Abstract
Primary aldosteronism is characterized by aldosterone secretion that is independent of renin and angiotensin II and sodium status. The deleterious effects of primary aldosteronism are mediated by excessive activation of the mineralocorticoid receptor that results in the well-known consequences of volume expansion, hypertension, hypokalemia, and metabolic alkalosis, but it also increases the risk for cardiovascular and kidney disease, as well as death. For decades, the approaches to defining, diagnosing, and treating primary aldosteronism have been relatively constant and generally focused on detecting and treating the more severe presentations of the disease. However, emerging evidence suggests that the prevalence of primary aldosteronism is much greater than previously recognized, and that milder and nonclassical forms of renin-independent aldosterone secretion that impart heightened cardiovascular risk may be common. Public health efforts to prevent aldosterone-mediated end-organ disease will require improved capabilities to diagnose all forms of primary aldosteronism while optimizing the treatment approaches such that the excess risk for cardiovascular and kidney disease is adequately mitigated. In this review, we present a physiologic approach to considering the diagnosis, pathogenesis, and treatment of primary aldosteronism. We review evidence suggesting that primary aldosteronism manifests across a wide spectrum of severity, ranging from mild to overt, that correlates with cardiovascular risk. Furthermore, we review emerging evidence from genetic studies that begin to provide a theoretical explanation for the pathogenesis of primary aldosteronism and a link to its phenotypic severity spectrum and prevalence. Finally, we review human studies that provide insights into the optimal approach toward the treatment of primary aldosteronism.
Essential Points
Primary aldosteronism is characterized by aldosterone secretion that is independent of renin and angiotensin II and sodium status
The deleterious effects of primary aldosteronism are mediated by excessive activation of the mineralocorticoid receptor, which results in volume expansion, hypertension, hypokalemia, and metabolic alkalosis and also increases the risk for cardiovascular and kidney disease and death
Primary aldosteronism may manifest across a wide spectrum of severity, ranging from mild to overt, that correlates with cardiovascular risk
Emerging evidence suggests that the prevalence of primary aldosteronism is much greater than previously recognized, and that milder and nonclassic forms of renin-independent aldosterone secretion that impart heightened cardiovascular risk may be common
Genetic and basic studies have suggested potential explanations for the pathogenesis of primary aldosteronism and theoretical links to its phenotypic severity spectrum and prevalence
Simply normalizing blood pressure in primary aldosteronism may not be sufficient to optimally reduce incident cardiovascular and kidney disease; rather, efforts to neutralize the effects of pathologic aldosterone–mineralocorticoid receptor interactions may be necessary
A Brief History of Aldosterone and Primary Aldosteronism
Thomas Addison published his seminal observations in 1855 in Disease of the Supra-Renal Capsules (1), in which he described a series of patients who almost universally died following a prolonged and progressive illness characterized by fatigue, circulatory collapse, and hyperpigmentation:
“The patient, in most of the cases I have seen, has been observed gradually to fall off in general health; he becomes languid and weak, indisposed to either bodily or mental exertion, the pulse small and feeble, or perhaps somewhat large, but excessively soft and compressible … and there is occasionally actual vomiting, which in one instance was both urgent and distressing; and it is by no means uncommon for the patient to manifest indications of disturbed cerebral circulation. Notwithstanding these unequivocal signs of feeble circulation, anaemia, and general prostration, neither the most diligent inquiry, nor the most careful physical examination, tends to throw the slightest gleam of light upon the precise nature of the patient’s malady ... but with a more or less manifestation of the symptoms already enumerated, we discover the most remarkable, and, so far as I know, characteristic discoloration taking place in the skin.”
Although many of Addison’s patients likely had more than one autoimmune endocrine disorder, the likely fatal condition was primary adrenal insufficiency, now termed Addison disease. Undoubtedly, most of the observations he noted were due to the deficiencies of glucocorticoid (cortisol) and mineralocorticoid (aldosterone and cortisol); however, for much of the next century, the major breakthroughs in research focused on improving the understanding and characterization of glucocorticoid chemistry and biology. It was in the 1930s and 1940s that assays on adrenocortical extracts provided more convincing evidence of unexplained mineralocorticoid activity that was attributed to a hormone called “electrocortin” (2, 3). In 1952, Grundy, Simpson, and Tait (4) reported that the mineralocorticoid activity of beef adrenal extract was due to a compound that was not cortisone. This finding was questioned by many researchers in the field, who postulated that cortisol and cortisone may account for all mammalian mineralocorticoid activity (5). Ultimately, Simpson, Tait, and Bush (5) reported the detection of a highly potent adrenocortical salt-retaining hormone in both adrenal extracts and venous blood of dogs and monkeys that was distinct from previously described glucocorticoids and displayed mineralocorticoid activity. Within a few years, these findings were confirmed, the structure of electrocortin as the 18-aldehyde of corticosterone was determined and termed “aldosterone” (6), and aldosterone was recognized as a vital adrenocortical mineralocorticoid.
In 1954, Jerome Conn first noted a syndrome with periodic muscular weakness, tetany, paresthesias, hypokalemia, hypernatremia, alkalosis, and hypertension without Cushing syndrome, with markedly elevated urinary aldosterone (7, 8). These findings were reported in 1955 as a new clinical syndrome of “primary aldosteronism” (7), and by the end of that year, at least 30 similar cases were recognized (8). Importantly, the concept of primary vs secondary aldosteronism was rapidly recognized. Secondary aldosteronism was recognized as a physiologic or normal increase in aldosterone in response to “changes in extracellular fluid volume,” “tissue electrolyte concentrations,” and “renal dynamics” that was triggered by an increase in renin and generation of angiotensin II and, to a lesser degree, by an increase in ACTH administration (8, 9). In contrast, primary aldosteronism was characterized as inappropriate aldosterone secretion and mineralocorticoid activity, as well as volume expansion despite the suppression (or lack of detection) of renin and angiotensin II (9). Within 10 years of the original description of primary aldosteronism, it had become clear that surgical resection of aldosterone-producing adenomas could cure the aldosterone excess, improve blood pressure, and result in an increase in renin from a suppressed state to a detectable level (9).
By 1975, primary aldosteronism was still recognized as a “rare cause of hypertension,” with reported incidence rates of 0.46% to 1.0% within the hypertensive population, and these rates were considered to be overestimates (10). However, it is notable that Jerome Conn, based on his own clinical and research experiences, postulated that primary aldosteronism need not be recognized as only a severe phenotype of hypertension with hypokalemia, but rather that it may originate much earlier, among normotensive patients with normokalemia (9–11); therefore, he prophetically postulated (as will become evident later in this review) that the prevalence of primary aldosteronism could be quite high, and that many milder/subclinical or nonclassical cases may have gone unrecognized. Ultimately, as it is still today, the shifting definitions and diagnostic criteria for the primary aldosteronism syndrome dictate its prevalence and relevance. In this regard, the 1960s and 1970s marked an era of dramatic progress in hypertension research, including the recognition of a highly prevalent phenotype of essential hypertension with a low or suppressed renin, without supranormal aldosterone levels (thereby steering diagnosticians away from the primary aldosteronism diagnosis), that displayed features suggestive of a mild mineralocorticoid excess syndrome (12–17). This period of research into the “low-renin hypertension” phenotype marks the beginning of a long line of provocative and illuminating research that has helped to characterize the prevalence of autonomous and renin-independent aldosteronism that appears to exist across an expanded severity spectrum reflecting the shades of primary aldosteronism.
Approach in This Review Article
This review takes a unique approach at comprehensively discussing primary aldosteronism, from overt manifestations of the phenotype to milder and nonclassical or subclinical forms of the disease. This review is framed by a triad of seemingly simple questions that have complicated, and at times, unresolved answers:
What is primary aldosteronism?
First, we review the normal physiology of aldosterone regulation to juxtapose the nature of the pathophysiology that characterizes primary aldosteronism and how it may be detected at its most nascent manifestations. The ability to detect subtle dysregulation in aldosterone physiology may enable the recognition of an expanded severity spectrum of autonomous aldosteronism, of which we currently only detect a small proportion in the clinical setting. Since the initial description by Conn, we now understand that primary aldosteronism is not rare; rather, it may be a common contributor to the pathogenesis of hypertension and cardiovascular disease. However, currently accepted diagnostic criteria for primary aldosteronism are not used as frequently as they should, and, moreover, diagnostic methodologies to detect milder forms of primary aldosteronism, when the disease is early in its course, are not well established. The subsections in this section include:
Normal aldosterone physiology
The pathophysiology of primary aldosteronism
Cardiometabolic consequences of primary aldosteronism in humans
The prevalence and severity spectrum of primary aldosteronism in hypertension
The prevalence and severity spectrum of a primary aldosteronism phenotype in normotension
The current and future approaches to diagnosing primary aldosteronism
What causes primary aldosteronism?
Our understanding of the pathogenesis of primary aldosteronism has undergone a renaissance in the last decade. This section only briefly reviews the genetic advances that have reshaped our knowledge of the pathogenesis of primary aldosteronism because this topic has been comprehensively reviewed in several recent publications (18–21). Furthermore, we review how observations from adrenal histopathology are reshaping our perception of the pathogenesis and progression of primary aldosteronism. The subsections in this section include:
Introduction to the genetic and molecular pathogenesis of primary aldosteronism
Genetics of familial primary hyperaldosteronism
Genetics of sporadic primary aldosteronism
Epigenetics of primary aldosteronism
Morphologies of primary aldosteronism and aldosterone-producing cell clusters
A proposed model for primary aldosteronism pathogenesis and epidemiology
How should primary aldosteronism be treated?
For decades, the answer to this question seemed straightforward: surgery, when possible, to remove a unilateral source of aldosterone excess, or lifelong mineralocorticoid receptor (MR) antagonist therapy to block aldosterone when surgery was not possible or when there was a bilateral source of aldosterone excess. Recently, however, it has become clearer that medical therapy with MR antagonists may not adequately mitigate the excessive risk for developing adverse cardiovascular, renal, and mortality outcomes. Herein, we review this evidence and propose modified treatment approaches for abrogating cardiovascular risk in primary aldosteronism. The subsections in this section include:
Dietary sodium restriction
Medical vs surgical therapy for primary aldosteronism
Optimizing therapy using available evidence
Some topics are not thoroughly addressed in this review, including updates in localization and imaging modalities for primary aldosteronism, as well as the utility and efficacy of adrenal venous sampling. Although these are important topics that have been thoroughly reviewed recently (22–26), with the goal of focusing on the updates in diagnosis, pathogenesis, and treatment, they are not discussed herein.
What Is Primary Aldosteronism?
Normal aldosterone physiology
Understanding the physiologic regulation of the renin–angiotensin–aldosterone system is essential to recognize how the pathophysiology of primary aldosteronism deviates from normal regulation and contributes to disease.
The renin–angiotensin–aldosterone system is a crucial regulator of intravascular volume in terrestrial animals that evolved in a milieu of scarce dietary sodium. The postulated evolution of aldosterone and the MR suggest an intimate connection to the transition from oceanic to land-based life. Phylogenetic and sequence analysis studies suggest that long before the development of aldosterone as a mineralocorticoid, oceanic vertebrates likely expressed a single ancestral steroid receptor that gradually evolved into specific receptors: the MR, glucocorticoid receptor, progesterone receptor, estrogen receptor, and androgen receptor (27–29). Prior to the evolutionary development of aldosterone, cortisol served as a nonspecific ligand for the MR (30). The lungfish, a primitive freshwater fish species (31–33), is one of the first creatures known to synthesize aldosterone. Interestingly, the lungfish has both gills and a mature set of lungs, imparting it the ability to breathe underwater and breathe air (28, 29). Thus, an overarching hypothesis is that to transition from an oceanic world to a terrestrial world that is scarce in dietary sodium, an organism must develop the capability to maintain and protect its intravascular volume and osmolality. In this regard, the emergence of aldosterone, the MR, and 11β-hydroxysteroid dehydrogenase type 2 (11βHSD2) (which functions as a gatekeeper to facilitate a specific high-affinity relationship between the MR and aldosterone, rather than cortisol) played a crucial role in the evolution of terrestrial mammals.
Aldosterone secretion is regulated by three dominant actors: angiotensin II, extracellular hyperkalemia, and ACTH (34). A decrease in intravascular volume and glomerular filtration is sensed by a reduction in the delivery of sodium and chloride to the macula densa, and it results in an adaptive tubuloglomerular feedback and the release of renin by juxtaglomerular cells. Renin catalyzes the cleavage of angiotensinogen to angiotensin I, which is further modified by angiotensin converting enzyme to angiotensin II. Angiotensin II is an important peptide for blood pressure and volume homeostasis because it induces a number of vital actions: (1) vasoconstriction to maintain arterial pressure; (2) marked increases in sodium reabsorption in the proximal convoluted tubule (which is paired with isotonic water reabsorption), loop of Henle, and distal convoluted tubule, such that the majority of filtered sodium (and water) is reabsorbed prior to the distal nephron, resulting in decreased delivery of sodium to the distal nephron; (3) stimulation of vasopressin release, which in turn functions as an arterial vasopressor and via endocrine-renal mechanisms increases water reabsorption in the distal nephron; and (4) stimulation of adrenal aldosterone synthesis and secretion. Therefore, the “secondary” hyperaldosteronism induced by intravascular volume contraction is a physiologic and “renin-dependent aldosteronism” that serves to isotonically expand intravascular volume and maintain blood pressure homeostasis [Fig. 1(a)].
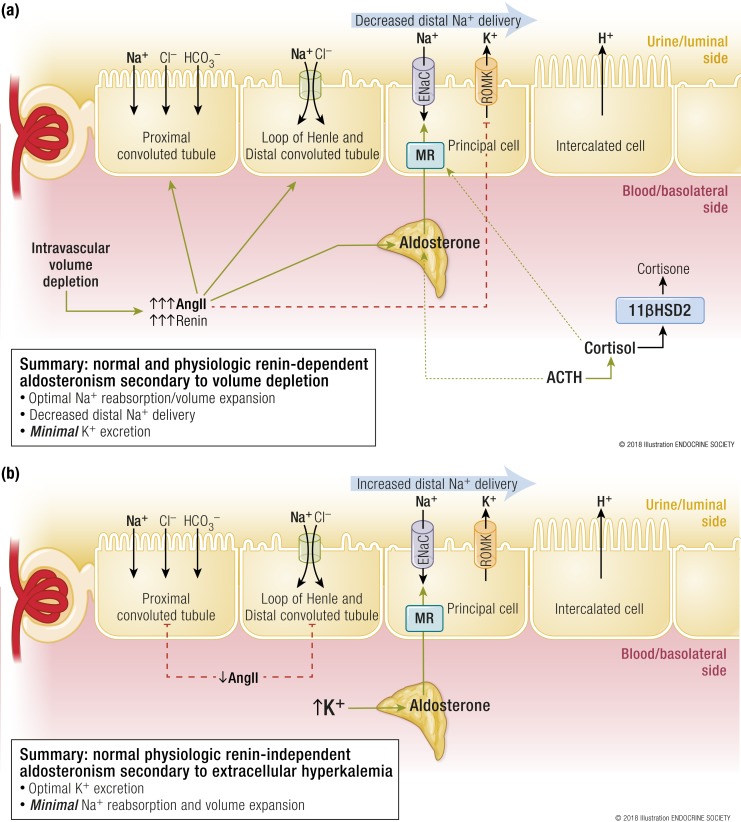
Figure 1.
(a) Normal and physiologic renin-dependent aldosteronism secondary to intravascular volume depletion. In the setting of hypovolemia and renal hypoperfusion, juxtaglomerular cells secrete renin, which initiates the activation of the renin–angiotensin–aldosterone system. The generation of angiotensin II is crucial because it serves as a vasoconstrictor to maintain blood pressure and plays an important role in counteracting the volume depletion. Angiotensin II increases sodium reabsorption in the proximal convoluted tubule, loop of Henle, and distal convoluted tubule such that the delivery of sodium to the distal nephron is decreased. Angiotensin II stimulates the secretion of aldosterone from zona glomerulosa cells, which then activates the MR in principal cells. MR activation in principal cells results in ENaC-mediated sodium reabsorption and, to maintain electroneutrality in the urinary lumen, the excretion of potassium and hydrogen ions. Potassium excretion is minimized by limiting distal sodium delivery and by the direct antagonist actions of angiotensin II in the principal cell. In this manner, the net effect of this physiological renin-dependent aldosteronism is to maximize sodium reabsorption (and water is reabsorbed isotonically) to expand circulating volume while minimizing potassium excretion. Importantly, note that ACTH is also an independent secretagogue of aldosterone and stimulates the secretion of cortisol, a potent mineralocorticoid that is metabolized to the inactive cortisone by 11βHSD2. (b) Normal physiologic renin-independent aldosteronism secondary to extracellular hyperkalemia (presuming a state of replete intravascular volume status). Extracellular hyperkalemia is an independent secretagogue of aldosterone secretion. High extracellular potassium concentrations result in depolarization of zona glomerulosa cells and consequent secretion of aldosterone, which activates the MR and stimulates ENaC-mediated sodium reabsorption in the principal cell. This process is paired with potassium ion excretion. In the setting of volume expansion, angiotensin II is suppressed and proximal tubular sodium reabsorption is decreased. Thus, the delivery of sodium to the distal nephron increases and permits the excretion of more potassium. The net effect is an increased excretion of urinary potassium while minimizing the reabsorption of sodium. AngII, angiotensin II. [© 2018 Illustration ENDOCRINE SOCIETY]
The interaction of aldosterone with the MR in the principal cell of the distal nephron (or aldosterone-sensitive distal nephron) results in an increase in luminal epithelial sodium channels (ENaCs) and consequent increased reabsorption of sodium (35). The negative intraluminal potential induced by Na+ ion reabsorption is neutralized by the excretion of potassium via renal outer medullary potassium channels (ROMKs) or hydrogen ions from α-intercalated cells. However, the physiologic actions of angiotensin II mitigate the potassium excretion by minimizing the amount of sodium delivered to the principal cell, by decreasing ROMK-mediated potassium secretion, and by dephosphorylating the MR in α-intercalated cells to promote hydrogen, rather than potassium, secretion (35–37) [Fig. 1(a)]. Therefore, the net effect of physiologic renin-dependent aldosteronism in the setting of intravascular volume depletion is maximal sodium reabsorption and volume expansion, while minimizing urinary potassium excretion: a sensible and appropriate solution to hypovolemia without inducing unnecessary hypokalemia.
There are other ligands for the MR, perhaps none as dominant as cortisol. Cortisol is a mineralocorticoid that can bind and activate the renal MR with similar potency to aldosterone and circulates in nearly 1000-fold the concentration of aldosterone. However, the coexpression of 11βHSD2 with the renal MR ensures that the vast majority of this cortisol is converted to the inactive cortisone [Fig. 1(a)]. Finally, it is important to remember that there are other aldosterone secretagogues. Extracellular hyperkalemia and ACTH can stimulate the secretion of aldosterone independently of angiotensin II (34) and are discussed further below.
Because the aldosterone response to hyperkalemia is independent of renin and angiotensin II, it represents a physiologic and renin-independent hyperaldosteronism [Fig. 1(b)]. In states of volume expansion, when renin and angiotensin II are suppressed, increased extracellular potassium can still directly stimulate secretion of aldosterone from zona glomerulosa cells (34). In the absence of angiotensin II, proximal tubular sodium reabsorption and volume expansion are diminished and the delivery of sodium to the aldosterone-sensitive distal nephron is increased. At the principal cells, potassium is excreted via ROMK in response to aldosterone–MR interactions until the potassium stimulus for aldosterone secretion is neutralized [Fig. 1(b)], a sensible physiologic solution to excreting a large dietary potassium load without unnecessarily expanding circulating volume (35).
The difference in potassium handling between the renin-dependent aldosteronism in hypovolemia and the renin-independent aldosteronism in hyperkalemia is often referred to as the “aldosterone paradox” and is largely explained by the changes in the delivery of sodium to the distal nephron (35, 38). In settings of volume depletion, renin- and angiotensin II–dependent hyperaldosteronism results in maximal volume expansion, decreased distal sodium delivery, and minimal potassium excretion. In contrast, in settings of volume expansion and hyperkalemia, renin- and angiotensin II–independent hyperaldosteronism results in decreased proximal tubular sodium reabsorption and volume expansion, increased distal delivery of sodium, and maximal potassium excretion. Thus, different forms of physiologic hyperaldosteronism serve to address specific physiologic perturbations (Table 1).
Table 1.
