Abstract
Control of bacterial infection-induced inflammatory responses is one of the effective therapeutic approaches of periodontal diseases. Natural products such as lipid mediators and metabolites from microorganisms have been used for decreasing inflammation. We previously reported that (+)-terrein inhibited activation of STAT3 and ERK1/2 in interleukin-6 (IL-6) signaling cascade, leading to prevent vascular endothelial growth factor (VEGF) secretion in human gingival fibroblasts (HGFs). However, little is still known about the role of (+)-terrein on inflammatory responses. In this study, we provided the possibility of novel action that (+)-terrein inhibits activation of Janus-activated kinase 1 (JAK1), which has a central function in IL-6 signaling cascade, and alters expression of mRNAs and proteins induced by IL-6/soluble IL-6 receptor (sIL-6R) stimulation in HGFs. First, we performed PCR array to examine IL-6/sIL-6R-induced mRNA expression, and then expression of mRNA and protein of colony stimulating factor-1 (CSF1) and VEGF were clearly determined by quantitative RT-PCR and ELISA, respectively. Treatment with (+)-terrein suppressed expression of mRNA and protein of CSF1 and VEGF by IL-6/sIL-6R stimulation. Next, to test the effect of (+)-terrein on IL-6/sIL-6R signaling cascade, we demonstrated whether (+)-terrein affects phosphorylation of JAK1 and its downstream proteins, Akt and SHP-2. Western blotting revealed that (+)-terrein inhibited IL-6/sIL-6R-induced phosphorylation of JAK1, Akt, and SHP-2. Therefore, (+)-terrein suppresses IL-6/sIL-6R-induced expression of CSF1 and VEGF via inhibition of JAK1, Akt, and SHP-2. Based on our results, we suggest that (+)-terrein is a candidate compound for anti-inflammatory effect associated with IL-6 signaling.
Keywords: Dentistry, Molecular biology
1. Introduction
Periodontitis is a chronic inflammatory disease characterized by the destruction of periodontal tissue and loss of alveolar bones [1], and caused by inflammation-associated dysbacteriosis of commensal microbiota; however, the temporal sequence of and interplay between specific bacteria and inflammation is not well understood [2]. Mechanical debridement, e.g., scaling and root planning and/or flap surgery, has long been the standard protocol for treating moderate-to-severe periodontitis [3]. Removal of subgingival plaque and calculus is sufficient to reduce bacteria-induced host inflammatory response regulated by multiple inflammatory cytokines, such as tumor necrosis factor-α, interleukin (IL)-1β and IL-6, and to suppress periodontal tissue destruction. However, subgingival debridement has limitations because of anatomical variations, including existing root furcation involvement, which results in the incomplete removal of diseased lesions [4]. Irrespective of the completeness of debridement, residual bacteria grow back gradually after mechanical treatment in the absence of additional treatment or maintenance, resulting in the recurrence of disease-associated inflammation [5]. Antibacterial therapy improves clinical symptoms but is associated with antibiotic resistance and other side effects [6]. Therefore, novel host-modulation treatment approach instead of antibiotics is required.
IL-6 is one of the most important pro-inflammatory cytokines and exerts various biological effects and plays an important role in the progression of inflammatory diseases including periodontitis [12, 13]. The IL-6/soluble IL-6 receptor (sIL-6R) complex activates glycoprotein 130 (gp130) that, in turn, up-regulates Janus-activated kinase (JAK)/signal transducers and activators of transcription-3 (STAT3) signaling, Ras/mitogen activator of protein kinase (MAPK) signaling and phosphoinositide 3-kinase (PI3k)/Akt signaling pathway. Blocking IL-6 cascade was recently shown to have therapeutic effects in several autoimmune and inflammatory disease models such as rheumatoid arthritis [44] and periodontitis [45]. Thus, control of IL-6 signaling is lead to control of chronic inflammation [12].
(+)-Terrein was isolated from Aspergillus terreus as a secondary bioactive fungal metabolite by Raistrick and Smith in 1935 [7]. (+)-Terrein exerts various biological effects, including antibacterial effect [8], inhibition of angiogenin secretion in prostate cancer cells [9], and modulation of pulpal inflammation [10]. In our previous study, we established the synthesis of (+)-terrein, and reported that (+)-terrein inhibited IL-6/sIL-6R-induced phosphorylation of STAT3 and extracellular signal-regulated kinase 1/2 (ERK1/2) in human gingival fibroblasts (HGFs), resulting in suppression of vascular endothelial growth factor (VEGF) secretion [11]. Therefore, we think that (+)-terrein might be a useful tool to regulate IL-6 signaling and to suppress IL-6-associated inflammatory disease progression [11]. However, the anti-inflammatory effects of (+)-terrein and the underlying functional mechanism are unclear.
The present study examined the effect of (+)-terrein on IL-6/sIL-6R-induced protein secretion using PCR array and determined the target molecules of (+)-terrein in the IL-6 signaling pathway in HGFs. Our results can be used to establish a novel host-modulation treatment approach by using (+)-terrein for preventing and treating inflammatory diseases, including periodontitis.
2. Material and methods
2.1. Reagents
Synthetic (+)-terrein was prepared from dimethyl L-tartrate, as described previously [11]. Recombinant human IL-6 and sIL-6R were purchased from R&D Systems (Minneapolis, MN). Rabbit anti-phosphorylated Akt and Src homology 2 domain-containing phosphatase-2 (SHP-2) polyclonal antibodies were purchased from Cell Signaling Technology (Danvers, MA). Rabbit anti-phosphorylated Janus-activated kinase 1 (JAK1) polyclonal antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-β-actin monoclonal antibody was obtained from Sigma (St. Louis, MO). JAK1/2 inhibitor, baricitinib, was obtained from Chemscene (Monmouth Junction, NJ).
2.2. Cell culture
HGFs were generated as described previously [14] and were cultured in DMEM (Thermo Fisher Scientific, Waltham, MA) containing 10% fetal bovine serum (FBS; Biowest, Riverside, MO), 20 mM HEPES (Sigma), 100 U/mL penicillin, and 100 μg/mL streptomycin (Thermo Fisher Scientific) at 37 °C in an atmosphere of 5% CO2. The Research Ethics Committee of Okayama University Graduate School of Medicine, Dentistry and Pharmaceuticals Sciences approved this study (approval number: 661), and we obtained signed informed consent from all volunteers before the study. Cells were subcultured up to 5–8 passages. For the specific experiments, the cells (5.0 × 104 cells/cm2) were seeded in a 35-mm dish and were cultured until they reached subconfluence. The cells were pretreated with (+)-terrein (10 μM) or baricitinib (0.1–5 μM) for 30 mins, followed by stimulation with IL-6/sIL-6R (50 ng/ml each) for specific duration. Cytotoxity of (+)-terrein on HGFs has been reported previously [11], and less than 10 μM of (+)-terrein has no effect on cell viability in HGFs.
