maintained in bronchial epithelial basal medium (BEBM; Lonza Walkersville, Walkersville, MD) containing fetal bovine serum (Gibco, Grand Island, NY, USA). All cells were incubated in a humidified atmosphere of 5% CO2 at 37°C.
Cell transfection
The miR-133a mimics, mimics negative control (mimics NC), miR-133a inhibitor, and inhibitor NC were bought from GenePharm (Shanghai, China). The efficiency of inhibitor and mimics was validated by qRT-PCR (data not shown). In addition, AFAP1-AS1 siRNA, IGF1R siRNA and si-scramble were purchased from Applied Biosystems (Foster City, CA, USA). The effective sequences were as follows: 5'-CCCTTTGAGGCACACGGCTTATAAT −3' (AFAP1-AS1-siRNA1), 5'- TGTCTGAAATTTGCTTCCTTCTCTA −3' (AFAP1-AS1-siRNA2), 5'- GCCATGTCATCTGACTGGCTCTGAA −3' (AFAP1-AS1-siRNA3), 5'- CCCGAGTCACGGGCAATTCTTTAAT – 3' (Scramble siRNA); 5'-GGACGAGATGGAGGCGGGCTTCCGG −3' (IGF1R-siRNA1), 5'-AGGCGGGCTTCCGGGAGGTCTCCTT −3' (IGF1R-siRNA2), 5'- GCGGGCTTCCGGGAGGTCTCCTTCT-3' (IGF1R-siRNA3), 5'-GGAAGAGGTGGAGGCTCGCTCGCGG −3' (Scramble siRNA). BxPC-3, PaCa-2 and SW1990 cells (1.0 × 106 per well) were seeded and grown overnight in six-well plates. The next day, transfection was performed using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) following manufacturer’s instructions.
Lentivirus production and infection
To overexpress AFAP1-AS1 in PaCa-2 and SW1990 cells, the lentiviral packaging kit was used (Thermo Fisher Scientific). Lentivirus carrying AFAP1-AS1 or negative control was packaged using HEK293T cells following the manufacturer’s manual. The lentiviral vector has red fluorescent protein (RFP) tag which can be used to check the efficiency of packaging using microscope. After 48 h, the supernatants of the cell culture were harvested and filtered through a 0.22-μm-pore-size membrane, and the filtrate was ultracentrifuged to concentrate the recombinant lentiviruses, and they are named as Lv-AFAP1-AS1 and Lv-control. PaCa-2 and SW1990 cells were transduced with the lentiviruses at an MOI of 10 transducing units (TU) and the cells were harvested 72 h later and analyzed for qRT-PCR and Western Blot.
Cell proliferation
PaCa-2 and SW1990 cells (5 × 103 per well) were suspended in DMEM medium (Invitrogen, Carlsbad, CA, USA) containing 10% FBS and cultured in 96-well plates overnight and then transfected with si-AFAP1-AS1, si-AFAP1-AS1 plus miR-133a inhibitor or negative control oligonucleotides for 1, 2, 3 and 4 days, respectively. The cell viability was determined by using a cell counting Kit-8 (Beyotime, Jiangsu, China). Briefly, 10 μl CCK-8 solution was added to each well and incubated at 37°C in a CO2 cell incubator for 90 min, then the absorbance rates were measured at 450 nm using a microplate reader (Infinite M200; Tecan, Austria). All experiments were performed in triplicate.
Cell apoptosis
PaCa-2 and SW1990 cells (1.0 × 106 per well) were suspended in DMEM medium (100 μl) containing 10% FBS and cultured in 96-well plates overnight and then transfected with si-AFAP1-AS1, si-AFAP1-AS1 plus miR-133a inhibitor or negative control oligonucleotides for 48 h, and apoptosis assay was performed with Annexin V-FITC Apoptosis Detection Kit (Abcam, Cambridge, UK) according to the manufacturer’s instructions. The cells were harvested and washed twice with PBS, and then the cells were stained with Annexin V and propidium iodide. After incubation at room temperature in the dark for 15 minutes, cell apoptosis was analyzed on a FACScan flow cytometer (FCM; Bechman Coulter, CA).
Wound-healing assay
PaCa-2 and SW1990 cells (1.0 × 106 per well) were cultured in six-well plates overnight and then transfected with si-AFAP1-AS1, si-AFAP1-AS1 plus miR-133a inhibitor or negative control oligonucleotides for 24 h. Upon reaching ~ 70–80% confluence, the cell layer was gently and slowly scratched with a new 1 ml pipette tip across the center of the well and then immediately washed with growth medium twice to remove the detached cells and cultured again in DMEM medium at 37°C in a humidified incubator with 5% CO2 for another 48 h. Then, the cells were fixed with 3.7% paraformaldehye for 30 min after washing twice with PBS, and then stained with 1% crystal violet in 2% ethanol for 30 min. The wound area was measured and the percentage of the wound healing was calculated by Image J software (NIH, Bethesda, MD, USA).
Transwell invasion assays
Cell invasion assays were performed using 24-well transwell chambers (pore size 8 μm; Corning, Inc., Corning, NY). Briefly, the transfected PaCa-2 and SW1990 cells were seeded on the top side of the membrane pre-coated with Matrigel (BD, Franklin Lakes, NJ, USA). At the end of the experiments, the cells on the upper surface of the membrane were removed using cotton buds, and the cells on the lower surface of the insert were fixed and stained with 5% crystal violet. Five visual fields of each insert were randomly chosen and photographed under a light microscope at 200 × magnification. The cells in the photographs were counted, and the data were summarized and presented as a percentage of controls.
Luciferase reporter assay
Two luciferase reporters containing wild-type AFAP1-AS1 (psiCHECK2-AFAP1-AS1-WT, which encompassed the binding sites for miR-133a-5p: ACCAGCU) or mutant AFAP1-AS1 (psiCHECK2-AFAP1-AS1-Mu, which encompassed the mutant sequence of the binding sites for miR-133a-5p: GAUCCAG) were constructed to validate the interaction between AFAP1-AS1 and miR-133a-5p (as shown in Figure 1(a)). HEK-293 cells were cultured in six-well plates and transfected with miR-133a mimics or mimics NC (50 nM) and wild-type (WT) or mutant (Mut) AFAP1-AS1 using Lipofectamine 2000. After 48 h of transfection, a Dual-Luciferase Reporter System (Promega, Madison, WI, USA) was used to evaluate the relative luciferase activity of HEK-293 cells according to the manufacturer’s protocol. Luciferase activity was normalized to Renilla luciferase activity.
Figure 1.
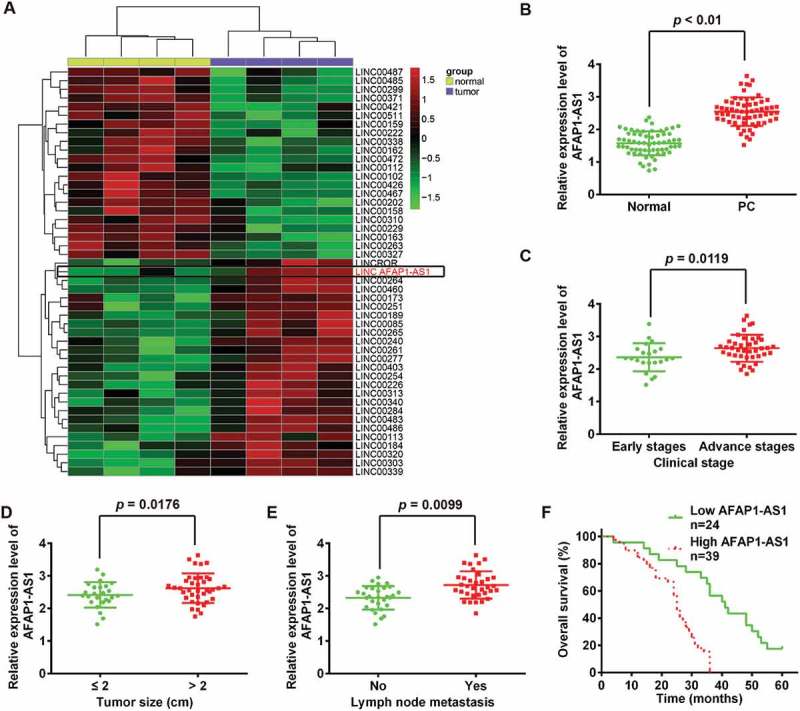
LncRNA AFAP1-AS1 was upregulated in pancreatic cancer (PC) tissues and correlated with poor prognosis in PC patients. (a) Heatmap of 48 lncRNAs from two human PC microarray GEO DataSets (GSE16515 and GSE32688). (b) Relative expression levels of AFAP1-AS1 in 63 paired cancerous and peritumoral normal tissues samples. p < 0.01 vs. Normal tissue group. (c) Relative expression levels of AFAP1-AS1 in early stages of PC patients and advanced stage of PC patients. (early stages vs. advanced stages, p = 0.0119). (d) Association between AFAP1-AS1 expression level and tumor size (< 2 vs. ≥ 2 cm, p = 0.0176). (e) Relative expression level of AFAP1-AS1 in PC patients with/without lymph node metastasis. (with vs. without, p = 0.0099). (f) Kaplan-Meier analysis of overall survival time in PC patients with high and low AFAP1-AS1 levels (n = 39 and n = 24, respectively).
