Abstract
Background
It is not only to evaluate the efficacy and safety of mace (Arils of Myristica fragrans Houtt) but also to compare pelvic floor muscle training vs. pelvic floor muscle training (PFMT) for improving symptoms and health-related quality of life (HRQoL) of woman with mixed urinary incontinence (MUI).
Methods
A prospective, single-blind randomized controlled study was conducted. Patients (n = 60) were randomly allocated (1:1) to receive either mace powder or placebo (1.5 g) orally twice daily along with pelvic floor muscle training in both groups for three consecutive months. The primary outcomes included symptom evaluation with Patient Global Impression Index of Improvement (PGI-I) and the Patient Global Impression Index of Severity (PGI-S) for MUI. For safety, clinical examination and biochemical parameters were assessed. Secondary outcomes included Short form of Urogenital Distress Inventory Questionnaire-6 (UDI-6) and quality of life assessment with questionnaire tools such as Short form of Incontinence Impact Questionnaire-7 (IIQ-7), Short form of the Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12) and ICIQ-SF. The data were statistically interpreted with 5% level of significance.
Results
After treatment (at third month), the patient reported cure for PGI-I and PGI-S was 46.66% and 90% for the mace group, whereas 0% and 16.66% for the control group, respectively (P < 0.001), statistically significant. No side effects were reported in the mace group. The mean difference noted in terms of scores, at the third month from baseline for UDI-6 (51.09 vs. 24.78), IIQ-7 (45.48 vs. 23.49), PISQ (11.33 vs. 5.40), and ICIQ-SF (8.10 vs. 2.43) scores were higher in the mace than that in the control group (P < 0.001).
Conclusion
Mace is effective and safe for the subjective improvement of mixed urinary incontinence symptoms and for the improvement of women's HRQoL than the placebo.
Clinical Trial Registry No.: CTRI/2017/04/008342
Keywords: Aril of Myristica fragrans Houtt (mace), Mixed urinary incontinence, Pelvic floor muscle training
1. Introduction
Urinary incontinence is not life threatening; however, it is associated with significant reduction in health-related quality of life (HRQoL).1 Mixed urinary incontinence (MUI) is the presence of both stress urinary incontinence (SUI) and urge urinary incontinence (UUI) symptoms.2 Therefore, mixed urinary incontinence has been defined symptomatically by the International Continence Society (ICS) as “the complaint of involuntary leakage associated with urgency and also with exertion, effort, sneezing or coughing”.3 MUI is an important disease with high prevalence in everyday clinical practice. It is not only under-reported and under-treated3 but it also significantly does impair the quality of life in women.4 MUI accounts for approximately 33% of all cases of incontinence in women.5 More recently authors of the previous study argued that “the laxity of the anterior vagina wall leads to activation of stretch receptors in the bladder neck and proximal urethra, resulting in dissipation of urethral closure pressure and inappropriate activation of micturition reflex”. This integral theory has been advocated to explain both SUI and MUI.3 MUI is the result of sphincter failure and over activity of the bladder detrusor muscle.6 The management of MUI combines many methods such as behavioral modification, pelvic floor muscle training (PFMT), pharmacotherapy,7 anti-incontinence devices, and biofeedback, and complementary therapies can be used for treating mixed symptoms.3 “A recent Cochrane review of pelvic floor muscle exercise found that these treatments were equally effectual for MUI and SUI compared to no treatment or placebo”.7, 8 Few studies of antimuscarinic drugs and serotonin/norepinephrine reuptake inhibitors had a positive effect on MUI.3 However, the incidences of side effects of conventional medicines are increasing; hence, worldwide people are turning toward complementary and alternative medicine.9 Many women seek alternatives to conventional medicine for the treatment of urinary incontinence.