Physiologic and Pathophysiologic Forms of Hyperaldosteronism
| Cause | Physiologic or Pathophysiologic | Renin–Angiotensin–Aldosterone System Phenotypes | Net Effects | Comments |
|---|---|---|---|---|
| Intravascular volume depletion | Physiologic | Renin- and angiotensin II–dependent hyperaldosteronism | • Increased sodium reabsorption and volume expansion | Homeostatic to maintain plasma volume and blood pressure |
| • Minimization of urinary potassium excretion | ||||
| Extracellular hyperkalemia | Physiologic | Renin- and angiotensin II–independent hyperaldosteronism | • Increased urinary potassium excretion | Highly dependent on volume status and distal sodium delivery |
| • Minimization of sodium reabsorption and volume expansion | ||||
| ACTH stimulation | Physiologic | Renin- and angiotensin II–independent hyperaldosteronism | • Depends on volume status, duration, and amplitude of ACTH stimulation | Often considered to be a milder contribution to aldosterone secretion compared with angiotensin II and potassium stimuli |
| Primary aldosteronism | Pathophysiologic | Renin- and angiotensin II–independent hyperaldosteronism | • Vicious cycle of increased sodium reabsorption and volume expansion | Contributes to increased risk for hypertension, cardiovascular disease, kidney disease, and death |
| • Increased glomerular filtration and distal delivery of sodium | ||||
| • Increased urinary potassium and hydrogen ion excretion |
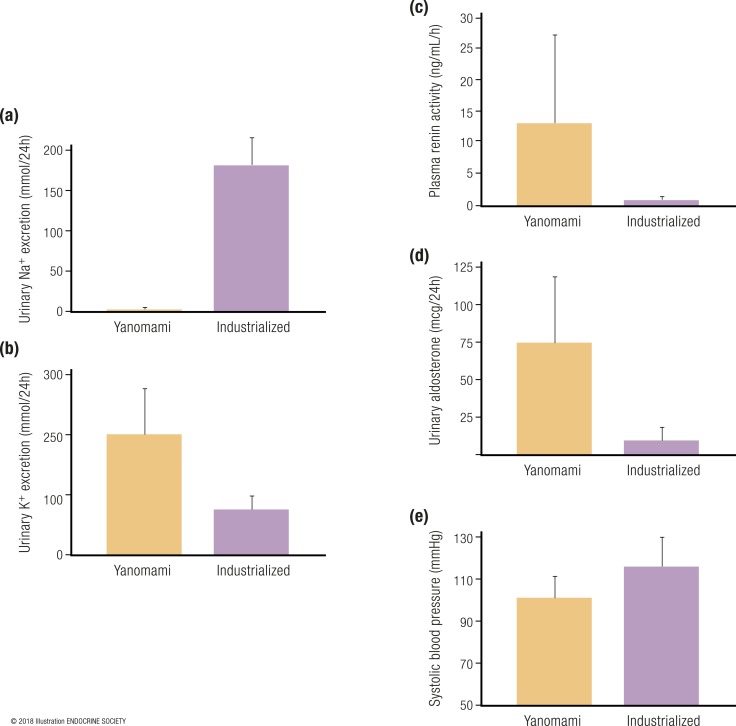
Of course, in reality, volume depletion and increased potassium balance need not be distinct states, rather, there is a constant flux in volume and potassium status, and the combined and integrated physiological pathways ensure a state of homeostasis. To exemplify the nature of aldosterone physiology and its intimate relationship to dietary sodium and potassium intake in humans, we can look to what has been learned from native populations in Papua New Guinea and the Yanomami tribe of the Amazon, when compared with humans living in modern industrialized societies (39–44). The Yanomami are an indigenous population living in the tropical rainforests of Brazil and Venezuela who were studied in the 1970s and found to be naive to the influences of dietary sodium in industrialized societies. Importantly, the Yanomami lived a hunter–gatherer lifestyle and consumed minute amounts of dietary sodium; therefore, their physiology at the time characterized an example to understand the native aldosterone physiology of terrestrial mammals, particularly humans (40). The 24-hour urinary sodium excretion of the Yanomami in 1975 was merely 1 mmol (40); in contrast, the estimated 24-hour urinary sodium excretion of the average healthy normotensive American free of cardiovascular disease in the year 2000 was ∼180 mmol (41) [Fig. 2(a)]. Conversely, the Yanomami consumed much more dietary potassium than did humans living in industrialized societies (40, 42, 43) [Fig. 2(b)]. Therefore, the chronic physiology of the Yanomami (and presumptively most wild terrestrial mammals) was perfectly designed to maximally stimulate aldosterone secretion to cope with scarce dietary sodium intake, consequent intravascular volume depletion, and superimposed high dietary potassium intake. Indeed, the mean plasma renin activity in the Yanomami was markedly elevated at 13 ng/mL/h with a corresponding elevated 24-hour urinary aldosterone excretion rate >70 μg (40); this is in stark contrast to modern American and European populations where renin and aldosterone are relatively low, owing to chronic high dietary sodium intake and a state of replete or expanded intravascular volume (41, 44) [Fig. 2(c) and 2(d)]. Despite this chronic (and likely lifelong) state of “hyperaldosteronism,” the Yanomami are reported to not develop hypertension or age-related increases in blood pressure (40). In fact, their mean blood pressure is remarkably lower than normotensive healthy Americans who consume a sodium-rich diet (41) [Fig. 2(e)].
Figure 2.
Comparison of sodium and potassium balance, as well as renin and aldosterone activity, between populations from industrialized societies with high dietary sodium intake vs the Yanomami tribe with low dietary sodium intake. (a) Twenty-four–hour urinary sodium excretion. (b) Twenty-four–hour urinary potassium excretion. (c) Plasma renin activity. (d) Twenty-four–hour urinary aldosterone excretion. (e) Systolic blood pressure. The yellow bars represent data from the Yanomami in the Amazon of Brazil and Venezuela [data extracted from Oliver et al. (40)]. The purple bars represent participants from studies in the United States and Europe that evaluated population level electrolyte balance and renin–angiotensin–aldosterone system parameters [data extracted from Brown et al. (41), Sun et al. (43), and Jin et al. (44)]. [© 2018 Illustration ENDOCRINE SOCIETY]
This latter point highlights that all hyperaldosteronism is not the same; physiological hyperaldosteronism is adaptive and necessary to maintain blood pressure and potassium homeostasis, whereas hyperaldosteronism in the setting of volume expansion and sodium sufficiency/excess is pathologic to cardiovascular and kidney tissues, a point that is underscored in the subsequent section of this review (Table 1).
Although it took centuries for us to realize this paradox of hyperaldosteronism and normal physiology, nature recognized the vital importance of physiologic hyperaldosteronism much earlier. The Brazilian pit viper, Bothrops jararaca, inhabits the same geography as the Yanomami and contains in its venom a toxin that is a primordial angiotensin-converting enzyme inhibitor. The fact that B. jararaca evolved to express this predatory advantage, allowing it to exploit a vital hormonal regulator of blood pressure to kill its prey, led to the pharmacologic discovery and development of angiotensin-converting enzyme inhibitors (45, 46).
The pathophysiology of primary aldosteronism
The pathophysiology of primary aldosteronism is best understood in the context of the aforementioned normal aldosterone physiology. For practical reasons, primary aldosteronism is often defined by diagnostic thresholds, specific laboratory criteria, and biochemical cutoffs. However, in its simplest form, primary aldosteronism can be considered to be any deviation from normal physiology whereby aldosterone is secreted autonomously or independent of its dominant regulators, and in a manner that is inappropriate to the status of volume balance.
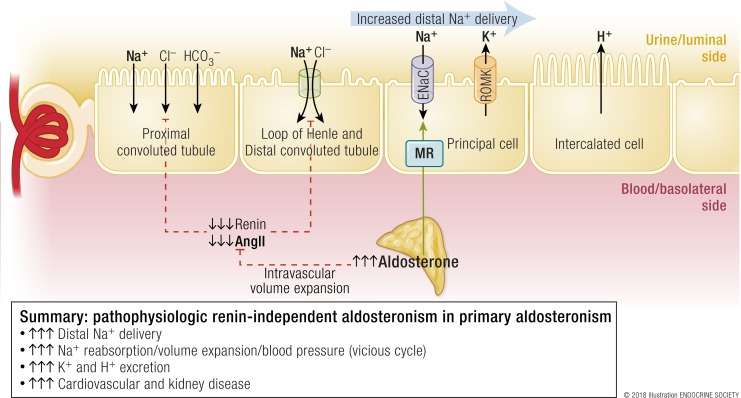
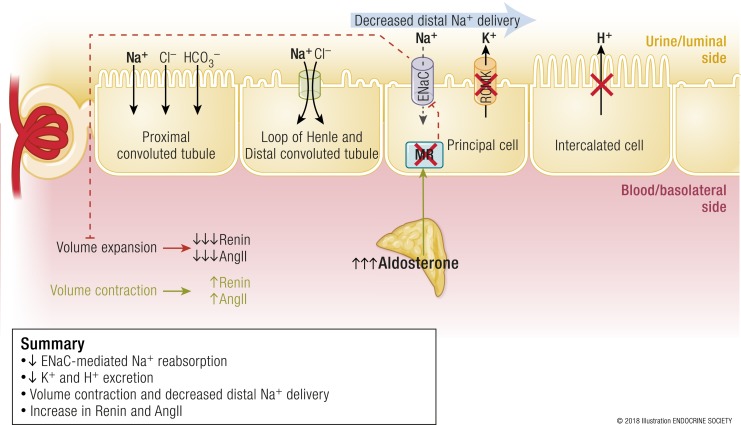
As discussed later in this review, the initial insult in primary aldosteronism is the development of one or multiple foci of autonomous aldosterone secretion in either one or both adrenal glands. Thus, the secretion of aldosterone is independent of renin and angiotensin II, and often in the face of hypokalemia, which should otherwise suppress aldosterone secretion. In fact, renin and angiotensin II are either appropriately suppressed or undetectable (Fig. 3). In the absence of angiotensin II, proximal tubular sodium reabsorption is diminished and, instead, a high flow of urine and sodium is delivered to the aldosterone-sensitive distal nephron. In the context of this increased distal sodium delivery, hyperaldosteronism and aldosterone–MR interactions in the principal cell induce increased ENaC–mediated sodium reabsorption, and, in exchange, to maintain urinary lumen electroneutrality, the excretion of potassium and hydrogen ions (Fig. 3). Increased sodium reabsorption is paired with water reabsorption that results in isotonic volume expansion, which increases glomerular hyperfiltration, and induces a vicious and self-propagating cycle of distal sodium delivery and reabsorption, and further volume expansion. Any impairment in vascular compliance and/or the renal handling of this excess volume and sodium will manifest as an increase in arterial blood pressure and, ultimately, hypertension. In stark contrast to the physiologic hyperaldosteronism in volume depletion, where urinary potassium excretion is minimized, the pathophysiology of primary aldosteronism is characterized by the suppression of angiotensin II and, thus, enhanced excretion of potassium and hydrogen in the distal nephron resulting in hypokalemia and metabolic alkalosis (35, 37) (Fig. 3).
Figure 3.
Pathophysiologic renin-independent aldosteronism in primary aldosteronism. The primary problem is that one or both adrenal glands contain foci of autonomous aldosterone secretion. There is increased stimulation of the MR in principal cells, despite the fact that circulating volume is expanded and renin and angiotensin II are suppressed. This results in increased and inappropriate sodium reabsorption as well as a vicious cycle of further volume expansion and greater distal delivery of sodium. Because potassium and hydrogen ion excretion are paired with sodium reabsorption in the principal cell, these processes are also increased. The net effect is volume expansion, increases in blood pressure, hypokalemia, and metabolic alkalosis. AngII, angiotensin II. [© 2018 Illustration ENDOCRINE SOCIETY]
This last point has important health implications. The renin-dependent hyperaldosteronism in the setting of intravascular volume depletion is physiologic and necessary for blood pressure homeostasis and survival. In contrast, the renin-independent hyperaldosteronism and MR activation in primary aldosteronism occurs in the setting of intravascular volume expansion and sodium excess, and consequently contributes to cardiovascular disease, atrial fibrillation, renal disease, diabetes, and death. Similarly, when intravascular volume depletion or renal hypoperfusion is perceived in states of total body hypervolemia (such as in heart failure and renal artery stenosis), the resultant renin-dependent hyperaldosteronism and MR activation is pathologic (47). In fact, the same absolute circulating aldosterone concentration in each of these settings might impart entirely different effects. This paradox again underscores why all hyperaldosteronism is not the same. A high aldosterone level is physiologic in the setting of hypovolemia, whereas the same high aldosterone level may be pathologic in the setting of volume expansion or total body volume overload. The precise nature of this paradox is not fully resolved, yet the experimental evidence discussed next adds support for this phenomenon.
Animal studies have observed that the administration of a high-sodium diet to rats increases blood pressure while suppressing aldosterone. When these rats were simultaneously treated with a continuous infusion of angiotensin II and N-nitro-l-arginine methyl ester (an inhibitor of nitric oxide synthase), they experienced a rise in aldosterone, marked increases in blood pressure, and myocardial necrosis and fibrosis (48, 49). This myocardial pathology was mediated by aldosterone–MR interactions, as both surgical adrenalectomy and treatment with eplerenone could mitigate the severity of myocardial fibrosis and necrosis (48, 49). Most remarkably, when these rats were sodium restricted to induce volume contraction and a physiologic renin-dependent aldosteronism, infusion of angiotensin II and N-nitro-l-arginine methyl ester still dramatically increased blood pressure and further raised aldosterone; however, little to no myocardial disease was induced (49). Similarly, induction of cardiac fibrosis by infusion of aldosterone into uninephrectomized rats occurs when the animals consume a high-sodium diet, but not when dietary sodium intake is restricted (50). Thus, aldosterone-mediated MR activation is pathologic and induces cardiovascular injury, independent of blood pressure, only in the context of volume expansion and high dietary sodium intake. The exact mechanism accounting for this dichotomy is not clear; however, in the presence of a high sodium intake, cardiac and vascular fibrosis is regulated by activation of the MR in multiple cell types, including macrophages, cardiomyocytes, vascular endothelial cells, and vascular smooth muscle cells (51). Thus, another growing line of evidence suggests that activation of the MR in vascular endothelial and smooth muscle cells can induce a direct pathogenic effect that may result in vascular fibrosis and stiffness (51–56). One older hypothesis implicates endogenous ouabain-like factors that are secreted by adrenocortical cells and act to inhibit Na+/K+-ATPase activity in vascular endothelium and cardiac myocytes to induce cardiovascular injury (57–60). However, the validity of this theory has been debated because of controversies regarding the ability to detect and measure endogenous ouabain or its synthetic pathways (61–64).
Finally, it is worth mentioning that even though ACTH is often considered to be a lesser stimulant of aldosterone when compared with angiotensin II and hyperkalemia, it may play an important role in the regulation of aldosterone secretion in primary aldosteronism. Studies have shown that aldosterone-producing adenomas (APAs) can overexpress melanocortin type 2 receptors, and patients with primary aldosteronism can display a diurnal variation in aldosterone secretion and a hyperresponsiveness to exogenous ACTH administration (65). Furthermore, nearly one-third of hypertensives without primary aldosteronism exhibit an exaggerated aldosterone secretory response to exogenous ACTH administration (66). Therefore, even in states of renin- and angiotensin II–independent aldosteronism, aldosterone secretion may not be entirely “autonomous,” as it may still be influenced by ACTH and potentially other secretagogues (65, 67).
Cardiometabolic consequences of primary aldosteronism in humans
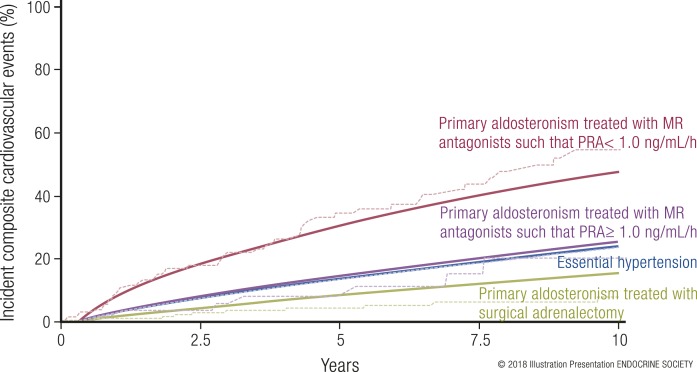
The number of human observational studies suggesting an association between primary aldosteronism and risk for heart disease (coronary artery disease, left ventricular hypertrophy, heart failure), atrial fibrillation, stroke, kidney disease, metabolic syndrome/diabetes, and skeletal disease has steadily grown. Although evidence linking primary aldosteronism and cognitive function has not been investigated as thoroughly, the MR is implicated as an important factor in memory and cognition (68–73). Given the large number of studies, it is not feasible to discuss each one individually here, rather, an overarching summary of the findings is provided. Most of the human studies that have educated us on the cardiometabolic consequences of primary aldosteronism were retrospective cross-sectional or retrospective cohort studies, often with blood pressure–matched essential hypertensive patients as comparators, and in fewer instances, prospective cohort studies. Collectively, these studies have shown that when compared with essential hypertension, primary aldosteronism is associated with a higher risk for coronary artery disease (74–82), atrial fibrillation that is lone or in context of other heart disease (74–78, 81–84), stroke (74–79, 82), left ventricular hypertrophy and/or heart failure (74, 75, 78, 79, 81, 82), metabolic syndrome and/or diabetes (74, 82, 85–87), kidney disease (decline in glomerular filtration rate and/or albuminuria) (88–91), and decreased bone density and fracture (92–95). Furthermore, primary aldosteronism increases the risk of death when compared with similar essential hypertensives, even after initiation of medical treatment to control blood pressure and block the MR (80, 82).
Since our understanding of the risk associated with primary aldosteronism stems from multiple observational studies with unique study designs, variable durations of follow-up, and variable degrees of matching with comparable populations without primary aldosteronism, Monticone et al. (96) attempted to provide a unifying assessment of cardiometabolic risk by conducting a systematic review and meta-analysis of available data. From a total of 31 aggregated studies including 3838 patients with primary aldosteronism and 9284 patients with essential hypertension and a median duration of 8.8 years from the initial diagnosis of hypertension, they reported summative odds ratios for those with untreated primary aldosteronism when compared with patients with essential hypertension for stroke (OR, 2.58; 95% CI, 1.93 to 3.45), coronary artery disease (OR, 1.77; 95% CI, 1.10 to 2.83), atrial fibrillation (OR, 3.52; 95% CI, 2.06 to 5.99), heart failure (OR, 2.05; 95% CI, 1.11 to 2.78), left ventricular hypertrophy (OR, 2.29; 95% CI, 1.65 to 3.17), diabetes (OR, 1.33; 95% CI, 1.01 to 1.74), and metabolic syndrome (OR, 1.53; 95% CI, 1.22 to 1.91).
Thus, it is now clear that primary aldosteronism, independent of the influences of blood pressure, is associated with a much higher risk for cardiometabolic, renal, and mortality outcomes when compared with essential hypertension. This excess risk is presumptively attributed to the effect of MR activation by aldosterone in the setting of volume expansion. Because targeted treatments to block or eliminate the source of autonomous aldosterone secretion exist, the public health emphasis should be on increasing the early recognition and treatment of primary aldosteronism. However, to implement, or improve, such a strategy, a better understanding of how to detect primary aldosteronism early in its course, before it has caused cardiometabolic disease, is critical.
The prevalence and severity spectrum of primary aldosteronism in hypertension
How common is primary aldosteronism? The answer to this question depends on which population is being referred to and how primary aldosteronism is defined and detected. The most commonly used and consensus opinion recommendation for screening of primary aldosteronism is use of the aldosterone-to-renin ratio (ARR) (97). The ARR has been used for decades and has been shown to be superior to the isolated reliance on potassium or aldosterone or renin alone (97–101). However, there are a number of limitations with using the ARR. Even after controlling known confounders of the ARR such as posture, dietary sodium intake, time of day, timing of the menstrual cycle, and influence of antihypertensive medications, the issue of how to interpret the results of the ARR are debated (97). In the absence of a histopathological gold standard for diagnosing primary aldosteronism, the default diagnostic gold standard is a substantial improvement in blood pressure following targeted therapy. As a result, the diagnostic cutoffs for the ARR vary by practice and geographical location, and there are no prospective clinical trials that have validated specific ARR thresholds over others (97, 98). Debate exists regarding which threshold of the ARR is the most sensitive and specific, cost-effective, and, furthermore, which absolute values of aldosterone and renin within the ARR are most indicative of primary aldosteronism (97) (Table 2).
Table 2.
Criteria and Interpretations of Biochemical Screening Results for Primary Aldosteronism
| Criteria | ARR, ng/dL per ng/mL/h | Serum Aldosterone, ng/dL | Plasma Renin Activity, ng/mL/h | Comments |
|---|---|---|---|---|
| Most conservative | ≥40 | ≥20 | ≤0.50 | Highest risk of missing mild-to-moderate severity cases (i.e., more false-negatives) |
| Conservative and most widely accepted | ≥30 | ≥15 | ≤1.0 | Some risk of false-negatives |
| More permissive but less widely accepted | ≥20 or ≥25 | ≥9–10 | ≤1.0 | Some risk of false-positives |
| Most permissive | ≥20 | ≥6 | ≤0.50 | Highest risk of positive screens that are not true cases (i.e., more false-positives) |
The most widely accepted international consensus for a positive screen for primary aldosteronism is an ARR >30 ng/dL per ng/mL/h in the context of a suppressed renin and an aldosterone concentration >15 ng/dL (97, 102); however, studies have shown that in the context of an elevated ARR, less conservative thresholds for a suppressed renin activity, <1.0 ng/mL/h (103), and plasma aldosterone levels, >6 ng/dL (104, 105) or >9 ng/dL (97, 106), can improve the detection of milder forms of primary aldosteronism. Because one objective of a screening test is traditionally to be as close to 100% sensitive as possible, as to not miss any true cases, the use of less conservative screening criteria seems desirable. Of course, while the use of more permissive screening criteria will increase the detection of true primary aldosteronism cases, it will also increase the number of false-positives (decreased specificity), resulting in increased cost, labor, time, and risk to the patient. Conversely, the use of more conservative screening criteria may limit the risk of false-positive case detection at the expense of missing true cases (decreased sensitivity) and detecting treatable disease (Table 2).
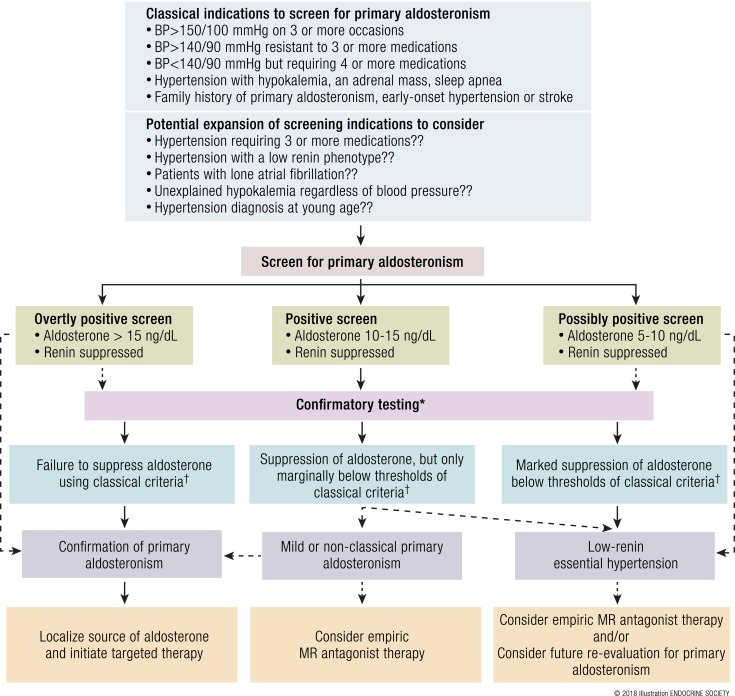
To compound the limitations of the ARR test is the issue of how and when primary aldosteronism should be suspected and in which populations to conduct screening for the disease. The recommendations by major endocrine societies (97, 107) have typically suggested that screening for primary aldosteronism should occur in patients who have one or more of the following indications:
-
Severe hypertension, defined as one of the following:
Sustained blood pressure >150/100 mm Hg, confirmed on separate days;
Blood pressure >140/90 mm Hg on three antihypertensive medications, including a diuretic;
Blood pressure <140/90 mm Hg requiring four or more antihypertensive medications.