Western blot
Total protein was extracted using radio immunoprecipitation assay (RIPA) lysis buffer (Beyotime Biotechnology, Shanghai, China). Concentrations of total cellular protein were determined using a BCA assay kit (Pierce, Rockford, IL, USA). Total protein samples (40 μg) were analyzed by 8% SDS-PAGE gel and transferred to polyvinylidene difluoride (PVDF) membranes (GE Healthcare, Freiburg, DE) by electroblotting. Primary antibodies against IGF1R (Abcam, Cambridge, MA, USA, 1:1,000 dilution), E-cadherin (Cell Signaling Technology, 1:1,000 dilution), N-cadherin (Thermo Fisher Scientific, 1:1,000 dilution), fibronectin and β-actin (Santa Cruz Biotechnology, 1:2000 dilution) were probed with proteins on the membrane at 4°C overnight. After incubating with secondary antibodies (1:10,000, Cell Signaling Technology, Danvers, MA), Bands were detected by enhanced chemiluminescence (ECL) kit (GE Healthcare, Freiburg, DE). The intensity of the bands of interest was analyzed by ImageJ software (Rawak Software, Inc. Munich, Germany).
Establishment of tumor xenografts in nude mice
For animal experiment, 6 week old female nude mice were used, which from the Weitong Lihua Laboratory Animal Center (Beijing, China). Mice were fed for one week in a specific pathogen-free animal cage before intervention. PaCa-2 and SW1990 cells were transfected with si-AFAP1-AS1 or si-Scramble using Lipofectamine 2000. After 48 h, PaCa-2 and SW1990 cells were collected and subcutaneously injected into the right flank of each mouse at a density of 5 × 106 (100 μL). Mouse weights were measured at 21 days after injection. All animal experiments were approved by the Institutional Committee for Animal Research and followed the national guidelines for the care and use of laboratory animals (GB14925-2010).
Statistical analysis
Statistical analysis was performed using the SPSS program (version 18.0; SPSS, Chicago, IL, USA). Data were presented as mean ± S.D. Student’s t-test or one-way ANOVA were used to analyze the difference among/between sample groups. Pearson’s or Spearman’s analysis was used in correlation analysis. P ≤ 0.05 was considered as statistically significant.
Results
LncRNA AFAP1-AS1 was upregulated in pancreatic cancer tissues and correlated with clinicopathological features of pancreatic cancer
We first investigated and sought to identify lncRNAs in pancreatic cancer (PC) tissues via retrieving the relevant microarray data from the GEO dataset. Two human PC DataSets (Accession Nos. GSE16515 and GSE32688) were selected and analyzed for consistently aberrantly expressed lncRNAs between pancreatic tumor tissues and normal (healthy) pancreatic tissues using the Affymetrix HG U133 Plus 2.0 gene chip platform [18,19]. As shown in Figure 2(a), of 48 lncRNAs the 48 lncRNAs that were identified as inconsistently expressed between these two patient groups, 26 were upregulated and 22 were downregulated in pancreatic tumor tissues. Of the upregulated lncRNAs, AFAP1-AS1 was identified as being one of the most markedly upregulated lncRNAs in both DataSets. Of relevance, AFAP1-AS1 has previously been reported to function as an oncogene in a variety of cancers, including lung cancer [20] and nasopharyngeal carcinoma [21]. A recent study by Ye et al. showed that elevated expression of AFAP1-AS1 was associated with poor survival and short-term recurrence in pancreatic ductal adenocarcinoma (being present in 90% of PC cases) [22]. However, the function and molecular mechanisms of AFAP1-AS1 in regulating tumor cells remains unclear. These existing findings underpinned our decision to select AFAP1-AS1 as a promising candidate for further research in relation to its involvement in the pathogenesis of PC. To further verify the dysregulation of AFAP1-AS1, we further performed qRT-PCR analysis based on 63 paired cancerous and peritumoral normal tissues samples. The results showed that AFAP1-AS1 was significantly upregulated in PC tissues (Figure 2(b)).
Figure 2.
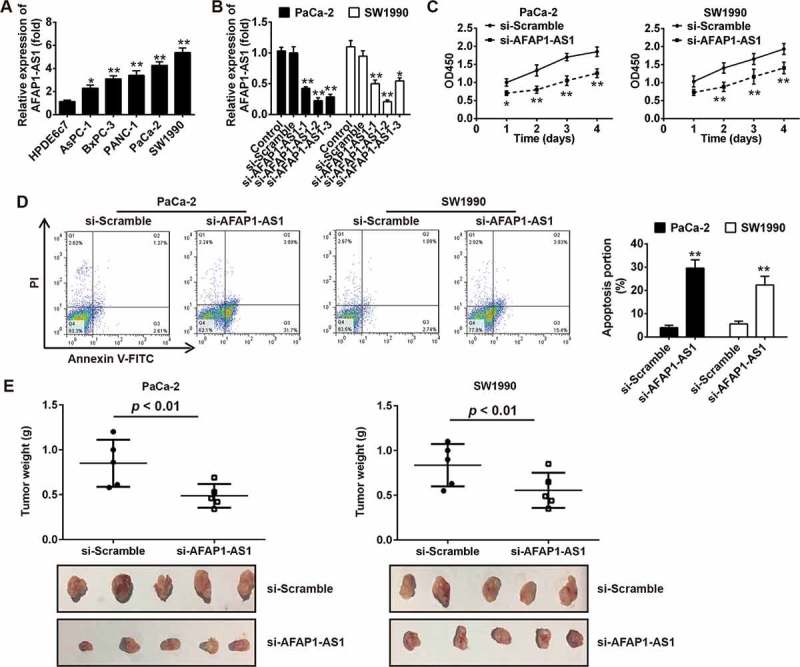
Knockdown of AFAP1-AS1 suppressed tumor growth in vitro and in vivo. (a) RT-PCR analysis of AFAP1-AS1 expression levels in PC cells (AsPC-1, BxPC-3, PANC-1, PaCa-2 and SW1990) and primary cultures of normal human pancreatic duct epithelial cells (HPDE6c7) used as a control. Data are represented as means ± S.D. from three independent experiments, *p < 0.05, **p < 0.01 vs. HPDE6c7. (b) qRT-PCR analysis of AFAP1-AS1 expression levels in PaCa-2 and SW1990 cells after siRNA transfection. Data are represented as the mean ± S.D. from three independent experiments, *p < 0.05, **p < 0.01 vs. si-Scramble or control. (c) The effect of AFAP1-AS1 knockdown on the proliferation of pancreatic cancer cells was determined by CCK-8 assays. Data are represented as the mean ± S.D. from three independent experiments, *p < 0.05, **p < 0.01 vs. si-Scramble. (d) The effect of AFAP1-AS1 knockdown on the cell apoptosis was measured by flow cytometry. Data are represented as the mean ± S.D. from three independent experiments, **p < 0.01 vs. si-Scramble. (e) Tumor weight changes in the mice bearing PaCa-2 and SW1990 cells with si-AFAP1-AS1 or si-Scramble. si-AFAP1-AS1 vs. si-Scramble, p < 0.01.
