Abstract
Introduction
The user experience and clinical effectiveness with wearable global positioning system (GPS) devices for persons with dementia (PwDs) and caregivers (CGs) remain unclear although many are available.
Methods
Using a crossover design, 20 dyads tested two similar commercial GPS watches (products A and B) at home for 4 weeks each. Usability, product functions, design features and product satisfaction at home and the clinic were investigated. Caregiver burden and quality of life assessed clinical effectiveness.
Results
The final 17 dyads rated the usability, telephone function, overall design features, font, buttons, and battery life of B significantly better than A. PwDs rated the overall design features and buttons of A significantly better than CGs. Product satisfaction with both products was significantly lower at home. Clinical effectiveness was not found.
Discussion
User experience can be improved by optimizing specific product details. This might translate to clinical effectiveness. Social desirability bias may explain different product satisfaction ratings.
Keywords: Assistive technology, Caregiving, Clinical effectiveness, Dementia, GPS, Home dementia care, Locating systems, Monitoring, Product satisfaction, Tracking systems, Usability, User experience, Wandering, Wearables
Highlights
-
•
Usability ratings differed between two similar global positioning system watches.
-
•
Users preferred less buttons, a clear font with limited text, and a long battery life.
-
•
Different ratings by persons with dementia and caregivers were found.
-
•
Clinical effectiveness resulting from product use was not found.
-
•
Product satisfaction was rated better at the clinical research setting than at home.
1. Introduction
Assistive technologies intended to aid persons with dementia (PwDs) and their primary caregivers (CGs) can be seen as promising, potentially cost-effective tools that could help optimize the amount of care provided in informal care settings [1], [2]. “Tracking” or locating systems are specific assistive technologies that enable the location of PwDs through a global positioning system (GPS) technology. Accordingly, GPS devices aim to promote the safety of PwDs who exhibit wandering behaviors [3], [4]. Therefore, these products can also reduce the stress and burden experienced by PwDs and CGs that is typically associated with wandering behaviors [5], [6].
To date, studies on prototype and commercial GPS devices in dementia care have used different product types with similar functions and design features. Typically, products are small (e.g., pager sized or watch sized), discrete or nonvisible (e.g., worn around the waist, neck, wrist, or placed inside a pocket or shoe sole), have two to six main functions (e.g., location, telephone, geofencing, alarm, fall detection, and speed alert), no buttons (i.e., passive systems) or one to three buttons (i.e., active systems), and are lightweight [5], [6], [7], [8], [9], [10], [11]. These functions and features are in line with most expert recommendations [7], [12].
However, research has also shown that assistive technologies for dementia care lack clear-cut quality standards regarding their design. Moreover, these technologies are rarely developed using techniques of user-centered design [13], [14]. It is therefore likely that most products exhibit a suboptimal design, which impedes technology acceptance and long-term use [7], [13]. This may partly explain why commercial GPS devices for dementia are rarely used outside clinical research settings [7], [10], [15], although many are commercially available [14], [16]. Furthermore, most studies on GPS devices focus on feasibility, acceptability, and usability [5], [6], [7], [8], [9], [10], [17], [18] without providing a more holistic understanding of end users' experience with such devices. In addition, it is unclear whether the use of such products results in clinical effectiveness [14], [19], [20]. As it stands, findings pertaining to the clinical effectiveness of assistive technologies are shockingly sparse [13], [21], [22]. For GPS devices, it is not clear whether they provide measurable aid in home dementia care beyond being accepted and feasible.
For these reasons, this study is based on the construct of user experience (UX), which is typically defined as “a person's perceptions and responses that result from the use or anticipated use of a product, system or service” [23]. In other words, it is a multidimensional construct that includes affective, cognitive, and behavioral dimensions that users exhibit before, during, and after product use [24], [25]. The theoretical background of UX overlaps with key aspects of the technology acceptance model [26], as a recent review analyzed [27]. In short, this model states that perceived ease of use and usefulness of a product, as well as external factors (e.g., technological affinity) contribute to actual acceptance and use. In contrast, UX focuses more on specific product characteristics and their associations with outcomes, including satisfaction or acceptance, which are moderated by specific usage situations [28].
Therefore, the central aim of this study was to perform an in-depth comparison of the UX of PwDs and their informal CGs with two similar commercial GPS watches in home dementia care. To date, only three studies have compared more than one commercial GPS device, yet the product types differed [5], [11], [18]. Of these studies, one did not collect data from PwDs [11], one did not focus on UX [5], and the other focused on acceptability without describing the devices used [18]. A direct comparison of GPS watches is particularly warranted given that it has been recommended that products take the form of familiar, everyday objects (e.g., watch) to help ensure their adoption [7], [10]. Furthermore, comparing commercial GPS products could provide additional information on their possible effectiveness [14] and on functions and features that lead to a more favorable UX. In addition, we examined whether product satisfaction differs when reported at home versus a clinical setting. One could hypothesize that PwDs and CGs report a more positive product satisfaction in clinical settings based on a social desirability bias [29], [30]. A second aim was to examine whether product use would result in clinical effectiveness for PwDs and CGs.
2. Methods
2.1. Sample
Twenty dyads (n = 20 PwDs, n = 20 CGs) were recruited following a convenience sampling technique from memory clinic patients in 2016. PwDs who could move about outside the home were included. Years since diagnosis and a Mini-Mental State Examination score assessed up to 1 month before baseline were available for all PwDs. All participants provided their written informed consent for participation in this UX study.
2.2. Materials
Two similar commercial GPS watches marketed for people with orientation impairments (hereafter products A and B) were selected (see Table 1). These were the only two GPS watches on the German market with a location and telephone function. In combination with products A and B CGs received a study-specific smartphone to prevent bias with two native Android applications preinstalled to be able to locate PwDs. By pressing one defined button of product A or the button of product B, a call is sent out to the smartphone and PwDs could accept an incoming call by pressing this button. With both smartphone applications, CGs could view the last recognized position of product A or positions of product B on an online map. To support product learning, we developed a 60-minute technological training session based on dementia communication guidelines [31], [32]. This included hands-on exercises by having PwD practice calling and accepting a call from CG, and CG calling and locating PwD on a map.
Table 1.