2.3. PCR array and quantitative reverse transcription-PCR
After stimulation for 12 h, total RNA was extracted from the cells using RNeasy® Mini Kit (Qiagen, Hilden, Germany), and DNA contamination was removed using RNase-Free DNase Kit (Qiagen). Next, 1 μg high-quality total RNA was reverse transcribed using SuperScript® III First-Strand Synthesis System (Thermo Fisher Scientific) and was loaded onto RT2 Profiler™ PCR Array Human Growth Factors (Qiagen; Table 1), according to the manufacturer's instructions. Qiagen's online Web analysis tool (http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php) was used to analyze PCR array data, and fold change was calculated by determining the ratio of mRNA levels to control values by using Δ threshold cycle (Ct) method (2−ΔΔCt). All data were normalized to an average of five housekeeping genes, namely, GUSB, HPRT, HSP90AB1, GAPDH, and ACTB. PCR conditions used are as follows: hold for 10 min at 95 °C, followed by 45 cycles of 15 s at 95 °C and 60 s at 60 °C. In addition, we considered significant changes in gene expression values of ±5.0-fold change compared to control. After PCR array analysis, we confirmed the mRNA expression pattern of target genes by performing quantitative PCR. Gene-specific primers [colony-stimulating factor-1 (CSF-1), brain-derived neutrophic factor (BDNF), bone morphogenetic protein-1 (BMP-1), dickkopf WNT signaling pathway inhibitor 1 (DKK1), endoplasmic reticulum aminopeptidase 1 (ERAP1)] were designed using Primer3 Plus (http://primer3plus.com/cgi-bin/dev/primer3plus.cgi) and NCBI Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). PCR primer sets used in this study are listed in Table 2. Quantitative PCR was performed to amplify up to 1 ng cDNA by using specific primers. Each sample was examined in a 10 μl reaction mixture by using SYBR Green PCR Master Mix (Thermo Fisher Scientific) and 7300 Real-Time PCR System (Thermo Fisher Scientific). Ratios of mRNA levels to control values were calculated using the ΔCt method (2−ΔΔCt). All data were normalized using the mRNA level of the housekeeping gene GAPDH. PCR conditions used are as follows: hold for 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 60 s at 60 °C.
Table 1.
84 genes on PCR Array (RT2 ProfilerTM PCR Array Human Growth Factors, Qiagen).
| AMH | BDNF | BMP1 | BMP10 | BMP2 | BMP3 | BMP4 | BMP5 | BMP6 | BMP7 | BMP8B | CECR1 |
| CLC | CSF1 | CSF2 | CSF3 | CSPG5 | CXCL1 | DKK1 | ERAP1 | EREG | FGF1 | FGF11 | FGF13 |
| FGF14 | FGF17 | FGF19 | FGF2 | FGF22 | FGF23 | FGF5 | FGF6 | FGF7 | FGF9 | FIGF | GDF10 |
| GDF11 | GDNF | GPI | HBEGF | IGF1 | IGF2 | IL10 | IL11 | IL12B | IL18 | IL1A | IL1B |
| IL2 | IL3 | IL4 | INHA | INHBA | INHBB | JAG1 | JAG2 | LEFTY1 | LEFTY2 | LIF | LTBP4 |
| MDK | MSTN | NDP | NGF | NODAL | NRG1 | NRG2 | NRG3 | NRTN | NTF3 | OSGIN1 | PDGFC |
| PGF | PSPN | PTN | SLCO1A2 | SPP1 | TDGF1 | TGFB1 | THPO | TNNT1 | TYMP | VEGFA | VEGFC |
| ACTB | B2M | GAPDH | HPRT1 | RPLP0 | HGDC | RTC | RTC | RTC | PPC | PPC | PPC |
AMH: anti-Mullerian hormone BDNF: brain derived neurotrophic factor BMP: bone morphogenetic protein CECR: cat eye syndrome chromosome region, candidate CLC: Charcot-Leyden crystal galectin CSF: colony stimulating factor CSPG5: chondroitin sulfate proteoglycan CXCL: C-X-C motif chemokine ligand DKK: dickkopf WNT signaling pathway inhibitor ERAP: endoplasmic reticulum aminopeptidase EREG: epiregulin FGF: fibroblast growth factor FIGF (VEGFD): vascular endothelial growth factor D GDF: growth differentiation factor GDNF: glial cell derived neurotrophic factor GPI: glucose-6-phosphate isomerase HBEGF: heparin binding EGF like growth factor IGF: insulin like growth factor IL: interleukin INH: inhibin JAG: jagged LEFTY: left-right determination factor LIF: leukemia inhibitory factor LTBP: latent transforming growth factor beta binding protein MDK: midkine MSTN: myostatin NDP: NDP, norrin cystine knot growth factor NGF: nerve growth factor NODAL: nodal growth differentiation factor NRG: neuregulin NRTN: neurturin NTF3: neurotrophin OSGIN: oxidative stress induced growth inhibitor PDGF: platelet derived growth factor PGF: placental growth factor PSPN: persephin PTN: pleiotrophin SLCO: solute carrier organic anion transporter family member SPP: secreted phosphoprotein TDGF: teratocarcinoma-derived growth factor TGFB: transforming growth factor beta THPO: thrombopoietin TNNT: troponin T, slow skeletal type TYMP: thymidine phosphorylase VEGF: vascular endothelial growth factor ACTB: beta actin B2M: beta 2 microgrobulin GAPDH: glyceraldehyde 3 phosphate dehydrogenase HPRT1: Hypoxanthine-guanine phosphoribosyltransferase RPLP0: ribosomal protein large P0 HGDC: Human Genomic DNA Contamination RTC: Reverse Transcription Control PPC: Positive PCR Control.
Table 2.
Genes and their respective primer sequence and amplicon size used in this study.
| Gene | primer sequence | Amplicon size (bp) |
|---|---|---|
| VEGF-A | 5′-AGGGCAGAATCATCACGAAGT-3' (forward) | 75 |
| 5′-AGGGTCTCGATTGGATGGCA-3' (reverse) | ||
| CSF1 | 5′-AGACCTCGTTGCCAAATTACATT-3' (forward) | 249 |
| 5′-AGGTGTCTCATAGAAAGTTCGGA-3' (reverse) | ||
| BDNF | 5′-AATCAGTTGCGCGTTCTGAA-3' (forward) | 185 |
| 5′-TAGCCATGATTTACCCAAATG-3' (reverse) | ||
| DKK1 | 5′-ATAGCACCTTGGATGGGTATTCC-3' (forward) | 96 |
| 5′-CTGATGACCGGAGACAAACAG-3' (reverse) | ||
| BMP1 | 5′-GGGGTGAAACCTCCCATTGG-3' (forward) | 170 |
| 5′-CACACGCAGTGCATGTGAG-3' (reverse) | ||
| ERAP1 | 5′-GGCAATCTTTCGGAGACTTTC-3' (forward) | 141 |
| 5′-GAAGGCAGGTTCATCAAAGC-3' (reverse) | ||
| GAPDH | 5′-TGGCAAATTCCATGGCA-3' (forward) | 164 |
| 5′-CCTTCTCCATGGTGGT-3' (reverse) |
2.4. Enzyme-linked immunosorbent assay
After stimulation for 24 h, culture supernatant was collected and stored at -80 °C for further use. VEGF and CSF1 levels were measured using sandwich enzyme-linked immunosorbent assay (ELISA) kits containing human anti-VEGF and anti-CSF1 antibodies (R&D Systems), according to the manufacturer's instructions.