To determine whether the expression of AFAP1-AS1 was correlated with the clinical outcome in PC patients, tissue samples from 63 PC patients were divided into two groups based on their (relative) expression levels of AFAP1-AS1: AFAP1-AS1 high expression group and AFAP1-AS1 low expression group. The relationships between the level of AFAP1-AS1 (high or low) and certain patient clinicopathological features are summarized in Table 1. High AFAP1-AS1 expression was found to be associated with advanced TNM stage, tumor size and lymph node metastasis (Figure 2(c–e)), whilst no significant correlations with gender, age, number of tumors, differentiation, T classification or distant metastasis were observed (Table 1). Further, compared with the patients in the low AFAP1-AS1 expression group, patients in high AFAP1-AS1 expression group were found to have a shorter 5-year overall survival (OS) rate (Figure 2(f)). Collectively, these results indicated that AFAP1-AS1 could potentially serve as an effective biomarker for the clinical prognosis of PC patients.
Table 1.
Correlation between lncRNA AFAP1-AS1 expression and clinicopathological features of pancreatic cancer patients.
| Feature | Total n = 63 |
LncRNA AFAP1-AS1 |
P value | |
|---|---|---|---|---|
| High No. cases | Low No. cases | |||
| Gender | 0.7666 | |||
| Male | 30 | 18 | 12 | |
| Female | 33 | 21 | 12 | |
| Age at presentaion (years) | 0.2278 | |||
| ≤ 60 | 23 | 12 | 11 | |
| > 60 | 40 | 27 | 13 | |
| Tumor number | 0.0987 | |||
| Single | 21 | 10 | 11 | |
| Multiple | 42 | 29 | 13 | |
| T classification | 0.0961 | |||
| T1-2 | 14 | 6 | 8 | |
| T3-4 | 49 | 33 | 16 | |
| Clinical stage | 0.0119* | |||
| Early stages (≤ IIa) | 22 | 9 | 13 | |
| Advanced stages (> IIa) | 41 | 30 | 11 | |
| Differentiation | 0.2557 | |||
| Well and moderate | 31 | 17 | 14 | |
| Poorly | 32 | 22 | 10 | |
| Tumor size (cm) | 0.0176* | |||
| ≤ 2 | 25 | 11 | 14 | |
| > 2 | 38 | 28 | 10 | |
| Lymph node metastasis | ||||
| No | 29 | 13 | 16 | 0.0099** |
| Yes | 34 | 26 | 8 | |
| Distant metastasis | ||||
| Absent | 49 | 28 | 21 | 0.1454 |
| Present | 14 | 11 | 3 | |
*P < 0.05, **P < 0.01
Knockdown of AFAP1-AS1 suppressed tumor growth in vitro and in vivo
Given the apparent up-regulation of AFAP1-AS1 in PC tissues, we predicted that AFAP1-AS1 may function as an oncogene in PC. To verify our hypothesis, we first analyzed the expression of AFAP1-AS1 in five human PC cell lines (AsPC-1, BxPC-3, PANC-1, PaCa-2 and SW1990) and in primary cultures of normal human pancreatic duct epithelial cells (HPD6c7) used as a control. Consistent with our findings from the analysis of AFAP1-AS1 expression in PC tissue samples, the expression of AFAP1-AS1 was found to be markedly upregulated in all PC cell lines compared with expression in HPD6c7 cells (Figure 3(a)). This suggested that AFAP1-AS1 may play an oncogenic role in the initiation and pathological progression of PC. To further evaluate the function of AFAP1-AS1 in PC, we knocked down AFAP1-AS1 expression in PaCa-2 and SW1990 cells using three AFAP1-AS1 targeting short interfering RNAs (siRNAs) that were associated with the highest AFAP1-AS1 level among the five PC cell lines. The results demonstrated that all three siRNAs were effective at knocking down the expression of AFAP1-AS1 and that the most effective siRNA was si-AFAP1-AS1-2. On this basis, this siRNA was selected for further study (Figure 3(b)). We next examined the proliferation and apoptosis of PaCa-2 and SW1990 cells after AFAP1-AS1 knockdown by si-AFAP-AS1-2. As expected, AFAP1-AS1 knockdown significantly decreased the cell proliferation and increased the proportion of apoptotic cells in both PaCa-2 and SW1990 cells (Figure 3(c,d)).
Figure 3.
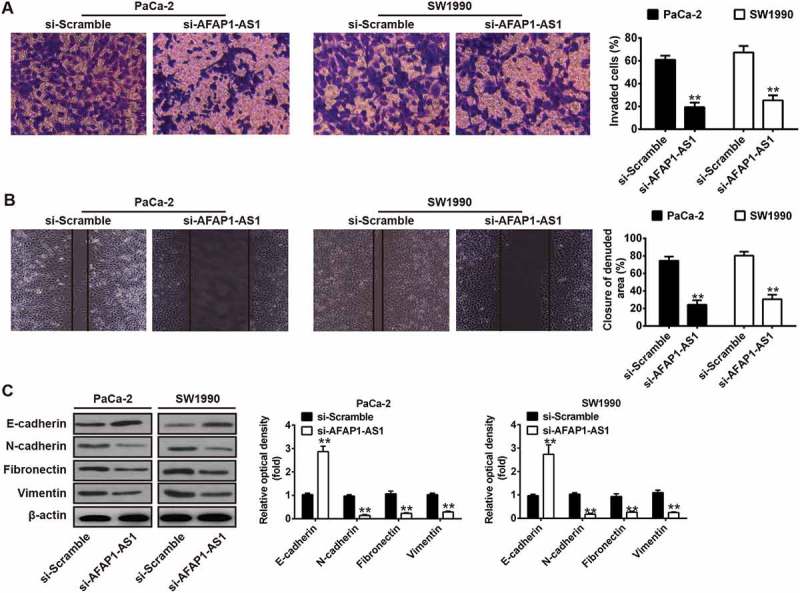
Knockdown of AFAP1-AS1 inhibited tumor metastasis in vitro. PaCa-2 and SW1990 cells were transfected with si-AFAP1-AS1 for 24 h, and then cells were used for further analyses. (a) Cell invasion was determined by transwell assays. (b) Cell migration was detected by wound healing assays. (c) The expressions of EMT markers including E-cadherin, N-cadherin, Vimentin and Fibronectin were measured by Western Blotting. β-actin was used as an internal loading control. Bands were quantitatively compared between groups. Data are represented as means ± S.D. from three independent experiments, **p < 0.01 vs. si-Scramble.
To evaluate the function of AFAP1-AS1 in the pathogenesis of PC in vivo, a PC xenograft mouse model was used. Consistent with findings of the results in vitro, the tumor weight was found to be significantly decreased in mice bearing PaCa-2 and SW1990 cells in which AFAP1-AS1 expression had been inhibited by AFAP1-AS1-2 (Figure 3(e)). Collectively, these data together suggested that AFAP1-AS1 was able to suppress tumor growth both in vitro and in vivo.
Knockdown of AFAP1-AS1 inhibited pancreatic tumor metastasis in vitro
Since the metastatic ability of PC is a critical factor in the poor prognosis of patients [23], we examined whether AFAP1-AS1 could modulate the metastatic ability of PC cells. Transwell invasion assays demonstrated that AFAP1-AS1 siRNA transfected PaCa-2 and SW1990 cells had lower invasive capability (Figure 4(e)). Wound healing assays demonstrated that wound recovery of AFAP1-AS1 siRNA transfected PC cells was significantly delayed when compared with that of si-Scramble treated PC cells (Figure 4(e)). Since it is already known that AFAP1-AS1 plays a crucial role in mediating the epithelial-mesenchymal transition (EMT) progress of colorectal cancer cells [24]. Western blotting was performed to explore whether AFAP1-AS1 also regulates EMT in PC cells. The results demonstrated that knockdown of AFAP1-AS1 in PaCa-2 and SW1990 cells was associated with upregulation of the epithelial marker E-cadherin and with downregulation of certain mesenchymal markers, including N-cadherin, Vimentin and Fibronectin. These results demonstrate that knockdown of AFAP1-AS1 suppressed cell metastasis and the cell motility ability of PC cells by inhibiting the EMT process.