Product description of GPS watches for PwDs
| A | B | |
|---|---|---|
| Product name | HIMATIC GPS Uhr Alpha [HIMATIC GPS Alpha watch] | ReSOS-2—Die Notfalluhr [ReSOS-2–the emergency watch] |
| Product picture |  |
 |
| Size | 45.5 mm × 64.5 mm × 17.5 mm | 43.0 mm × 43.0 mm × 19.0 mm |
| Weight | 70 g | 66 g |
| Main colors | Black and blue | Black and red |
| Buttons | Five (Ø: 0.5 mm) | One (1.8 mm × 0.5 mm) |
| Band type | Silicone strap | Silicone strap |
| Battery | Li-ion (3.7 V, 500 mAh) | Li-ion (850 mAh) |
| Charging method | DC 5 V USB charger cable | Charging station with USB cable |
| Software/application | Native Android App: HIMATIC GPS Uhr Alpha | Native Android and iOS App: ReSOS-2 |
| Website | https://himaticmobile.de/personenortung.html | http://notfall-uhr.de/ |
Abbreviations: A, product A; B, product B; GPS, global positioning system; PwDs, persons with dementia; USB, Universal Serial Bus.
NOTE. Size noted as width by length by depth; websites last accessed on August 23, 2018; both watches have a SIM card that allows for two-way communication and GPS connection.
2.3. Study design
Products A and B were compared using a 2 × 2 crossover design (sequences AB|BA, two study periods, four assessment points). Each product was tested at home for 4 weeks. The first study period lasted from baseline at T1 to T2.1, and the second study period lasted from T2.2 to T3.
2.3.1. First study period
At T1, standard demographic data were measured. Also, CGs and PwDs were independently asked to report on a history of wandering events. Then, four secondary outcome measures assessed clinical effectiveness: CG burden (Zarit Burden Interview, range 0–48) [33], quality of life of PwDs and CGs (European Health Interview Surveys-quality of life, range 0–48) [34], orientation impairments, and subjective burden of getting lost (self-developed six-point Likert scales ranging from 0 = no impairments/not at all worried to 5 = very impaired/very worried, with CGs appraising PwDs). Higher scores represent more negative endpoints, except for the European Health Interview Surveys-quality of life. The covariate technological affinity was assessed for PwDs and CGs with the technological affinity scale for electronic products (TA-EG, range 19–95) [35]. Dyads then randomly received their first product and completed the technological training session. The primary outcome measure usability was then assessed with the International Standardization Organisation Norm (ISONORM) 9241/10 scale (range 0–210) [36], [37]. This scale relies on principles of the International Standardization Organisation [38] and is recommended for UX studies [39]. The scale measures seven usability domains, including suitability for the task, self-descriptiveness, controllability, conformity with user expectations, error tolerance, suitability for individualization, and suitability for learning. For the TA-EG and ISONORM 9241/10 scales, higher scores represent more positive endpoints.
At T2.1, dyads returned to the clinic and first performed the same tasks as at T1 during the technological training session as a way to control product learning, followed by a reassessment of usability with the ISONORM 9241/10 scale. Thereafter, dyad members rated two further primary outcome measures, namely the main product functions (i.e., telephone and location) and design features (i.e., color, font, size, weight, buttons, and battery life) on five-point Likert scales ranging from 0 = not at all good to 4 = very good. Font ratings assessed the font's typeface, size, and color, and ratings of buttons measured amount, size, sound, haptic feedback, and color. Then, dyads jointly rated their overall product satisfaction using the same five-point Likert scale. In addition, the covariate subjective frequency of use of the location function was assessed by CGs on a five-point Likert scale ranging from 0 = not at all to 4 = very often. All custom items are displayed in Supplementary Table 1. Also, secondary outcome measures and the TA-EG were readministered. Finally, participants were asked whether any wandering events and technical difficulties with the product had occurred.
2.3.2. Second study period
T2.2 directly followed T2.1. Here, dyads received their second product and completed the technological training session, followed by filling out the ISONORM 9241/10 scale. At T3, dyad members' UX with the second product was assessed following the same methodological procedure as at T2.1.
2.3.3. Home assessments
At the end of each week, dyads jointly rated their overall weekly product satisfaction with product A or B at home with the same question as at the clinic.
2.4. Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences Statistics 24. Because of the nonindependence between PwDs and CGs within a dyad [40], PwDs and CGs data were analyzed with paired samples t tests for primary and secondary outcome measures. Independent samples t tests between products were additionally performed for primary outcome measures. Furthermore, secondary outcome measures and covariates were analyzed with Spearman's rank-order correlations and one-way repeated measures analysis of variances. Potential order effects between sequences AB and BA were examined with independent samples t tests.
3. Results
3.1. Participant characteristics
Baseline data were first inspected for outliers, and one dyad with a PwD Mini-Mental State Examination score more than 2 SDs (standard deviations) below the mean was excluded [41]. Two dyads dropped out at T2.1 (dropout rate 10%) stating product dissatisfaction because of technical difficulties, with no other significant differences compared with nondropouts, and both dyads received different products. In the final sample of 17 dyads (n = 17 PwDs, n = 17 CGs), seven received product A and 10 received product B at T1 and vice versa at T2.2. Ten CGs and three PwDs reported a history of wandering events. All participant characteristics are displayed in Table 2.
Table 2.
Participant characteristics at baseline (T1)
| Characteristics | PwDs (n = 17) | CGs (n = 17) |
|---|---|---|
| Age, mean ± SD [range] | 71.7 ± 6.9 [56–80] | 67.7 ± 8.0 [51–77] |
| Gender ratio (% female) | 8/9 (53) | 9/8 (47) |
| Education | ||
| High school, n (%) | 7 (41) | 7 (41) |
| College, n (%) | 2 (12) | 1 (6) |
| University, n (%) | 8 (47) | 9 (53) |
| MMSE score, mean ± SD [range] | 18.2 ± 4.3 [12–25] | — |
| Mild dementia (20–25), n (%) | 8 (47) | — |
| Moderate dementia (12–19), n (%) | 9 (53) | — |
| Years since diagnosis | ||
| >1, n (%) | 1 (6) | — |
| 1–2, n (%) | 5 (29) | — |
| 2–3, n (%) | 4 (24) | — |
| 3–4, n (%) | 2 (12) | — |
| >5, n (%) | 5 (29) | — |
| History of wandering events | ||
| None, n (%) | 14 (82) | 7 (41) |
| 1–3 times, n (%) | 3 (18) | 8 (47) |
| 4–6 times, n (%) | 0 | 2 (12) |
| >7 times, n (%) | 0 | 0 |
| TA-EG (19–95) | 71.2 ± 7.3 [58–85] | 74.1 ± 6.1 [63–83] |
Abbreviations: CGs, caregivers; MMSE, Mini-Mental State Examination; PwDs, persons with dementia; SD, standard deviation; TA-EG, technological affinity scale for electronic products.