2.5. Western blotting
After stimulation for 1 or 5 min, the cells were lysed rapidly in ice-cold cell lysis buffer (50 mM NaCl, 10 mM Tris-HCl [pH 7.2], 1% Nonidet P-40, 5 mM EDTA-Na, 1 mM sodium orthovanadate, 1% sodium dodecyl sulfate [SDS], and protease inhibitor cocktail [Sigma]) for 10 min. Protein concentration was determined using Bradford method [15], with bovine serum albumin faction V (Sigma) as the standard. Cell lysates (30 μg each for determining phosphorylated Akt and SHP-2, and 50 μg for determining phosphorylated JAK1) were mixed with SDS sample buffer (1% [w/v] SDS, 45 mM Tris-HCl [pH 6.8], 15% [v/v] glycerol, 144 mM 2-mercaptoethanol, and 0.002% bromophenol blue) and were boiled for 5 min. Proteins present in the samples were separated by performing SDS-PAGE on polyacrylamide gels (12% [v/v] gel for phosphorylated Akt and SHP-2, and 7.5% [v/v] gel for phosphorylated JAK1) and were transferred electrophoretically onto polyvinylidene difluoride membranes. The membranes were blocked with 5% skim milk in TBST (20 mM Tris-HCl [pH 7.6] containing 150 mM NaCl and 0.1% [v/v] Tween 20) for 1 h and were incubated with the appropriate primary antibodies overnight at 4 °C. Next, the membranes were incubated with secondary antibodies (goat anti-mouse or rabbit horseradish peroxidase [HRP]-conjugated IgG; dilution, 1:2,000; GE Healthcare Bioscience, Pittsburgh, PA) for 1 h. HRP activity was visualized by incubating the membranes in an ECL detection system (SuperSignal® West Dura Extended Duration Substrate; Thermo Fisher Scientific) and by performing autoradiography. Finally, the immunodetection system and bound antibodies were removed from the blots by incubating the membranes with a reprobing buffer (Restore™ Western Blot Stripping Buffer; Thermo Fisher Scientific). The blots were then stained with anti-β-actin antibody (dilution, 1:10,000) to confirm the presence of equal amounts of proteins in each lane of the gel.
2.6. Statistical analysis
Experimental results are presented as mean ± SD. Multiple groups were compared using one-way ANOVA/Scheffe's test, and p < 0.05 was considered statistically significant.
3. Results
3.1. Effect of (+)-terrein on IL-6/sIL-6-induced gene expression by using PCR array
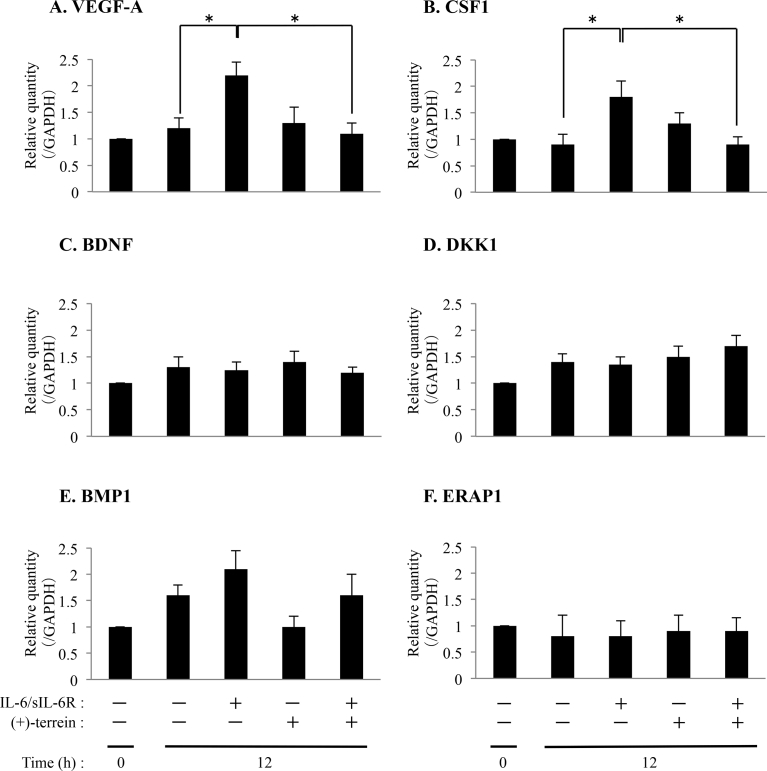
Specific effects of (+)-terrein on IL-6/sIL-6R-induced secretion of growth factors were evaluated at a steady-state mRNA level by using RT2 Profiler™ PCR Array Human Growth Factors. Gene expression patterns were compared among each group (without stimulation vs. IL-6/sIL-6R stimulation, without stimulation vs. (+)-terrein pretreatment, IL-6/sIL-6R stimulation vs. IL-6/sIL-6R and (+)-terrein treatment), and five-fold change in gene expression was used as the experimental cut-off value for implying significance (Table 3). Of the 84 genes on the array, six genes were upregulated by more than five fold (VEGF-A, +15.4325; BDNF, +9.1343; DKK1, +8.5309; BMP1, +7.9222; ERAP1, +7.5491; and CSF1, +6.6821]) in IL-6/sIL-6R-stimulated cells compared with those in control (unstimulated) cells. (+)-Terrein did not affect the expression of these six genes (VEGF-A, -2.7824; BDNF, -3.0509; DKK1, -1.7486; BMP1, -1.2963; ERAP1, -1.1127; and CSF1, -1.2033). However, expression of these six genes was downregulated to <20% of the baseline level (VEGF-A, -7.894; BDNF, -7.7316; DKK1, -4.5982; BMP1, -8.8046; ERAP1, -5.8798; and CSF1, -11.0331) in cells pretreated with (+)-terrein and stimulated with IL-6/sIL-6R compared with that in cells stimulated with only IL-6/sIL-6R.
Table 3.