Figure 4.
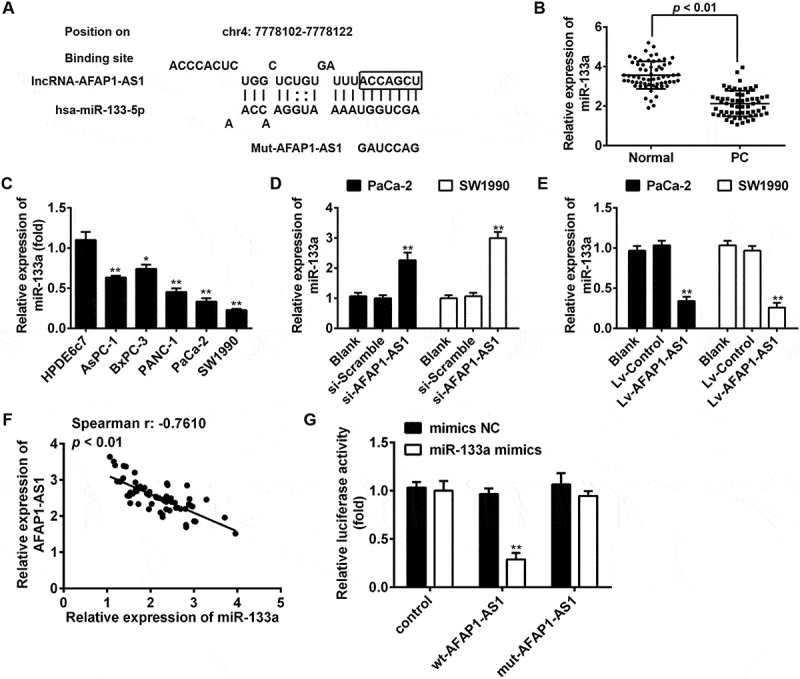
AFAP1-AS1 acts as a ceRNA for miR-133a in PC cells. (a) Schematic representation of the predicted target site for miR-133a in AFAP1-AS1. (b) Relative expression levels of miR-133a in 63 paired cancerous and peritumoral normal tissues. p < 0.01 vs. Normal tissue group. (c) Relative expression levels of miR-133a in pancreatic cancer cells (AsPC-1, BxPC-3, PANC-1, PaCa-2 and SW1990) and primary cultures of normal human pancreatic duct epithelial cells (HPDE6c7) used as a control. Data are represented as the mean ± S.D. from three independent experiments, *p < 0.05, **p < 0.01 vs. HPDE6c7. (d) Relative expression level of miR-133a in PaCa-2 and SW1990 cells after siRNA transfection. Data are represented as the mean ± S.D. from three independent experiments, **p < 0.01 vs. si-Scramble. (e) Relative expression level of miR-133a in PaCa-2 and SW1990 cells after Lv-AFAP1-AS1 infection. Data are represented as the mean ± S.D. from three independent experiments, **p < 0.01 vs. Lv-control. (F) The Spearman’s rank test was used to analyze the relationship between AFAP1-AS1 and miR-133a expression levels in PC tissues (r:-0.7610, p < 0.01). (g) Luciferase reporter assay in human embryonic kidney (HEK) 293T cells, co-transfected with the reporter plasmid (or the corresponding mutant reporter) and the indicated miRNAs. miR-133a mimics significantly decreased the luciferase activity in wt-AFAP1-AS1 but not in mut-AFAP1-AS1. Data are represented as means ± S.D. from three independent experiments, **p < 0.01 vs. mimics NC.
AFAP1-AS1 acted as a competing endogenous RNA (ceRNA) for miR-133a
Accumulating evidence has suggested that lncRNAs might function as competing endogenous RNAs (ceRNAs) by binding to miRNAs and functionally liberating other RNA transcripts (the target genes of the bound miRNAs) [25]. To examine whether AFAP1-AS1 functions by a similar mechanism in PC cells, we used Starbase v2.0 (http://starbase.sysu.edu.cn/) to predict the potential miRNA binding sites in AFAP1-AS1. Among several predicted target genes, miR-133a was identified as a potential candidate miRNA. As shown in Figure 1(a), AFAP1-AS1 contains a sequence that is complementary to that of miR-133a. Moreover, miR-133a has previously been shown to be an important tumor suppressor in many types of cancers, including PC [26,27,28,29]. On this basis, miR-133a was selected for further investigation. Subsequently, miR-133a expression levels were assessed in PC tissues and cell lines. The results of qRT-PCR indicated that miR-133a was significantly downregulated in cancerous tissues and human PC cell lines. The results of qRT-PCR analysis indicated that the expression of miR-133a was significantly downregulated in cancerous tissues and PC cell lines (Figure 1(b,c)), which is consistent with the findings of previous studies [26,30,31]. We subsequently tested the effects of AFAP1-AS1 expression on miR-133a expression. Notably, miR-133a was upregulated in si-AFAP1-AS1-treated PaCa-2 and SW1990 cells compared with negative si-Scramble-treated cells (Figure 1(d)), but was downregulated in lv-AFAP1-AS1-infected PaCa-2 and SW1990 cells compared with lv-control infected cells (Figure 1(e)). Furthermore, we found that the expression of AFAP1-AS1 was negatively associated with the expression of miR-133a in PC tissues (n = 63) (Figure 1(f)).
To confirm our hypothesis that AFAP1-ASl directly binds to miR-133a, a luciferase activity assay was conducted. The results showed that co-transfection of miR-133a mimics and wt-AFAP1-ASl strongly decreased the luciferase activity of human embryonic kidney (HEK) 293T cells compared with co-transfection with mimics-NC and wt-AFAP1-ASl, while co-transfection of miR-133a mimics and mut-AFAP1-ASl did not alter the luciferase activity of HEK cells (Figure 1(g)). These findings strongly suggest that AFAP1-ASl can act as a miRNA decoy for miR-133a.
AFAP1-ASl increases the expression of IGF1R oncogene through acting as a ceRNA of miR-133a
Insulin-like growth factor 1 receptor (IGF1R), a transmembrane heterotetrameric protein, is frequently overexpressed in various cancers and has been shown to act as an important oncogene in the development and maintenance of several cancers through activation of MEK/ERK signaling pathway [32,33]. Notably, several studies have reported that miR-133a functions as a tumor suppressor in a variety of human cancers by targeting IGF1R [26,27,34]. Thus, we hypothesized that AFAP1-ASl might modulate IGF1R by competing with miR-133a. Detection of miR-133a expression level in PC cell lines indicated that expression levels of miR-133a were showed the highest level in BxPC-3 cells and lowest in SW1990 cells. Therefore, BxPC-3 cells were chosen for use in loss-of-function experiments and SW1990 cells for gain-of-function experiments. The results of these experiments demonstrated that the protein expression level of IGF1R was significantly increased after knockdown of miR-133a in BxPC-3 cells, and decreased after overexpression of miR-133a in SW1990 cells. Conversely, knockdown of AFAP1-AS1 decreased the expression of IGF1R and overexpression of AFAP1-AS1 increased the expression of IGF1R. Interestingly, it was also found that the decreased expression of IGF1R induced by knockdown of AFAP1-AS1 could be restored by co-transfecting with si-AFAP1-AS1 and miR-133a inhibitor (Figure 5(a)). In parallel, the enhanced expression of IGF1R that was apparently induced by overexpression of AFAP1-AS1 could be reversed by co-transfecting with lv-AFAP1-AS1 and miR-133a mimics (Figure 5(b)). Furthermore, the expressions of IGF1R in miR-133a inhibitor and si-AFAP1-AS1-transfected AsPC-1 cells and in cells transfected with miR-133a mimics and in Lv-AFAP1-AS1 transfected-PaCa-2 cells were also analyzed by Western Blotting The results showed that the expression of IGF1R was significantly increased in the miR-133a inhibitor group compared with the blank (control) group, while it was markedly decreased in the si-AFAP1-AS1 group compared with the blank (control) group. However, the inhibitory effect of si-AFAP1-AS1 was found to be attenuated by miR-133a inhibitor AsPC-1-transfected cells. Interestingly, the increased expression of IGF1R in lv-AFAP1-AS1 transfected PaCa-2 cells was found to be decreased after transfection with miR-133a mimics (supplement Figure 1). Moreover, we also found that IGF1R was significantly upregulated in PC tissues compared with adjacent normal (healthy) tissues (n = 63) (Figure 5(c)). Furthermore, a positive correlation between the levels of expression of AFAP1-ASl and IGF1R was observed in tissues samples from PC patients (Figure 5(d)). Collectively, these data indicate that AFAP1-ASl increases the expression of IGF1R through acting as a ceRNA for miR-133a.