Hypertension with spontaneous or diuretic-induced hypokalemia;
Hypertension with an adrenal mass;
Hypertension with sleep apnea;
Hypertension and a family history of early onset hypertension or stroke at a young age (<40 years of age);
Hypertension with a first-degree relative who has primary aldosteronism.
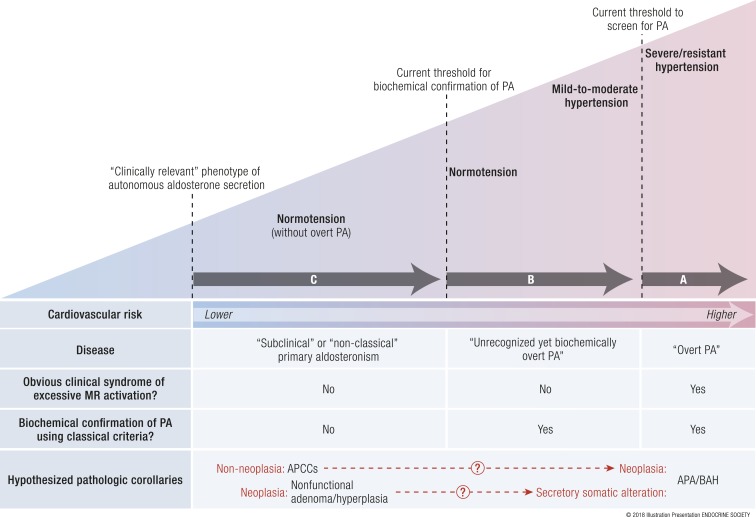
By restricting the impetus to search for primary aldosteronism to patients who have relatively severe and resistant hypertension, these indications relegate the detection of primary aldosteronism to cases that are likely to be long-standing and may have already incurred years of untreated aldosterone- and MR-mediated cardiovascular injury. Specifically, as discussed in “The pathophysiology of primary aldosteronism” and “Cardiometabolic consequences of primary aldosteronism in humans” above, aldosterone-mediated cardiovascular injury can develop independent of blood pressure. The current recommended indications to screen for primary aldosteronism place higher emphasis on detecting “overt primary aldosteronism,” a syndrome of relatively severe hypertension and/or hypokalemia that portends high cardiovascular risk and is usually attributed to an aldosterone-producing adenoma or bilateral adrenal hyperplasia (Fig. 4A).
Figure 4.
The severity spectrum of primary aldosteronism. (A) Overt primary aldosteronism. The Endocrine Society clinical practice guidelines recommend screening for primary aldosteronism using the aldosterone-to-renin ratio in severe or resistant hypertension. This practice of screening for a high aldosterone-to-renin ratio (>20 to 30 ng/dL per ng/dL per hour in conventional units or >750 to 830 pmol/L per μg/L per hour in SI units) is highly sensitive for detecting patients with severe hypertensive primary aldosteronism, that is, those with an obvious clinical syndrome of excessive MR activation (hypertension and/or hypokalemia) who are confirmed to have biochemically overt primary aldosteronism and likely to have an APA or bilateral adrenal hyperplasia as the cause of their disease. These cases of overt primary aldosteronism have the highest risk for incident cardiovascular disease. (B) Unrecognized, yet biochemically overt primary aldosteronism. Using confirmatory testing thresholds recommended by the Endocrine Society (see Table 3), human studies have demonstrated that a substantial portion of normotensive and mild-to-moderate hypertensive persons, populations for whom primary aldosteronism screening is not routinely recommended, have unrecognized, yet biochemically overt primary aldosteronism. (C) Subclinical or nonclassical primary aldosteronism. Even below the Endocrine Society recommended thresholds of what is currently considered biochemical confirmation of primary aldosteronism, a continuum of renin-independent aldosterone secretion can be detected among healthy normotensive and moderately hypertensive persons, in whom no obvious clinical syndrome of MR overactivation is apparent. These persons have subtle biochemical evidence of renin-independent aldosteronism (renin suppression with inappropriately “normal” or high aldosterone levels) and higher risk for developing hypertension. This phenotype may best be described as subclinical or nonclassical primary aldosteronism (41). The hypothesized pathologic corollaries remain theoretical and are discussed in “What Causes Primary Aldosteronism?” One theory suggests that the newly described finding of APCCs may represent an aldosterone-secretory abnormality wherein a subset may then acquire neoplastic alterations to transform into APAs or bilateral adrenal hyperplasia. An alternative theory suggests that nonfunctional adrenocortical neoplasia may acquire secretory somatic alterations to become APAs or bilateral adrenal hyperplasia. A combination of both or neither theories may also be possible. BAH, bilateral adrenal hyperplasia; PA, primary aldosteronism. Adapted with permission from Brown JM, Robinson-Cohen C, Luque-Fernandez,MA, et al. The spectrum of subclinical primary aldosteronism and incident hypertension: A cohort study. Ann Intern Med. 2017;167(9):630–641. http://annals.org/aim/article-abstract/2657166/spectrum-subclinical-primary-aldosteronism-incident-hypertension-cohort-study ©American College of Physicians. [© 2018 Illustration Presentation ENDOCRINE SOCIETY]
However, it is now evident that overt primary aldosteronism can be detected even below the clinical thresholds for screening that are commonly recommended. For example, Monticone et al. (74) systematically screened nearly 1700 consecutive referrals to a hypertension center in Italy for primary aldosteronism using the relatively conservative and strict binary criteria of an ARR >30 ng/dL per ng/mL/h in addition to an aldosterone level >10 ng/dL. The 14% of patients who had a positive screening result underwent a confirmatory aldosterone suppression test with either an intravenous saline suppression test or captopril challenge; 6% of their total population had a confirmed diagnosis of primary aldosteronism. Perhaps more impressive than the total prevalence of disease was the observed distribution of primary aldosteronism. Among patients who had a clear indication to be screened for primary aldosteronism (an untreated blood pressure of >180/110 or 160 to 179/100 to 109 mm Hg), the prevalence of disease was very high, 10% to 12% (74). These patients can be considered to have overt primary aldosteronism: they had severe hypertension warranting a screen for primary aldosteronism and in this context 10% to 12% were confirmed to have the disease. More surprising was the fact that 4% of patients with an untreated blood pressure of 140 to 159/90 to 99 mm Hg were also confirmed to have primary aldosteronism. These patients can be considered to have “unrecognized, yet biochemically overt primary aldosteronism.” Despite having a confirmed diagnosis of primary aldosteronism, these patients generally did not meet the current clinical indications to be screened for the disease, and therefore, in the absence of a systematic research investigation as was conducted by Monticone et al., these patients would not have been diagnosed with primary aldosteronism (Fig. 4B). It is worth considering that this 4% prevalence of unrecognized, yet biochemically overt primary aldosteronism was observed while using fixed and firm screening and confirmatory diagnostic criteria; allowing slightly more flexible and permissive criteria may have increased this prevalence (Tables 2 and 3 ).
Importantly, the findings by Monticone et al. are not in isolation. More than a decade earlier, Mosso et al. (106) reported similar findings from Chile. Using an ARR >25 ng/dL per ng/dL/h and fludrocortisone suppression testing on 609 consecutive participants, they too reported that the prevalence of primary aldosteronism was 6%. Again, the distribution of disease was 13% among the most hypertensive individuals, and 2% to 8% in those with milder hypertension, thus affirming the concept that a substantial proportion of mild-to-moderate hypertensives, who do not meet the current indications to undergo screening for primary aldosteronism, do in fact have unrecognized, yet biochemically overt primary aldosteronism according to well-accepted confirmatory criteria. Similarly, Rossi et al. (108) reported an overall prevalence of primary aldosteronism of 11.2% in a cohort of patients evaluated at an Italian referral center; however, they also noted that the prevalence ranged from ∼7% in those with stage I hypertension to 19.5% in those with stage III hypertension. Finally, in a Japanese cohort with hypertension, screening and confirmatory testing revealed an overall primary aldosteronism prevalence of 8.1% (109). Thus, the prevalence of primary aldosteronism is highly dependent on the population being targeted by screening, the specific diagnostic criteria used to define a positive screen, and the mode of confirmation. As a testament to this variability and lack of standardization, the prevalence rates of primary aldosteronism have been reported to range from 3.2% to 7.2% in mild-to-moderate hypertension (108, 110, 111), >15% in moderate-to-severe hypertension (112), and ≥20% in resistant hypertension and/or sleep apnea (113–116).
We strongly suggest that it is important to avoid excessive focus on specific prevalence statistics, because without a diagnostic histopathologic gold standard the somewhat arbitrary and varying criteria for positive screening and confirmatory testing can lead to wide prevalence ranges. Even though a large body of literature has investigated the prevalence of primary aldosteronism in resistant hypertension, the methods and criteria used can dramatically alter the prevalence estimates. For example, the PATHWAY-2 study was a randomized intervention study that investigated which antihypertensive would be the most effective for patients with resistant hypertension already treated with three antihypertensives (117). The investigators focused on resistant hypertensives in whom secondary hypertension, including primary aldosteronism, had been excluded by expert hypertension centers using conventional criteria. After a randomized crossover intervention that included 12 weeks of treatment with doxazosin (α-antagonist), bisoprolol (β-antagonist), spironolactone (MR antagonist), and placebo, the most effective fourth antihypertensive agent was spironolactone (117). Importantly, the greatest blood pressure lowering effect by spironolactone was seen when renin was the most suppressed. In a subsequent substudy of the original PATHWAY-2 participants, aldosterone levels were measured and a trial of amiloride was assessed (118). The results showed that spironolactone exerted the greatest blood pressure lowering effect in resistant hypertension when renin was suppressed and aldosterone and the ARR levels were the highest, and furthermore, that amiloride (an ENaC inhibitor) could exert a similar effect to spironolactone (118). Thus, the overall results of the PATHWAY-2 studies raised the possibility that a substantial proportion of resistant hypertension may not be “essential hypertension” but rather may be enriched for autonomous and renin-independent aldosterone secretion that falls below our current diagnostic standards for primary aldosteronism. The role of other potential MR agonists (such as cortisol and deoxycorticosterone) and factors that could result in a low-renin hypertension or increased MR signaling (119–121) were not reported in the PATHWAY-2 studies, but they may have also played a role.
Several important questions about the role of aldosterone in resistant hypertension arise from this study. When did the renin-independent aldosterone secretion begin? Could it have contributed to the development of worsening and resistant hypertension? What cutoffs of the ARR in resistant hypertension indicate a condition that would benefit from an MR antagonist? Is the suppression of renin in hypertension sufficient to consider using an MR antagonist or amiloride? Could identification and treatment (with an MR antagonist or amiloride) of this nonclassical phenotype of primary aldosteronism have averted years of severe hypertension?
The answers to the questions may lie in how expansive the detectable spectrum of primary aldosteronism is and how well we can assess the parallel chronology of its nature with blood pressure trajectories. For example, overt primary aldosteronism can be detected in mild-to-moderate hypertension. Baudrand et al. (104) evaluated the prevalence of primary aldosteronism in 241 mild-to-moderate hypertensives (blood pressure range, 140 to 159/80 to 99 mm Hg) who had a plasma renin activity <1.0 ng/mL/h using more permissive screening thresholds, ARR >20 ng/dL per ng/mL/h, and an aldosterone level of at least >6 ng/dL, but with subsequent employment of standard and conservative thresholds for a confirmatory oral sodium suppression test, where the 24-hour urine aldosterone excretion rate was measured following oral sodium loading (Table 3). By focusing on a population with low-renin hypertension, and by relaxing the screening thresholds to minimize the chances of missing true cases, they observed a primary aldosteronism prevalence of 19% (104). Thus, despite having seemingly unimpressive plasma aldosterone levels in a single cross-sectional measurement, many of these participants had nonsuppressible integrated 24-hour urinary aldosterone excretion rates, an important distinction that may be explained by the fact that plasma aldosterone is extracted by the kidneys and metabolized and excreted into the urine in a remarkably rapid and stable equilibrium (122). This study underscores two important points: the prevalence of a disease can be modulated by the risk of the population that is being tested and by how strictly the screening and confirmatory criteria are interpreted and evaluated. These points are particularly relevant for a disease such as primary aldosteronism, where a universal diagnostic gold standard to calibrate diagnostic testing is lacking.
Table 3.
Interpretations of Biochemical Dynamic Confirmation Results for Primary Aldosteronism
| Test | Brief Methodology | Most Conservative Positive Interpretation | More Permissive Positive Interpretation |
|---|---|---|---|
| Oral sodium suppression | • Increase dietary sodium to >200 mmol/d for 3-4 d | Twenty-four–hour urinary aldosterone excretion rate >12–14 μg | Twenty-four–hour urinary aldosterone excretion rate >10 μg |
| • Measure 24-h urine sodium, creatinine, and aldosterone excretion rate on day 3 or 4 | |||
| Supine intravenous saline suppression | • Following 1 h of supine rest, infuse 2 L of normal saline during 4 h | Postinfusion aldosterone level >10 ng/dL | Postinfusion aldosterone level >5 ng/dL |
| • Measure PRA and serum aldosterone before and after infusion | |||
| Fludrocortisone suppression | • Administer 0.10 mg of fludrocortisone every 6 h for 4 d, while maintaining normal serum potassium and high dietary sodium intake | Seated aldosterone >6 ng/dL with PRA <1.0 ng/mL/h | |
| • Measure PRA and serum aldosterone while seated on morning of day 4 | |||
| • Dexamethasone suppression can also be performed to minimize confounding by ACTH | |||
| Captopril challenge | • Administer 25 mg of oral captopril after 1 h of seated posture | • Less than 30% suppression of aldosterone from baseline while PRA remains suppressed (97) | • ARR postcaptopril >20 ng/dL per ng/mL/h |
| • Measure PRA, serum aldosterone, and ARR before and 1 h and 2 h after captopril in seated position | alternatively• ARR postcaptopril >30 ng/dL per ng/mL/h (108) |
Interpretations are based on the Endocrine Society guidelines (97).
Abbreviation: PRA, plasma renin activity.
The prevalence and severity spectrum of the primary aldosteronism phenotype in normotension
The detection of unrecognized, yet biochemically overt primary aldosteronism is not limited to hypertensives, but it extends to individuals with normal blood pressure as well (Fig. 4B). Markou et al. (123) indiscriminately (regardless of baseline ARR) conducted fludrocortisone-dexamethasone suppression testing on 100 normotensive individuals in Greece and demonstrated that 13% of their normotensive population had aldosterone secretion meeting the definition of confirmed primary aldosteronism. Similarly, Baudrand et al. (124) indiscriminately conducted oral sodium suppression tests on 210 normotensive individuals with low renin activity (<1.0 ng/mL/h) in the United States and observed a primary aldosteronism prevalence of 14%. Importantly, normotensive participants in both studies had ARR and serum potassium values in the normal range, suggesting that these participants not only lacked any indication to be screened for primary aldosteronism, but even if they had been screened with an ARR, it would not have been interpreted as positive or have provoked further confirmatory testing.
How can a normotensive individual have primary aldosteronism? One potential explanation is that healthy normotensive individuals have little to no vascular disease or kidney disease; thus, their arterial compliance can handle the excess volume and millions of healthy nephrons can excrete the excess sodium/volume to prevent substantial increases in blood pressure. However, it can then be presumed that exposure to intravascular volume expansion in combination with excess aldosterone–MR interactions over time could induce reductions in arterial compliance and age- or pressure-dependent reductions in kidney function that could then gradually manifest as increases in blood pressure and incident hypertension. Indeed, Markou et al. (123) conducted a 5-year longitudinal assessment in their study and showed that 85% of patients with normotensive primary aldosteronism developed frank hypertension; in contrast, only 23% of those without normotensive primary aldosteronism developed hypertension (OR, 18.4; 95% CI, 3.8 to 90.1).
If less severe cases of primary aldosteronism can be detected in mild-to-moderate hypertension and normotension, how far does this severity spectrum of primary aldosteronism extend? On the basis of logical biological continuums, it can be hypothesized that this severity spectrum could extend further, and that the above-mentioned studies were limited in detecting only primary aldosteronism cases that emerged by employing currently accepted thresholds for confirmatory tests (Table 3) (97). There are four well-known dynamic confirmatory tests for primary aldosteronism, and all employ a similar mantra: can aldosterone secretion be suppressed? A fifth confirmatory test is nondynamic and involves the observation that in the setting of a very high aldosterone level (>20 ng/dL), suppressed renin, and hypertension and hypokalemia, the diagnosis is confirmed (97). The method of aldosterone suppression in dynamic tests varies, that is, oral sodium or intravenous saline or fludrocortisone or captopril, each with its own pros and cons, and so do the relatively arbitrary thresholds that define when a confirmatory test is “positive” and when a test is “negative.” Furthermore, the concordance (sensitivity and specificity) between these confirmatory tests can vary greatly (125, 126). Therefore, the diagnostic thresholds of these confirmatory tests are not written in stone, and milder autonomous aldosterone secretion that fails to reach the currently accepted confirmatory thresholds (Table 3) may not always be entirely “normal.”
Is there a clinically relevant phenotype of autonomous aldosterone secretion that exists even below the current thresholds used to confirm primary aldosteronism? If so, this phenotype of primary aldosteronism might be referred to as “subclinical” (to imply that this phenotype is mild enough that it falls below the currently accepted diagnostic thresholds) or “nonclassical” (to imply that this phenotype is not recognized by the classical syndrome of severe hypertension and hypokalemia) (Fig. 4C). The idea that mild and nonclassical forms of primary aldosteronism exist and evade detection is not new. Jerome Conn suspected that primary aldosteronism likely originated in normokalemic normotensives, prior to progressing to its more overt manifestations of severe hypertension and hypokalemia (9–11). Shortly thereafter, in the 1960s and 1970s, low-renin hypertension was described as a phenotype of hypertension, low renin, but without overtly high aldosterone levels to qualify as primary aldosteronism (12, 13, 119, 127). However, evidence that low-renin hypertension could include a spectrum of excess aldosterone and/or MR activation accrued. Patients with low-renin hypertension were observed to have low salivary sodium-to-potassium ratios and notable blood pressure decrements when treated with spironolactone or inhibitors of adrenal steroids (14, 15, 17, 128).
“One of the major transformative breakthroughs in the last decade has been the increase in our understanding of the genetics of primary aldosteronism.”
Adlin (129) reported an interesting study in 1975 wherein 42 healthy normotensive individuals were evaluated after 3 days of dietary sodium restriction (mean 24-hour urinary sodium balance of only 20.5 mmol). These experimental conditions mimic the dietary sodium status of the Yanomami, discussed earlier in this review, whereby plasma renin activity was maximally stimulated (40). Subjects in Adlin’s study had their maximally stimulated plasma renin activity measured after 4 hours of ambulation where it was observed that stimulated plasma renin activity was inversely correlated with blood pressure (129). Adlin speculated from these results that some of these individuals may have subtle mineralocorticoid excess manifesting as an inability to maximally stimulate renin (“a more sensitive indicator of renin suppression”) and higher blood pressure within the normal range (129). Hundemer et al. (90) effectively repeated this experiment on a much larger scale by studying 663 normotensive and untreated mild hypertensive participants without any known cardiovascular or renal disease and without overt primary aldosteronism (based on current confirmatory testing). Maximally stimulated plasma renin activity was evaluated in the standing position after 5 days of dietary sodium restriction and the maximally stimulated renin values were hypothesized to inversely correlate with autonomous aldosterone secretion and MR activation. Indeed, participants with the least ability to physiologically increase renin (or most suppressed renin activity) when sodium restricted had higher blood pressure, greater salt sensitivity of blood pressure, higher ARR, greater autonomous aldosterone secretion in the context of an oral sodium suppression test, lower renal plasma flow, and trends suggesting greater urinary potassium excretion and lower serum potassium within the normal range (90). Importantly, none of these participants had primary aldosteronism by conventional criteria, thereby demonstrating a continuum of autonomous aldosterone secretion and renin suppression that was associated with cardiovascular and renal disease even below the current diagnostic confirmatory thresholds.
Cross-sectional studies have repeatedly observed a continuum of greater autonomous aldosterone secretion that exists below the current definitions of primary aldosteronism, and correlates with older age, adverse cardiometabolic profiles, and potassium regulation suggestive of increased MR activation (90, 124, 130–133). The significance of these cross-sectional findings is best appreciated in the context of longitudinal cohort studies that included outcomes assessments. In prospective studies of healthy normotensives from the Framingham Offspring cohort, Vasan et al. (134) showed that higher aldosterone levels were associated with greater increases in blood pressure and risk for incident hypertension. In subsequent studies they showed that the development of increases in blood pressure among normotensives may have been driven by participants with the lowest renin concentrations and highest aldosterone levels, in other words, those with a phenotype of normotensive renin-independent aldosteronism (135). Similarly, Brown et al. (41) reported that among untreated normotensive and normokalemic participants from the Multi-Ethnic Study of Atherosclerosis, those with suppressed plasma renin activity (≤0.50 ng/mL/h) had a significantly higher risk for developing incident hypertension (85.4 hypertension events per 1000 person-years) when compared with normotensives with unsuppressed plasma renin activity (0.51 to 0.99 and ≥1.0 ng/mL/h) (each with ∼54 hypertension events per 1000 person-years). Importantly, the risk for increases in systolic blood pressure and incident hypertension among normotensives with a suppressed renin was progressively associated with higher aldosterone levels, such that even higher aldosterone levels in the normal range were associated with increased risk for incident hypertension when renin was suppressed (41).