NOTE. Percentages rounded to the nearest whole number.
3.2. Primary outcome measures
Results of the ISONORM 9241/10 usability scale for both products were fair to good and are presented in Table 3 and Supplementary Fig. 1. Independent samples t tests showed that dyads rated product B better than A at all assessments. At T1, t(32) = −2.11, P < .05 (MA = 146, SD = 40.7, MB = 171, SD = 27.7); at T2.1, t(32) = −2.29, P < .05 (MA = 131, SD = 45.5, MB = 164, SD = 38.6); at T2.2, t(32) = −4.32, P < .001 (MA = 123, SD = 42.9, MB = 175, SD = 16.6); and at T3, t(32) = −2.47, P < .05 (MA = 117, SD = 60.7, MB = 162, SD = 35.7). To assess differences between PwD and CG ratings, paired samples t tests revealed only one significant result at T1 for sequence AB, where the ratings of PwDs were lower than those of CGs: t(6) = −4.77, P < .01. Paired samples t tests to test changes in usability scores over time revealed that neither the usability ratings of PwDs and CGs nor of dyads with either product differed after each study period.
Table 3.
ISONORM 9241/10 usability ratings over the course of the study
| Sequence | Study period 1 |
Study period 2 |
||
|---|---|---|---|---|
| T1 | T2.1 | T2.2 | T3 | |
| AB (n = 7 dyads)∗ | ||||
| PwDs | 126 ± 37.3† | 116 ± 58.8 | 170 ± 15.3 | 168 ± 32.9 |
| CGs | 165 ± 36.2† | 147 ± 20.9 | 179 ± 17.7 | 155 ± 39.8 |
| BA (n = 10 dyads)∗ | ||||
| PwDs | 170 ± 28.2 | 153 ± 48.8 | 117 ± 49.6 | 111 ± 67.0 |
| CGs | 171 ± 28.8 | 176 ± 21.5 | 128 ± 36.7 | 123 ± 56.7 |
Abbreviations: A, product A; B, product B; CGs, caregivers; PwDs, persons with dementia; ISONORM, International Standardization Organisation Norm; SD, standard deviation.
NOTE. Continuous variables are displayed as mean value ± SD.
Independent samples t tests for dyads: B rated better than A at all time points, P value <.05.
Paired samples t tests for within-dyad effects: PwDs rated A worse than CGs, P value <.01.
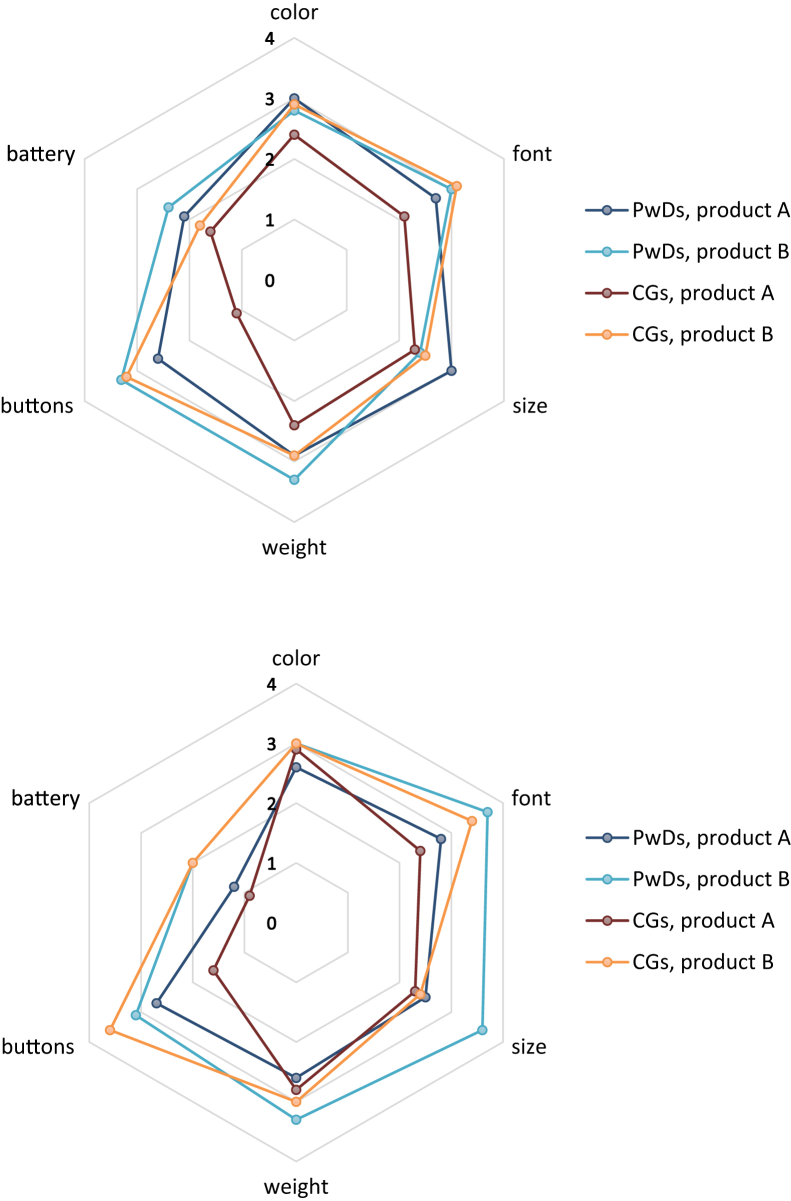
Results concerning product function and design feature ratings are presented in Table 4 and Fig. 1. Independent samples t tests revealed a significant difference in dyads' rating of the telephone function at T2.1 between products, where t(32) = −2.63, P < .05 (MA = 2.1, SD = 1.0, MB = 3.0, SD = 0.8). For overall design features, dyads rated product A worse than B at T3, where t(32) = −3.18, P < .01 (MA = 13.7, SD = 4.3, MB = 18.4, SD = 4.4) and CGs at T2.1, where t(15) = −2.28, P < .05, similar to PwDs at T3, where t(15) = −2.56, P < .05. Concerning individual design features, dyads rated the font and buttons of product A worse than those of B at T2.1, t(32) = −2.24, P < .05 (MA = 2.4, SD = 0.7, MB = 3.1, SD = 0.9) and t(32) = −4.03, P < .001 (MA = 1.9, SD = 1.2, MB = 3.3, P < .05), respectively. At T3, the font, t(32) = −3.20, P < .01 (MA = 2.6, SD = 1.0, MB = 3.6, SD = 0.7), buttons, t(32) = −2.67, P < .05 (MA = 2.2, SD = 1.5, MB = 3.4, SD = 0.8), and battery life, t(32) = −3.12, P < .01 (MA = 1.1, SD = 1.1, MB = 2.4, SD = 1.3) of product A were rated worse than those of B by dyads. Independent samples t tests for CGs at T2.1 revealed significant differences in the ratings of font, t(15) = −2.70, P < .05 and buttons, t(15) = −3.76, P < .01, and at T3 for