Figure 5.
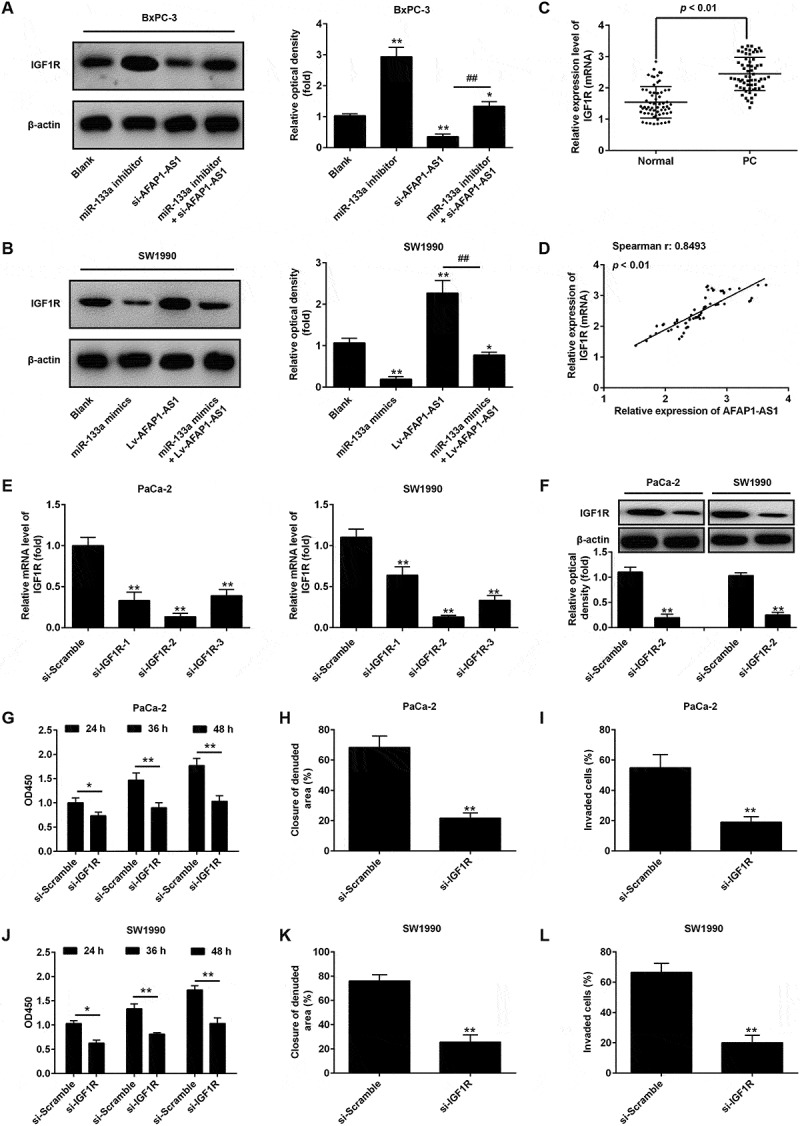
AFAP1-AS1 increases the expression of IGF1R by acting as a ceRNA of miR-133a. BxPC-3 cells were co-transfected with miR-133a inhibitor and si-AFAP1-AS1, while SW1990 cells were co-transfected with miR-133a mimics and Lv-AFAP1-AS1. Cells were then harvested for Western Blotting. (a) The expression of IGF1R protein was measured by Western Blotting in BxPC-3 cells after co-transfection with miR-133a inhibitor and si-AFAP1-AS1. Bands were quantitatively compared between groups. Data are represented as means ± S.D. from three independent experiments, **p < 0.01 vs. Blank group; ##p < 0.01 vs. si-AFAP1-AS1. (b) The expression of IGF1R protein was measured by Western Blotting in SW1990 cells after co-transfection with miR-133a mimics and Lv-AFAP1-AS1. Bands were quantitatively compared between groups. Data are represented as the mean ± S.D. from three independent experiments, **p < 0.01 vs. Blank group; ##p < 0.01 vs. Lv-AFAP1-AS1. (c) Relative expression level of IGF1R in 60 paired cancerous and peritumoral normal tissues. p < 0.01 vs. Normal tissue group. (D) Spearman’s analysis was used to analyze the relationship between AFAP1-AS1 and IGF1R expression levels in PC tissues (r: 0.8493, p < 0.01). (e, f) qRT-PCR and Western Blot analysis of IGF1R expression levels in PaCa-2 and SW1990 cells after siRNA transfection. Data are represented as the mean ± S.D. from three independent experiments, **p < 0.01 vs. si-Scramble. (g, j) The effect of IGF1R knockdown on cell proliferation was determined by CCK-8 assays. Data are represented as the mean ± S.D. from three independent experiments, *p < 0.05, **p < 0.01 vs. si-Scramble. (H, K) The effect of IGF1R knockdown on cell migration was assessed by wound healing assays. Data are represented as the mean ± S.D. from three independent experiments, **p < 0.01 vs. si-Scramble. (i, l) The effect of IGF1R knockdown on cell invasion was assessed by transwell assays. Data are represented as the mean ± S.D. from three independent experiments, **p < 0.01 vs. si-Scramble.
In order to further confirm whether IGF1R mediates the tumorigenic effects of AFAP1-AS1, we used three IGF1R-specific siRNAs to investigate the effects of knocking down IGF1R in PaCa-2 and SW1990 cells that are high expressors of AFAP1-AS1, on cell growth and invasive capability. As shown in Figure 5(e), the results verified that the three siRNAs were effective at knocking down IGF1R expression, with si-IGF1R-2 being the most effective of the three. This siRNA was therefore selected for further studies. The expression of IGF1R protein levels was similarly found to be markedly reduced in PaCa-2 and SW1990 cells after siRNA transfection (Figure 5(f)). The CCK-8 assay was performed and showed that knockdown of IGF1R markedly inhibited cell proliferation in PaCa-2 and SW1990 cells (Figure 5(g,j)). Moreover, wound healing assays showed a significant reduction in cell migration (a measure of the invasive capability of cells) after knockdown of IGF1R in PaCa-2 and SW1990 cells (Figure 5(h,k), and transwell assays indicated that IGF1R-knockdown also inhibited cell invasion (Figure 5(i,l). These data strongly suggest that IGF1R functions as an oncogene in PC cells, and that AFAP1-AS1 may exert its tumorigenic effects via the modulation of IFG1R expression.
AFAP1-AS1 regulates miR‑133a to suppress PC cell proliferation and invasion
Although the preceding experiments had confirmed a functional interaction between AFAP1-AS1 and miR-133a was confirmed, the biological behaviors of PC cells when regulated by AFAP1-AS1 and miR-133a remained unclear. In order to investigate this, PaCa-2 and SW1990 cells were co-transfected with si-AFAP1-AS1 and miR-133a inhibitor, and then cell proliferation, apoptosis, migration and invasion were assessed. The CCK8 assay revealed that the proliferation of PaCa-2 and SW1990 cells in si-AFAP1-AS1 group was decreased in the si-AFAP1-AS1 transfected group compared with the blank (control) group, and that this reduction could be restored by co-transfecting with si-AFAP1-AS1 and miR-133a inhibitor (Figure 6(a,b)). As shown in Figure 6(c,d), the apoptosis rates of PaCa-2 and SW1990 cells in the si-AFAP1-AS1 group were found to be increased compared with the blank (control) group, and this effect could be ameliorated by co-transfecting cells with both si-AFAP1-AS1 and miR-133a inhibitor. Similarly, cell migration and invasion in the si-AFAP1-AS1 transfected groups of PaCa-2 and SW1990 cells were shown to be inhibited, and this inhibition could be reversed by co-transfecting cells with both si-AFAP1-AS1 and miR-133a inhibitor (Figure 6(e–h)). The above data showed that the tumor suppressive effects of AFAP1-AS1 knockdown were mediated by miR-133a in PC cells.