Up- and down-regulated mRNA profiling in HGFs treated with IL-6 and (+)-terrein.
| # | Conditions |
(-) vs IL6 |
(-) vs (+)-terrein |
IL6 vs IL6+(+)-terrein |
|
|---|---|---|---|---|---|
| Description | Symbol | Fold Regulation | Fold Regulation | Fold Regulation | |
| 1 | Vascular endothelial growth factor A | VEGFA | 15.4325 | -2.7824 | -7.894 |
| 2 | Brain-derived neurotrophic factor | BDNF | 9.1343 | -3.0509 | -7.7316 |
| 3 | Dickkopf homolog 1 (Xenopus laevis) | DKK1 | 8.5309 | -1.7486 | -4.5982 |
| 4 | Bone morphogenetic protein 1 | BMP1 | 7.9222 | -1.2963 | -8.8046 |
| 5 | Endoplasmic reticulum aminopeptidase 1 | ERAP1 | 7.5491 | -1.1127 | -5.8798 |
| 6 | Colony stimulating factor 1 (macrophage) | CSF1 | 6.6821 | -1.2033 | -11.0331 |
| 7 | Fibroblast growth factor 1 (acidic) | FGF1 | 4.8878 | -3.2819 | -4.6511 |
| 8 | Oxidative stress induced growth inhibitor 1 | OSGIN1 | 4.8848 | 6.2341 | -32.6398 |
| 9 | Bone morphogenetic protein 4 | BMP4 | 4.3678 | -1.2073 | -5.433 |
| 10 | Leukemia inhibitory factor (cholinergic differentiation factor) | LIF | 3.7203 | -1.0707 | -3.0476 |
| 11 | Glial cell derived neurotrophic factor | GDNF | 3.1468 | 1.9254 | -5.2903 |
| 12 | Heparin-binding EGF-like growth factor | HBEGF | 3.1052 | -1.7615 | -1.1679 |
3.2. Expression of mRNA and protein for CSF1 and VEGF was determined by quantitative RT-PCR and ELISA
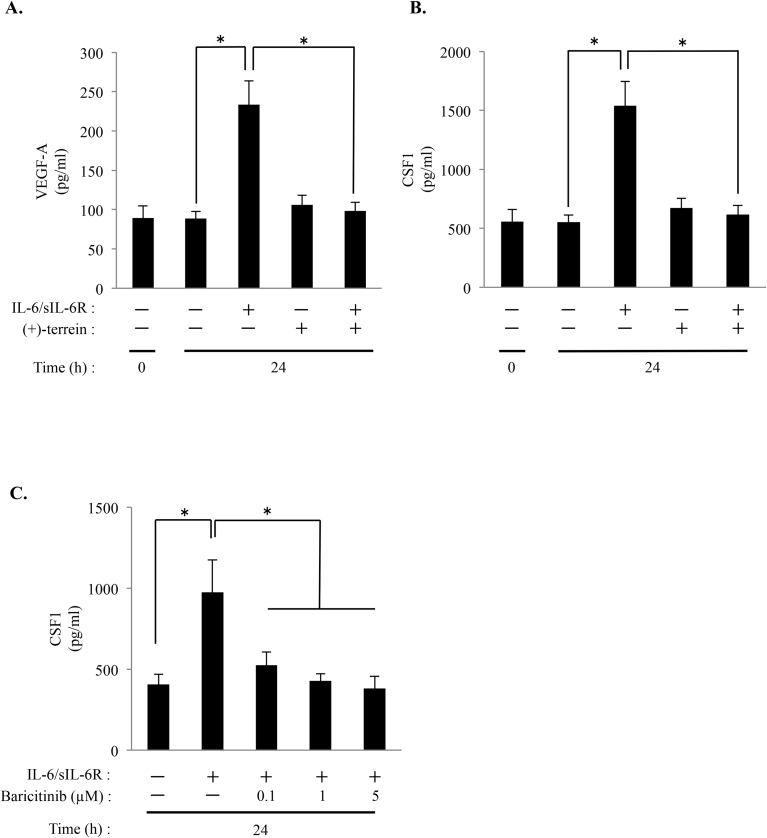
Changes in the expression levels six genes (VEGF-A, BDNF, DKK1, BMP1, ERAP1 and CSF1) observed in the PCR arrays were validated by performing quantitative RT-PCR by using mRNA obtained from the same samples as those used for performing PCR arrays (Fig. 1). Results of quantitative RT-PCR showed a correlation between VEGF-A and CSF1 expression (p < 0.05; Fig. 1A and B). However, quantitative RT-PCR did not show a correlation of other 4 genes (BDNF, DKK1, BMP1, and ERAP1) expression (Fig. 1C–F). In addition, we examined the secretion of VEGF-A and CSF1 into the culture supernatant by performing ELISA. We demonstrated that (+)-terrein suppresses IL-6/sIL-6R-induced expression of VEGF-A and CSF1 (p < 0.05; Fig. 2A and B). On the other hand, JAK1/2 inhibitor, baricitinib, suppressed IL-6/sIL-6R-induced CSF1 secretion as well as (+)-terrein (p < 0.05; Fig. 2C).
Fig. 1.
Accumulation of CSF1 and VEGF-A mRNAs in HGFs treated with IL-6/sIL-6R, and (+)-terrein. HGFs (5.0 × 104 cells/cm2) were cultured until they reached subconfluence. Next, the cells were pretreated with (+)-terrein (10 μM) for 30 min, followed by stimulation with IL-6/sIL-6R (50 ng/mL each) for 12 h. Total RNA was extracted, as described in Materials and Methods, and was analyzed by performing quantitative RT-PCR with specific primers shown in Table 2. GAPDH was used as an internal control. Data are expressed as mean ± SD and are representative of three independent experiments; *p < 0.05 (ANOVA/Scheffe's test).
Fig. 2.
Secretion of CSF1 and VEGF-A in HGFs treated with IL-6/sIL-6R, and (+)-terrein. HGFs (5.0 × 104 cells/cm2) were cultured until they reached subconfluence. Next, the cells were pretreated with (+)-terrein (10 μM) or baricitinib (0.1–5 μM) for 30 min, followed by stimulation with IL-6/sIL-6R (50 ng/mL each) for 24 h. After stimulation, culture supernatants were collected, and levels of VEGF-A (A) and CSF1 (B) treated with (+)-terrein, level of CSF1 treated with baricitinib (C) were measured using ELISA kits. Data are expressed as mean ± SD and are representative of three independent experiments; *p < 0.05 (ANOVA/Scheffe's test).