buttons, t(15) = −3.03, P < .01, all in favor of product B. Likewise, ratings of PwDs at T2.1 were significantly different for buttons, t(15) = −2.38, P < .05, and at T3 for font, t(15) = −2.71, P < .05, size, t(15) = −2.41, P < .05, and battery life, t(15) = −2.73, P < .05. Furthermore, paired samples t tests showed that the overall design features of product A were rated worse by CGs than by PwDs at T2.1, where t(6) = 2.44, P = .05. For individual design features, PwDs rated the buttons of product A better than CGs at T2.1, t(6) = 2.71, P < .05 and at T3, t(9) = 2.70, P < .05. To test potential order effects based on receiving product A or B first, independent samples t tests were performed and yielded nonsignificant differences in primary outcome measures.
Table 4.
Product function and design feature ratings over the course of the study
| Variables | T2.1 |
T3 |
||||||
|---|---|---|---|---|---|---|---|---|
| A (n = 7 dyads) |
B (n = 10 dyads) |
A (n = 10 dyads) |
B (n = 7 dyads) |
|||||
| PwDs | CGs | PwDs | CGs | PwDs | CGs | PwDs | CGs | |
| Product functions | ||||||||
| Telephone∗ | 2.1 ± 0.9 | 2.1 ± 1.1 | 3.0 ± 0.8 | 2.9 ± 0.9 | 2.1 ± 1.0 | 2.7 ± 1.1 | 2.7 ± 1.1 | 2.9 ± 1.2 |
| Location | — | 2.9 ± 0.9 | — | 2.7 ± 1.2 | — | 2.7 ± 0.8 | — | 2.9 ± 0.7 |
| Design features | ||||||||
| Overall†,‡,§ | 16.3 ± 4.2¶ | 12.0 ± 4.3¶ | 17.2 ± 4.2 | 16.4 ± 3.6 | 14.4 ± 3.7 | 12.9 ± 4.8 | 19.4 ± 4.4 | 17.4 ± 4.5 |
| Color | 3.0 ± 0.8 | 2.4 ± 1.3 | 2.8 ± 0.9 | 2.9 ± 0.9 | 2.6 ± 1.0 | 2.9 ± 0.7 | 3.0 ± 0.8 | 3.0 ± 0.8 |
| Font∗,†,‡,§ | 2.7 ± 0.5 | 2.1 ± 0.7 | 3.0 ± 1.1 | 3.1 ± 0.7 | 2.8 ± 0.8 | 2.4 ± 1.2 | 3.7 ± 0.5 | 3.4 ± 0.8 |
| Size§ | 3.0 ± 1.2 | 2.3 ± 0.8 | 2.4 ± 1.2 | 2.5 ± 0.7 | 2.5 ± 1.1 | 2.3 ± 1.1 | 3.6 ± 0.5 | 2.4 ± 1.3 |
| Weight | 2.9 ± 0.7 | 2.4 ± 1.0 | 3.3 ± 0.8 | 2.9 ± 1.0 | 2.6 ± 1.2 | 2.8 ± 1.1 | 3.3 ± 1.1 | 3.0 ± 0.8 |
| Buttons∗,†,‡,#,∗∗ | 2.6 ± 0.5†† | 1.1 ± 1.2†† | 3.3 ± 0.7 | 3.2 ± 1.0 | 2.7 ± 1.3†† | 1.6 ± 1.7†† | 3.1 ± 1.1 | 3.6 ± 0.5 |
| Battery life†,§ | 2.1 ± 1.2 | 1.6 ± 1.5 | 2.4 ± 1.1 | 1.8 ± 1.1 | 1.2 ± 1.1 | 0.9 ± 1.1 | 2.7 ± 1.1 | 2.0 ± 1.5 |
Abbreviations: CGs, caregivers; PwDs, persons with dementia; SD, standard deviation.
NOTE. Continuous variables are displayed as mean value ± SD (range 0–5); overall stands for mean of all design features (range 0–24).
Independent samples t tests for dyads: B rated better than A at T2.1.
Independent samples t tests for dyads: B rated better than A at T3.
Independent samples t tests for PwDs and CGs: B rated better than A by CGs at T2.1 (P value <.01 for buttons. All other P values <.05).
Independent samples t tests for PwDs and CGs: B rated better than A by PwDs at T3.
Paired samples t tests for within-dyad effects: A rated better by PwDs than CGs.
Independent samples t tests for PwDs and CGs: B rated better than A by CGs at T3.
Independent samples t tests for PwDs and CGs: B rated better than A by PwDs at T2.1.
Paired samples t tests for within-dyad effects: A rated better by PwDs than CGs.
Fig. 1.
Radar charts for design feature ratings at T2.1 (top) and T3 (bottom). Product A rated by seven dyads at T2.1 and by 10 dyads at T3, and vice-versa for product B. Abbreviations: CGs, caregivers; PwDs, persons with dementia.