In Unani medicine, mixed urinary incontinence comes under salas al-bawl. Concepts and practices of the Unani system of medicine are several thousand years old and till date are in use to treat salsas al-bawl (urinary incontinence). However, the Unani medicines should be investigated to better understand their properties, safety, and efficacy. Unani scholars surmised that Boswellia serrata L., Cyperus scariosus L., arils of Myristica fragrans Houtt (mace), Alpinia khulanjan rhizome, flower of Punica granatum L., Coriander sativum L, and Rosa damascene are useful for urinary incontinence with the concept that these plants have astringent property and tones up the muscles.10, 11 Hence, mace (Arils of M. fragrans Houtt) was selected as experiment drug. In Unani medicine, the vernacular name of mace is bisbasah/javetri.10 It has astringent property, and phytochemical constituent analysis showed that it has tannins.12 Pharmacologically, it has been proven for antidepressive and anticholinergic activities.13B. serrata and C. scariosus have been evaluated for stress urinary incontinence.14 However, till date, no randomized placebo-controlled studies have proven the efficacy of mace in improvement of symptoms of MUI. Hence, this study was conducted to evaluate the efficacy and safety of mace plus PFMT vs. PFMT in improving symptoms of mixed urinary incontinence (MUI) and improving woman's health-related quality of life.
2. Methods
2.1. Study design
A single center, parallel, single-blind, prospective, simple randomized placebo controlled study was conducted at the outpatient department of Amraze Niswan (Gynecology), National Institute of Unani Medicine Hospital, Bengaluru, India. The scientific review committee of the Institute and Institutional Ethical Committee (IEC No.: NIUM/IEC/2013-14/015/ANQ/07) approved this study. Both written and oral information about the reasons for this study were given to women and requested to participate. Written informed consent was obtained from all randomized patients. The Declaration of Helsinki and GCP guidelines issued by the Ministry of AYUSH, India was followed to conduct the study.
2.2. Participants
2.2.1. Participant's selection
Diagnosed women (n = 60; including 10% dropout) with mixed urinary incontinence for at least 2 months were recruited.
2.2.1.1. Inclusion criteria
Parous women aged ≥21 years with symptoms of MUI as evidenced by stress and urge symptoms reported on MESA (Medical, Epidemiologic and Social Aspects of Aging) questionnaire were included.8
2.2.1.2. Exclusion criteria
Women with known systemic and endocrine diseases such as uncontrolled hypertension, diabetes mellitus, bronchial asthma, known malignancies, pregnant, and lactating women were excluded.8 Further, incontinence of urine due to neurological defect, urinary tract infection, peripheral nerve disturbances, continuous leakage, post void residual urine volume >200 mL, medication for urinary incontinence, and advanced uterine prolapse (grade ≥3 by half way system) were also excluded.14
2.3. Procedure for data collection
At pre-study screening, MUI was diagnosed in a woman as evidenced by stress and urge symptoms reported on MESA questionnaire. All included participants provided written informed consent for this study. At the baseline visit, first researcher collected relevant socio-demographic data as well as clinical information and conducted general physical and gynecological examination with cough stress test. Patients were enquired for monthly income, education, and occupation as per Kuppuswamy's socioeconomic scale.14 Mental status assessment was performed. Pelvic examination included observation for any vaginal or cervical discharge, full bladder and supine empty bladder cough stress test, pelvic floor muscular strength (PFMS), vaginal wall, cervix, uterine size, and genital prolapse assessment. The pelvic floor muscle strength was rated as per previous study,15 and to assess the extent of genital prolapse, Baden-Walker Halfway scoring system was used. General diseases were excluded by routine investigations such as random blood sugar, hemogram erythrocyte sedimentation rate (ESR), and routine urine examination at baseline. For safety assessment, the tests such as random blood sugar, hemogram, ESR, serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), alkaline phosphatase, serum creatinine and blood urea were conducted at Baseline and at 3rd month (post-intervention). Thyroid profile, urine culture, and sensitivity and abdomino-pelvis ultrasonography test were conducted to exclude thyroid dysfunction, urinary tract infection, and pelvic pathology, respectively. Participants returned to the research institute once in every 15 days during the treatment period of 12 weeks, and one follow-up was performed after 4 weeks without treatment. At each visit, the first researcher recorded the improvement or worsening in their symptoms and side effects, change in PGI-I, PGI-S, UDI-6, IIQ-7, PISQ-12, and ICIQ-SF questionnaires. Side effects were reviewed by the researcher, who determined whether the event was study-related. The patients were withdrawn, who failed to follow the protocol and the cases in which side effects were observed.