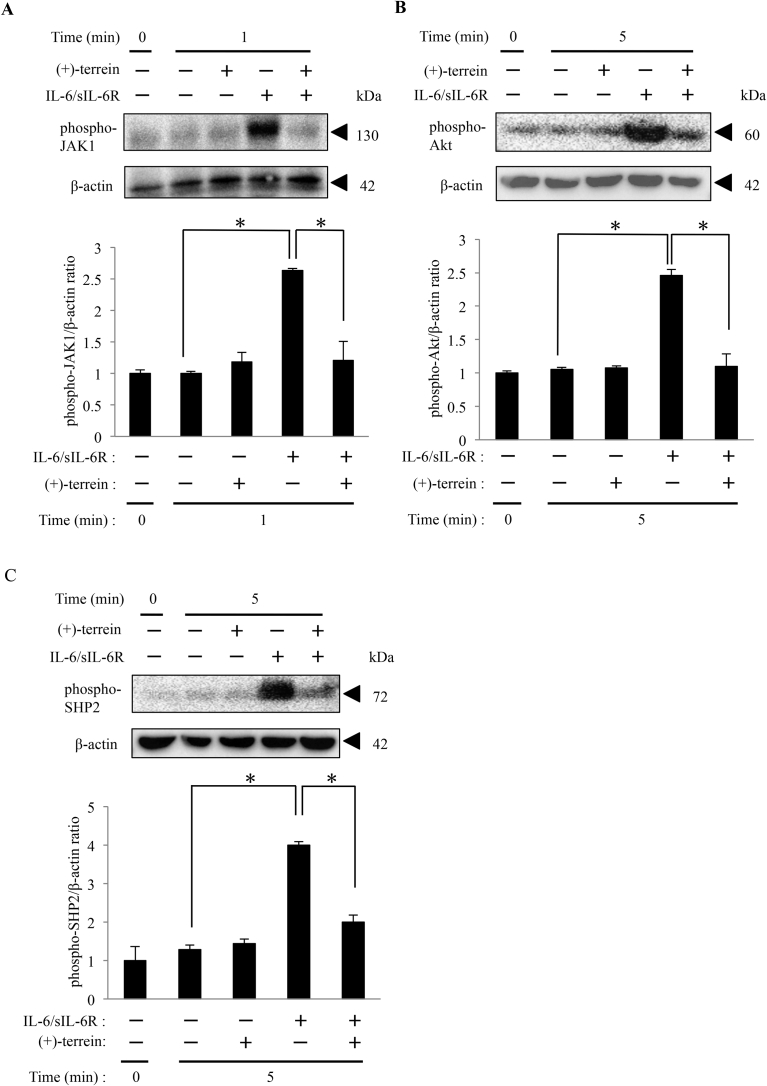
3.3. (+)-Terrein suppressed IL-6/sIL-6R-induced JAK1 phosphorylation
JAK1 is the upstream kinase of them in IL-6 signaling pathway, and hence we examined whether (+)-terrein affects phosphorylation of JAK1 induced by IL-6/sIL-6R stimulation. HGFs were treated with or without (+)-terrein at 10 μM for 30 min, and stimulated with IL-6/sIL-6R for up to 5 min. After stimulation, phosphorylation of JAK1 was detected with western blotting. (+)-Terrein notably decreased in phosphorylation of JAK1 in IL-6/sIL-6R-stimulated HGFs compared with that of IL-6/sIL-6R-stimulated cells without (+)-terrein treatment (Fig. 3A). Furthermore, we found that Akt and SHP-2, which are downstream molecules of JAK1 in IL-6 signaling pathway, were markedly reduced their phosphorylation by treatment with (+)-terrein (Fig. 3B and C). These results indicated that (+)-terrein might have an inhibitory effect on IL-6/sIL-6R-induced cellular responses via JAK1 inhibition.
Fig. 3.
(+)-Terrein suppresses IL-6/sIL-6R-induced phosphorylation of JAK1, Akt, and SHP-2 in HGFs. HGFs (5.0 × 104 cells/cm2) were cultured until they reached subconfluence. Next, the cells were pretreated with (+)-terrein (10 μM) for 30 min, followed by stimulation with IL-6/sIL-6R (50 ng/mL each) for 5 min. After stimulation, total cell lysates were collected. Phosphorylation of JAK1 (A), Akt (B), and SHP-2 (C) were determined by Western blotting using phospho-specific antibodies, respectively. Relative band density of each phosphorylation level is compared with β-actin using Image J software. β-Actin was detected by reprobing the blots after detecting SHP-2. Data are expressed as mean ± SD and are representative of three independent experiments; *p < 0.05 (ANOVA/Scheffe's test). Representative full, non-adjusted blot images are shown as supplementary figure (file name: 20181115supplementaryfigure_Heliyon_YamamotoS.pdf).
4. Discussion
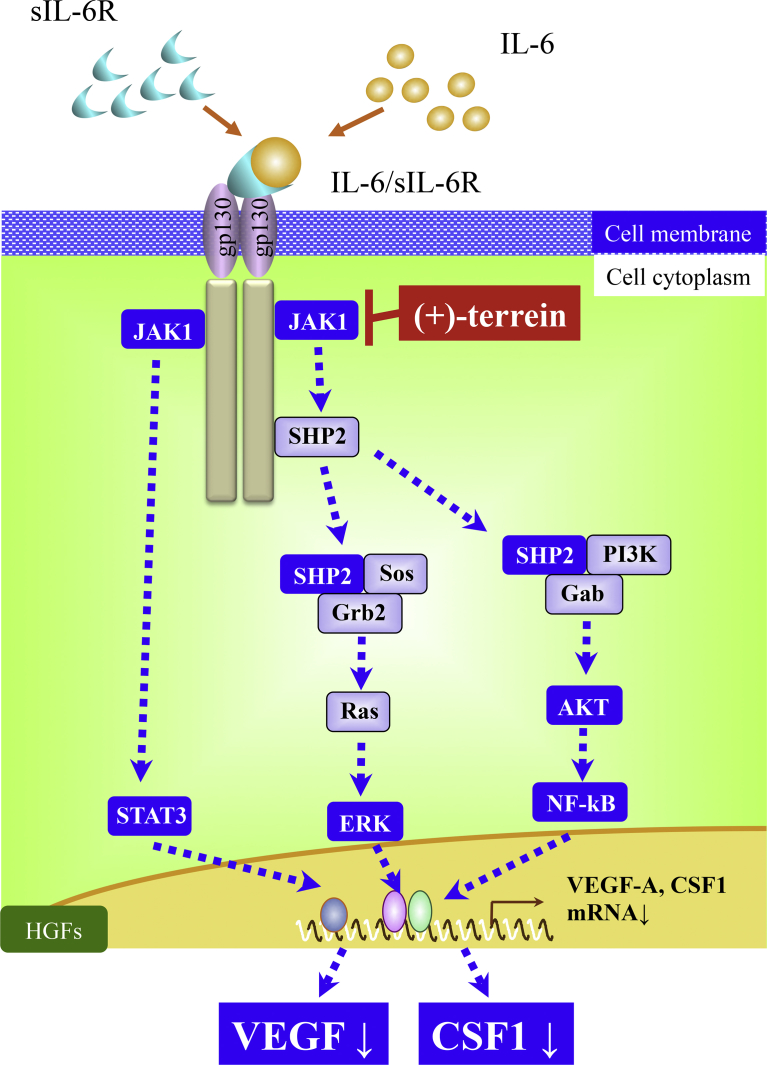
In the present study, we found that treatment with (+)-terrein suppressed IL-6/sIL-6R-induced secretion of CSF1 and phosphorylation of JAK1, a JAK family protein present just below the cell membrane in HGFs. Together, these findings suggest that (+)-terrein may play an important role by suppressing JAK1 phosphorylation to regulate IL-6 signaling and CSF1- and VEGF-induced inflammation in HGFs (Fig. 4).