Finally, results of paired samples t tests for product satisfaction ratings at the clinic versus home revealed several significant differences, with consistently higher ratings at the clinic. Specifically, a significant difference was found for product A at T3, where t(19) = −5.08, P < .001 (Mclinic = 2.2, SD = 1.0, Mhome = 1.1, SD = 0.9) and for product B at T2.1, t(19) = −2.90, P < .01 (Mclinic = 2.8, SD = 0.8, Mhome = 2.1, SD = 1.0) and at T3, t(13) = −3.31, P < .01 (Mclinic = 2.3, SD = 1.4, Mhome = 1.1, SD = 1.0).
3.3. Secondary outcome measures and covariates
Secondary outcome measures and covariates obtained over the course of the study are presented in Table 5. At baseline, Spearman's rank-order correlations revealed that quality of life and subjective burden of getting lost significantly correlated (rs = 0.40, P < .05), and that the latter significantly correlated with orientation impairments (rs = 0.73, P < .001) and age (rs = −0.36, P < .05). Furthermore, paired samples t tests for secondary outcome measures were performed to test any within-dyad effects between PwDs and CGs. Significant results were found for orientation impairments at T1, t(16) = 4.01, P < .001, T2.1, t(16) = 3.85, P < .001, and T3, t(16) = 2.31, P < .05, as well as for subjective burden of getting lost at T1, t(16) = 12.26, P < .001, T2.1, t(16) = 4.82, P < .001, and T3, t(16) = 5.13, P < .001. A significant difference was also found for the European Health Interview Surveys-quality of life at T1, t(16) = −2.21, P < .001. In all cases, PwDs rated themselves significantly better than CGs.
Table 5.
Secondary outcome measures and covariates over the course of the study, PwDs (n = 17) and CGs (n = 17)
| Variables | T1 | T2.1 | T3 |
|---|---|---|---|
| ZBI (0–48), CGs | 14.5 ± 6.4 [3–25] | 16.3 ± 9.2 [2–41] | 17.2 ± 8.5 [5–36] |
| EUROHIS-QOL (0–48) | |||
| PwDs | 10.2 ± 6.6 [1–27]† | 11.9 ± 9.1 [2–33] | 10.3 ± 8.5 [0–34] |
| CGs | 14.7 ± 5.7 [6–23]† | 14.4 ± 6.6 [5–26] | 14.6 ± 6.2 [6–28] |
| TA-EG (19–95)∗ | |||
| PwDs | 71.2 ± 7.3 [58–85] | 72.2 ± 10.0 [46–87] | 58.7 ± 7 [43–71] |
| CGs | 74.1 ± 6.1 [63–83] | 74.7 ± 7.0 [58–82] | 56.7 ± 10.7 [38–75] |
| Orientation impairments (0–5) | |||
| PwDs | 1.4 ± 1.3 [0–5]† | 1.5 ± 0.9 [0–3]† | 1.9 ± 1.1 [0–5]† |
| CGs | 3.0 ± 1.1 [1–4]† | 3.1 ± 1.5 [0–5]† | 2.7 ± 1.5 [0–5]† |
| Subjective burden of getting lost (0–5) | |||
| PwDs | 0.5 ± 0.8 [0–3]† | 1.1 ± 1.5 [0–5]† | 1.1 ± 1.4 [0–4]† |
| CGs | 3.6 ± 1.0 [1–5]† | 3.4 ± 1.2 [1–5]† | 3.5 ± 1.2 [1–5]† |
Abbreviations: CGs, caregivers; EUROHIS-QOL, European Health Interview Surveys-quality of life; PwDs, persons with dementia; SD, standard deviation; TA-EG, technological affinity scale for electronic products; ZBI, Zarit Burden Interview.
NOTE. Continuous variables are displayed as mean value ± SD, with minimum and maximum scores in brackets.
One-way repeated measures analysis of variances: PwDs and CGs ratings at T1 and T2.1 higher than at T3, P value <.001.
Paired samples t tests for within-dyad effects: CGs ratings higher than PwDs (P value <.05 for orientation impairments at T3. All other P values <.001).
Examination of clinical effectiveness with one-way repeated measures analysis of variances revealed no significant changes over the study duration. For the covariate TA-EG, a significant main effect of time for PwDs, F (2, 32) = 16.03, P < .001 and for CGs, F (1.11, 17.73) = 23.64, P < .001 was found, where scores at T1 and T2.1 were significantly higher than T3 scores. Finally, results of CGs' subjective frequency of use of the location function of product A or B showed that CGs used it a moderate amount of times at T2.1 (MA = 3.0, SD = 1.4, MB = 2.6, SD = 1.1) and T3 (MA = 2.4, SD = 0.8, MB = 2.6, SD = 1.6).
Over the course of the study, product A was able to assist in locating PwD in three wandering events (i.e., lost during shopping outing, lost during hiking, and lost during walk out of home). In two cases, the telephone function assisted and in the third case the PwD was located with the location function. Regarding technical difficulties, nine cases were reported for each product (i.e., problems with the charging cable or dock, the software, and the telephone function). Of these 18 cases, CGs reported more difficulties than PwDs (i.e., CGs: n = 7 for A, n = 6 for B; PwDs: n = 2 for A, n = 3 for B).
4. Discussion
The present study reports on the UX with and the clinical effectiveness resulting from the use of two similar commercial GPS watches used for a period of 4 weeks each in home dementia care. Although the selected products were similar, usability ratings by dyads of product B were significantly better than ratings of product A throughout the study. Differences in ratings of usability and design features within dyads are in line with previous studies, which have suggested that the needs and preferences of PwDs and CGs with GPS devices need to be taken into consideration as they may differ [7], [8], [42], [43]. Also, the finding that usability ratings with both products decreased after 4 weeks of use, but not significantly, seems to imply that users' expectations could not be fully met, but that they were not entirely dissatisfied. The finding that end users' subjective technological affinity significantly decreased at the end of the study may be indirectly associated with decreased usability ratings. Indeed, it is possible that dissatisfaction with either product left users to rate themselves as being less technologically savvy. Furthermore, technical difficulties may have also contributed to the decrease in ratings of usability and technological affinity, and are often cited as factors for low product acceptance and use [15], [44].
Regarding product functions, we expected CG to prefer the location function of product B since the last visited positions of PwD could be viewed. However, it is possible that users viewed this extra information as nice-to-have, but not essential or too infringing on their personal privacy. Ethical considerations regarding privacy constraints, data protection, autonomy, and personal dignity need to be taken into account when it comes to product development and use [45], [46]. The finding that dyads preferred product B's telephone function could be associated with end users' more favorable ratings of the buttons of product B. On the other hand, at all assessments, PwDs preferred the buttons of product A compared with CGs. This may speak to the need to design discrete wearable devices to avoid stigmatization of PwDs [42], [47]. Dyads' better ratings of product B's font might be best explained by the concept of “less is more.”