2.4. Intervention
Mace group: Mace (Arils of M. fragrans Houtt) powder plus PFMT was advised.
Placebo group: Placebo plus PFMT was advised. Microcrystalline edible cellulose powder was given as placebo.
Mace is the arils of M. fragrans Houtt belongs to family Myristicaceae. It is native to the Moluccas Islands and grown in the Nilgris, Kerala, Karnataka and West Bengal. It has a stimulant, carminative, and narcotic in high doses. The hexane extract contains “myristicin, an anti-inflammatory principle, and licarin-B and dehydrodiisoeugenol exhibited CNS depressant properties. The anti-inflammatory activity observed in carrageenan-induced edema in rats and enhanced vascular permeability in mice is attributed to myristicin present in mace”.10 Assa et al., proved that the seed and mace extract of nutmeg contain quite high tannins, flavonoids, and terpenoids.12
2.4.1. Selection and identification
The experiment drug, mace, was selected from the Unani pharmacopeia of India.11 The pharmacy of National Institute of Unani Medicine (NIUM) supplied mace, and pharmacognonist identified it (Identification No. RRCBI-Mus.155). For further reference, the specimen of the mace was submitted in the Department of Pharmacology, NIUM with Voucher specimen no. 38/UQ/Res/2016.
2.4.2. Method of preparation, route of administration and dosage
Mace was cleaned, pounded, and powdered in hammer mill to make fine powder. Mesh no. 100 was used to sieve the powder. The powder was filled in capsules (500 mg) with manual capsule filling machine. Patients were advised to take three capsules orally, twice daily for twelve weeks. In placebo group, microcrystalline edible cellulose powder was filled in the capsules and administered in the same dose and duration as that of the mace. Fortnightly, 90 capsules (500 mg) were dispensed in an individual pack to each patient, and its compliance was checked after each course of the treatment.
2.4.3. Method of pelvic floor muscle training (PFMT)
Each participant of both groups was instructed for specific pelvic-floor exercises and met with the physiotherapist of this Institute for 30-minute training session at baseline, later at each visit of follow-up with the researcher for 12 consecutive weeks. Participants were advised to perform a set of progressive specific exercises along with Kegel's exercise daily at home for 12 weeks as per previous study.14
2.5. Outcomes
The primary outcomes were subjective improvement in symptoms of MUI measured with PGI-I instrument and PGI-S from baseline to third month and fourth month. In this study, the outcomes were dichotomously considered as ‘cured’ and ‘not cured’. Cured defined “as a participant report of incontinence as ‘much better’ or ‘very much better’, on PGI-I and a participant report of ‘normal’ or ‘mild’ urinary symptoms on PGI-S. Not cured was defined as participant report of incontinence as ‘a little better’, ‘no change’, ‘a little worse’, ‘much worse’ or ‘very much worse’ on PGI-I or patient report of ‘moderate’ or ‘severe’ urinary symptoms on PGI-S”.16 The safety assessment of the mace group included clinical examination and biochemical parameters (laboratory investigations) at baseline and at third month. The secondary outcomes included are the change in UDI-6, and impact on women's quality of life (QoL) assessed by short form of IIQ-7, PISQ-12, and ICIQ-SF questionnaires from baseline to third month and fourth month post-intervention.
2.5.1. Data collection tools
The data collection tools were MESA questionnaire, Questionnaire-Based Voiding Diary (QVD), Incontinence severity index (ISI),19 PGI-I, PGI-S, UDI-6, IIQ-7, PISQ-12, and ICIQ-SF. For the diagnosis of MUI at baseline condition-specific measures included the medical, epidemiologic, and social aspects of aging (MESA) scale, ISI, and QVD.