Fig. 4.
JAK1 may be the target molecule of (+)-terrein in the IL-6 signaling pathway in HGFs. (+)-Terrein regulates IL-6 signaling by suppressing phosphorylation of JAK1, the upstream molecule of the IL-6 signaling pathway.
Recent studies have shown that susceptibility to and pathogenesis of periodontitis are mediated by host immune response [16, 17]. Patients with severe periodontitis exhibit hyper-inflammation, which is characterized by multiple cytokines production [18] and oxidative stress [19]. These host-immune response markers also can be used to predict diseases progression [20]. Previous animal and clinical studies assessing the regulation of host inflammatory response have shown that cyclooxygenase inhibitors suppress disease progression without modifying bacterial composition [21, 22]. However, long-term use of cyclooxygenase inhibitors is associated with serious side effects such as increased risk of gastrointestinal tract bleeding and cardiovascular diseases [23] that discourage the persistent clinical use of these inhibitors for treating periodontitis. In addition, periodontitis is associated with increased prevalence of several systemic diseases such as diabetes and cardiovascular disease as well as with preterm birth [24, 25]. Therefore, there is a critical need to develop reasonable and safe host modulation treatments for preventing and treating periodontitis.
IL-6 is an important proinflammatory cytokine that performs multiple functions in various cell types, and blockade of IL-6 signaling is an effective strategy for managing chronic inflammatory diseases and cancer progression [26]. We previously showed that (+)-terrein suppressed VEGF secretion by inhibiting phosphorylation of STAT3 and ERK1/2 [11], suggesting that synthetic (+)-terrein-induced inhibition of IL-6 signaling is a novel approach for treating IL-6-associated inflammatory diseases. In addition, (+)-terrein therapy might be suggested to be more economical and convenient than conventional anti-IL-6 antibody therapies. However, little is known about the effects of (+)-terrein on IL-6/sIL-6R-induced protein synthesis and target molecules of (+)-terrein. We first examined the effect of (+)-terrein on IL-6/sIL-6R-induced gene expression pattern by using PCR arrays (Table 3). PCR array data showed that (+)-terrein did not exert significant effects on gene expression pattern, indicating that (+)-terrein is not a cytotoxic compound. However, (+)-terrein suppressed IL-6/sIL-6R-induced expression of several mRNAs, including VEGF mRNA (Table 3). Moreover, we found that (+)-terrein significantly suppressed IL-6/sIL-6R-induced CSF1 and VEGF mRNA and protein expression (Figs. 1 and 2).
CSF1, also known as macrophage colony-stimulating factor (M-CSF), is a secreted cytokine that performs various biological functions, including differentiation of hematopoietic stem cells into macrophages [27, 28] and differentiation of precursor cells into osteoclasts [29]. In mice lipopolysaccharide-induced periodontitis model, the expression M-CSF is enhanced in inflamed periodontal tissue [30], indicate that CSF1 (M-CSF) is one of key players to increase osteoclastogenesis and alveolar bone resorption [31, 32]. In addition, macrophages induce immune responses by promoting foreign body phagocytosis, antigen presentation, and proinflammatory cytokine production. Remaining of infection source, such as subgingival calculus, causes cytokine network-centered endless negative cycle of chronic inflammation. Thus, (+)-terrein-induced suppression of CSF1 secretion can abolish this negative cycle regulated by IL-6. Previous studies have shown that (+)-terrein performs various biological functions, including intracellular signaling. In mouse melanocytes, treatment with 10–100 μM (+)-terrein inhibits melanogenesis by reducing tyrosinase production through MAPK activation [33]. (+)-Terrein reduces intracellular adhesion molecule-1 and vascular cell adhesion molecule-1 expression in dental pulp cells by blocking nuclear factor-kappa B (NF-κB) and Akt activation [10]. We previously reported that treatment with 10 μM synthetic (+)-terrein suppressed VEGF secretion in HGFs by blocking STAT3 and ERK1/2 activation [11]. (+)-Terrein promotes the differentiation of osteoblast-like MC-3T3E1 cells grown on a titanium surface by blocking the nuclear translocation of NF-κB [34]. Moreover, (+)-terrein exerts antioxidant effect by upregulating MAPK and focal adhesion kinase activity [34]. In epidermal keratinocytes, (+)-terrein suppresses MAPK activation and cell proliferation without exerting cytotoxicity [35]. Thus, the results of these studies indicate that (+)-terrein suppress intracellular signaling molecules, including MAPKs, STAT3, Akt, and NF-κB. However, precise targets of (+)-terrein have not been reported to date. The present study is the first to show that (+)-terrein suppresses phosphorylation of JAK1, the most upstream molecule in the IL-6 signaling pathway, in the cytosol (Fig. 3A).
JAK1 belongs to JAK protein family, which includes JAK1, JAK2, JAK3, and tyrosine kinase 2. JAK family proteins are located just below the cell membrane and are involved in signaling induced by several cytokines [36], including IL-2, IL-4, IL-6, interferon-α (IFN- α), IFN-β, tumor necrosis factor-α, and leukemia inhibitory factor [37]. An abnormality in JAK1 induces rheumatoid arthritis [38], atopic diathesis [39], and hematologic malignancy [40]. Furthermore, absence of JAK1 is associated with a disorder in neural stem cell differentiation [41], and diseased activity of JAK2 is associated with mutations in hematopoietic stem cells [42]. Recently, JAK inhibitor is focused on and developed for those intractable diseases [43]. JAK inhibitor has a lot of significant features, being available for oral administration owing to low-molecular compound like (+)-terrein, short half-life, and low cost compared to biological products. The results in the present study provide new insights on the potential of (+)-terrein to inhibit JAK1. However, additional studies should be performed to examine how to interact with (+)-terrein and JAK1 in cytosol, and the effect of (+)-terrein on other JAK family proteins like JAK2 for future experiments. In addition, the side effects of (+)-terrein like immunosuppression also need to be considered as well as other JAK inhibitors.