These longitudinal studies have suggested that the pathophysiology of primary aldosteronism may perhaps be best defined as any inappropriate or dysregulated secretion of aldosterone from its normal and physiologic regulators, and that this pathology may begin in seemingly healthy normotensives. Greater degrees of renin-independent aldosterone secretion in normotension are associated with a higher risk for developing hypertension, and more severe renin-independent aldosteronism in hypertensives may increase the risk for developing resistant hypertension and evident cardiovascular disease (Fig. 4) (17, 41, 118). The implications of this expanding spectrum of primary aldosteronism are that our current diagnostic definitions and practices may be suboptimal at detecting mild and nonclassical forms of primary aldosteronism, and consequently, missing opportunities to mitigate pathologic renin-independent aldosterone–MR interactions that increase the risk for cardiovascular disease. This was recently underscored by Dr. Funder (67) who suggested that “the strict definition of primary aldosteronism is no longer tenable” and that progress will require that we “recognize the true prevalence of primary aldosteronism to include dysregulated aldosterone secretion and inappropriate aldosterone production.”
Importantly, no prospective study has robustly evaluated whether a phenotype of renin-independent aldosteronism in normotension progressively worsens to become a more apparent phenotype of primary aldosteronism in hypertension. Therefore, whether these forms of inappropriate and dysregulated autonomous aldosteronism represent a single continuum, or multiple unique phenotypes that collectively form a severity spectrum, is unknown. Prospective studies are needed to better understand the longitudinal natural history and progression of autonomous aldosterone secretion to determine its origins and prevalence.
Current and future approaches to diagnosing primary aldosteronism
What interventions can be made to improve the earlier detection of primary aldosteronism? First and foremost, greater public health awareness and outreach efforts to encourage screening for primary aldosteronism based on the current and classical indications need to be made (97, 107, 136). The current clinical practice guidelines recommend “classical indications” to screen for primary aldosteronism using the ARR, that is, when patients have severe hypertension and/or hypokalemia, as well as hypertension with an adrenal mass, sleep apnea, or suspicious family history (Fig. 5). In this context, a very high aldosterone (such as >15 ng/dL) and a suppressed renin are highly suggestive of overt primary aldosteronism. However, future studies should prospectively evaluate the efficacy and cost-effectiveness of expanding the screening criteria to include nonclassical indications that could capture a broader population of individuals who may be enriched for primary aldosteronism, such as hypertensives using three or more antihypertensives, hypertensives with a low-renin phenotype, lone or unexplained atrial fibrillation, and patients with unexplained hypokalemia regardless of blood pressure (Fig. 5). Given that >1.3 billion people worldwide are estimated to have hypertension (137, 138), prospective studies will be needed to assess the efficacy and cost-effectiveness of expanded screening (139). Missing cases of primary aldosteronism carries a risk of increased patient morbidity and excess costs related to consequent cardiovascular disease. Alternatively, overtesting for primary aldosteronism can carry a risk of excessive costs and wasteful and unnecessary diagnostic testing.
Figure 5.
Proposed modifications to the diagnostic approach to detect overt and milder forms of primary aldosteronism. Biochemical screening for primary aldosteronism is generally pursued when classical indications are observed, as recommended by the Endocrine Society (97). Consideration of expanded screening indications may increase the probability of detecting more cases of primary aldosteronism. A positive screen for primary aldosteronism should suggest renin-independent aldosterone secretion, whereby aldosterone levels are relatively high in the context of a suppressed renin. In the absence of overt evidence for renin-independent aldosteronism on screening, confirmatory testing can be used to affirm the diagnosis (Table 3) (97). Failure or relative failure to suppress aldosterone on dynamic testing may confirm the diagnosis, whereas marked suppression of aldosterone may instead suggest a diagnosis of low-renin hypertension. The diagnosis of primary aldosteronism need not rely on binary thresholds, rather it may exist across a continuum of severity whereby mild and nonclassical cases may be detected as well. Solid arrows indicate recommended decision pathways; dashed arrows indicate the authors’ proposals to consider in the appropriate clinical context. BP, blood pressure. [© 2018 Illustration ENDOCRINE SOCIETY]
Furthermore, we propose that to maximize detection of true positives, screening criteria may be relaxed. We suggest that patients with hypertension and/or hypokalemia who have suppressed renin activity in combination with aldosterone levels >10 ng/dL should be considered to have a positive screening test, whereas those with lower aldosterone levels of 5 to 10 ng/dL can also be considered to have a potentially positive test result (Fig. 5) (74). Aldosterone levels can vary substantially throughout the day, and therefore a single value that falls below 10 ng/dL should not necessarily exclude the possibility of primary aldosteronism (140). The testing can be repeated and/or confirmatory testing can be performed. The inability to adequately suppress aldosterone on a dynamic confirmatory test would validate the screening testing, whereas the ability to markedly suppress aldosterone with sodium/saline or fludrocortisone or captopril could exclude primary aldosteronism and steer the diagnosis toward low-renin essential hypertension (Fig. 5). Evidence of only marginal aldosterone suppression below the conventional thresholds of recommended confirmatory testing (Table 3) requires careful clinical judgment, and may still be interpreted as a mild or nonclassical form of autonomous aldosterone secretion. These latter patients could undergo localization studies, or be empirically treated with MR antagonists, depending on the clinician’s confidence and judgment, because the thresholds for confirmatory tests are relatively arbitrary and there is likely a continuum of primary aldosteronism that exists below classical diagnostic thresholds (104, 124, 141). The use of MR antagonists in low-renin hypertension and resistant hypertension has been shown to be efficacious and may represent a targeted treatment that mitigates aldosterone-MR–mediated cardiovascular injury (16, 17, 117, 118). Alternatively, ENaC inhibitors may also be an effective alternative to MR antagonists, particularly in resistant hypertension (118).
As our understanding of the spectrum of primary aldosteronism expands, we will require better diagnostic tools. Novel biomarkers of aldosterone excess and MR activation, such as urinary exosomes that reflect MR and ENaC activity (142–144) or highly sensitive liquid chromatography–tandem mass spectroscopy steroid profiling (145, 146), may represent new diagnostic tools that provide greater confidence in detecting milder cases of primary aldosteronism.
What Causes Primary Aldosteronism?
Introduction to genetic and molecular pathogenesis of primary aldosteronism
As reviewed previously, emerging evidence suggests that the prevalence of autonomous aldosterone secretion is relatively high. Given the known physiologic role of aldosterone in human evolution, why and how would autonomous and pathophysiological aldosterone secretion develop? This section reviews the current evidence that has illuminated our understanding of how primary aldosteronism may develop, with an emphasis on recent discoveries. These discoveries show that genetic alterations, and potentially also ectopic and nonneoplastic expression of aldosterone synthase, underlie primary aldosteronism, and how these two findings might be related.
Genetics of familial primary hyperaldosteronism
One of the major transformative breakthroughs in the last decade has been the increase in our understanding of the genetics of primary aldosteronism. Although inheritable forms of primary aldosteronism [familial hyperaldosteronism (FH)] are extremely rare (147), the discovery and comprehension of familial forms of the disease have transformed our understanding of aldosterone secretion in the more common and sporadic forms of primary aldosteronism. This section only briefly overviews the history of genetic discoveries to allow contextualizing other findings discussed later; the genetics of primary aldosteronism have been comprehensively described in several recent reviews (18–21).
FH-I, also known as glucocorticoid remediable aldosteronism, was first described nearly 30 years ago (148). Patients classically present with early onset primary aldosteronism and have a higher incidence of stroke; however, the phenotype and penetrance of FH-I are highly variable and can often be difficult to detect (149). The molecular cause of FH-I is a chimeric gene whereby the promoter for 11β-hydroxylase (CYP11B1) is translocated to the coding region of aldosterone synthase (CYP11B2). This fusion gene, and aldosterone synthase expression and aldosterone production, is therefore under the regulation of ACTH and occurs in the zona fasciculata with resultant production of 18-hydroxycortisol and 18-oxocortisol. For this reason, glucocorticoids can be an effective therapy; the suppression of ACTH secretion can diminish excessive aldosterone secretion.
FH-II has been described for >20 years. FH-II has traditionally been defined as an inheritable form of primary aldosteronism whereby FH-I has been excluded. Early studies implicated an association between FH-II and a 7p22 chromosomal locus (150, 151); however, the molecular and genetic mechanisms underlying FH-II have been largely unknown, until recently. Studies, discussed below, suggest that we may be able to reclassify some cases of FH-II as a result of one, or possibly more, newly discovered genetic alterations.
Recent discoveries have identified numerous mechanisms that alter the cell membrane potential and intracellular calcium concentration of zona glomerulosa cells to result in autonomous aldosterone secretion (18–21). In general, membrane depolarization of zona glomerulosa cells leads to opening of voltage-gated calcium channels, increasing intracellular calcium concentrations. This calcium increase stimulates calcium signaling leading to increased expression of steroidogenic genes and aldosterone production. FH-III and FH-IV are the consequence of germline mutations that affect this signaling process. In 2008, Lifton and colleagues (152) described a father and his two daughters who each had early-onset primary aldosteronism requiring bilateral adrenalectomy for cure, and they had pathology revealing massive hyperplasia of the zona fasciculata. A few years later, using next-generation sequencing, they reported that this family, and others with inheritable primary aldosteronism (i.e., FH), had a germline mutation in the KCNJ5 gene, which encodes the G-protein–activated inward rectifier potassium channel 4 (GIRK4) (153). The wild-type channel is responsible for an outward potassium current that plays an important role in maintaining the hyperpolarized membrane potential of zona glomerulosa cells. In contrast, the mutant channel permits an inward leak of sodium ions, thereby raising the cell membrane potential toward the depolarization threshold, increasing opening of voltage-gated calcium channels and intracellular calcium influx, and increasing aldosterone production (153–155). This syndrome is now termed FH-III, and although it is exceedingly rare, it has shaped our understanding of how elements that regulate cell membrane potential may modulate aldosterone secretion.
Subsequently, FH-IV was identified from germline gain-of-function mutations in the CACNA1H gene, a T-type voltage–gated calcium channel, that when mutant, increases calcium influx and aldosterone production (156–158). FH-IV results not only in a syndrome of primary aldosteronism, but also neurocognitive disorders, epilepsy, and autism (159, 160). Similarly, in screening patients with early-onset primary aldosteronism, gain-of-function mutations were discovered in the CACNA1D gene, encoding an l-type voltage–gated calcium channel that when mutant results in increased calcium influx and aldosterone production (161). Germline CACNA1D mutations cause primary aldosteronism but also a phenotype of cerebral palsy, seizures, and possibly autism spectrum disorder (also referred to as PASNA, i.e., primary aldosteronism with seizures and neurologic abnormalities) (162, 163). Most recently, germline gain-of-function mutations in the CLCN2 gene were described, which encodes an outward chloride channel in zona glomerulosa cells (164, 165). Mutant CLCN2 increases chloride efflux resulting in depolarization of the cell, opening of voltage-gated calcium channels, and increases in aldosterone production (164, 165). Notably, these germline CLCN2 mutations were found among patients and families with the presumptive diagnosis of FH-II, whereby no known mechanism had been previously attributed (165); therefore, CLCN2 alterations may represent at least one potential explanation for the FH-II syndrome.
Genetics of sporadic primary aldosteronism
Although the aforementioned knowledge that elucidated mechanisms for familial primary aldosteronism have been groundbreaking, it is important to note that these germline mutations are very rare causes of primary aldosteronism. However, this knowledge has been particularly instructive for understanding sporadic, or noninheritable, forms of primary aldosteronism.
Numerous studies have now been performed examining tissue from surgically resected APAs. More than half of all APAs harbor at least one somatic mutation known to increase aldosterone production, and in one recent study that utilized a CYP11B2 immunohistochemistry-guided approach, up to 88% of APAs had a somatic mutation (166). The most frequent somatic alteration is gain-of-function mutations in KCNJ5, for which more than a dozen pathogenic mutations have been described (18–21). The exact frequency of somatic KCNJ5 mutations in APAs varies by geography and is limited to the bias of surgically resected specimens. They have been observed in 40% to 45% of APAs in European and American populations, and even higher in Asian populations (21, 166–174), while also displaying a higher prevalence in women (166, 174, 175). The high prevalence of KCNJ5 mutations in sporadic primary aldosteronism provides a common mechanism for the disease (cell membrane depolarization) that may provide insights for treatment. For example, a recent study demonstrated that macrolide antibiotics could selectively inhibit mutant GIRK4 and consequent aldosterone production (177). Furthermore, patients with APAs that harbor KCNJ5 mutations tend to present at young age with pronounced severity of biochemical primary aldosteronism, and have been observed to have substantial clinical improvement/cure following adrenalectomy, including regression of left-ventricular mass (167, 173, 178–180).
Following somatic KCNJ5 mutations, a broad spectrum of somatic mutations in CACNA1D are the next most commonly detected in sporadic primary aldosteronism with a prevalence of 9-21% among APAs (166, 175). Selective antagonists of voltage-gated calcium channels are currently available and may provide targeted therapy for primary aldosteronism driven by CACNA1D mutations (180, 181). In 4-17% of APAs, loss of function somatic mutations in ATP1A1 (encoding Na+/K+-ATPase 1) and ATP2B3 (encoding Ca2+-ATPase 3) have been reported (166, 175, 183–186); however, germline mutations in these genes have not yet been observed in primary aldosteronism. Loss-of-function mutations in these ATPases is postulated to result in zona glomerulosa cell membrane depolarization and consequent increased calcium influx and aldosterone production.
Importantly, although firm evidence has directly implicated these aforementioned “channelopathies” in increasing aldosterone production, evidence to support their role in the development of adrenal neoplasia or APA has been inconsistent and inconclusive (21, 155, 187). Finally, although the aforementioned studies focused on unilateral APAs, one recent study of 15 adrenal specimens from patients with bilateral primary aldosteronism due to presumed hyperplasia demonstrated the presence of pathogenic somatic mutations in every specimen, and these mutations were almost exclusively in CACNA1D (188). Larger studies will be needed to validate this finding; however, this early evidence implicates somatic mutations, and specifically CACNA1D mutations, in the pathogenesis of autonomous aldosterone secretion in bilateral primary aldosteronism.
“Simply normalizing blood pressure in primary aldosteronism may not be sufficient to optimally reduce incident cardiovascular events.”
Epigenetics of primary aldosteronism
In parallel with the surge in genetic research in primary aldosteronism, there have been studies implicating epigenetic factors that occur in APAs. Although the field of epigenetics can comprise a vast array of genetic modulators, the most consistent observations have related to methylation of the CYP11B2 gene.
When the renin–angiotensin–aldosterone system is activated with conditions of sodium restriction, hypomethylation and increased expression of CYP11B2 have been observed, whereas inhibition of the angiotensin type 1 receptor results in hypermethylation and decreased expression of CYP11B2 (189). The APA tissue from patients with primary aldosteronism exhibits global CpG hypomethylation compared with normal adrenal tissue, including hypomethylation of the Purkinje cell protein 4 gene (190), which is associated with increased gene expression of CYP11B2 (189, 191). Integrated analyses of genome-wide methylation have demonstrated distinct methylation patterns between APAs, nonfunctioning adrenocortical tumors, and normal adrenal cortex tissue (192), where the pattern of CYP11B2 hypomethylation associated with CYP11B2 overexpression in APAs is consistently observed. Continued research into the role of epigenetic regulation in inducing hyperaldosteronism and adrenal neoplasia is likely to evolve in the future.
Morphologies of primary aldosteronism and aldosterone-producing cell clusters
Another transformative breakthrough in the last decade has been in relation to our understanding of the phenotypic morphology of the adrenal glands in primary aldosteronism. It is conventionally assumed that primary aldosteronism is either caused by a unilateral neoplastic process (adenoma, hyperplasia, or carcinoma) or via a bilateral neoplastic process (adenomas or hyperplasia). However, recent studies focused on the histopathology of adrenal specimens from patients with and without primary aldosteronism have provided evidence that may refine this view.
Aldosterone production occurs in zona glomerulosa cells and requires expression of aldosterone synthase (CYP11B2). The availability of highly specific antibodies to CYP11B2 to conduct immunohistochemistry has enhanced our ability to visualize where aldosterone expression may be occurring (193–196). As expected, APAs have been shown to express CYP11B2, thereby confirming the relationship of adrenal neoplasia with the biochemical phenotype of primary aldosteronism (197). However, clusters of CYP11B2-expressing cells have also been observed to exist adjacent to APAs in regions without apparent neoplasia (197, 198). These clusters have been termed aldosterone-producing cell clusters (APCCs) and have several features that suggest they represent nonneoplastic foci of autonomous aldosterone production. The observation that APCCs can be visualized in adrenal tissue adjacent to APAs suggests that CYP11B2 expression in these foci is independent of renin and angiotensin II, which are physiologically suppressed in patients with primary aldosteronism (154, 198). Importantly, APCCs do not express CYP11B1 or CYP17A1, which are necessary for cortisol production (197, 199). When isolated and sequenced, more than a third of APCCs have been found to harbor known aldosterone-driver mutations in CACNA1D and ATP1A1 (199, 200), further supporting APCCs as foci of autonomous aldosterone secretion. An interesting observation has been that APCCs are more frequently found to harbor CACNA1D mutations (in both adrenal glands of patients with hypertension and normotension) rather than the KCNJ5 mutations that are most frequently observed in APAs (199, 200). Moreover, APCCs extend from the subcapsular area into the zona fasciculata, but they are not overtly neoplastic in nature, as they can be detected using CYP11B2 immunohistochemistry in morphologically normal adrenal glands without evident tumor or hyperplasia (201).
A causal link between the histopathology of APCCs and a biochemical phenotype of primary aldosteronism has not yet been confirmed. Because APCCs have largely been identified from postmortem adrenal tissue, they have mostly been described without the benefit of correlative biochemical phenotyping of aldosterone physiology.
A proposed model for primary aldosteronism pathogenesis and epidemiology
The pathogenesis of primary aldosteronism and APAs remains unknown. However, the aforementioned discoveries have resulted in the development of two hypothetical models for the development of neoplastic primary aldosteronism (21).
Broadly speaking, one theory suggests that the initial insult may be abnormal adrenocortical growth (a “proliferative abnormality”) that is than superimposed with the acquisition of a somatic mutation in an aldosterone-driver gene (a “secretory abnormality”) (21). In this regard, one can imagine that common adrenocortical proliferative abnormalities would give rise to nonfunctional adrenocortical adenomas, a well-observed and common phenomenon, and that a subset of these neoplastic lesions may then acquire somatic mutations that also result in abnormal secretion of aldosterone (and/or cortisol for the case of cortisol-producing adenomas).
An alternative proposal suggests that the initial insult may be a secretory abnormality resulting in ectopic and nonphysiologic aldosterone secretion. In this case, the suspected culprit may be the APCCs, which often harbor mutations in aldosterone-driver genes. These APCCs may display a phenotype of mild and autonomous aldosterone secretion that may also be vulnerable to acquiring further alterations that permit transformation to neoplastic and more severe autonomous aldosterone secretion (i.e., APA or bilateral adrenal hyperplasia) (21, 201–203) (Fig. 4).
It is possible that both theories, and perhaps others, may be valid. In this regard, the heterogeneous multitude of permutations that can combine nonneoplastic “secretory alterations” (i.e., mutations in aldosterone-driver genes and APCCs) with “proliferative/neoplastic alterations” [i.e., alternations in the Wnt/β-catenin pathway (204–211) and other potential progrowth alterations] may dictate the biochemical and clinical phenotype of primary aldosteronism (21, 201–203, 212).
This latter point has important implications for public health. Postmortem studies suggest that APCCs are relatively common (199, 200), and that nearly 30% of normotensive individuals harbor APCCs (200). These findings may provide a histopathologic explanation for the expansive spectrum of autonomous aldosteronism that is described (Fig. 4), and they suggest that aldosterone-mediated hypertension may be more common than perceived (41, 134, 200). Furthermore, APCCs are observed to be more prevalent with older age (133, 213, 214). Postmortem studies describe two notable adrenal histopathologic changes that are observed with older age: (1) the normal and contiguous expression of CYP11B2 appears to decline, and (2) the number of APCCs appears to be higher (133, 214). Collectively, these histopathologic observations suggest that aging may be associated with a blunted ability to physiologically secrete aldosterone, as well as an increase in autonomous and pathophysiologic aldosterone secretion, as has been suggested in prior human physiology studies (133, 215). These observations from separate histopathology and human physiology studies seem concordant: foci of autonomous aldosterone secretion (i.e., APCCs) may manifest clinically as a phenotype of nonsuppressible aldosterone secretion in the face of a sodium load, which under normal physiologic conditions would result in suppression of endogenous renin, angiotensin II, and thereby CYP11B2 expression (133, 214). To what extent this phenotype of autonomous aldosterone secretion contributes to age-related hypertension is not clear; however, given the global prevalence of hypertension and its rising incidence trajectory, this emerging field of investigation is relevant and potentially consequential.
How Should Primary Aldosteronism Be Treated?
Given the adverse effects of pathologic aldosterone–MR interactions on blood pressure, cardiometabolic and kidney disease, and death, treatment to eliminate or block aldosterone is recommended. The aforementioned studies by Monticone et al. (96) and those of others (76, 77, 80) that have used blood pressure–matched essential hypertensives as comparators have shown that the excess risk for cardiovascular disease in primary aldosteronism is independent of the influence of blood pressure. Therefore, simply normalizing blood pressure in primary aldosteronism may not be sufficient to optimally reduce incident cardiovascular events. Rather efforts to neutralize the effects of pathologic aldosterone-mediated MR activation may be necessary.