2.5.2. MESA questionnaire
This questionnaire was used only for the diagnosis of MUI in the present study. It is useful to record urinary incontinence severity and incontinence subtype (stress or urge or MUI). “The MESA is a self-reported questionnaire with nine questions on stress incontinence and six questions on urge incontinence. The four response categories ranged from “never” (0 points), “rarely” (1 point), “sometimes” (2 points) and “often” (3 points)”.17
2.5.3. PGI-I questionnaire
The PGI-I instrument is a global, patient-oriented outcome measure that assesses components of both SUI and UUI.8 The PGI-I response has been shown “to correlate significantly with the frequency of incontinence episodes, cough-test results, pad-test results, and scores on several Incontinence Quality of Life questionnaires”.18
2.5.4. PGI-S questionnaire
The “Patient Global Impression of Severity (PGI-S) index was used to assess changes in the perceived severity of incontinence on a 4-point Likert scale”.18
2.5.5. The Incontinence Impact (IIQ-7) and the Urogenital Distress Inventory Questionnaire (UDI-6)
In the present study, short form of IIQ-7 and UDI-6 was used. One of the studies reported that both “instruments showed a high internal consistency (Cronbach's alpha for the IIQ-7 and UDI-6 was 0.87 and 0.74, respectively) and test–retest reliability (Spearman's rho was 0.99 for both of the scales (P < 0.001)”.19 The average, which ranges from 0 to 3, is multiplied by 33 1/3 to put scores on a scale of 0 to 100. Utomo et al., show that the internal consistency was good in the IIQ-SF baseline scores; however, it was moderate for the UDI-6. The test–retest for both measures was good. “Construct was adequate with 75% confirmed hypotheses of urodynamic data with measure scores”.20
2.5.6. PISQ-12 Questionnaire
The PISQ-12 is a short-form of the PISQ-31 measure. It is a condition-specific measure that evaluates sexual function in heterosexual women who suffer from Urinary incontinence (UI) and/or pelvic organ prolapse (POP).
2.5.7. ICIQ-SF Questionnaire
The International Consultation on Incontinence Questionnaire-Short Form (ICIQ-SF) is a new subjective measure for evaluating the severity of urinary loss and condition-specific quality of life.14 Assessments of internal consistency and stability have demonstrated that the ICIQ-SF is highly reliable.21
2.6. Sample size estimation
On the basis of proportion value of decrease in urinary incontinence was 59% and 41% achieved from an previous study,22 a total of 53 participants (n1 = 26, n2 = 27) would be required to have 80% power with an alpha = 0.05 to detect improvement in UI. Hence, a total sample size of 60 patients was taken with 10% dropout rate.
2.7. Randomization, allocation and blinding
Patients who fulfilled the inclusion criteria and willing to participate after pre-study screening were randomly allocated in 1:1 ratio into the mace and control group. The intervention was started according to randomization. Randomization was performed by a computer generated random list (http://graphpad.com/quickcalcs/randomize2/) in single block with a single sequence of random assignment. An open list of random number was used; however, the sequence was concealed from the first researcher (data collector) until the interventions were assigned to each patient. Both mace and cellulose powder were filled in the same color capsules without any differentiation and with no detectable odor for masking and matching for participants blinding.
2.8. Statistical analysis
“The Statistical software Graph Pad Instat version 3.00 for window (Graph Pad Software, San Diego, Calif, USA) was used”.
2.8.1. Statistical analysis
Results on continuous measurements were presented on mean ± SD, and results on categorical measurements were presented in number (%). For all statistical tests, a two-sided P value was used and the type 1 error was set as 0.05 with 95% confidence interval. For comparison of the proportions, the chi-square test or Fisher's exact tests were utilized, when necessary. Pre and post scores were compared using paired Student's t-test and Wilcoxon matched paired test for normally distributed data and skewed data, respectively. Between-group comparisons of scores were performed using unpaired Student's t-test and Mann–Whitney U test for normally distributed data and skewed data, respectively. All efficacy variables were analyzed according to the intention-to-treat principle using data from all randomized subjects with at least two post-randomization outcome measures. Missing data were imputed using the last observation carried forward method. Change from baseline for primary efficacy parameters was calculated for the test and control groups at every month during treatment and at one month post-intervention. Changes from baseline for secondary efficacy parameters were calculated for the mace and control group at third month and fourth month.