5. Conclusion
In summary, we performed a comprehensive analysis to show that (+)-terrein suppressed IL-6/sIL-6R-induced CSF1 secretion in HGFs. Moreover, we identified JAK1 as a putative target molecule of (+)-terrein in HGFs. These data indicate that (+)-terrein might be useful for regulating IL-6 signaling in chronic inflammatory diseases including periodontitis, and be able to be applied to not only anti-inflammatory but also variety of fields by regulating JAK1 phosphorylation.
Declarations
Author contribution statement
Satoshi Yamamoto: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Kazuhiro Omori: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Hiroki Mandai, Seiji Suga: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Masaaki Nakayama, Hiroya Kobayashi, Soichiro Ibaragi, Tadashi Yamamoto, Hiroshi Maeda: Analyzed and interpreted the data.
Saki Nakagawa, Kyosuke Sakaida, Hidefumi Sako: Performed the experiments.
Tadashi Kunimine, Hiroshi Yoshimura: Contributed reagents, materials, analysis tools or data.
Shogo Takashiba: Conceived and designed the experiments; Wrote the paper.
Funding statement
This work was supported by a Grant-in-Aid for Scientific Research (C) (No. 16K11549 to KO) from the Japan Society for the Promotion of Science, Ryobi Memorial Foundation, and Wesco Memorial Foundation (to KO and HM).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Kazuhiro Omori, Email: kazu@cc.okayama-u.ac.jp.
Shogo Takashiba, Email: stakashi@okayama-u.ac.jp.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Philstrom B.L., Michalowicz B.S., Johnson N.W. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 2.Hajishengallis G., Darveau R.P., Curtis M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012;10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heitz-Mayfield L.J., Lang N.P. Surgical and nonsurgical periodontal therapy. Learned and unlearned concepts. Periodontol. 2000. 2013;62:218–231. doi: 10.1111/prd.12008. [DOI] [PubMed] [Google Scholar]
- 4.Slots J. Selection of antimicrobial agents in periodontal therapy. J. Periodontal. Res. 2002;37:389–398. doi: 10.1034/j.1600-0765.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 5.Haffajee A.D., Socransky S.S. Introduction to microbial aspects of periodontal biofilm communities, development and treatment. Periodontol. 2000. 2006;42:7–12. doi: 10.1111/j.1600-0757.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 6.Feres M., Figueiredo L.C., Soares G.M., Faveri M. Systemic antibiotics in the treatment of periodontitis. Periodontol. 2000. 2015;67:131–186. doi: 10.1111/prd.12075. [DOI] [PubMed] [Google Scholar]
- 7.Raistrick H., Smith G. Studies in the biochemistry of micro-organisms: the metabolic products of Aspergillus terreus Thom. A new mould metabolic product-terrein. Biochem. J. 1935;29:606–611. doi: 10.1042/bj0290606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qureshi I., Begum T., Noorani R. Isolation and identification of the metabolic products of Aspergillus pulvinus Kwon and Fennel. Comparative studies of production of terrein and ergosterol in different media. J. Sci. Industr. Res. 1976:120–122. [Google Scholar]
- 9.Arakawa M., Someno T., Kawada M., Ikeda D. A new terrein glucoside, a novel inhibitor of angiogenin secretion in tumor angiogenesis. J. Antibiot. (Tokyo) 2008;61:442–448. doi: 10.1038/ja.2008.60. [DOI] [PubMed] [Google Scholar]
- 10.Lee J.C., Yu M.K., Lee R., Lee Y.H., Jeon J.G., Lee M.H., Jhee E.C., Yoo I.D., Yi H.K. Terrein reduces pulpal inflammation in human dental pulp cells. J. Endod. 2008;34:433–437. doi: 10.1016/j.joen.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Mandai H., Omori K., Yamamoto D., Tsumura T., Murota K., Yamamoto S., Mitsudo K., Ibaragi S., Sasaki A., Maeda H., Takashiba S., Suga S. Synthetic (+)-terrein suppresses interleukin-6/soluble interleukin-6 receptor induced-secretion of vascular endothelial growth factor in human gingival fibroblasts. Bioorg. Med. Chem. 2014;22:5338–5344. doi: 10.1016/j.bmc.2014.07.047. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka T., Kishimoto T. Targeting interleukin-6: all the way to treat autoimmune and inflammatory diseases. Int. J. Biol. Sci. 2012;8:1227–1236. doi: 10.7150/ijbs.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omori K., Naruishi K., Nishimura F., Yamada-Naruishi H., Takashiba S. High glucose enhances interleukin-6-induced vascular endothelial growth factor 165 expression via activation of gp130-mediated p44/42 MAPK-CCAAT/enhancer binding protein signaling in gingival fiboroblasts. J. Biol. Chem. 2004;279:6643–6649. doi: 10.1074/jbc.M311688200. [DOI] [PubMed] [Google Scholar]
- 14.Naruishi K., Takashiba S., Nishimura F., Chou H.H., Arai H., Yamada H., Murayama Y. Impairment of gingival fibroblast adherence by IL-6/sIL-6R. J. Dent. Res. 2001;80:1421–1424. doi: 10.1177/00220345010800050701. [DOI] [PubMed] [Google Scholar]
- 15.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Moutsopoulos N.M., Konkel J., Sarmadi M., Eskan M.A., Wild T., Dutzan N., Abusleme L., Zenobia C., Hosur K.B., Abe T., Uzel G., Chen W., Chavakis T., Holland S.M., Hajishengallis G. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Sci. Transl. Med. 2014;6:229–240. doi: 10.1126/scitranslmed.3007696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Dyke T.E. Commentary: periodontitis is characterized by an immuno-inflammatory host-mediated destruction of bone and connective tissues that support the teeth. J. Periodontol. 2014;85:509–511. doi: 10.1902/jop.2014.130701. [DOI] [PubMed] [Google Scholar]
- 18.Graves D. Cytokines that promote periodontal tissue destruction. J. Periodontol. 2008;79:1585–1591. doi: 10.1902/jop.2008.080183. [DOI] [PubMed] [Google Scholar]
- 19.Omori K., Ohira T., Uchida Y., Ayilavarapu S., Batista E.L., Yagi M., Iwata T., Liu H., Hasturk H., Kantarci A., Van Dyke T.E. Priming of neutrophil oxidative burst in diabetes requires preassembly of the NADPH oxidase. J. Leukoc. Biol. 2008;84:292–301. doi: 10.1189/jlb.1207832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinney J.S., Morelli T., Oh M., Braun T.M., Ramseier C.A., Sugai J.V., Giannobile W.V. Crevicular fluid biomarkers and periodontal disease progression. J. Clin. Periodontol. 2014;41:113–120. doi: 10.1111/jcpe.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams R.C., Jeffcoat M.K., Howell T.H., Reddy M.S., Johnson H.G., Hall C.M., Goldhaber P. Ibuprofen: an inhibitor of alveolar bone resorption in beagles. J. Periodontal. Res. 1988;23:225229. doi: 10.1111/j.1600-0765.1988.tb01363.x. [DOI] [PubMed] [Google Scholar]