Dietary sodium restriction
Directly addressing blood pressure is paramount. As many patients with primary aldosteronism have resistant hypertension, the use of a multidrug regimen including an MR antagonist is common and recommended clinical practice (117). As with essential hypertension, dietary sodium restriction is to be recommended in primary aldosteronism (216). Dietary sodium restriction in primary aldosteronism is effective at lowering blood pressure and may render the effects of MR antagonists more effective (217, 218). The effects of dietary sodium restriction can be considered from a physiologic perspective. Marked reductions of sodium intake can induce volume contraction and further reduce the distal delivery of sodium in the nephron, thereby decreasing sodium reabsorption and excretion of potassium and hydrogen ions. The amount of dietary sodium restriction is often specified for essential hypertension. For example, organizations such as the American Heart Association (216) recommend consuming <100 mmol of sodium a day, and for patients with essential hypertension, <65 mmol of sodium a day. Despite these recommendations, the average individual in the United States still consumes >150 mmol of sodium daily (41, 219, 220), highlighting the pervasiveness of dietary sodium in everyday life and the difficulty in translating recommendations to practice and societal norms.
Whether these sodium restriction recommendations should be similar for patients with primary aldosteronism is less clear. In one study, among 79 participants with a confirmed diagnosis of primary aldosteronism, a sodium-restricted diet of <50 mmol per day for nearly 1 week, which had to be prepared and provided by professional clinical research center dieticians, resulted in a substantial rise in renin in >50% of participants such that they had a normalization of their aldosterone-to-renin ratio and therefore a negative screen for primary aldosteronism (104). Thus, when there is marked dietary sodium restriction in primary aldosteronism, it may limit distal sodium delivery sufficiently to reverse volume expansion, substantially increase renin, normalize the ARR, and reduce hypokalemia and potentially abrogate the pathological consequences of the disease (110). However, this degree of dietary sodium restriction is challenging to practically implement and adhere to.
“Despite normalizations in blood pressure and/or potassium, the persistent suppression of renin may serve as a biomarker of sub-optimal MR blockade.”
Medical vs surgical therapy for primary aldosteronism
The Endocrine Society clinical practice guidelines recommend laparoscopic adrenalectomy for patients with unilateral primary aldosteronism, and lifelong medical therapy with MR antagonists for patients who have bilateral primary aldosteronism or who are unwilling or unable to undergo surgery (97). This general dogma has been prescribed for decades and assumes that both surgical and medical treatments may mitigate or reverse the cardiovascular disease induced by aldosterone–MR interactions (221–223). Notably, there have been no robust randomized clinical trials to confirm this presumed efficacy of treatments. However, growing evidence from cohort studies suggests that removing the source of autonomous aldosterone secretion with surgery may result in superior cardiovascular risk reduction (91, 224, 225) and quality of life (226, 227) when compared with blocking the effect of aldosterone medically.
Surgical adrenalectomy has been shown to be highly effective at curing aldosteronism and hypokalemia, and either curing hypertension or substantially decreasing its severity (83, 228–235). Studies evaluating the comparative efficacy and/or disparities between medical and surgical therapy for primary aldosteronism are always somewhat challenging because of inherent differences in these patient populations that may influence future risk. Patients with unilateral disease often present younger in age and with more severe hypertension. In contrast, patients with bilateral primary aldosteronism often present older in age, with more insidious and possibly longer duration of hypertension and aldosteronism, and are more enriched with patients who are nonwhite. Therefore, the most illuminating studies are those that have attempted to account for these differences when comparing and contrasting outcomes of efficacy.
For example, in a recent large cohort study of 602 patients with primary aldosteronism treated with MR antagonists (spironolactone and/or eplerenone) and 41,853 age-matched patients with essential hypertension with similar blood pressure and antihypertensive medication use, the risk for incident cardiovascular outcomes was substantially greater among patients with medically treated primary aldosteronism (82). Despite having similar longitudinal blood pressure control to essential hypertensives, that was on average <140/90 mm Hg, primary aldosteronism patients treated with MR antagonists had a twofold higher adjusted risk for incident myocardial infarction, heart failure hospitalization, or stroke, with a relatively high composite 10-year adjusted cumulative incidence of 14 excess events per 100 people (82). Furthermore, primary aldosteronism patients treated with MR antagonists had a 93% higher risk for incident atrial fibrillation, 26% higher risk for incident diabetes, and 34% higher risk for incident mortality. Importantly, the excess risk for these cardiovascular events, as well as death and atrial fibrillation, was associated with renin activity: primary aldosteronism patients treated with higher doses of MR antagonists whose renin activity substantially increased had no excess risk for these outcomes, whereas primary aldosteronism patients treated with lower doses of MR antagonists whose renin activity remained suppressed/undetectable had a nearly threefold excess risk for cardiovascular events and atrial fibrillation and a 63% higher risk for death when compared with age-matched and blood pressure–similar essential hypertensives (82, 236) (Fig. 6). In contrast, among 205 patients with unilateral primary aldosteronism patients treated with curative surgical adrenalectomy, the risk for incident cardiovascular events, atrial fibrillation, and death was no different than that observed among age-matched and blood pressure–similar essential hypertensives without primary aldosteronism (Fig. 6) (82, 236). Thus, longitudinal data do support MR blockade as a valuable and effective therapy in primary aldosteronism; however, its efficacy may depend on whether adequate MR blockade is achieved. Despite normalizations in blood pressure and/or potassium, the persistent suppression of renin may serve as a biomarker of suboptimal MR blockade, whereas a rise in renin with MR antagonist therapy may reflect optimal blockade of the MR (renal and extrarenal MR) that corresponds with a lower risk for incident cardiovascular outcomes, death, and atrial fibrillation, similar to that achieved with surgical adrenalectomy to cure primary aldosteronism (82, 236) (Fig. 7).
Figure 6.
Incident composite cardiovascular events in medically and surgically treated primary aldosteronism when compared with essential hypertension. When compared with age-matched and blood pressure–similar patients with essential hypertension, patients with primary aldosteronism treated with MR antagonists had a nearly threefold higher risk for incident cardiovascular events (myocardial infarction, stroke, heart failure hospitalization) when renin remained suppressed (82). In contrast, patients with primary aldosteronism treated with MR antagonists such that their renin increased, as well as patients with unilateral primary aldosteronism treated with surgical adrenalectomy, had no significant difference in the risk for incident cardiovascular events when compared with essential hypertension (82). Similar findings were observed with respect to the risk for atrial fibrillation (236) and death (82). Dashed lines represent unadjusted cumulative incidence curves; solid lines represent adjusted cumulative incidence curves. PRA, plasma renin activity. Adapted with permission from Hundemer GL, Curhan GC, Yozamp N, et al. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol 2018;6(1):51–59. [© 2018 Illustration Presentation ENDOCRINE SOCIETY]
Figure 7.
Optimal medical treatment with MR antagonists in primary aldosteronism. The action of MR antagonists in the principal cell results in decreased ENaC-mediated urinary sodium reabsorption, and consequently decreased volume expansion and potassium and hydrogen ion excretion. If the effect of this action is sufficient, the contraction of the intravascular volume may result in a relative renal hypoperfusion and increased secretion of renin by juxtaglomerular cells. Thus, the rise in renin, from suppressed to unsuppressed, may serve as a biomarker of optimal MR antagonism in primary aldosteronism. AngII, angiotensin II. [© 2018 Illustration ENDOCRINE SOCIETY]
These findings are echoed in other cohort studies that have shown that either adrenalectomy or more intensive MR antagonist therapy can reduce the risk for diabetes and death when compared with essential hypertension (237, 238), and that adrenalectomy in primary aldosteronism can mitigate the excess risk for incident atrial fibrillation whereas medical treatment with MR antagonists may not (84, 239). Cohort studies have also shown that treatment of primary aldosteronism with MR antagonists and surgery lowers glomerular filtration rate as volume expansion and glomerular hyperfiltration are mitigated (91, 240); in fact, the higher the pretreatment aldosterone, the greater the decline in postsurgical kidney function (241). However, patients treated with MR antagonists have a much higher risk for incident chronic kidney disease when compared with age- and glomerular filtration rate–matched patients with essential hypertension and those who underwent surgical adrenalectomy (240). In fact, the age-related decline in glomerular filtration rate has been observed to be similar between essential hypertension patients and surgically cured patients with primary aldosteronism, whereas this decline is accelerated among primary aldosteronism patients who were medically treated with MR antagonists alone (240). This underscores another point: although aggressive MR antagonist therapy in primary aldosteronism seems ideal, it is often challenging to accomplish. Some patients, despite intensive MR antagonist dosing, have difficult-to-control blood pressure and suppressed renin activity. Furthermore, declines in glomerular filtration rate increase the risk for hyperkalemia when using MR antagonists, thereby potentially limiting the aggressiveness with which MR antagonists are dosed, and potentially perpetuating a vicious cycle of inadequate MR blockade and renal injury (240). Finally, the antiandrogenic effects of spironolactone may result in side effects in men that limit maximal dosing.
Note that ENaC antagonists, such as amiloride and triamterene, are also effective for blood pressure and potassium control in primary aldosteronism (97, 118, 242). This is not surprising given that the pathophysiology of primary aldosteronism is mediated through ENaCs (Fig. 7). However, these agents also may induce hyperkalemia when renal function is impaired, and furthermore, they do not block the MR; therefore, whether long-term use of ENaC inhibitors provides similar cardiovascular risk reductions to MR antagonists, beyond blood pressure control, is unknown.
Optimizing therapy using available evidence
The recent Primary Aldosteronism Surgical Outcome study developed standardized outcome criteria to grade the success of surgical treatment in primary aldosteronism (243). The standards set by this international consensus provide an objective framework and nomenclature to determine the clinical and biochemical success of interventional therapy in primary aldosteronism. In general, complete clinical cure after surgery was defined as a complete resolution of hypertension and cessation of antihypertensive medication use, whereas the progressive need for antihypertensive medications or residual degrees of hypertension suggests persistent clinical ramifications of primary aldosteronism (243). Biochemical cure was inferred with normalization of potassium and the aldosterone-to-renin ratio, whereas continued hypokalemia and the lack of aldosterone suppression on confirmatory testing postoperatively suggested persistent biochemical primary aldosteronism (243). This distinction between clinical and biochemical improvement (or cure) is important to develop an international nomenclature because even when biochemical cure is achieved, most patients will continue to have some degree of hypertension owing to the long duration of antecedent high blood pressure and vascular remodeling. For example, in the Primary Aldosteronism Surgical Outcome study, even though 94% of surgically treated patients with primary aldosteronism achieved a complete biochemical cure, only 37% of these patients had normal postoperative blood pressures without the aid of an antihypertensive medication (243). These criterion have also been used to show that patients with primary aldosteronism who undergo surgery after reliance on only computed tomography–based localization had a lower likelihood of achieving biochemical cure than when adrenal venous sampling-based localization was employed (244).
The accumulating data that patients more commonly benefit from eliminating the source of excess aldosterone as compared with blocking the MR raise the possibility that inadequate MR blockade leads to continued exposure to aldosterone and thus continued (though attenuated) cardiometabolic injury. This raises a question related to the medical management of bilateral primary aldosteronism with lifelong MR antagonist therapy: Should physicians consider unilateral adrenalectomy to attenuate the aldosterone exposure in selected patients with bilateral disease? Robust studies to address this question do not exist. Furthermore, the aforementioned studies implicating the benefits of surgical therapy over medical therapy should be considered “level 2” evidence emerging from cohort studies that are vulnerable to bias and confounding; there is no “level 1” evidence emerging from randomized, placebo-controlled, and blinded studies in primary aldosteronism. Because such prospective level 1 evidence does not seem to be on the horizon in the near future, clinicians must use their judgment and appraisal of the current cohort studies to appropriately manage their patients and optimize cardiovascular risk reduction.
We propose a modified approach toward the treatment of primary aldosteronism as outlined in Fig. 8. The classical approach is represented in red, whereby unilateral primary aldosteronism is best treated surgically and bilateral disease is treated with lifelong medical therapy; proposals to consider and systematically evaluate in the future are represented in blue. Currently, bilateral disease is diagnosed via adrenal venous sampling using varying criteria for lateralization, or the lack thereof. However, adrenal venous sampling results also provide quantified information on the relative degree of autonomous aldosterone secretion from each adrenal vein. The presence of grossly asymmetric bilateral disease presents a clinical challenge for which high-grade clinical trial data do not exist; however, we suggest that it may be useful to consider a combined medical/surgical approach: unilateral adrenalectomy to attenuate the severity of primary aldosteronism followed by MR antagonist therapy. This combined approach may be most appropriate in younger patients and those with established cardiovascular and renal disease, or those at high risk for developing these outcomes (Fig. 8). Choosing this treatment strategy assumes that there is long-term benefit in attenuating the severity of primary aldosteronism and thereby a greater possibility for clinical success with lifelong MR antagonist therapy; however, this approach has not been evaluated in prospective clinical studies. When prescribing lifelong MR antagonists, the objective is not only to normalize blood pressure and potassium, but also to prevent incident cardiovascular and metabolic diseases by blocking pathologic MR activation, for which a rise in renin may serve as a useful physiologic biomarker of success (82). The inability to adequately control blood pressure or potassium, or the inability to maximize MR antagonist dosing due to impaired renal function (240), again raises the option of unilateral adrenalectomy to attenuate the severity of disease coupled with long-term MR antagonist therapy. The alternative option is a lifelong sentence to inadequate MR antagonist therapy, which is already known to be associated with poorer outcomes (Fig. 8). Although this proposed model provides therapeutic suggestions based on currently available data and extrapolations of data, ultimately, prospective intervention studies are needed to ascertain the efficacy of this approach for reducing adverse cardiometabolic outcomes.
Figure 8.
Modified proposal toward the treatment of primary aldosteronism. The conventional approach to treating primary aldosteronism dictates that surgical adrenalectomy is preferred when there is unilateral disease, and lifelong MR antagonist therapy is preferred for bilateral disease. The pink boxes represent the conventional approach to treatment. The blue boxes represent the authors’ modified proposal toward treatment. Adrenal venous sampling provides results for lateralization, but it can also quantify the degree of relative autonomous aldosterone secretion from each adrenal vein. For patients with known cardiovascular or renal disease and who have grossly asymmetric bilateral primary aldosteronism, unilateral adrenalectomy to attenuate the severity of disease may be considered. When lifelong MR antagonist therapy is employed, the objective is to normalize blood pressure and potassium, and when possible, titrate the dose of the medication to achieve a rise in renin as a biomarker for sufficient MR blockade. When blood pressure or potassium cannot be effectively normalized with MR antagonist therapy, or chronic kidney disease limits the aggressiveness with which these medications can be used, unilateral adrenalectomy and/or additional antihypertensives could be considered. BP, blood pressure. [© 2018 Illustration ENDOCRINE SOCIETY]
Perspectives: Important Questions for the Future
Herein, we have reviewed some of the current knowledge and ongoing trajectories of thought in the understanding of how the primary aldosteronism phenotype can manifest, the newly understood molecular and genetic underpinnings of the disease, and recent findings informing the treatment of primary aldosteronism. Furthermore, we have proposed models to consider the pathogenesis, diagnosis, and treatment strategies for primary aldosteronism that should be systematically investigated in the future. However, much more work is needed to refine these concepts so that the care of patients can be optimized and decision-making is based on the highest levels of evidence. Here, we outline some important topics and questions that will require dedicated focus in the near future.
Diagnosis of primary aldosteronism
How should primary aldosteronism be defined to optimally detect cases of renin-independent aldosterone secretion that increase the risk for incident cardiovascular and kidney disease? Given recent studies suggesting milder (potentially also referred to as nonclassical or subclinical) forms of primary aldosteronism in resistant hypertension (118), mild-to-moderate hypertension (74, 106, 108), and even normotension (41, 123, 134), the paradigm of primary aldosteronism as synonymous with severe hypertension and hypokalemia should be modified (67). New efforts to build consensus for defining and diagnosing primary aldosteronism are needed.
What new biomarkers can be used to detect primary aldosteronism? Beyond measuring renin and aldosterone, recent evidence suggests that new biomarkers, such as urinary exosomes (142–144) and novel steroid metabolites (145, 245–248), may change the way we test for the disease. Additionally, although plasma renin activity has been used for decades, a trend toward replacing it with renin concentration measurements is growing, and a recalibration of diagnostic standards and practices will be needed (176).
An increased effort to improve public health awareness, especially at the primary care level, about the high prevalence of primary aldosteronism and its modifiable cardiovascular risk is necessary.
Pathogenesis of primary aldosteronism
What are the genetic underpinnings of bilateral primary aldosteronism? Although our understanding of genetic alterations that drive aldosterone secretion in surgically resected aldosterone-producing adenomas has been transformative, a parallel discovery in bilateral forms of primary aldosteronism is much needed. The differences and similarities between the different forms and morphologies or primary aldosteronism may dictate novel approaches to diagnosis, prognosis, and potentially treatment.
How common are APCCs and what triggers their development? The preliminary studies suggesting that these ectopic foci of aldosterone synthase expression are common and are sources of autonomous aldosterone secretion has informed how we think about the pathogenesis of primary aldosteronism (133, 197, 199, 200, 212, 214). However, little is known about their pathogenesis or risk factors for development, other than that they may be more common with older age (133, 214). Furthermore, because APCCs were identified using postmortem specimens, parallel evidence to support that they induce a biochemical phenotype of autonomous aldosterone secretion has not yet been established.
Treatment of primary aldosteronism
There is a need for robust intervention studies (i.e., randomized clinical trials) focused on therapeutic approaches in primary aldosteronism. The recommended targeted therapies have had little high-grade evidence to support them, and the best evidence to date stems from cohort studies.
Is there a role for unilateral noncurative adrenalectomy in patients with bilateral primary aldosteronism? The conventional answer to this question has been “no.” However, because recent cohort studies have suggested that unilateral adrenalectomy to eliminate the source of aldosterone secretion may be more effective at preventing adverse outcomes when compared with lifelong MR antagonist medical therapy to block the aldosterone excess (82, 84, 236–238, 240), this question becomes important to consider. Prospective studies to evaluate whether unilateral adrenalectomy in bilateral disease to attenuate the severity of disease, or strategic MR antagonist therapy to maximally block the adverse effects of aldosterone, are needed.
To what extent, and in what situations, can empiric MR antagonist therapy (or treatment with ENaC inhibitors) be initiated for patients with new-onset and low-renin hypertension? Given the large prevalence of the low-renin hypertension phenotype, and the large scale at which primary aldosteronism screening is not being conducted, what is the cost-to-benefit ratio of increasing the empiric usage of these medications rather than insisting on primary aldosteronism diagnostics? The findings of the PATHWAY-2 study (117, 118) suggest that empiric MR antagonist or ENaC inhibitor therapy may be effective in those with low-renin phenotypes (even when it is presumed that secondary hypertension has been excluded).
Finally, we await the evaluation of novel therapies that might provide greater efficacy and safety; for example, nonsteroidal MR antagonists (249) and therapies that target known genetic mechanisms of aldosterone secretion (177).
Acknowledgments
Financial Support: This work was supported in part by funding from the National Institutes of Health to A.V.
Disclosure Summary: In the last five years, A.V. has provided consulting services for Selenity Therapeutics, HRA Pharma, Corcept Inc, Ionis Pharmaceuticals, and Orphagen Pharmaceuticals Inc. A.V. has also received honoraria for speaking at educational conferences from The Endocrine Society, the American Association of Clinical Endocrinologists, and Harvard Medical School.