3. Results
3.1. Participant flow
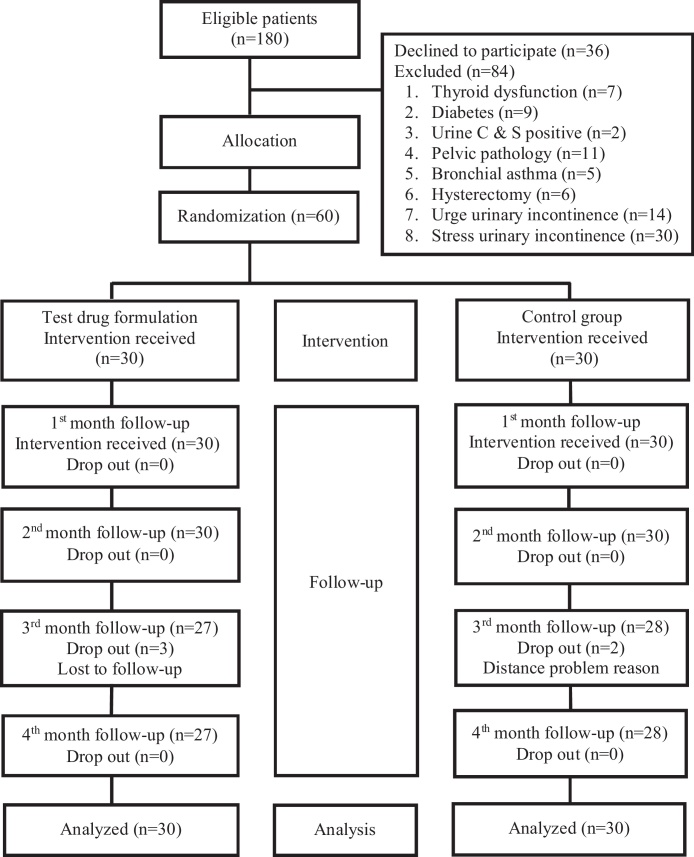
A total of 180 patients were screened for mixed urinary incontinence. Eighty four were excluded from the study and sixty patients were subjected to investigations and randomly allocated (Fig. 1).
Fig. 1.
Flow chart of participants as per CONSORT statement.
3.2. Recruitment
Patients were recruited from February 24, 2015. The last patient follow-up was completed on December 2, 2015.
3.3. Baseline characteristics
The patients of both groups were assessed for various baseline variables (age, religion, habitat, addiction frequency of voiding day and night, duration of incontinence, Incontinence Severity Index (ISI), MESA score, age of marriage, menstrual cycle, contraceptive history, and body mass index). The inter group comparison for the baseline variables showed no statistical difference, showing that groups were homogenous (Table 1).
Table 1.