Figure 6.
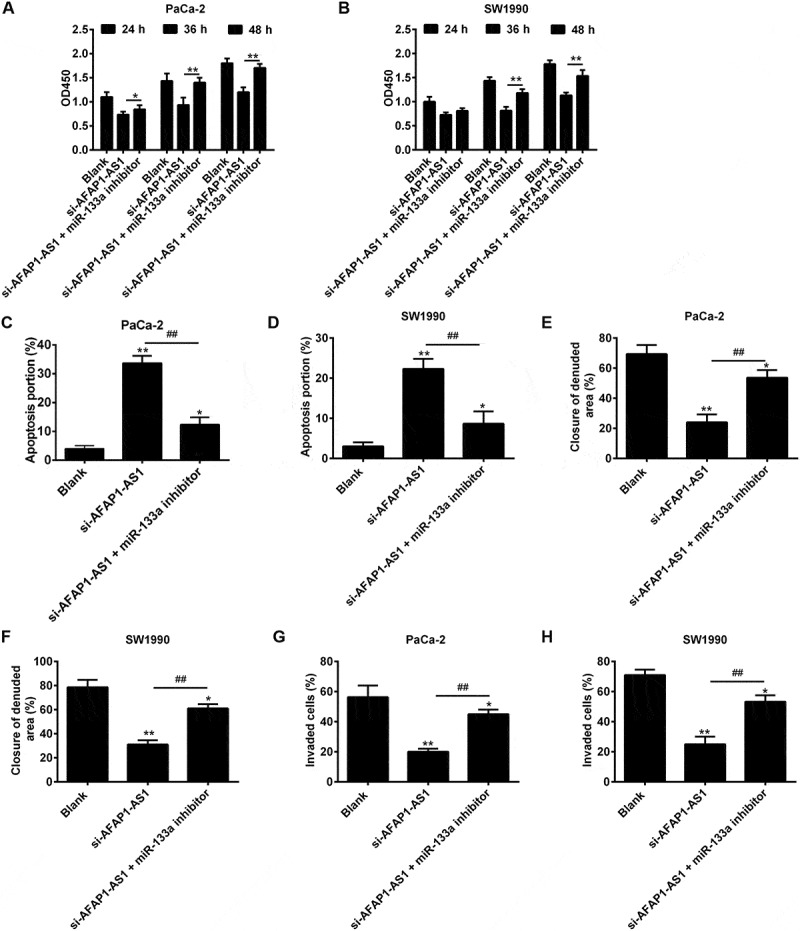
AFAP1-AS1 regulates miR‑133a to suppress PC cell proliferation and invasion. PaCa and SW1990 cells were co-transfected with miR-133a inhibitor and si-AFAP1-AS1. Cells were then harvested for further analyses. (a, b) Cell proliferation was determined by CCK-8 assays. (c, d) Cell apoptosis was measured by flow cytometry. (e, f) Cell migration was detected by wound healing assays. (g, h) Cell invasion was determinned by transwell assays. Data are represented as means ± S.D. from three independent experiments, *p < 0.05, **p < 0.01 vs. Blank group; ##p < 0.01 vs. si-AFAP1-AS1.
Discussion
The present study has demonstrated that AFAP1-AS1 is upregulated in the tumor tissues of PC patients, and that its high expression is correlated with poor prognosis in PC patients. We have also demonstrated, in vitro, that knockdown of AFAP1-AS1 inhibited the proliferation, migration and invasion of PaCa-2 and SW1990 PC cells, and induced apoptosis by acting as a ceRNA for miR-133a. The sponging of miR-133a by AFAP1-AS1 resulted in the loss of the suppressive effects of miR-133a on the downstream target IGF1R oncogene. In this way, it appears that AFAP1-AS1 influences PC progression by regulating cellular expression of IGF1R. On this basis, AFAP1-AS1 may serve as a survival indicator and a potential therapeutic target for PC patients.
Increasing evidence indicates that lncRNAs play important roles in tumor pathology and might be useful as diagnostic and therapeutic target. For example, it has been shown that NORAD can act as a diagnostic and prognostic biomarker in PC [14]. Also, the lncRNA XIST may serve as a potential therapeutic target in PC [35]. Zhao et al. revealed that lncRNA PVT1 can function as an endogenous ‘sponge’ by competing for miR-448 and thereby regulating its miRNA target SERBP1, which promoted growth and metastasis in PC [36]. In the present study, by retrieving and screening the relevant PC microarray data from the GEO dataset, we identified that AFAP1-AS1 was significantly upregulated in PC tissues and cell lines. High AFAP1-AS1 expression was associated with advanced TNM stage, tumor size and lymph node metastasis. Moreover, upregulation of AFAP1-AS1 found to be correlated with poor overall survival and therefore was considered to have potential as an independent prognostic indicator for patients with PC and to be involved in the progression of PC.
The AFAP1-AS1 RNA protein is encoded by the antisense strand of the AFAP1 (Actin Filament Associated Protein) gene and was initially discovered in 2013 in esophageal adenocarcinoma [37]. Recently, a large number of studies have investigated AFAP1-AS1 expression in human cancers and have reported it to be upregulated in many cancers and to play an important role in tumor progression [21,22,37]. For example, AFAP1-AS1 expression was increased in esophageal squamous cell carcinoma (ESCC) and knockdown of AFAP1-AS1 in ESCC cells suppressed cell proliferation and colony formation and induced cell apoptosis [38,39]. In addition, it has been shown that AFAP1-AS1 knockdown significantly inhibited the nasopharyngeal carcinoma (NPC) cell migration and invasive capability [21]. However, little previous research attention has been paid to the role of AFAP1-AS1 in PC. In the present study, we found that knockdown of AFAP1-AS1 by transfection with siRNA inhibited PC cell proliferation, and induced cell apoptosis in vitro as well as suppressing tumor growth in vivo. Furthermore, AFAP1-AS1 knockdown inhibited cell migration and invasion by influencing the expression of EMT-related genes, including E-cadherin, N-cadherin, Vimentin and Fibronectin. This finding is consistent with previous studies that have demonstrated that EMT plays a central role in tumor progression and metastasis [23,40]. Taken together, our results suggest that AFAP1-AS1 knockdown has a tumor-suppressive effect in PC. Recently, Ye et al. demonstrated that AFAP1-AS1 was upregulated in pancreatic ductal adenocarcinoma (PDAC), which accounts for more than 90% of PC and that knockdown of AFAP1-AS1 reduced proliferation and induced G2/M phase arrest in PDAC cells [22]. However, that study was did not identify the biomolecular mechanism of these effects.
Recently, it has been proposed that several lncRNAs can function as ceRNAs by sponging miRNAs to influence post-transcriptional regulation of their target genes in PC. For example, Cai et al. showed that HOTAIR functioned as a ceRNA to regulate notch3 expression via sponging miR-613 in PC [41]. Wang et al. found that colorectal neoplasia contained differentially expressed lncRNA CRNDE that sponged miR-384 to promote proliferation and metastasis of pancreatic cancer cells through upregulating the expression of IRS1 [42]. Whether AFAP1-AS1 also functions as a ceRNA to regulate pancreatic cancer progression was unknown. In this study, we used a bioinformatics databases (Starbase v2.0) to identify several miRNAs that might interact with AFAP1-AS1. Our findings indicated miR-133a as a potential miR target that could be regulated by AFAP1-AS1, and since it has been previously implicated as a tumor suppressor in many human tumors including PC [35,43,44,45], it was selected for further study. We further investigated the regulatory relationship between AFAP1-AS1 and miR-133a in PC. We showed that AFAP1-AS1 negatively regulated the expression levels of miR-133a in PC cells and that there was an inverse association existed between the expression levels of AFAP1-AS1 and miR-133a in the tumor tissues of PC patients. More importantly, our results showed for the first time that AFAP1-AS1 sponged miR-133a to inhibit cell proliferation and metastasis. On this basis it was hypothesized that AFAP1-AS1/miR-133a axis might play an important role in the progression of PC.