Glossary
Abbreviations
- 11βHSD2
11β-hydroxysteroid dehydrogenase type 2
- APCC
aldosterone-producing cell cluster
- APA
aldosterone-producing adenoma
- ARR
aldosterone-to-renin ratio
- ENaC
epithelial sodium channel
- FH
familial hyperaldosteronism
- MR
mineralocorticoid receptor
- ROMK
renal outer medullary potassium channel
References
- 1. Addison T. On the Constitutional and Local Effects of Disease of the Supra-Renal Capsules. London, UK: Leslie B. Adams, Jr.; 1855. [Google Scholar]
- 2. Kuizenga MH, Cartland GF. Fractionation studies on adrenal cortical extract with notes on the distribution of biological activity among the crystalline and amorphous fractions. Endocrinology. 1939;24(4):526–535. [Google Scholar]
- 3. Deane HW, Shaw JH, Greep RO. The effect of altered sodium or potassium intake on the width and cytochemistry of the zona glomerulosa of the rat’s adrenal cortex. Endocrinology. 1948;43(3):133–153. [DOI] [PubMed] [Google Scholar]
- 4. Grundy HM, Simpson SA, Tait JF. Isolation of a highly active mineralocorticoid from beef adrenal extract. Nature. 1952;169(4306):795–796. [DOI] [PubMed] [Google Scholar]
- 5. Simpson SA, Tait JF, Bush IE. Secretion of a salt-retaining hormone by the mammalian adrenal cortex. Lancet. 1952;2(6727):226–228. [DOI] [PubMed] [Google Scholar]
- 6. Williams JS, Williams GH. 50th Anniversary of aldosterone. J Clin Endocrinol Metab. 2003;88(6):2364–2372. [DOI] [PubMed] [Google Scholar]
- 7. Conn JW. Presidential address. I. Painting background. II. Primary aldosteronism, a new clinical syndrome. J Lab Clin Med. 1955;45(1):3–17. [PubMed] [Google Scholar]
- 8. Conn JW. Aldosterone in clinical medicine; past, present, and future. AMA Arch Intern Med. 1956;97(2):135–144. [DOI] [PubMed] [Google Scholar]
- 9. Conn JW. Plasma renin activity in primary aldosteronism. Importance in differential diagnosis and in research of essential hypertension. JAMA. 1964;190(3):222–225. [PubMed] [Google Scholar]
- 10. Ross EJ. Aldosterone and Aldosteronism. London, UK: Whitefriars Press; 1975. [Google Scholar]
- 11. Conn JW, Rovner DR, Cohen EL. Normal and altered function of the renin-angiotensin-aldosterone system in man: applications in clinical and research medicine. Ann Intern Med. 1965;63(2):266–284. [DOI] [PubMed] [Google Scholar]
- 12. Jose A, Crout JR, Kaplan NM. Suppressed plasma renin activity in essential hypertension. Roles of plasma volume, blood pressure, and sympathetic nervous system. Ann Intern Med. 1970;72(1):9–16. [DOI] [PubMed] [Google Scholar]
- 13. Channick BJ, Adlin EV, Marks AD. Suppressed plasma renin activity in hypertension. Arch Intern Med. 1969;123(2):131–140. [PubMed] [Google Scholar]
- 14. Adlin EV, Marks AD, Channick BJ. The salivary sodium/potassium ratio in hypertension: relation to race and plasma renin activity. Clin Exp Hypertens A. 1982;4(9–10):1869–1880. [DOI] [PubMed] [Google Scholar]
- 15. Woods JW, Liddle GW, Michelakis AM, Brill AB. Effect of an adrenal inhibitor in hypertensive patients with suppressed renin. Arch Intern Med. 1969;123(4):366–370. [PubMed] [Google Scholar]
- 16. Adlin EV, Marks AD, Channick BJ. Spironolactone and hydrochlorothiazide in essential hypertension. Blood pressure response and plasma renin activity. Arch Intern Med. 1972;130(6):855–858. [PubMed] [Google Scholar]
- 17. Adlin EV. Subclinical primary aldosteronism. Ann Intern Med. 2017;167(9):673–674. [DOI] [PubMed] [Google Scholar]
- 18. Faillot S, Assie G. Endocrine tumours: the genomics of adrenocortical tumors. Eur J Endocrinol. 2016;174(6):R249–R265. [DOI] [PubMed] [Google Scholar]
- 19. Monticone S, Buffolo F, Tetti M, Veglio F, Pasini B, Mulatero P. Genetics in endocrinology: the expanding genetic horizon of primary aldosteronism. Eur J Endocrinol. 2018;178(3):R101–R111. [DOI] [PubMed] [Google Scholar]
- 20. Prada ETA, Burrello J, Reincke M, Williams TA. Old and new concepts in the molecular pathogenesis of primary aldosteronism. Hypertension. 2017;70(5):875–881. [DOI] [PubMed] [Google Scholar]
- 21. Zennaro MC, Boulkroun S, Fernandes-Rosa F. Genetic causes of functional adrenocortical adenomas. Endocr Rev. 2017;38(6):516–537. [DOI] [PubMed] [Google Scholar]
- 22. Reincke M. Adrenal vein sampling for subtyping in primary aldosteronism. Lancet Diabetes Endocrinol. 2016;4(9):718–719. [DOI] [PubMed] [Google Scholar]
- 23. Stowasser M. Adrenal venous sampling for differentiating unilateral from bilateral primary aldosteronism: still the best, but could be better. Hypertension. 2015;65(4):704–706. [DOI] [PubMed] [Google Scholar]
- 24. Rossi GP, Auchus RJ, Brown M, Lenders JW, Naruse M, Plouin PF, Satoh F, Young WF Jr. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014;63(1):151–160. [DOI] [PubMed] [Google Scholar]
- 25. Rossi GP, Funder JW. Adrenal venous sampling versus computed tomographic scan to determine treatment in primary aldosteronism (the SPARTACUS trial): a critique. Hypertension. 2017;69(3):396–397. [DOI] [PubMed] [Google Scholar]
- 26. Stowasser M, Gordon RD. Primary aldosteronism: changing definitions and new concepts of physiology and pathophysiology both inside and outside the kidney. Physiol Rev. 2016;96(4):1327–1384. [DOI] [PubMed] [Google Scholar]
- 27. Kassahn KS, Ragan MA, Funder JW. Mineralocorticoid receptors: evolutionary and pathophysiological considerations. Endocrinology. 2011;152(5):1883–1890. [DOI] [PubMed] [Google Scholar]
- 28. Funder JW. Aldosterone and mineralocorticoid receptors—physiology and pathophysiology. Int J Mol Sci. 2017;18(5):1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baker ME, Katsu Y. 30 Years of the mineralocorticoid receptor: evolution of the mineralocorticoid receptor: sequence, structure and function. J Endocrinol. 2017;234(1):T1–T16. [DOI] [PubMed] [Google Scholar]
- 30. Baudrand R, Vaidya A. Cortisol dysregulation in obesity-related metabolic disorders. Curr Opin Endocrinol Diabetes Obes. 2015;22(3):143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahlberg PE, Smith MM, Johanson Z. Developmental plasticity and disparity in early dipnoan (lungfish) dentitions. Evol Dev. 2006;8(4):331–349. [DOI] [PubMed] [Google Scholar]
- 32. Rossier BC, Baker ME, Studer RA. Epithelial sodium transport and its control by aldosterone: the story of our internal environment revisited. Physiol Rev. 2015;95(1):297–340. [DOI] [PubMed] [Google Scholar]
- 33. Joss JMP, Arnold-Reed DE, Balment RJ. The steroidogenic response to angiotensin II in the Australian lungfish, Neoceratodus forsteri. J Comp Physiol. 1994;164(5):378–382. [Google Scholar]
- 34. Kronenberg H, Melmed S, Polonsky K, Larsen P.. Williams Textbook of Endocrinology. 11th ed. Philadelphia, PA: Saunders; 2008.
- 35. Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol. 2015;10(6):1050–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roy A, Al-bataineh MM, Pastor-Soler NM. Collecting duct intercalated cell function and regulation. Clin J Am Soc Nephrol. 2015;10(2):305–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shibata S, Rinehart J, Zhang J, Moeckel G, Castañeda-Bueno M, Stiegler AL, Boggon TJ, Gamba G, Lifton RP. Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab. 2013;18(5):660–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arroyo JP, Ronzaud C, Lagnaz D, Staub O, Gamba G. Aldosterone paradox: differential regulation of ion transport in distal nephron. Physiology (Bethesda). 2011;26(2):115–123. [DOI] [PubMed] [Google Scholar]
- 39. Rikimaru T, Fujita Y, Okuda T, Kajiwara N, Miyatani S, Alpers MP, Koishi H. Responses of sodium balance, blood pressure, and other variables to sodium loading in Papua New Guinea highlanders. Am J Clin Nutr. 1988;47(3):502–508. [DOI] [PubMed] [Google Scholar]
- 40. Oliver WJ, Cohen EL, Neel JV. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a “no-salt” culture. Circulation. 1975;52(1):146–151. [DOI] [PubMed] [Google Scholar]
- 41. Brown JM, Robinson-Cohen C, Luque-Fernandez MA, Allison MA, Baudrand R, Ix JH, Kestenbaum B, de Boer IH, Vaidya A. The spectrum of subclinical primary aldosteronism and incident hypertension: a cohort study. Ann Intern Med. 2017;167(9):630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carvalho JJ, Baruzzi RG, Howard PF, Poulter N, Alpers MP, Franco LJ, Marcopito LF, Spooner VJ, Dyer AR, Elliott P. Blood pressure in four remote populations in the INTERSALT Study. Hypertension. 1989;14(3):238–246. [DOI] [PubMed] [Google Scholar]
- 43. Sun Q, Bertrand KA, Franke AA, Rosner B, Curhan GC, Willett WC. Reproducibility of urinary biomarkers in multiple 24-h urine samples. Am J Clin Nutr. 2017;105(1):159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jin Y, Kuznetsova T, Maillard M, Richart T, Thijs L, Bochud M, Herregods MC, Burnier M, Fagard R, Staessen JA. Independent relations of left ventricular structure with the 24-hour urinary excretion of sodium and aldosterone. Hypertension. 2009;54(3):489–495. [DOI] [PubMed] [Google Scholar]
- 45. Bomback AS, Bove RM, Klemmer PJ. Of snakes and men: the evolution of ACE inhibitors. J Renin Angiotensin Aldosterone Syst. 2007;8(1):1–2. [DOI] [PubMed] [Google Scholar]
- 46. Smith CG, Vane JR. The discovery of captopril. FASEB J. 2003;17(8):788–789. [DOI] [PubMed] [Google Scholar]
- 47. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J; Randomized Aldactone Evaluation Study Investigators . The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341(10):709–717. [DOI] [PubMed] [Google Scholar]
- 48. Rocha R, Stier CT Jr, Kifor I, Ochoa-Maya MR, Rennke HG, Williams GH, Adler GK. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141(10):3871–3878. [DOI] [PubMed] [Google Scholar]
- 49. Martinez DV, Rocha R, Matsumura M, Oestreicher E, Ochoa-Maya M, Roubsanthisuk W, Williams GH, Adler GK. Cardiac damage prevention by eplerenone: comparison with low sodium diet or potassium loading. Hypertension. 2002;39(2 Pt 2):614–618. [PubMed] [Google Scholar]
- 50. Brilla CG, Weber KT. Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. J Lab Clin Med. 1992;120(6):893–901. [PubMed] [Google Scholar]
- 51. Tesch GH, Young MJ. Mineralocorticoid receptor signaling as a therapeutic target for renal and cardiac fibrosis. Front Pharmacol. 2017;8:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18(9):1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Diaz-Otero JM, Fisher C, Downs K, Moss ME, Jaffe IZ, Jackson WF, Dorrance AM. Endothelial mineralocorticoid receptor mediates parenchymal arteriole and posterior cerebral artery remodeling during angiotensin II–induced hypertension. Hypertension. 2017;70(6):1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mueller KB, Bender SB, Hong K, Yang Y, Aronovitz M, Jaisser F, Hill MA, Jaffe IZ. Endothelial mineralocorticoid receptors differentially contribute to coronary and mesenteric vascular function without modulating blood pressure. Hypertension. 2015;66(5):988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barrett KV, McCurley AT, Jaffe IZ. Direct contribution of vascular mineralocorticoid receptors to blood pressure regulation. Clin Exp Pharmacol Physiol. 2013;40(12):902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim SK, McCurley AT, DuPont JJ, Aronovitz M, Moss ME, Stillman IE, Karumanchi SA, Christou DD, Jaffe IZ. Smooth muscle cell–mineralocorticoid receptor as a mediator of cardiovascular stiffness with aging. Hypertension. 2018;71(4):609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hasegawa T, Masugi F, Ogihara T, Kumahara Y. Increase in plasma ouabainlike inhibitor of Na+, K+-ATPase with high sodium intake in patients with essential hypertension. J Clin Hypertens. 1987;3(4):419–429. [PubMed] [Google Scholar]
- 58. Lorenz JN, Loreaux EL, Dostanic-Larson I, Lasko V, Schnetzer JR, Paul RJ, Lingrel JB. ACTH-induced hypertension is dependent on the ouabain-binding site of the α2-Na+-K+-ATPase subunit. Am J Physiol Heart Circ Physiol. 2008;295(1):H273–H280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Laredo J, Shah JR, Lu ZR, Hamilton BP, Hamlyn JM. Angiotensin II stimulates secretion of endogenous ouabain from bovine adrenocortical cells via angiotensin type 2 receptors. Hypertension. 1997;29(1 Pt 2):401–407. [DOI] [PubMed] [Google Scholar]
- 60. Hamlyn JM, Blaustein MP. Endogenous ouabain: recent advances and controversies. Hypertension. 2016;68(3):526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lewis LK, Yandle TG, Hilton PJ, Jensen BP, Begg EJ, Nicholls MG. Endogenous ouabain is not ouabain. Hypertension. 2014;64(4):680–683. [DOI] [PubMed] [Google Scholar]
- 62. Baecher S, Kroiss M, Fassnacht M, Vogeser M. No endogenous ouabain is detectable in human plasma by ultra-sensitive UPLC-MS/MS. Clin Chim Acta. 2014;431:87–92. [DOI] [PubMed] [Google Scholar]
- 63. Doris PA, Jenkins LA, Stocco DM. Is ouabain an authentic endogenous mammalian substance derived from the adrenal? Hypertension. 1994;23(5):632–638. [DOI] [PubMed] [Google Scholar]
- 64. Naruse K, Naruse M, Tanabe A, Yoshimoto T, Watanabe Y, Kurimoto F, Horiba N, Tamura M, Inagami T, Demura H. Does plasma immunoreactive ouabain originate from the adrenal gland? Hypertension. 1994;23(1, Suppl):I102–I105. [DOI] [PubMed] [Google Scholar]
- 65. El Ghorayeb N, Bourdeau I, Lacroix A. Role of ACTH and other hormones in the regulation of aldosterone production in primary aldosteronism. Front Endocrinol (Lausanne). 2016;7:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Markou A, Sertedaki A, Kaltsas G, Androulakis II, Marakaki C, Pappa T, Gouli A, Papanastasiou L, Fountoulakis S, Zacharoulis A, Karavidas A, Ragkou D, Charmandari E, Chrousos GP, Piaditis GP. Stress-induced aldosterone hyper-secretion in a substantial subset of patients with essential hypertension. J Clin Endocrinol Metab. 2015;100(8):2857–2864. [DOI] [PubMed] [Google Scholar]
- 67. Funder JW. Primary aldosteronism: the next five years. Horm Metab Res. 2017;49(12):977–983. [DOI] [PubMed] [Google Scholar]
- 68. Saha S, Bornstein SR, Graessler J, Chakrabarti S, Kopprasch S. Aldosterone hypothesis for cognitive impairment in diabetes mellitus. Horm Metab Res. 2017;49(9):716–718. [DOI] [PubMed] [Google Scholar]
- 69. Gomez-Sanchez EP. Brain mineralocorticoid receptors in cognition and cardiovascular homeostasis. Steroids. 2014;91:20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Horiuchi M. [Brain renin-angiotensin-aldosterone system and cognitive function]. Nihon Rinsho. 2014;72(4):641–647. [PubMed] [Google Scholar]
- 71. Loprinzi PD, Frith E. Obesity and episodic memory function. J Physiol Sci. 2018;68(4):321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. de Kloet ER, Meijer OC, de Nicola AF, de Rijk RH, Joëls M. Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front Neuroendocrinol. 2018;49:124–145. [DOI] [PubMed] [Google Scholar]
- 73. Rotenstein LS, Sheridan M, Garg R, Adler GK. Effect of mineralocorticoid receptor blockade on hippocampal-dependent memory in adults with obesity. Obesity (Silver Spring). 2015;23(6):1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, Gabetti L, Mengozzi G, Williams TA, Rabbia F, Veglio F, Mulatero P. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69(14):1811–1820. [DOI] [PubMed] [Google Scholar]
- 75. Murata M, Kitamura T, Tamada D, Mukai K, Kurebayashi S, Yamamoto T, Hashimoto K, Hayashi RD, Kouhara H, Takeiri S, Kajimoto Y, Nakao M, Hamasaki T, Otsuki M, Shimomura I. Plasma aldosterone level within the normal range is less associated with cardiovascular and cerebrovascular risk in primary aldosteronism. J Hypertens. 2017;35(5):1079–1085. [DOI] [PubMed] [Google Scholar]
- 76. Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, Sechi LA. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008;168(1):80–85. [DOI] [PubMed] [Google Scholar]
- 77. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45(8):1243–1248. [DOI] [PubMed] [Google Scholar]
- 78. Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A, Crudo V, Burrello J, Milan A, Rabbia F, Veglio F. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98(12):4826–4833. [DOI] [PubMed] [Google Scholar]
- 79. Takeda R, Matsubara T, Miyamori I, Hatakeyama H, Morise T; The Research Committee of Disorders of Adrenal Hormones in Japan . Vascular complications in patients with aldosterone producing adenoma in Japan: comparative study with essential hypertension. J Endocrinol Invest. 1995;18(5):370–373. [DOI] [PubMed] [Google Scholar]
- 80. Reincke M, Fischer E, Gerum S, Merkle K, Schulz S, Pallauf A, Quinkler M, Hanslik G, Lang K, Hahner S, Allolio B, Meisinger C, Holle R, Beuschlein F, Bidlingmaier M, Endres S; German Conn’s Registry-Else Kröner-Fresenius-Hyperaldosteronism Registry . Observational study mortality in treated primary aldosteronism: the German Conn’s registry. Hypertension. 2012;60(3):618–624. [DOI] [PubMed] [Google Scholar]
- 81. Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2013;62(2):331–336. [DOI] [PubMed] [Google Scholar]
- 82. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(1):51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rossi GP, Cesari M, Cuspidi C, Maiolino G, Cicala MV, Bisogni V, Mantero F, Pessina AC. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension. 2013;62(1):62–69. [DOI] [PubMed] [Google Scholar]
- 84. Rossi GP, Maiolino G, Flego A, Belfiore A, Bernini G, Fabris B, Ferri C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Muiesan ML, Mannelli M, Negro A, Palumbo G, Parenti G, Rossi E, Mantero F; PAPY Study Investigators . Adrenalectomy lowers incident atrial fibrillation in primary aldosteronism patients at long term. Hypertension. 2018;71(4):585–591. [DOI] [PubMed] [Google Scholar]
- 85. Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, Rabbia F, Federspil G, Mulatero P. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab. 2006;91(2):454–459. [DOI] [PubMed] [Google Scholar]
- 86. Chen W, Li F, He C, Zhu Y, Tan W. Elevated prevalence of abnormal glucose metabolism in patients with primary aldosteronism: a meta-analysis. Ir J Med Sci. 2014;183(2):283–291. [DOI] [PubMed] [Google Scholar]
- 87. Hanslik G, Wallaschofski H, Dietz A, Riester A, Reincke M, Allolio B, Lang K, Quack I, Rump LC, Willenberg HS, Beuschlein F, Quinkler M, Hannemann A; participants of the German Conn’s Registry . Increased prevalence of diabetes mellitus and the metabolic syndrome in patients with primary aldosteronism of the German Conn’s Registry. Eur J Endocrinol. 2015;173(5):665–675. [DOI] [PubMed] [Google Scholar]
- 88. Rossi GP, Bernini G, Desideri G, Fabris B, Ferri C, Giacchetti G, Letizia C, Maccario M, Mannelli M, Matterello MJ, Montemurro D, Palumbo G, Rizzoni D, Rossi E, Pessina AC, Mantero F; PAPY Study Participants . Renal damage in primary aldosteronism: results of the PAPY Study. Hypertension. 2006;48(2):232–238. [DOI] [PubMed] [Google Scholar]
- 89. Reincke M, Rump LC, Quinkler M, Hahner S, Diederich S, Lorenz R, Seufert J, Schirpenbach C, Beuschlein F, Bidlingmaier M, Meisinger C, Holle R, Endres S; Participants of German Conn’s Registry . Risk factors associated with a low glomerular filtration rate in primary aldosteronism. J Clin Endocrinol Metab. 2009;94(3):869–875. [DOI] [PubMed] [Google Scholar]
- 90. Hundemer GL, Baudrand R, Brown JM, Curhan G, Williams GH, Vaidya A. Renin phenotypes characterize vascular disease, autonomous aldosteronism, and mineralocorticoid receptor activity. J Clin Endocrinol Metab. 2017;102(6):1835–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sechi LA, Novello M, Lapenna R, Baroselli S, Nadalini E, Colussi GL, Catena C. Long-term renal outcomes in patients with primary aldosteronism. JAMA. 2006;295(22):2638–2645. [DOI] [PubMed] [Google Scholar]
- 92. Salcuni AS, Palmieri S, Carnevale V, Morelli V, Battista C, Guarnieri V, Guglielmi G, Desina G, Eller-Vainicher C, Beck-Peccoz P, Scillitani A, Chiodini I. Bone involvement in aldosteronism. J Bone Miner Res. 2012;27(10):2217–2222. [DOI] [PubMed] [Google Scholar]
- 93. Salcuni AS, Carnevale V, Battista C, Palmieri S, Eller-Vainicher C, Guarnieri V, Pugliese F, Guglielmi G, Desina G, Minisola S, Chiodini I, Scillitani A. Primary aldosteronism as a cause of secondary osteoporosis. Eur J Endocrinol. 2017;177(5):431–437. [DOI] [PubMed] [Google Scholar]
- 94. Ceccoli L, Ronconi V, Giovannini L, Marcheggiani M, Turchi F, Boscaro M, Giacchetti G. Bone health and aldosterone excess. Osteoporos Int. 2013;24(11):2801–2807. [DOI] [PubMed] [Google Scholar]