It is important to note that product functions and design features were assessed with non-standardized self-developed Likert scales. Currently, no standardized measures exist to assess these variables, except for parts of the QUEST 2.0 questionnaire [48]. However, this scale focuses on satisfaction with assistive technologies. Also, the use of the ISONORM 9241/10 scale with PwDs is debatable, as the psychometric properties of this scale have not been evaluated with PwDs.
Encouragingly, we did not find any order effects. Thus, receiving product A or B first did not significantly influence ratings of the second product received. The comparison of product satisfaction at home versus the clinic showed that users may bias their answers in clinical settings to avoid appearing too critical [30]. Note, however, that only one question assessed product satisfaction, and dyads jointly rated this variable. Future studies may benefit from the implementation of standardized home assessments with digital or paper-based user diaries [39].
Clinical effectiveness was not found. The short study duration and the small sample size might explain this finding. Nevertheless, this assessment is an overarching goal in research on assistive technologies, with too little information currently available [19], [21]. In practice, no established guidelines on GPS devices exist, which makes it difficult for end users to select and for clinicians to recommend any GPS technology. Nonetheless, recommendations that support the decision-making process for professionals in an ethically responsible manner [45] and that define who will more likely benefit from such technologies [47] exist. Also, the results on differences between PwD and CG ratings of orientation impairments and burden of getting lost could be associated with anosognosia in PwDs and needs to be addressed sensitively.
A central limitation is the small sample size and the two dropouts after 4 weeks. It should be noted that most studies on intelligent assistive technologies have a sample size of less than 20 participants [13]. Nonetheless, the two dropouts, as well as the reported technical difficulties and the decrease in participants' technological affinity suggest that low product satisfaction and adoption are real concerns and may add an additional burden on end users. Also, the selected products were not specifically designed for PwDs and CGs, but rather for a heterogeneous population, which is typical for most commercial GPS devices. Proactive ways to address these issues include designing products that are adaptive to the changing needs of specific end users and that are tested in large, randomized controlled studies that follow a user-centered design [8], [20], [43]. We also did not focus on the cost of either product, although this has been found to influence product acceptability [49]. Furthermore, history of wandering behavior, which frequently occurs in later disease stages [50], but that can happen at any disease stage [51], was not an inclusion criterion. Indeed, most CGs reported PwDs as having a history of wandering behavior. Another limitation was the lack of objective data on users' frequency of use. Finally, the crossover design did not include a washout period to avoid having carryover effects (e.g., learning effects) confound outcome measures. However, no order effects were detected, which indicates that potential learning effects did not influence UX.
Study strengths include the dyadic design, pre-assessments and post-assessments, and the development and implementation of a technological training session. Furthermore, the assessment of the telephone function was deemed important as devices featuring this function are less passive, making them likely better suited for PwDs in earlier disease stages. Two of three PwDs who wandered were located by using the telephone function. Also, self-developed Likert scales were visualized to make them easier to understand. Overall, the detailed UX findings highlight the need to perform in-depth and dyadic analyses.
In conclusion, the results presented here support specific design recommendations for GPS watches in home dementia care. Specifically, devices should contain few buttons, display a clear font with parsimonious text, and have a battery life of at least 24 hours. Indeed, “for any kind of product or service, it's the little things that count” [52]. In spite of the mentioned limitations, this study provides a starting point for research on UX and clinical effectiveness with wearable GPS devices. Future studies using a randomized mixed-method dyadic approach with standardized and validated outcome measures are needed [43], [53].
Research in Context.
-
1.
Systematic review: The authors reviewed all relevant publications on the user experience and clinical effectiveness with wearable global positioning system (GPS) devices for persons with dementia (PwDs) and caregivers (CGs) using PubMed, meeting abstracts, and presentations. This is the first user experience study comparing two commercial GPS watches in home dementia care with data from PwDs and CGs, and reporting on clinical effectiveness resulting from product use.
-
2.
Interpretation: Our results support specific design recommendations for GPS watches in home dementia care. Namely, devices should contain few buttons, display a clear font with limited text, and have a battery life of at least 24 hours. Addressing these recommendations may contribute to measurable clinical effectiveness.
-
3.
Future directions: Given the number of commercially available GPS watches and their low use in home dementia care, our results highlight the importance of optimizing products. Future studies with a randomized mixed-method dyadic approach and with standardized and validated outcome measures are warranted.
Acknowledgments
The authors would like to thank the research participants with dementia and their caregivers for participating in this study.
Funding: The German Alzheimer's Association (Deutsche Alzheimer Gesellschaft e.V. Selbsthilfe Demenz, DAlzG) supported this work (Research grant 2014). The German Alzheimer's Association was not involved in data collection, analysis, or in manuscript preparation and final submission. We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité - Universitätsmedizin Berlin.
Ethics approval: The Charité Ethics Board number EA4/033/16 approved of the study (clinicaltrials.gov identifier: NCT02893800).
Author contributions: H.M., S.D.F., and O.P. were involved in study design. H.M. and C. R. were involved in data collection. H.M., S.D.F., and C.R. performed data analyses. All authors critically reviewed and edited the article.
Footnotes
Conflict of interest: None to report.
Supplementary data to this article can be found online at doi.org/10.1016/j.trci.2018.10.002.