The IGF1R gene is a well-known oncogene that has been found to play important roles in the pathogenesis of many human tumors including PC [46,47,48]. For example, Yeo et al. showed that IGF1R exacerbated malignant transformation and tumor cell proliferation in lung adenocarcinoma [49]. MacEwen et al. found that lowering IGF1R levels could reduce primary tumor growth and metastasis of murine osteosarcoma [50]. Recently, IGF1R was found to be highly expressed in PC tissues and in both tumor and stromal cells, and was associated with poor prognosis of PC [52]. Ramadevi et al. found that silencing IGF1R inhibits pancreatic cancer growth and metastasis by blocking key signaling pathways such AKT/PI3K, MAPK, JAK/STAT and EMT [53]. Importantly, it has also been shown in several types of cancers that miR-133a exerted its tumor-suppressive effects by targeting IGF1R [28,54]. For example, miR-133a was observed to inhibit osteosarcoma cells proliferation and invasion via targeting IGF-1R [26]. Guo et al. demonstrated that miR-133a suppressed ovarian cancer cell proliferation by directly targeting IGF1R [29]. Given the relationship between AFAP1-AS1 and miR-133a, we hypothesized that, in PC, the oncogene function of AFAP1-AS1 was achieved through reversing miR-133a-mediated IGF1R repression. We found that miR-133a negatively regulated the protein expression levels of IGF1R in PaCa-2 and SW1990 PC cell lines, which was reversed by AFAP1-AS1. Moreover, we demonstrated that knockdown of IGF1R in these cells by transfecting with si-IGF1R suppressed cell proliferation, invasion and migration, suggesting that IGF1R functioned as an oncogene in PC cells. When taken together, these data indicate that AFAP1-ASl acts as a ceRNA by binding to miR-133a and thereby liberating the expression of IGF1R. In addition, IGF1R was found to be an important regulator of several downstream pathways, including the MEK/ERK signaling pathways, which play crucial roles in tumorigenesis and tumor development [55,56]. Further experiments are needed to assess if MEK/ERK signaling pathways are correlated with AFAP1-AS1 expression levels.
In conclusion, we have provided new evidence suggesting that AFAP1-AS1 acts as an oncogene in PC by sponging miR-133a and thereby functionally releasing IGF1R mRNA transcripts targeted by miR-133a in PC cells (Figure 7). The findings of this study suggest that the AFAP1-AS1/miR-133a/IGF1R axis could be an effective target for the development of effective PC therapies.
Figure 7.
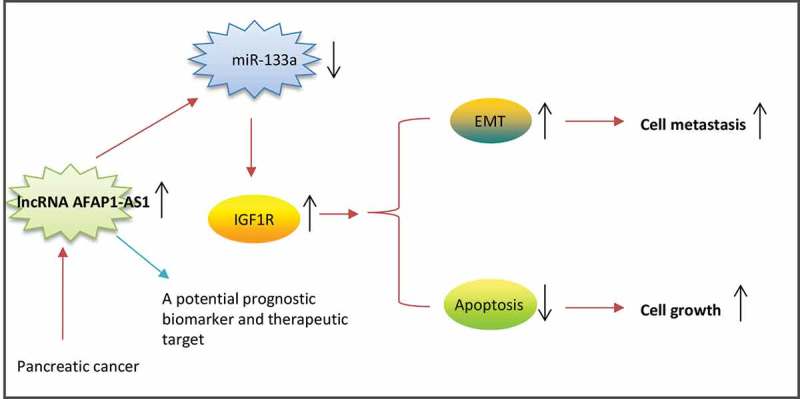
Schematic diagrams showing that AFAP1-AS1 is upregulated in PC tissues and cell lines, and AFAP1-AS1 acts as an oncogene by sponging miR-133a, functionally releasing IGF1R mRNA transcripts targeted by miR-133a, and activating the downstream AKT/ERK signaling pathways.
Biography
Xiaohua Jiang and Wujun Xiong was responsiblefor the main conceive of the study and the draft of the manuscript. Bo chen, Qinhua Li, Yongping Zhou, XujingWang, Qiqi zhang, Yongkun Wang and Huiren Zhuang helped to design the study and performed the statistical analysis. Bo chen, Qinhua Li and Yongping Zhou helped to revise the manuscript and participated in its design. All authors have read and approved the final manuscript
Funding Statement
This work was funded by Pudong New District Science and Technology Development Fund (No.PKJ2015-Y15) and Key Specialty Construction Project of Pudong Health and Family Planning Commission of Shanghai (No.PWZz2013-05)Pudong New District Science and Technology Development Fund [PKJ2015-Y15].
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplementary material can be accessed here.
References
- [1].Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Steeg PS. Targeting metastasis. Nature Reviews Cancer. 2016;16:201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yang Z, Li X, Yang Y, et al. Long noncoding RNAs in the progression, metastasis, and prognosis of osteosarcoma. Cell Death Dis. 2016;7:e2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Khaitan D, Dinger ME, Mazar J, et al. The melanoma-upregulated long noncoding RNA SPRY4-IT1 modulates apoptosis and invasion. Cancer Res. 2011;71:3852–3862. [DOI] [PubMed] [Google Scholar]
- [5].Zhou X, Liu S, Cai G, et al. Long non coding RNA MALAT1 promotes tumor growth and metastasis by inducing epithelial-mesenchymal transition in oral squamous cell carcinoma. Sci Rep. 2015;5:15972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhou X, Ye F, Yin C, et al. The interaction between MiR-141 and lncRNA-H19 in regulating cell proliferation and migration in gastric cancer. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology. 2015;36:1440–1452. [DOI] [PubMed] [Google Scholar]
- [7].Dhamija S, Diederichs S. From junk to master regulators of invasion: lncRNA functions in migration, EMT and metastasis. Int J Cancer Manag. 2016;139:269–280. [DOI] [PubMed] [Google Scholar]
- [8].Li C, Miao R, Liu S, et al. Down-regulation of miR-146b-5p by long noncoding RNA MALAT1 in hepatocellular carcinoma promotes cancer growth and metastasis. Oncotarget. 2017;8:28683–28695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Luan W, Li R, Liu L, et al. Long non-coding RNA HOTAIR acts as a competing endogenous RNA to promote malignant melanoma progression by sponging miR-152-3p. Oncotarget. 2017;8:85401–85414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang YY, Feng HM. MEG3 suppresses human pancreatic neuroendocrine tumor cells growth and metastasis by down-regulation of Mir-183. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology. 2017;44:345–356. [DOI] [PubMed] [Google Scholar]
- [11].Sauvageau M, Goff LA, Lodato S, et al. Multiple knockout mouse models reveal lincRNAs are required for life and brain development. eLife. 2013;2:e01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shi X, Sun M, Liu H, et al. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 2013;339:159–166. [DOI] [PubMed] [Google Scholar]
- [14].Li H, Wang X, Wen C, et al. Long noncoding RNA NORAD, a novel competing endogenous RNA, enhances the hypoxia-induced epithelial-mesenchymal transition to promote metastasis in pancreatic cancer. Mol Cancer. 2017;16:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gao ZQ, Wang JF, Chen DH, et al. Long non-coding RNA GAS5 antagonizes the chemoresistance of pancreatic cancer cells through down-regulation of miR-181c-5p. Biomed Pharmacother. 2017;97:809–817. [DOI] [PubMed] [Google Scholar]
- [16].Oda D, Savard CE, Nguyen TD, et al. Culture of human main pancreatic duct epithelial cells. Vitro Cellular & Developmental Biology Animal. 1998;34:211–216. [DOI] [PubMed] [Google Scholar]
- [17].Yamaguchi H, Kojima T, Ito T, et al. Transcriptional control of tight junction proteins via a protein kinase C signal pathway in human telomerase reverse transcriptase-transfected human pancreatic duct epithelial cells. Am J Pathol. 2010;177:698–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Donahue TR, Tran LM, Hill R, et al. Integrative survival-based molecular profiling of human pancreatic cancer. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research. 2012;18:1352–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pei H, Li L, Fridley BL, et al. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16:259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zeng Z, Bo H, Gong Z, et al. AFAP1-AS1, a long noncoding RNA upregulated in lung cancer and promotes invasion and metastasis. Tumour Biology: Journal Int Soc Oncodevelopmental Biol Med. 2016;37:729–737. [DOI] [PubMed] [Google Scholar]