- 95. Wu VC, Chang CH, Wang CY, Lin YH, Kao TW, Lin PC, Chu TS, Chang YS, Chen L, Wu KD, Chueh SJ. Risk of fracture in primary aldosteronism: a population-based cohort study. J Bone Miner Res. 2017;32(4):743–752. [DOI] [PubMed] [Google Scholar]
- 96. Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, Mulatero P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6(1):41–50. [DOI] [PubMed] [Google Scholar]
- 97. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. [DOI] [PubMed] [Google Scholar]
- 98. Montori VM, Young WF Jr. Use of plasma aldosterone concentration-to-plasma renin activity ratio as a screening test for primary aldosteronism. A systematic review of the literature. Endocrinol Metab Clin North Am. 2002;31(3):619–632, xi. [DOI] [PubMed] [Google Scholar]
- 99. Hannemann A, Bidlingmaier M, Friedrich N, Manolopoulou J, Spyroglou A, Völzke H, Beuschlein F, Seissler J, Rettig R, Felix SB, Biffar R, Döring A, Meisinger C, Peters A, Wichmann HE, Nauck M, Wallaschofski H, Reincke M. Screening for primary aldosteronism in hypertensive subjects: results from two German epidemiological studies. Eur J Endocrinol. 2012;167(1):7–15. [DOI] [PubMed] [Google Scholar]
- 100. Hiramatsu K, Yamada T, Yukimura Y, Komiya I, Ichikawa K, Ishihara M, Nagata H, Izumiyama T. A screening test to identify aldosterone-producing adenoma by measuring plasma renin activity. Results in hypertensive patients. Arch Intern Med. 1981;141(12):1589–1593. [PubMed] [Google Scholar]
- 101. McKenna TJ, Sequeira SJ, Heffernan A, Chambers J, Cunningham S. Diagnosis under random conditions of all disorders of the renin-angiotensin-aldosterone axis, including primary hyperaldosteronism. J Clin Endocrinol Metab. 1991;73(5):952–957. [DOI] [PubMed] [Google Scholar]
- 102. Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007;66(5):607–618. [DOI] [PubMed] [Google Scholar]
- 103. Rye P, Chin A, Pasieka J, So B, Harvey A, Kline G. Unadjusted plasma renin activity as a “first-look” test to decide upon further investigations for primary aldosteronism. J Clin Hypertens (Greenwich). 2015;17(7):541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Baudrand R, Guarda FJ, Torrey J, Williams G, Vaidya A. Dietary sodium restriction increases the risk of misinterpreting mild cases of primary aldosteronism. J Clin Endocrinol Metab. 2016;101(11):3989–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Stowasser M, Ahmed AH, Pimenta E, Taylor PJ, Gordon RD. Factors affecting the aldosterone/renin ratio. Horm Metab Res. 2012;44(3):170–176. [DOI] [PubMed] [Google Scholar]
- 106. Mosso L, Carvajal C, González A, Barraza A, Avila F, Montero J, Huete A, Gederlini A, Fardella CE. Primary aldosteronism and hypertensive disease. Hypertension. 2003;42(2):161–165. [DOI] [PubMed] [Google Scholar]
- 107. Vaidya A, Malchoff CD, Auchus RJ; AACE Adrenal Scientific Committee . An individualized approach to the evaluation and management of primary aldosteronism. Endocr Pract. 2017;23(6):680–689. [DOI] [PubMed] [Google Scholar]
- 108. Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Mannelli M, Mattarello MJ, Moretti A, Palumbo G, Parenti G, Porteri E, Semplicini A, Rizzoni D, Rossi E, Boscaro M, Pessina AC, Mantero F; PAPY Study Investigators . A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48(11):2293–2300. [DOI] [PubMed] [Google Scholar]
- 109. Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res. 2004;27(3):193–202. [DOI] [PubMed] [Google Scholar]
- 110. Williams JS, Williams GH, Raji A, Jeunemaitre X, Brown NJ, Hopkins PN, Conlin PR. Prevalence of primary hyperaldosteronism in mild to moderate hypertension without hypokalaemia. J Hum Hypertens. 2006;20(2):129–136. [DOI] [PubMed] [Google Scholar]
- 111. Rossi E, Regolisti G, Negro A, Sani C, Davoli S, Perazzoli F. High prevalence of primary aldosteronism using postcaptopril plasma aldosterone to renin ratio as a screening test among Italian hypertensives. Am J Hypertens. 2002;15(10 Pt 1):896–902. [DOI] [PubMed] [Google Scholar]
- 112. Štrauch B, Zelinka T, Hampf M, Bernhardt R, Widimsky J Jr. Prevalence of primary hyperaldosteronism in moderate to severe hypertension in the Central Europe region. J Hum Hypertens. 2003;17(5):349–352. [DOI] [PubMed] [Google Scholar]
- 113. Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40(6):892–896. [DOI] [PubMed] [Google Scholar]
- 114. Goodfriend TL, Calhoun DA. Resistant hypertension, obesity, sleep apnea, and aldosterone: theory and therapy. Hypertension. 2004;43(3):518–524. [DOI] [PubMed] [Google Scholar]
- 115. Calhoun DA, Nishizaka MK, Zaman MA, Harding SM. Aldosterone excretion among subjects with resistant hypertension and symptoms of sleep apnea. Chest. 2004;125(1):112–117. [DOI] [PubMed] [Google Scholar]
- 116. Valaiyapathi B, Calhoun DA. Role of mineralocorticoid receptors in obstructive sleep apnea and metabolic syndrome. Curr Hypertens Rep. 2018;20(3):23. [DOI] [PubMed] [Google Scholar]
- 117. Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ; British Hypertension Society’s PATHWAY Studies Group . Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386(10008):2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Williams B, MacDonald TM, Morant SV, Webb DJ, Sever P, McInnes GT, Ford I, Cruickshank JK, Caulfield MJ, Padmanabhan S, Mackenzie IS, Salsbury J, Brown MJ; British Hypertension Society programme of Prevention and Treatment of Hypertension With Algorithm Based Therapy (PATHWAY) Study Group . Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY-2 mechanisms substudies. Lancet Diabetes Endocrinol. 2018;6(6):464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Baudrand R, Vaidya A. The low-renin hypertension phenotype: genetics and the role of the mineralocorticoid receptor. Int J Mol Sci. 2018;19(2):E546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Nagase M. Activation of the aldosterone/mineralocorticoid receptor system in chronic kidney disease and metabolic syndrome. Clin Exp Nephrol. 2010;14(4):303–314. [DOI] [PubMed] [Google Scholar]
- 121. Nagase M, Fujita T. Role of Rac1–mineralocorticoid-receptor signalling in renal and cardiac disease. Nat Rev Nephrol. 2013;9(2):86–98. [DOI] [PubMed] [Google Scholar]
- 122. Cheville RA, Luetscher JA, Hancock EW, Dowdy AJ, Nokes GW. Distribution, conjugation, and excretion of labeled aldosterone in congestive heart failure and in controls with normal circulation: development and testing of a model with an analog computer. J Clin Invest. 1966;45(8):1302–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Markou A, Pappa T, Kaltsas G, Gouli A, Mitsakis K, Tsounas P, Prevoli A, Tsiavos V, Papanastasiou L, Zografos G, Chrousos GP, Piaditis GP. Evidence of primary aldosteronism in a predominantly female cohort of normotensive individuals: a very high odds ratio for progression into arterial hypertension. J Clin Endocrinol Metab. 2013;98(4):1409–1416. [DOI] [PubMed] [Google Scholar]
- 124. Baudrand R, Guarda FJ, Fardella C, Hundemer G, Brown J, Williams G, Vaidya A. Continuum of renin-independent aldosteronism in normotension. Hypertension. 2017;69(5):950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Mulatero P, Monticone S, Bertello C, Mengozzi G, Tizzani D, Iannaccone A, Veglio F. Confirmatory tests in the diagnosis of primary aldosteronism. Horm Metab Res. 2010;42(6):406–410. [DOI] [PubMed] [Google Scholar]
- 126. Mulatero P, Milan A, Fallo F, Regolisti G, Pizzolo F, Fardella C, Mosso L, Marafetti L, Veglio F, Maccario M. Comparison of confirmatory tests for the diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 2006;91(7):2618–2623. [DOI] [PubMed] [Google Scholar]
- 127. Monticone S, Losano I, Tetti M, Buffolo F, Veglio F, Mulatero P. Diagnostic approach to low-renin hypertension. Clin Endocrinol (Oxf). 2018. [DOI] [PubMed]
- 128. Carey RM, Douglas JG, Schweikert JR, Liddle GW. The syndrome of essential hypertension and suppressed plasma renin activity. Normalization of blood pressure with spironolactone. Arch Intern Med. 1972;130(6):849–854. [PubMed] [Google Scholar]
- 129. Adlin EV. Letter: plasma-renin and blood-pressure. Lancet. 1975;1(7908):699. [DOI] [PubMed] [Google Scholar]
- 130. Vaidya A, Underwood PC, Hopkins PN, Jeunemaitre X, Ferri C, Williams GH, Adler GK. Abnormal aldosterone physiology and cardiometabolic risk factors. Hypertension. 2013;61(4):886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Brown JM, Underwood PC, Ferri C, Hopkins PN, Williams GH, Adler GK, Vaidya A. Aldosterone dysregulation with aging predicts renal vascular function and cardiovascular risk. Hypertension. 2014;63(6):1205–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bentley-Lewis R, Adler GK, Perlstein T, Seely EW, Hopkins PN, Williams GH, Garg R. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab. 2007;92(11):4472–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Nanba K, Vaidya A, Williams GH, Zheng I, Else T, Rainey WE. Age-related autonomous aldosteronism. Circulation. 2017;136(4):347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Vasan RS, Evans JC, Larson MG, Wilson PWF, Meigs JB, Rifai N, Benjamin EJ, Levy D. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351(1):33–41. [DOI] [PubMed] [Google Scholar]
- 135. Newton-Cheh C, Guo CY, Gona P, Larson MG, Benjamin EJ, Wang TJ, Kathiresan S, O’Donnell CJ, Musone SL, Camargo AL, Drake JA, Levy D, Hirschhorn JN, Vasan RS. Clinical and genetic correlates of aldosterone-to-renin ratio and relations to blood pressure in a community sample. Hypertension. 2007;49(4):846–856. [DOI] [PubMed] [Google Scholar]
- 136. Young JWF, Calhoun DA, Lenders JWM, Stowasser M, Textor SC. Screening for endocrine hypertension: an Endocrine Society scientific statement. Endocr Rev. 2017;38(2):103–122. [Google Scholar]
- 137. Bloch MJ. Worldwide prevalence of hypertension exceeds 1.3 billion. J Am Soc Hypertens. 2016;10(10):753–754. [DOI] [PubMed] [Google Scholar]
- 138. Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134(6):441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Maiolino G, Calò LA, Rossi GP. The time has come for systematic screening for primary aldosteronism in all hypertensives. J Am Coll Cardiol. 2017;69(14):1821–1823. [DOI] [PubMed] [Google Scholar]
- 140. Kowarski A, de Lacerda L, Migeon CJ. Integrated concentration of plasma aldosterone in normal subjects: correlation with cortisol. J Clin Endocrinol Metab. 1975;40(2):205–210. [DOI] [PubMed] [Google Scholar]
- 141. Cornu E, Steichen O, Nogueira-Silva L, Küpers E, Pagny JY, Grataloup C, Baron S, Zinzindohoue F, Plouin PF, Amar L. Suppression of aldosterone secretion after recumbent saline infusion does not exclude lateralized primary aldosteronism. Hypertension. 2016;68(4):989–994. [DOI] [PubMed] [Google Scholar]
- 142. Qi Y, Wang X, Rose KL, MacDonald WH, Zhang B, Schey KL, Luther JM. Activation of the endogenous renin-angiotensin-aldosterone system or aldosterone administration increases urinary exosomal sodium channel excretion. J Am Soc Nephrol. 2016;27(2):646–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Wolley MJ, Wu A, Xu S, Gordon RD, Fenton RA, Stowasser M. In primary aldosteronism, mineralocorticoids influence exosomal sodium-chloride cotransporter abundance. J Am Soc Nephrol. 2016;28(1):56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Pizzolo F, Chiecchi L, Morandini F, Castagna A, Zorzi F, Zaltron C, Pattini P, Chiariello C, Salvagno G, Olivieri O. Increased urinary excretion of the epithelial Na channel activator prostasin in patients with primary aldosteronism. J Hypertens. 2017;35(2):355–361. [DOI] [PubMed] [Google Scholar]
- 145. Arlt W, Lang K, Sitch AJ, Dietz AS, Rhayem Y, Bancos I, Feuchtinger A, Chortis V, Gilligan LC, Ludwig P, Riester A, Asbach E, Hughes BA, O’Neil DM, Bidlingmaier M, Tomlinson JW, Hassan-Smith ZK, Rees DA, Adolf C, Hahner S, Quinkler M, Dekkers T, Deinum J, Biehl M, Keevil BG, Shackleton CHL, Deeks JJ, Walch AK, Beuschlein F, Reincke M. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight. 2017;2(8):e93136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Williams TA, Peitzsch M, Dietz AS, Dekkers T, Bidlingmaier M, Riester A, Treitl M, Rhayem Y, Beuschlein F, Lenders JW, Deinum J, Eisenhofer G, Reincke M. Genotype-specific steroid profiles associated with aldosterone-producing adenomas. Hypertension. 2016;67(1):139–145. [DOI] [PubMed] [Google Scholar]
- 147. Mulatero P, Tizzani D, Viola A, Bertello C, Monticone S, Mengozzi G, Schiavone D, Williams TA, Einaudi S, La Grotta A, Rabbia F, Veglio F. Prevalence and characteristics of familial hyperaldosteronism: the PATOGEN study (primary aldosteronism in torino-genetic forms). Hypertension. 2011;58(5):797–803. [DOI] [PubMed] [Google Scholar]
- 148. Lifton RP, Dluhy RG, Powers M, Rich GM, Cook S, Ulick S, Lalouel JM. A chimaeric 11β-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992;355(6357):262–265. [DOI] [PubMed] [Google Scholar]
- 149. Carvajal CA, Campino C, Martinez-Aguayo A, Tichauer JE, Bancalari R, Valdivia C, Trejo P, Aglony M, Baudrand R, Lagos CF, Mellado C, Garcia H, Fardella CE. A new presentation of the chimeric CYP11B1/CYP11B2 gene with low prevalence of primary aldosteronism and atypical gene segregation pattern. Hypertension. 2012;59(1):85–91. [DOI] [PubMed] [Google Scholar]
- 150. Lafferty AR, Torpy DJ, Stowasser M, Taymans SE, Lin JP, Huggard P, Gordon RD, Stratakis CA. A novel genetic locus for low renin hypertension: familial hyperaldosteronism type II maps to chromosome 7 (7p22). J Med Genet. 2000;37(11):831–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Stowasser M, Gordon RD. Primary aldosteronism: learning from the study of familial varieties. J Hypertens. 2000;18(9):1165–1176. [DOI] [PubMed] [Google Scholar]
- 152. Geller DS, Zhang J, Wisgerhof MV, Shackleton C, Kashgarian M, Lifton RP. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab. 2008;93(8):3117–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Choi M, Scholl UI, Yue P, Björklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Åkerström G, Wang W, Carling T, Lifton RPK. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331(6018):768–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Hattangady NG, Olala LO, Bollag WB, Rainey WE. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol. 2012;350(2):151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Oki K, Plonczynski MW, Luis Lam M, Gomez-Sanchez EP, Gomez-Sanchez CE. Potassium channel mutant KCNJ5 T158A expression in HAC-15 cells increases aldosterone synthesis. Endocrinology. 2012;153(4):1774–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Scholl UI, Stölting G, Nelson-Williams C, Vichot AA, Choi M, Loring E, Prasad ML, Goh G, Carling T, Juhlin CC, Quack I, Rump LC, Thiel A, Lande M, Frazier BG, Rasoulpour M, Bowlin DL, Sethna CB, Trachtman H, Fahlke C, Lifton RP. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. eLife. 2015;4:e06315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Korah HE, Scholl UI. An update on familial hyperaldosteronism. Horm Metab Res. 2015;47(13):941–946. [DOI] [PubMed] [Google Scholar]
- 158. Daniil G, Fernandes-Rosa FL, Chemin J, Blesneac I, Beltrand J, Polak M, Jeunemaitre X, Boulkroun S, Amar L, Strom TM, Lory P, Zennaro MC. CACNA1H mutations are associated with different forms of primary aldosteronism. EBioMedicine. 2016;13:225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Eckle VS, Shcheglovitov A, Vitko I, Dey D, Yap CC, Winckler B, Perez-Reyes E. Mechanisms by which a CACNA1H mutation in epilepsy patients increases seizure susceptibility. J Physiol. 2014;592(4):795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Splawski I, Yoo DS, Stotz SC, Cherry A, Clapham DE, Keating MT. CACNA1H mutations in autism spectrum disorders. J Biol Chem. 2006;281(31):22085–22091. [DOI] [PubMed] [Google Scholar]
- 161. Scholl UI, Goh G, Stölting G, de Oliveira RC, Choi M, Overton JD, Fonseca AL, Korah R, Starker LF, Kunstman JW, Prasad ML, Hartung EA, Mauras N, Benson MR, Brady T, Shapiro JR, Loring E, Nelson-Williams C, Libutti SK, Mane S, Hellman P, Westin G, Åkerström G, Björklund P, Carling T, Fahlke C, Hidalgo P, Lifton RP. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013;45(9):1050–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Pinggera A, Mackenroth L, Rump A, Schallner J, Beleggia F, Wollnik B, Striessnig J. New gain-of-function mutation shows CACNA1D as recurrently mutated gene in autism spectrum disorders and epilepsy. Hum Mol Genet. 2017;26(15):2923–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Pinggera A, Lieb A, Benedetti B, Lampert M, Monteleone S, Liedl KR, Tuluc P, Striessnig J. CACNA1D de novo mutations in autism spectrum disorders activate Cav1.3 L-type calcium channels. Biol Psychiatry. 2015;77(9):816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Fernandes-Rosa FL, Daniil G, Orozco IJ, Göppner C, El Zein R, Jain V, Boulkroun S, Jeunemaitre X, Amar L, Lefebvre H, Schwarzmayr T, Strom TM, Jentsch TJ, Zennaro MC. A gain-of-function mutation in the CLCN2 chloride channel gene causes primary aldosteronism. Nat Genet. 2018;50(3):355–361. [DOI] [PubMed] [Google Scholar]
- 165. Scholl UI, Stölting G, Schewe J, Thiel A, Tan H, Nelson-Williams C, Vichot AA, Jin SC, Loring E, Untiet V, Yoo T, Choi J, Xu S, Wu A, Kirchner M, Mertins P, Rump LC, Onder AM, Gamble C, McKenney D, Lash RW, Jones DP, Chune G, Gagliardi P, Choi M, Gordon R, Stowasser M, Fahlke C, Lifton RP. CLCN2 chloride channel mutations in familial hyperaldosteronism type II. Nat Genet. 2018;50(3):349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Nanba K, Omata K, Else T, Beck PCC, Nanba AT, Turcu AF, Miller BS, Giordano TJ, Tomlins SA, Rainey WE. Targeted molecular characterization of aldosterone-producing adenomas in white americans. J Clin Endocrinol Metab. 2018;103(10):3869–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Lenzini L, Rossitto G, Maiolino G, Letizia C, Funder JW, Rossi GP. A meta-analysis of somatic KCNJ5 K+ channel mutations in 1636 patients with an aldosterone-producing adenoma. J Clin Endocrinol Metab. 2015;100(8):E1089–E1095. [DOI] [PubMed] [Google Scholar]
- 168. Åkerström T, Crona J, Delgado Verdugo A, Starker LF, Cupisti K, Willenberg HS, Knoefel WT, Saeger W, Feller A, Ip J, Soon P, Anlauf M, Alesina PF, Schmid KW, Decaussin M, Levillain P, Wängberg B, Peix JL, Robinson B, Zedenius J, Bäckdahl M, Caramuta S, Iwen KA, Botling J, Stålberg P, Kraimps JL, Dralle H, Hellman P, Sidhu S, Westin G, Lehnert H, Walz MK, Åkerström G, Carling T, Choi M, Lifton RP, Björklund P. Comprehensive re-sequencing of adrenal aldosterone producing lesions reveal three somatic mutations near the KCNJ5 potassium channel selectivity filter. PLoS One. 2012;7(7):e41926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Kitamoto T, Suematsu S, Yamazaki Y, Nakamura Y, Sasano H, Matsuzawa Y, Saito J, Omura M, Nishikawa T. Clinical and steroidogenic characteristics of aldosterone-producing adenomas with ATPase or CACNA1D gene mutations. J Clin Endocrinol Metab. 2016;101(2):494–503. [DOI] [PubMed] [Google Scholar]
- 170. Wu VC, Huang KH, Peng KY, Tsai YC, Wu CH, Wang SM, Yang SY, Lin LY, Chang CC, Lin YH, Lin SL, Chu TS, Wu KD. Prevalence and clinical correlates of somatic mutation in aldosterone producing adenoma-Taiwanese population. Sci Rep. 2015;5(1):11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Wang B, Li X, Zhang X, Ma X, Chen L, Zhang Y, Lyu X, Tang Y, Huang Q, Gao Y, Fan Y, Ouyang J. Prevalence and characterization of somatic mutations in Chinese aldosterone-producing adenoma patients. Medicine (Baltimore). 2015;94(16):e708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Zheng FF, Zhu LM, Nie AF, Li XY, Lin JR, Zhang K, Chen J, Zhou WL, Shen ZJ, Zhu YC, Wang JG, Zhu DL, Gao PJ. Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension. 2015;65(3):622–628. [DOI] [PubMed] [Google Scholar]
- 173. Kitamoto T, Suematsu S, Matsuzawa Y, Saito J, Omura M, Nishikawa T. Comparison of cardiovascular complications in patients with and without KCNJ5 gene mutations harboring aldosterone-producing adenomas. J Atheroscler Thromb. 2015;22(2):191–200. [DOI] [PubMed] [Google Scholar]
- 174. Taguchi R, Yamada M, Nakajima Y, Satoh T, Hashimoto K, Shibusawa N, Ozawa A, Okada S, Rokutanda N, Takata D, Koibuchi Y, Horiguchi J, Oyama T, Takeyoshi I, Mori M. Expression and mutations of KCNJ5 mRNA in Japanese patients with aldosterone-producing adenomas. J Clin Endocrinol Metab. 2012;97(4):1311–1319. [DOI] [PubMed] [Google Scholar]