Baseline characteristics
| Characteristics | Mace group (n = 30) | Control group (n = 30) | Total (n = 60) | P value |
|---|---|---|---|---|
| Age (y) | ||||
| Mean ± SD | 39.13 ± 8.34 | 42.53 ± 6.92 | 0.10a | |
| 21–30 | 5 (16.66) | 0 (0) | 5 (8.33) | |
| 31–40 | 14 (46.66) | 13 (43.33) | 27 (45) | |
| 41–50 | 8 (26.66) | 13 (43.33) | 21 (35) | |
| 51–60 | 3 (10) | 4 (13.33) | 7 (11.66) | |
| Religion | ||||
| Muslim | 25 (83.33) | 25 (83.33) | 50 (83.33) | 1.00d |
| Hindu | 5 (16.66) | 5 (16.66) | 10 (16.66) | |
| Habitat | ||||
| Urban | 30 (100) | 30 (100) | 60 (100) | 1.00c |
| Rural | 0 (0) | 0 (0) | 0 (0) | |
| Voiding and incontinence | ||||
| Frequency of voiding in day | 8.9 ± 1.90 | 9.13 ± 2.2 | 0.97a | |
| Frequency of voiding in night | 2.03 ± 0.66 | 1.9 ± 1.18 | 0.69a | |
| Duration of MUI (months) | 17.6 ± 17.8 | 18.4 ± 17.76 | 0.99a | |
| Incontinence Severity Index (ISI) | 11.06 ± 1.72 | 11.2 ± 1.627 | 0.37b | |
| MESA | ||||
| UUI | 15.43 ± 1.97 | 15.06 ± 2.31 | 0.25b | |
| SUI | 26.1 ± 1.60 | 25.7 ± 1.70 | 0.17b | |
| Age of marriage (y) | 16.9 ± 1.38 | 18.5 ± 3.43 | 0.06a | |
| Menstrual cycle | ||||
| Regular | 23 (76.66) | 17 (56.66) | 40 (66.66) | |
| Irregular | 2 (6.66) | 1 (3.33) | 3 (5) | 0.12c |
| Menopause | 5 (16.66) | 12 (40) | 17 (28.33) | |
| Contraceptive history | ||||
| Mechanical barrier | 4 (13.33) | 5 (13.33) | 9 (15) | 0.72c |
| Oral contraceptive pill | 1 (3.33) | 0 | 1 (1.66) | |
| Tubectomy | 24 (80) | 22 (73.33) | 46 (76.66) | |
| Others | 1 (3.33) | 3 (10) | 4 (6.66) | |
| Cough stress test | ||||
| Absent | 0 (0) | 0 (0) | 0 (0) | 1.00c |
| Present | 30 (100) | 30 (100) | 60 (100) | |
| BMI (kg/m2) | 26.59 ± 3.63 | 27.12 ± 2.57 | 0.25b | |
Data presented: mean ± SD or no. (%); P > 0.05, considered not significant; Test used: bunpaired Student's ‘t’ test and aMann–Whitney U test and cFisher's exact/dChi-square test. MUI, mixed urinary incontinence; MESA, Medical, Epidemiologic and Social Aspects of Aging; UUI, urge urinary incontinence; SUI, stress urinary incontinence; BMI, body mass index.
3.4. Primary outcome
The patients reported cured were 46.66% (n = 14/30) in the mace group, whereas none of the patients were cured in the control group at the third month, and the inter group comparison for PGI-I was statistically, extremely significant (P < 0.001). The patients reported cured were 90% (n = 27/30) and 16.66% (n = 5/30) in the mace and control groups, respectively, for PGI-S, and the between-groups comparison was statistically, extremely significant (P < 0.001) at third month (Table 2). The mean (SD) of PGI-I and PGI-S is summarized in Table 2.
Table 2.