- [21].Bo H, Gong Z, Zhang W, et al. Upregulated long non-coding RNA AFAP1-AS1 expression is associated with progression and poor prognosis of nasopharyngeal carcinoma. Oncotarget. 2015;6:20404–20418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ye Y, Chen J, Zhou Y, et al. High expression of AFAP1-AS1 is associated with poor survival and short-term recurrence in pancreatic ductal adenocarcinoma. J Transl Med. 2015;13:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nakaya Y, Sheng G. EMT in developmental morphogenesis. Cancer Lett. 2013;341:9–15. [DOI] [PubMed] [Google Scholar]
- [24].Han X, Wang L, Ning Y, et al. Long non-coding RNA AFAP1-AS1 facilitates tumor growth and promotes metastasis in colorectal cancer. Biol Res. 2016;49:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chen G, Fang T, Huang Z, et al. MicroRNA-133a inhibits osteosarcoma cells proliferation and invasion via targeting IGF-1R. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology. 2016;38:598–608. [DOI] [PubMed] [Google Scholar]
- [27].Guo J, Xia B, Meng F, et al. miR-133a suppresses ovarian cancer cell proliferation by directly targeting insulin-like growth factor 1 receptor. Tumour Biology: Journal Int Soc Oncodevelopmental Biol Med. 2014;35:1557–1564. [DOI] [PubMed] [Google Scholar]
- [28].Huang Y, Wu Y, Dong J, et al. MicroRNA-133a-3p exerts inhibitory effects on gallbladder carcinoma via targeting RBPJ. Am J Cancer Res. 2016;6:2448–2462. [PMC free article] [PubMed] [Google Scholar]
- [29].Zheng K, Liu W, Liu Y, et al. MicroRNA-133a suppresses colorectal cancer cell invasion by targeting Fascin1. Oncol Lett. 2015;9:869–874. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [30].Xiao Y, Tian Q, He J, et al. MiR-503 inhibits hepatocellular carcinoma cell growth via inhibition of insulin-like growth factor 1 receptor. Onco Targets Ther. 2016;9:3535–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang X, Liu S, Cao L, et al. miR-29a-3p suppresses cell proliferation and migration by downregulating IGF1R in hepatocellular carcinoma. Oncotarget. 2017;8:86592–86603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang W, Liu K, Liu S, et al. MicroRNA-133a functions as a tumor suppressor by targeting IGF-1R in hepatocellular carcinoma. Tumour Biology: Journal Int Soc Oncodevelopmental Biol Med. 2015;36:9779–9788. [DOI] [PubMed] [Google Scholar]
- [33].Gao S, Wassler M, Zhang L, et al. MicroRNA-133a regulates insulin-like growth factor-1 receptor expression and vascular smooth muscle cell proliferation in murine atherosclerosis. Atherosclerosis. 2014;232:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wei W, Liu Y, Lu Y, et al. LncRNA XIST promotes pancreatic cancer proliferation through miR-133a/EGFR. J Cell Biochem. 2017;118:3349–3358. [DOI] [PubMed] [Google Scholar]
- [35].Zhao L, Kong H, Sun H, et al. LncRNA-PVT1 promotes pancreatic cancer cells proliferation and migration through acting as a molecular sponge to regulate miR-448. J Cell Physiol. 2018;233:4044–4055. [DOI] [PubMed] [Google Scholar]
- [36].Wu W, Bhagat TD, Yang X, et al. Hypomethylation of noncoding DNA regions and overexpression of the long noncoding RNA, AFAP1-AS1, in Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterology. 2013;144:956–66 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhou XL, Wang WW, Zhu WG, et al. High expression of long non-coding RNA AFAP1-AS1 predicts chemoradioresistance and poor prognosis in patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Mol Carcinog. 2016;55:2095–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Luo HL, Huang MD, Guo JN, et al. AFAP1-AS1 is upregulated and promotes esophageal squamous cell carcinoma cell proliferation and inhibits cell apoptosis. Cancer Med. 2016;5:2879–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nature Reviews Cancer. 2013;13:97–110. [DOI] [PubMed] [Google Scholar]
- [40].Cai H, Yao J, An Y, et al. LncRNA HOTAIR acts a competing endogenous RNA to control the expression of notch3 via sponging miR-613 in pancreatic cancer. Oncotarget. 2017;8:32905–32917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wang G, Pan J, Zhang L, et al. Long non-coding RNA CRNDE sponges miR-384 to promote proliferation and metastasis of pancreatic cancer cells through upregulating IRS1. Cell Prolif. 2017;50:e12389. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [42].Cai J, Liu T, Huang P, et al. USP39, a direct target of microRNA-133a, promotes progression of pancreatic cancer via the AKT pathway. Biochem Biophys Res Commun. 2017;486:184–190. [DOI] [PubMed] [Google Scholar]
- [43].Qin Y, Dang X, Li W, et al. miR-133a functions as a tumor suppressor and directly targets FSCN1 in pancreatic cancer. Oncol Res. 2013;21:353–363. [DOI] [PubMed] [Google Scholar]
- [44].Zhou Y, Wu D, Tao J, et al. MicroRNA-133 inhibits cell proliferation, migration and invasion by targeting epidermal growth factor receptor and its downstream effector proteins in bladder cancer. Scand J Urol. 2013;47:423–432. [DOI] [PubMed] [Google Scholar]
- [45].Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther. 2007;6:1–12. [DOI] [PubMed] [Google Scholar]
- [46].Ioannou N, Dalgleish AG, Seddon AM, et al. Anti-tumour activity of afatinib, an irreversible ErbB family blocker, in human pancreatic tumour cells. Br J Cancer. 2011;105:1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nature Reviews Cancer. 2008;8:915–928. [DOI] [PubMed] [Google Scholar]
- [48].Park E, Park SY, Kim H, et al. Membranous insulin-like growth factor-1 receptor (IGF1R) expression is predictive of poor prognosis in patients with epidermal growth factor receptor (EGFR)-mutant lung adenocarcinoma. J Pathol Transl Med. 2015;49:382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].MacEwen EG, Pastor J, Kutzke J, et al. IGF-1 receptor contributes to the malignant phenotype in human and canine osteosarcoma. J Cell Biochem. 2004;92:77–91. [DOI] [PubMed] [Google Scholar]
- [50].Ouban A, Muraca P, Yeatman T, et al. Expression and distribution of insulin-like growth factor-1 receptor in human carcinomas. Hum Pathol. 2003;34:803–808. [DOI] [PubMed] [Google Scholar]
- [51].Valsecchi ME, McDonald M, Brody JR, et al. Epidermal growth factor receptor and insulinlike growth factor 1 receptor expression predict poor survival in pancreatic ductal adenocarcinoma. Cancer. 2012;118:3484–3493. [DOI] [PubMed] [Google Scholar]
- [52].Subramani R, Lopez-Valdez R, Arumugam A, et al. Targeting insulin-like growth factor 1 receptor inhibits pancreatic cancer growth and metastasis. PloS one. 2014;9:e97016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gong Y, Ren J, Liu K, et al. Tumor suppressor role of miR-133a in gastric cancer by repressing IGF1R. World J Gastroenterol. 2015;21:2949–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wang LK, Hsiao TH, Hong TM, et al. MicroRNA-133a suppresses multiple oncogenic membrane receptors and cell invasion in non-small cell lung carcinoma. PloS one. 2014;9:e96765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bertrand FE, Steelman LS, Chappell WH, et al. Synergy between an IGF-1R antibody and Raf/MEK/ERK and PI3K/Akt/mTOR pathway inhibitors in suppressing IGF-1R-mediated growth in hematopoietic cells. Leukemia. 2006;20:1254–1260. [DOI] [PubMed] [Google Scholar]
- [56].Shelton JG, Steelman LS, White ER, et al. Synergy between PI3K/Akt and Raf/MEK/ERK pathways in IGF-1R mediated cell cycle progression and prevention of apoptosis in hematopoietic cells. Cell Cycle. 2004;3:372–379. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