Supplementary data
References
- 1.Van der Roest H.G., Wenborn J., Pastink C., Dröes R.M., Orrell M. Assistive technology for memory support in dementia. Cochrane Database Syst Rev. 2017;6:1–27. doi: 10.1002/14651858.CD009627.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samus Q.M., Lyketsos C.G., Black B.S., Bovenkamp D., Buckley M., Callahan C. Home is where the future is: The BrightFocus Foundation consensus panel on dementia care. Alzheimers Dement. 2017;14:104–114. doi: 10.1016/j.jalz.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McShane R., Gedling K., Kenward B., Kenward R., Hope T., Jacoby R. The feasibility of electronic tracking devices in dementia: a telephone survey and case series. Int J Geriatr Psychiatry. 1998;13:556–563. doi: 10.1002/(sici)1099-1166(199808)13:8<556::aid-gps834>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Miskelly F. A novel system of electronic tagging in patients with dementia and wandering. Age Ageing. 2004;33:304–306. doi: 10.1093/ageing/afh084. [DOI] [PubMed] [Google Scholar]
- 5.Oderud T., Landmark B., Eriksen S., Fossberg A.B., Aketun S., Omland M. Persons with dementia and their caregivers using GPS. Stud Health Technol Inform. 2015;217:212–221. [PubMed] [Google Scholar]
- 6.Pot A.M., Willemse B.M., Horjus S. A pilot study on the use of tracking technology: feasibility, acceptability, and benefits for people in early stages of dementia and their informal caregivers. Aging Ment Health. 2012;16:127–134. doi: 10.1080/13607863.2011.596810. [DOI] [PubMed] [Google Scholar]
- 7.Robinson L., Brittain K., Lindsay S., Jackson D., Olivier P. Keeping in touch everyday (KITE) project: developing assistive technologies with people with dementia and their carers to promote independence. Int Psychogeriatr. 2009;21:494–502. doi: 10.1017/S1041610209008448. [DOI] [PubMed] [Google Scholar]
- 8.Megges H., Freiesleben S.D., Jankowski N., Haas B., Peters O. Technology for home dementia care: A prototype locating system put to the test. Alzheimers Dement. 2017;3:332–338. doi: 10.1016/j.trci.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsson A., Engström M., Asenlöf P., Skovdahl K., Lampic C. Effects of tracking technology on daily life of persons with dementia: Three experimental single-case studies. Am J Alzheimers Dis Other Demen. 2014;30:29–40. doi: 10.1177/1533317514531441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan L., Müller C., Wulf V., Randall D. Proceedings of the SIGCHI conference on human factors in computing systems. ACM; Toronto: 2014. Addressing the subtleties in dementia care: pre-study & evaluation of a GPS monitoring system; pp. 3987–3996. [Google Scholar]
- 11.Williamson B., Aplin T., de Jonge D., Goyne M. Tracking down a solution: exploring the acceptability and value of wearable GPS devices for older persons, individuals with a disability and their support persons. Disabil Rehabil Assist Technol. 2017;12:822–831. doi: 10.1080/17483107.2016.1272140. [DOI] [PubMed] [Google Scholar]
- 12.Asghar I., Cang S., Yu H. Usability evaluation of assistive technologies through qualitative research focusing on people with mild dementia. Comput Hum Behav. 2018;79:192–201. [Google Scholar]
- 13.Ienca M., Fabrice J., Elger B., Caon M., Pappagallo A.S., Kressig R.W. Intelligent assistive technology for Alzheimer's disease and other dementias: A systematic review. J Alzheimers Dis. 2017;56:1301–1340. doi: 10.3233/JAD-161037. [DOI] [PubMed] [Google Scholar]
- 14.Neubauer N.A., Lapierre N., Ríos-Rincón A., Miguel-Cruz A., Rousseau J., Liu L. What do we know about technologies for dementia-related wandering? A scoping review: Examen de la portée: que savons-nous à propos des technologies de gestion de l'errance liée à la démence? Can J Occup Ther. 2018;85:196–208. doi: 10.1177/0008417418777530. [DOI] [PubMed] [Google Scholar]
- 15.Bantry White E., Montgomery P., McShane R. Electronic tracking for people with dementia who get lost outside the home: a study of the experience of familial carers. Br J Occup Ther. 2010;73:152–159. [Google Scholar]
- 16.Sauer A. 2018. 10 Lifesaving Location Devices for Dementia Patients US: alzheimers.net.https://www.alzheimers.net/8-8-14-location-devices-dementia/ Available at: [Google Scholar]
- 17.Dale Ø. International conference on computers for handicapped persons. Springer; Vienna: 2010. Usability and usefulness of GPS based localization technology used in dementia care; pp. 300–307. [Google Scholar]
- 18.Liu L., Miguel Cruz A., Ruptash T., Barnard S., Juzwishin D. Acceptance of global positioning system (GPS) technology among dementia clients and family caregivers. J Technol Hum Serv. 2017;35:99–119. [Google Scholar]
- 19.Meiland F., Innes A., Mountain G., Robinson L., van der Roest H., García-Casal J.A. Technologies to support community-dwelling persons with dementia: a position paper on issues regarding development, usability, effectiveness and cost-effectiveness, deployment, and ethics. JMIR Rehabil Assist Technol. 2017;4:1–21. doi: 10.2196/rehab.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topfer L.A. Canadian Agency for Drugs and Technologies in Health; Ottawa: 2016. GPS locator devices for people with dementia. CADTH issues in emerging health technologies; pp. 1–14. [PubMed] [Google Scholar]
- 21.Livingston G., Sommerlad A., Orgeta V., Costafreda S.G., Huntley J., Ames D. Dementia prevention, intervention, and care. Lancet. 2017;390:1–62. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 22.Brims L., Oliver K. Effectiveness of assistive technology in improving the safety of people with dementia: a systematic review and meta-analysis. Aging Ment Health. 2018:1–10. doi: 10.1080/13607863.2018.1455805. [DOI] [PubMed] [Google Scholar]
- 23.ISO9241-210 . International Organization for Standardization ISO; Geneva, CH: 2010. Ergonomics of human-system interaction—Part 210: human-centred design for interactive systems. [Google Scholar]
- 24.Forlizzi J., Ford S. Proceedings of the 3rd conference on designing interactive systems: processes, practices, methods, and techniques. ACM; New York: 2000. The building blocks of experience: an early framework for interaction designers; pp. 419–423. [Google Scholar]