- 22.Jeffcoat M.K., Reddy M.S., Haigh S., Buchanan W., Doyle M.J., Meredith M.P., Nelson S.L., Goodale M.B., Wehmeyer K.R. A comparison of topical ketorolac, systemic flurbiprofen, and placebo for the inhibition of bone loss in adult periodontitis. J. Periodontol. 1995;66:329–338. doi: 10.1902/jop.1995.66.5.329. [DOI] [PubMed] [Google Scholar]
- 23.Yu Y., Ricciotti E., Scalia R., Tang S.Y., Grant G., Yu Z., Landesberg G., Crichton I., Wu W., Pure E., Funk C.D., FitzGerald G.A. Vascular COX-2 modulates blood pressure and thrombosis in mice. Sci. Transl. Med. 2012;4:132ra54. doi: 10.1126/scitranslmed.3003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otomo-Corgel J., Pucher J.J., Rethman M.P., Reynolds M.A. State of the science: chronic periodontitis and systemic health. J. Evid. Base Dent. Pract. 2012;12:20–28. doi: 10.1016/S1532-3382(12)70006-4. [DOI] [PubMed] [Google Scholar]
- 26.Nagasaki T., Hara M., Nakanishi H., Takahashi H., Sato M., Takeyama H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br. J. Cancer. 2014;110:469–478. doi: 10.1038/bjc.2013.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanley E.R. Action of the colony-stimulating factor, CSF-1, Ciba found. Symp. 1986;118:29–41. doi: 10.1002/9780470720998.ch3. [DOI] [PubMed] [Google Scholar]
- 28.Chomarat P., Banchereau J., Davoust J., Palucka A.K. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat. Immunol. 2000;1:510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 29.Kodama H., Nose M., Niida S., Yamasaki A. Essential role of macrophage colony-stimulating factor in the osteoclast differentiation supported by stromal cells. J. Exp. Med. 1991;173:12911294. doi: 10.1084/jem.173.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y., Lu Z., Zhang X., Kirkwood K.L., Lopes-Virella M.F., Huang Y. Metabolic syndrome exacerbates inflammation and bone loss in periodontitis. J. Dent. Res. 2015;94:362–370. doi: 10.1177/0022034514561658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taubman M.A., Valverde P., Han X., Kawai T. Immune response: the key to bone resorption in periodontal disease. J. Periodontol. 2005;76:2033–2041. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- 32.Lee M.S., Kim H.S., Yeon J.T., Choi S.W., Chun C.H., Kwak H.B., Oh J. GM-CSF regulates fusion of mononuclear osteoclasts into bone-resorbing osteoclasts by activating the Ras/ERK pathway. J. Immunol. 2009;183:3390–3399. doi: 10.4049/jimmunol.0804314. [DOI] [PubMed] [Google Scholar]
- 33.Park S.H., Kim D.S., Kim W.G., Ryoo I.J., Lee D.H., Huh C.H., Youn S.W., Yoo I.D., Park K.C. Terrein: a new melanogenesis inhibitor and its mechanism. Cell. Mol. Life Sci. 2004;61:2878–2885. doi: 10.1007/s00018-004-4341-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee Y.H., Lee N.H., Bhattarai G., Oh Y.T., Yu M.K., Yoo I.D., Jhee E.C., Yi H.K. Enhancement of osteoblast biocompatibility on titanium surface with Terrein treatment. Cell Biochem. Funct. 2010;28:678–685. doi: 10.1002/cbf.1708. [DOI] [PubMed] [Google Scholar]
- 35.Kim D.S., Lee H.K., Park S.H., Lee S., Ryoo I.J., Kim W.G., Yoo I.D., Na J.I., Kwon S.B., Park K.C. Terrein inhibits keratinocyte proliferation via ERK inactivation and G2/M cell cycle arrest. Exp. Dermatol. 2008;17:312–317. doi: 10.1111/j.1600-0625.2007.00646.x. [DOI] [PubMed] [Google Scholar]
- 36.Yamaoka K., Saharinen P., Pesu M., Holt V.E., Silvennoinen O., O'Shea J.J. The Janus kinases (Jaks) Genome Biol. 2004;5:253. doi: 10.1186/gb-2004-5-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onishi K., Zandstra P.W. LIF signaling in stem cells and development. Development. 2015;142:2230–2236. doi: 10.1242/dev.117598. [DOI] [PubMed] [Google Scholar]
- 38.Walker J.G., Smith M.D. The Jak-STAT pathway in rheumatoid arthritis. J. Rheumatol. 2005;32:1650–1653. [PubMed] [Google Scholar]
- 39.Yasuda T., Fukada T., Nishida K., Nakayama M., Matsuda M., Miura I., Dainichi T., Fukuda S., Kabashima K., Nakaoka S., Bin B.H., Kubo M., Ohno H., Hasegawa T., Ohara O., Koseki H., Wakana S., Yoshida H. Hyperactivation of JAK1 tyrosine kinase induces stepwise, progressive pruritic dermatitis. J. Clin. Invest. 2016;126:2064–2076. doi: 10.1172/JCI82887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan K.J., Gilliland D.G. A role for JAK2 mutations in myeloproliferative diseases. Annu. Rev. Med. 2008;59:213–222. doi: 10.1146/annurev.med.59.061506.154159. [DOI] [PubMed] [Google Scholar]
- 41.Taga T., Fukuda S. Role of IL-6 in the neural stem cell differentiation. Clin. Rev. Allergy Immunol. 2005;28:249–256. doi: 10.1385/CRIAI:28:3:249. [DOI] [PubMed] [Google Scholar]
- 42.Levine R.L., Wadleigh M., Cools J., Ebert B.L., Wernig G., Huntly B.J., Boggon T.J., Wlodarska I., Clark J.J., Moore S., Adelsperger J., Koo S., Lee J.C., Gabriel S., Mercher T., D'Andrea A., Fröhling S., Döhner K., Marynen P., Vandenberghe P., Mesa R.A., Tefferi A., Griffin J.D., Eck M.J., Sellers W.R., Meyerson M., Golub T.R., Lee S.J., Gilliland D.G. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 43.O'Shea J.J., Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–550. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishimoto N., Yoshizaki K., Miyasaka N., Yamamoto K., Kawai S., Takeuchi T., Hashimoto J., Azuma J., Kishimoto T. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:1761–1769. doi: 10.1002/art.20303. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi T., Ito S., Kobayashi D., Kojima A., Shimada A., Narita I., Murasawa A., Nakazono K., Yoshie H. Interelukin-6 receptor inhibitor tocilizumab ameliorates periodontal inflammation in patients with rheumatoid arthritis and periodontitis as well as tumor necrosis factor inhibitors. Clin. Exp. Dent. Res. 2015;1:63–73. doi: 10.1002/cre2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.