- 175. Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, Strom TM, Monticone S, Amar L, Meatchi T, Mantero F, Cicala MV, Quinkler M, Fallo F, Allolio B, Bernini G, Maccario M, Giacchetti G, Jeunemaitre X, Mulatero P, Reincke M, Zennaro MC. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. 2014;64(2):354–361. [DOI] [PubMed] [Google Scholar]
- 176. Baron S, Amar L, Faucon AL, Blanchard A, Baffalie L, Faucard C, Travers S, Pagny JY, Azizi M, Houillier P. Criteria for diagnosing primary aldosteronism on the basis of liquid chromatography–tandem mass spectrometry determinations of plasma aldosterone concentration. J Hypertens. 2018;36(7):1592–1601. [DOI] [PubMed] [Google Scholar]
- 177. Scholl UI, Abriola L, Zhang C, Reimer EN, Plummer M, Kazmierczak BI, Zhang J, Hoyer D, Merkel JS, Wang W, Lifton RP. Macrolides selectively inhibit mutant KCNJ5 potassium channels that cause aldosterone-producing adenoma. J Clin Invest. 2017;127(7):2739–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Rossi GP, Cesari M, Letizia C, Seccia TM, Cicala MV, Zinnamosca L, Kuppusamy M, Mareso S, Sciomer S, Iacobone M, Mantero F, Pessina AC. KCNJ5 gene somatic mutations affect cardiac remodelling but do not preclude cure of high blood pressure and regression of left ventricular hypertrophy in primary aldosteronism. J Hypertens. 2014;32(7):1514–1522. [DOI] [PubMed] [Google Scholar]
- 179. Ip JC, Pang TC, Pon CK, Zhao JT, Sywak MS, Gill AJ, Soon PS, Sidhu SB. Mutations in KCNJ5 determines presentation and likelihood of cure in primary hyperaldosteronism. ANZ J Surg. 2015;85(4):279–283. [DOI] [PubMed] [Google Scholar]
- 180. Arnesen T, Glomnes N, Strømsøy S, Knappskog S, Heie A, Akslen LA, Grytaas M, Varhaug JE, Gimm O, Brauckhoff M. Outcome after surgery for primary hyperaldosteronism may depend on KCNJ5 tumor mutation status: a population-based study from Western Norway. Langenbecks Arch Surg. 2013;398(6):869–874. [DOI] [PubMed] [Google Scholar]
- 181. Xie CB, Shaikh LH, Garg S, Tanriver G, Teo AE, Zhou J, Maniero C, Zhao W, Kang S, Silverman RB, Azizan EA, Brown MJ. Regulation of aldosterone secretion by Cav1.3. Sci Rep. 2016;6(1):24697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Kang S, Cooper G, Dunne SF, Dusel B, Luan CH, Surmeier DJ, Silverman RB. CaV1.3-selective L-type calcium channel antagonists as potential new therapeutics for Parkinson’s disease. Nat Commun. 2012;3(1):1146. [DOI] [PubMed] [Google Scholar]
- 183. Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, Walther A, Tauber P, Schwarzmayr T, Diener S, Graf E, Allolio B, Samson-Couterie B, Benecke A, Quinkler M, Fallo F, Plouin PF, Mantero F, Meitinger T, Mulatero P, Jeunemaitre X, Warth R, Vilsen B, Zennaro MC, Strom TM, Reincke M. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45(4):440–444, 444e1-2. [DOI] [PubMed] [Google Scholar]
- 184. Stindl J, Tauber P, Sterner C, Tegtmeier I, Warth R, Bandulik S. Pathogenesis of adrenal aldosterone-producing adenomas carrying mutations of the Na+/K+-ATPase. Endocrinology. 2015;156(12):4582–4591. [DOI] [PubMed] [Google Scholar]
- 185. Williams TA, Monticone S, Schack VR, Stindl J, Burrello J, Buffolo F, Annaratone L, Castellano I, Beuschlein F, Reincke M, Lucatello B, Ronconi V, Fallo F, Bernini G, Maccario M, Giacchetti G, Veglio F, Warth R, Vilsen B, Mulatero P. Somatic ATP1A1, ATP2B3, and KCNJ5 mutations in aldosterone-producing adenomas. Hypertension. 2014;63(1):188–195. [DOI] [PubMed] [Google Scholar]
- 186. Azizan EA, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, Maniero C, Garg S, Bochukova EG, Zhao W, Shaikh LH, Brighton CA, Teo AE, Davenport AP, Dekkers T, Tops B, Küsters B, Ceral J, Yeo GS, Neogi SG, McFarlane I, Rosenfeld N, Marass F, Hadfield J, Margas W, Chaggar K, Solar M, Deinum J, Dolphin AC, Farooqi IS, Striessnig J, Nissen P, Brown MJ. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. 2013;45(9):1055–1060. [DOI] [PubMed] [Google Scholar]
- 187. Scholl UI, Nelson-Williams C, Yue P, Grekin R, Wyatt RJ, Dillon MJ, Couch R, Hammer LK, Harley FL, Farhi A, Wang WH, Lifton RP. Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. Proc Natl Acad Sci USA. 2012;109(7):2533–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Omata K, Satoh F, Morimoto R, Ito S, Yamazaki Y, Nakamura Y, Anand S, Guo Z, Stowasser M, Sasano H, Tomlins SA, Rainey W. Cellular and genetic causes of idiopathic hyperaldosteronism. Hypertension. 2018;72:874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Takeda Y, Demura M, Wang F, Karashima S, Yoneda T, Kometani M, Hashimoto A, Aono D, Horike SI, Meguro-Horike M, Yamagishi M, Takeda Y. Epigenetic regulation of aldosterone synthase gene by sodium and angiotensin II. J Am Heart Assoc. 2018;7(10):e008281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Kobuke K, Oki K, Gomez-Sanchez CE, Ohno H, Itcho K, Yoshii Y, Yoneda M, Hattori N. Purkinje cell protein 4 expression is associated with DNA methylation status in aldosterone-producing adenoma. J Clin Endocrinol Metab. 2018;103(3):965–971. [DOI] [PubMed] [Google Scholar]
- 191. Murakami M, Yoshimoto T, Nakabayashi K, Tsuchiya K, Minami I, Bouchi R, Izumiyama H, Fujii Y, Abe K, Tayama C, Hashimoto K, Suganami T, Hata K, Kihara K, Ogawa Y. Integration of transcriptome and methylome analysis of aldosterone-producing adenomas. Eur J Endocrinol. 2015;173(2):185–195. [DOI] [PubMed] [Google Scholar]
- 192. Howard B, Wang Y, Xekouki P, Faucz FR, Jain M, Zhang L, Meltzer PG, Stratakis CA, Kebebew E. Integrated analysis of genome-wide methylation and gene expression shows epigenetic regulation of CYP11B2 in aldosteronomas. J Clin Endocrinol Metab. 2014;99(3):E536–E543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey W, Satoh F, Maekawa T, Nakamura Y, Sasano H, Gomez-Sanchez EP. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014;383(1–2):111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Gioco F, Seccia TM, Gomez-Sanchez EP, Rossi GP, Gomez-Sanchez CE. Adrenal histopathology in primary aldosteronism: is it time for a change? Hypertension. 2015;66(4):724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Ono Y, Nakamura Y, Maekawa T, Felizola SJ, Morimoto R, Iwakura Y, Kudo M, Seiji K, Takase K, Arai Y, Gomez-Sanchez CE, Ito S, Sasano H, Satoh F. Different expression of 11β-hydroxylase and aldosterone synthase between aldosterone-producing microadenomas and macroadenomas. Hypertension. 2014;64(2):438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Nakamura Y, Maekawa T, Felizola SJ, Satoh F, Qi X, Velarde-Miranda C, Plonczynski MW, Ise K, Kikuchi K, Rainey WE, Gomez-Sanchez EP, Gomez-Sanchez CE, Sasano H. Adrenal CYP11B1/2 expression in primary aldosteronism: immunohistochemical analysis using novel monoclonal antibodies. Mol Cell Endocrinol. 2014;392(1–2):73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, Ogishima T, Suematsu M, Mukai K. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 2010;95(5):2296–2305. [DOI] [PubMed] [Google Scholar]
- 198. Boulkroun S, Samson-Couterie B, Dzib JF, Lefebvre H, Louiset E, Amar L, Plouin PF, Lalli E, Jeunemaitre X, Benecke A, Meatchi T, Zennaro MC. Adrenal cortex remodeling and functional zona glomerulosa hyperplasia in primary aldosteronism. Hypertension. 2010;56(5):885–892. [DOI] [PubMed] [Google Scholar]
- 199. Nishimoto K, Tomlins SA, Kuick R, Cani AK, Giordano TJ, Hovelson DH, Liu C-J, Sanjanwala AR, Edwards MA, Gomez-Sanchez CE, Nanba K, Rainey WE. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci USA. 2015;112(33):E4591–E4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Omata K, Anand SK, Hovelson DH, Liu CJ, Yamazaki Y, Nakamura Y, Ito S, Satoh F, Sasano H, Rainey WE, Tomlins SA. Aldosterone-producing cell clusters frequently harbor somatic mutations and accumulate with age in normal adrenals. J Endocr Soc. 2017;1(7):787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Omata K, Tomlins SA, Rainey WE. Aldosterone-producing cell clusters in normal and pathological states. Horm Metab Res. 2017;49(12):951–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Lalli E, Barhanin J, Zennaro MC, Warth R. Local control of aldosterone production and primary aldosteronism. Trends Endocrinol Metab. 2016;27(3):123–131. [DOI] [PubMed] [Google Scholar]
- 203. Gomez-Sanchez CE, Kuppusamy M, Reincke M, Williams TA. Disordered CYP11B2 expression in primary aldosteronism. Horm Metab Res. 2017;49(12):957–962. [DOI] [PubMed] [Google Scholar]
- 204. Åkerström T, Maharjan R, Sven Willenberg H, Cupisti K, Ip J, Moser A, Stålberg P, Robinson B, Alexander Iwen K, Dralle H, Walz MK, Lehnert H, Sidhu S, Gomez-Sanchez C, Hellman P, Björklund P. Activating mutations in CTNNB1 in aldosterone producing adenomas. Sci Rep. 2016;6(1):19546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Teo AE, Garg S, Shaikh LH, Zhou J, Karet Frankl FE, Gurnell M, Happerfield L, Marker A, Bienz M, Azizan EA, Brown MJ. Pregnancy, primary aldosteronism, and adrenal CTNNB1 mutations. N Engl J Med. 2015;373(15):1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, Horne MJ, Dietrich D, Riemer J, Kücükköylü S, Reimer EN, Reis AC, Goh G, Kristiansen G, Mahajan A, Korah R, Lifton RP, Prasad ML, Carling T. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin Endocrinol (Oxf). 2015;83(6):779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Bonnet S, Gaujoux S, Launay P, Baudry C, Chokri I, Ragazzon B, Libé R, René-Corail F, Audebourg A, Vacher-Lavenu MC, Groussin L, Bertagna X, Dousset B, Bertherat J, Tissier F. Wnt/β-catenin pathway activation in adrenocortical adenomas is frequently due to somatic CTNNB1-activating mutations, which are associated with larger and nonsecreting tumors: a study in cortisol-secreting and -nonsecreting tumors. J Clin Endocrinol Metab. 2011;96(2):E419–E426. [DOI] [PubMed] [Google Scholar]
- 208. Berthon A, Sahut-Barnola I, Lambert-Langlais S, de Joussineau C, Damon-Soubeyrand C, Louiset E, Taketo MM, Tissier F, Bertherat J, Lefrançois-Martinez AM, Martinez A, Val P. Constitutive β-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum Mol Genet. 2010;19(8):1561–1576. [DOI] [PubMed] [Google Scholar]
- 209. Tissier F, Cavard C, Groussin L, Perlemoine K, Fumey G, Hagneré AM, René-Corail F, Jullian E, Gicquel C, Bertagna X, Vacher-Lavenu MC, Perret C, Bertherat J. Mutations of β-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005;65(17):7622–7627. [DOI] [PubMed] [Google Scholar]
- 210. Heikkilä M, Peltoketo H, Leppäluoto J, Ilves M, Vuolteenaho O, Vainio S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002;143(11):4358–4365. [DOI] [PubMed] [Google Scholar]
- 211. Wu VC, Wang SM, Chueh SJ, Yang SY, Huang KH, Lin YH, Wang JJ, Connolly R, Hu YH, Gomez-Sanchez CE, Peng KY, Wu KD. The prevalence of CTNNB1 mutations in primary aldosteronism and consequences for clinical outcomes. Sci Rep. 2017;7(1):39121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Omata K, Yamazaki Y, Nakamura Y, Anand SK, Barletta JA, Sasano H, Rainey WE, Tomlins SA, Vaidya A. Genetic and histopathologic intertumor heterogeneity in primary aldosteronism. J Clin Endocrinol Metab. 2017;102(6):1792–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Nishimoto K, Seki T, Hayashi Y, Mikami S, Al-Eyd G, Nakagawa K, Morita S, Kosaka T, Oya M, Mitani F, Suematsu M, Kabe Y, Mukai K.. Human adrenocortical remodeling leading to aldosterone-producing cell cluster generation. Int J Endocrinol. 2016; 2016:7834356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Nanba K, Vaidya A, Rainey WE. Aging and adrenal aldosterone production. Hypertension. 2018;71(2):218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Mulkerrin E, Epstein FH, Clark BA. Aldosterone responses to hyperkalemia in healthy elderly humans. J Am Soc Nephrol. 1995;6(5):1459–1462. [DOI] [PubMed] [Google Scholar]
- 216. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13–e115. [DOI] [PubMed] [Google Scholar]
- 217. Bravo EL, Dustan HP, Tarazi RC. Spironolactone as a nonspecific treatment for primary aldosteronism. Circulation. 1973;48(3):491–498. [DOI] [PubMed] [Google Scholar]
- 218. Takakuwa H, Shimizu K, Izumiya Y, Kato T, Nakaya I, Yokoyama H, Kobayashi K, Ise T. Dietary sodium restriction restores nocturnal reduction of blood pressure in patients with primary aldosteronism. Hypertens Res. 2002;25(5):737–742. [DOI] [PubMed] [Google Scholar]
- 219. Kalogeropoulos AP, Georgiopoulou VV, Murphy RA, Newman AB, Bauer DC, Harris TB, Yang Z, Applegate WB, Kritchevsky SB. Dietary sodium content, mortality, and risk for cardiovascular events in older adults: the Health, Aging, and Body Composition (Health ABC) Study. JAMA Intern Med. 2015;175(3):410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Cogswell ME, Loria CM, Terry AL, Zhao L, Wang CY, Chen TC, Wright JD, Pfeiffer CM, Merritt R, Moy CS, Appel LJ. Estimated 24-hour urinary sodium and potassium excretion in US adults. JAMA. 2018;319(12):1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Rossi GP, Bolognesi M, Rizzoni D, Seccia TM, Piva A, Porteri E, Tiberio GA, Giulini SM, Agabiti-Rosei E, Pessina AC. Vascular remodeling and duration of hypertension predict outcome of adrenalectomy in primary aldosteronism patients. Hypertension. 2008;51(5):1366–1371. [DOI] [PubMed] [Google Scholar]
- 222. Catena C, Colussi G, Lapenna R, Nadalini E, Chiuch A, Gianfagna P, Sechi LA. Long-term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension. 2007;50(5):911–918. [DOI] [PubMed] [Google Scholar]
- 223. Marzano L, Colussi G, Sechi LA, Catena C. Adrenalectomy is comparable with medical treatment for reduction of left ventricular mass in primary aldosteronism: meta-analysis of long-term studies. Am J Hypertens. 2015;28(3):312–318. [DOI] [PubMed] [Google Scholar]
- 224. Kline GA, Pasieka JL, Harvey A, So B, Dias VC. Medical or surgical therapy for primary aldosteronism: post-treatment follow-up as a surrogate measure of comparative outcomes. Ann Surg Oncol. 2013;20(7):2274–2278. [DOI] [PubMed] [Google Scholar]
- 225. Fourkiotis V, Vonend O, Diederich S, Fischer E, Lang K, Endres S, Beuschlein F, Willenberg HS, Rump LC, Allolio B, Reincke M, Quinkler M; Mephisto Study Group . Effectiveness of eplerenone or spironolactone treatment in preserving renal function in primary aldosteronism. Eur J Endocrinol. 2012;168(1):75–81. [DOI] [PubMed] [Google Scholar]
- 226. Velema M, Dekkers T, Hermus A, Timmers H, Lenders J, Groenewoud H, Schultze Kool L, Langenhuijsen J, Prejbisz A, van der Wilt GJ, Deinum J; SPARTACUS investigators . Quality of life in primary aldosteronism: a comparative effectiveness study of adrenalectomy and medical treatment. J Clin Endocrinol Metab. 2018;103(1):16–24. [DOI] [PubMed] [Google Scholar]
- 227. Velema MS, de Nooijer AH, Burgers VWG, Hermus ARMM, Timmers HJLM, Lenders JWM, Husson O, Deinum J. Health-related quality of life and mental health in primary aldosteronism: a systematic review. Horm Metab Res. 2017;49(12):943–950. [DOI] [PubMed] [Google Scholar]
- 228. Sukor N, Kogovsek C, Gordon RD, Robson D, Stowasser M. Improved quality of life, blood pressure, and biochemical status following laparoscopic adrenalectomy for unilateral primary aldosteronism. J Clin Endocrinol Metab. 2010;95(3):1360–1364. [DOI] [PubMed] [Google Scholar]
- 229. Celen O, O’Brien MJ, Melby JC, Beazley RM. Factors influencing outcome of surgery for primary aldosteronism. Arch Surg. 1996;131(6):646–650. [DOI] [PubMed] [Google Scholar]
- 230. Meyer A, Brabant G, Behrend M. Long-term follow-up after adrenalectomy for primary aldosteronism. World J Surg. 2005;29(2):155–159. [DOI] [PubMed] [Google Scholar]
- 231. Harris DA, Au-Yong I, Basnyat PS, Sadler GP, Wheeler MH. Review of surgical management of aldosterone secreting tumours of the adrenal cortex. Eur J Surg Oncol. 2003;29(5):467–474. [DOI] [PubMed] [Google Scholar]
- 232. Blumenfeld JD, Sealey JE, Schlussel Y, Vaughan ED Jr, Sos TA, Atlas SA, Müller FB, Acevedo R, Ulick S, Laragh JH. Diagnosis and treatment of primary hyperaldosteronism. Ann Intern Med. 1994;121(11):877–885. [DOI] [PubMed] [Google Scholar]
- 233. Proye CA, Mulliez EA, Carnaille BM, Lecomte-Houcke M, Decoulx M, Wémeau JL, Lefebvre J, Racadot A, Ernst O, Huglo D, Carré A. Essential hypertension: first reason for persistent hypertension after unilateral adrenalectomy for primary aldosteronism? Surgery. 1998;124(6):1128–1133. [DOI] [PubMed] [Google Scholar]
- 234. Lo CY, Tam PC, Kung AW, Lam KS, Wong J. Primary aldosteronism. Results of surgical treatment. Ann Surg. 1996;224(2):125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Stowasser M, Klemm SA, Tunny TJ, Storie WJ, Rutherford JC, Gordon RD. Response to unilateral adrenalectomy for aldosterone-producing adenoma: effect of potassium levels and angiotensin responsiveness. Clin Exp Pharmacol Physiol. 1994;21(4):319–322. [DOI] [PubMed] [Google Scholar]
- 236. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol. 2018;3(8):768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Wu VC, Wang SM, Chang CH, Hu YH, Lin LY, Lin YH, Chueh SC, Chen L, Wu KD. Long term outcome of aldosteronism after target treatments. Sci Rep. 2016;6(1):32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Wu VC, Chueh SJ, Chen L, Chang CH, Hu YH, Lin YH, Wu KD, Yang WS; TAIPAI Study Group . Risk of new-onset diabetes mellitus in primary aldosteronism: a population study over 5 years. J Hypertens. 2017;35(8):1698–1708. [DOI] [PubMed] [Google Scholar]
- 239. Calhoun DA. Medical versus surgical treatment of primary aldosteronism. Hypertension. 2018;71(4):566–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension. 2018;72(3):658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Kramers BJ, Kramers C, Lenders JW, Deinum J. Effects of treating primary aldosteronism on renal function. J Clin Hypertens (Greenwich). 2017;19(3):290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Lim PO, Young WF, MacDonald TM. A review of the medical treatment of primary aldosteronism. J Hypertens. 2001;19(3):353–361. [DOI] [PubMed] [Google Scholar]
- 243. Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, Satoh F, Amar L, Quinkler M, Deinum J, Beuschlein F, Kitamoto KK, Pham U, Morimoto R, Umakoshi H, Prejbisz A, Kocjan T, Naruse M, Stowasser M, Nishikawa T, Young WF Jr, Gomez-Sanchez CE, Funder JW, Reincke M; Primary Aldosteronism Surgery Outcome (PASO) investigators . Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5(9):689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Williams TA, Burrello J, Sechi LA, Fardella CE, Matrozova J, Adolf C, Baudrand R, Bernardi S, Beuschlein F, Catena C, Doumas M, Fallo F, Giacchetti G, Heinrich DA, Saint-Hilary G, Jansen PM, Januszewicz A, Kocjan T, Nishikawa T, Quinkler M, Satoh F, Umakoshi H, Widimský J Jr, Hahner S, Douma S, Stowasser M, Mulatero P, Reincke M. Computed tomography and adrenal venous sampling in the diagnosis of unilateral primary aldosteronism. Hypertension. 2018;72(3):641–649. [DOI] [PubMed] [Google Scholar]
- 245. Auchus RJ, Chandler DW, Singeetham S, Chokshi N, Nwariaku FE, Dolmatch BL, Holt SA, Wians FH Jr, Josephs SC, Trimmer CK, Lopera J, Vongpatanasin W, Nesbitt SD, Leonard D, Victor RG. Measurement of 18-hydroxycorticosterone during adrenal vein sampling for primary aldosteronism. J Clin Endocrinol Metab. 2007;92(7):2648–2651. [DOI] [PubMed] [Google Scholar]
- 246. Nakamura Y, Satoh F, Morimoto R, Kudo M, Takase K, Gomez-Sanchez CE, Honma S, Okuyama M, Yamashita K, Rainey WE, Sasano H, Ito S. 18-Oxocortisol measurement in adrenal vein sampling as a biomarker for subclassifying primary aldosteronism. J Clin Endocrinol Metab. 2011;96(8):E1272–E1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Satoh F, Morimoto R, Ono Y, Iwakura Y, Omata K, Kudo M, Takase K, Seiji K, Sasamoto H, Honma S, Okuyama M, Yamashita K, Gomez-Sanchez CE, Rainey WE, Arai Y, Sasano H, Nakamura Y, Ito S. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension. 2015;65(5):1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Satoh F, Morimoto R, Ono Y, Iwakura Y, Omata K, Kudo M, Tkase K, Seiji K, Gomez-Sanchez C, Rainey W, Nakamura Y, Sasano H, Ito S. 8d.02: Peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in primary aldosteronism patients. J Hypertens. 2015;33(Suppl 1):e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Bramlage P, Swift SL, Thoenes M, Minguet J, Ferrero C, Schmieder RE. Non-steroidal mineralocorticoid receptor antagonism for the treatment of cardiovascular and renal disease. Eur J Heart Fail. 2016;18(1):28–37. [DOI] [PubMed] [Google Scholar]