- 25.Law E., Roto V., Vermeeren A.P.O.S., Kort J., Hassenzahl M. ACM; Florence: 2008. Towards a shared definition of user experience. Extended abstracts on human factors in computing systems; pp. 2395–2398. [Google Scholar]
- 26.Davis F.D., Bagozzi R.P., Warshaw P.R. User acceptance of computer technology: a comparison of two theoretical models. Manage Sci. 1989;35:982–1003. [Google Scholar]
- 27.Hornbæk K., Hertzum M. Technology acceptance and user experience: A review of the experiential component in HCI. ACM Trans Comput-Hum Interact. 2017;24:33. [Google Scholar]
- 28.Hassenzahl M. The thing and I: Understanding the relationship between user and product. In: Mark A., Blythe A.F.M., Overbeeke K., Wright P.C., editors. Funology: From Usability to Enjoyment. Kluwer Academic Publishers; Netherlands: 2003. pp. 31–42. [Google Scholar]
- 29.Natesan D., Walker M., Clark S. Cognitive bias in usability testing. Proc Int Symp Hum Factors Ergon Healthc. 2016;5:86–88. [Google Scholar]
- 30.Edwards A.L. The social desirability variable in personality assessment and research. Acad Med. 1958;33:610–611. [Google Scholar]
- 31.Haberstroh J., Pantel J. [Communication in dementia: TANDEM training manual]. Springer; Heidelberg: 2011. Kommunikation bei Demenz: TANDEM Trainings manual. [Google Scholar]
- 32.Feil N., de Klerk-Rubin V. 3 ed. Health Professions Press; Baltimore: 2012. The Validation Breakthrough: Simple Techniques for Communicating With People With Alzheimer's and Other Dementias. [Google Scholar]
- 33.Bédard M., Molloy D.W., Squire L., Dubois S., Lever J.A., O'Donnell M. The Zarit Burden Interview: A new short version and screening version. Gerontologist. 2001;41:652–657. doi: 10.1093/geront/41.5.652. [DOI] [PubMed] [Google Scholar]
- 34.Brähler E., Mühlan H., Albani C., Schmidt S. Teststatistische Prüfung und Normierung der deutschen Versionen des EUROHIS-QOL Lebensqualität-Index und des WHO-5 Wohlbefindens-Index [Statistical testing and standardization of the German version of the EUROHIS-QOL quality of life index and the WHO-5 well-being index] Diagnostica. 2007;53:83–96. [Google Scholar]
- 35.Karrer K., Glaser C., Clemens C., Bruder C. Technikaffinität erfassen–der Fragebogen TA-EG [Assessment of technological affinity-the TA-EG] Mensch Mittelpunkt Technischer Systeme. 2009;8:196–201. [Google Scholar]
- 36.Prümper J. Der Benutzungsfragebogen ISONORM 9241/10: Ergebnisse zur Reliabilität und Validität [The Usability Questionnaire ISO (International Organization for Standardization) NORM 9241/10: results on reliability and validity] In: Liskowsky R., Velichkovsky B., Wünschmann W., editors. Software-Ergonomie '97. Vieweg+Teubner Verlag; Wiesbaden: 1997. pp. 253–262. [Google Scholar]
- 37.Prümper J. Springer; Berlin, Heidelberg: 1993. Software-evaluation based upon ISO 9241 Part 10; pp. 255–265. [Google Scholar]
- 38.ISO9241-110 . International Standard Organization ISO; Geneva, CH: 2006. Ergonomics of human-system interaction—Part 110: dialogue principles [Revision of ISO 9241-10:1996 Ergonomic requirements for office work with visual display terminals (VDTs)—Part 10: dialogue principles] [Google Scholar]
- 39.Sarodnick F., Brau H. 1 ed. Huber; Bern: 2006. Methoden der Usability Evaluation: Wissenschaftliche Grundlagen und praktische Anwendung [Methods of usability assessment: scientific foundations and practical application] [Google Scholar]
- 40.Kenny D.A., Kashy D.A., Cook W.L. Guilford Press; New York: 2006. The analysis of dyadic data. [Google Scholar]
- 41.Field A. 3 ed. Sage; London: 2009. Discovering statistics using SPSS. [Google Scholar]
- 42.Wood E., Ward G., Woolham J. The development of safer walking technology: a review. J Assist Technol. 2015;9:100–115. [Google Scholar]
- 43.MacAndrew M., Brooks D., Beattie E. Nonpharmacological interventions for managing wandering in the community: A narrative review of the evidence base. Health Soc Care Community. 2018:1–14. doi: 10.1111/hsc.12590. [DOI] [PubMed] [Google Scholar]
- 44.Olsson A., Engström M., Lampic C., Skovdahl K. A passive positioning alarm used by persons with dementia and their spouses—a qualitative intervention study. BMC Geriatr. 2013;13:11. doi: 10.1186/1471-2318-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bantry-White E. Supporting ethical use of electronic monitoring for people living with dementia: social work's role in assessment, decision-making, and review. J Gerontol Soc Work. 2018;61:261–279. doi: 10.1080/01634372.2018.1433738. [DOI] [PubMed] [Google Scholar]
- 46.Robillard J.M., Cleland I., Hoey J., Nugent C. Ethical adoption: A new imperative in the development of technology for dementia. Alzheimers Dement. 2018;14:1104–1113. doi: 10.1016/j.jalz.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y.-C., Leung C.-Y. Exploring functions of the lost seeking devices for people with dementia. Work. 2012;41:3093–3100. doi: 10.3233/WOR-2012-0568-3093. [DOI] [PubMed] [Google Scholar]
- 48.Demers L., Weiss-Lambrou R., Ska B. The Quebec user evaluation of satisfaction with assistive technology (QUEST 2.0): an overview and recent progress. Technol Disabil. 2002;14:101–105. [Google Scholar]
- 49.Peek S.T.M., Wouters E.J.M., van Hoof J., Luijkx K.G., Boeije H.R., Vrijhoef H.J.M. Factors influencing acceptance of technology for aging in place: A systematic review. Int J Med Inf. 2014;83:235–248. doi: 10.1016/j.ijmedinf.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Lai C.K.Y., Arthur D.G. Wandering behaviour in people with dementia. J Adv Nurs. 2003;44:173–182. doi: 10.1046/j.1365-2648.2003.02781.x. [DOI] [PubMed] [Google Scholar]
- 51.Association As . Alzheimer’s Association; Chicago: 2015. Behaviors: How to Respond When Dementia Causes Unpredictable Behaviors; pp. 1–13. [Google Scholar]
- 52.Garrett J.J. 2 ed. Pearson Education; Berkeley: 2010. The elements of User Experience: User-Centered Design for the Web and Beyond. [Google Scholar]
- 53.Patomella A.-H., Lovarini M., Lindqvist E., Kottorp A., Nygård L. Technology use to improve everyday occupations in older persons with mild dementia or mild cognitive impairment: A scoping review. Br J Occup Ther. 2018;0 0308022618771533. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.