Primary and secondary outcome parameters
| Primary and secondary outcomes | Baseline | Third month | Fourth month (FU with no Rx) |
|---|---|---|---|
| Patient Global Impression Index of Improvement (PGI-I) | |||
| Mace (n = 30) | 6.33 ± 0.49 | 2.76 ± 1.22b*** | 2.66 ± 1.26b*** |
| Control (n = 30) | 6.5 ± 0.62* | 5.4 ± 0.67b*** | 5.3 ± 0.6b*** |
| P value (Inter group) | >0.05a | <0.001a | <0.001a |
| Patient Global Impression of Severity (PGI-S) | |||
| Mace (n = 30) | 3.83 ± 0.37 | 2.33 ± 0.47b*** | 1.96 ± 0.49b*** |
| Control (n = 30) | 3.66 ± 0.54* | 2.93 ± 0.52b*** | 2.9 ± 0.48b*** |
| P value (Inter group) | >0.05a | <0.001a | <0.001a |
| Secondary outcomes | |||
| Urinary Distress Inventory Questionnaire (UDI-6) | |||
| Mace (n = 30) | 80.15 ± 10.26 | 29.06 ± 17.24b*** | 28.13 ± 17.74b*** |
| Control (n = 30) | 80.70 ± 12.07 | 55.18 ± 13.20b*** | 55.91 ± 13.20b*** |
| P value | 0.32a | <0.0001a | <0.0001a |
| Short form of Incontinence Impact Questionnaire-7 (IIQ-7) | |||
| Mace (n = 30) | 65.00 ± 9.75 | 19.51 ± 16.74b*** | 18.24 ± 16.96b*** |
| Control (n = 30) | 66.33 ± 13.28 | 42.84 ± 11.04b*** | 42.84 ± 11.04b*** |
| P value | 0.33a | <0.0001a | <0.0001a |
| Short form of the Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12) | |||
| Mace (n = 24) | 28.91 ± 4.09 | 40.25 ± 4.80c*** | 40.91 ± 5.19c*** |
| Control (n = 20) | 27.55 ± 1.87 | 32.95 ± 2.66c*** | 33 ± 2.61c*** |
| P value | 0.07d | <0.0001d | <0.0001d |
| The International Consultation on Incontinence Questionnaire-Short Form (ICIQ-SF) | |||
| Mace (n = 30) | 13.76 ± 1.83 | 5.66 ± 2.60b*** | 4.56 ± 2.71b*** |
| Control (n = 30) | 14.26 ± 1.72 | 11.83 ± 1.68b** | 11.8 ± 1.75b*** |
| P value | 0.16a | <0.0001a | <0.0001a |
Data presented: mean ± SD or no. (%); *P > 0.05, considered not significant; **P < 0.05 considered significant; ***P < 0.0001, extremely significant (baseline vs. third month) & (baseline vs. follow-up); FU with no Rx: follow-up with no treatment; Test used: aMann–Whitney U test/bWilcoxon Matched paired test,cPaired/dUnpaired Student's ‘t’ test.
At the third month, 5 (16.66%) and 9 (30%) patients described their improvement as “very much better” and “much better”, respectively, on the PGI-I score in the mace group (P < 0.001, extremely significant), whereas none of the patients showed improvement as “very much better” and “much better”, respectively, on the PGI-I score. At the third month, 4 (13.33%) and 23 (76.66%) patients described normal or no incontinence and mild incontinence, respectively, on the PGI-S score in the mace group (P < 0.001, extremely significant). Whereas in the control group at baseline 8 (26.66%) and 21 (70%) patients described their incontinence severity as moderate and severe, respectively, on the PGI-S score (Table 2).
3.5. Secondary outcome parameters
The mean ± SD of UDI-6, IIQ-7, PISQ-12, and ICIQ-SF scores, at the baseline, at the third month of treatment and one-month post-treatment follow-up is summarized in Table 2. At baseline, the between-groups comparison was statistically insignificant, whereas at third and at fourth, it was statistically significant for UDI, IIQ, PISQ, and ICIQ-SF questionnaire. The mean difference in UDI-6 (51.09 vs. 24.78), IIQ-7 (45.48 vs. 23.49), PISQ (11.33 vs. 5.40), and ICIQ-SF (8.10 vs. 2.43) scores at third month from baseline was higher in the mace group compared to the control group. The mean difference in UDI-6 (52.01 vs. 24.78), IIQ-7 (46.75 vs. 23.49), PISQ (12 vs. 5.45), and ICIQ-SF (9.2 vs. 2.46) scores fourth month from baseline was higher in the mace group compared to the control group.
3.6. Safety profile
All the biochemical parameters were comparable and statistically not significant when compared from baseline in both groups except hemoglobin (Hb%) and alkaline phosphatase in the mace and control groups which was statistically significant, but laboratory values were within normal range. Blood urea levels in the mace group and SGOT in the control group were statistically significant; however, laboratory values were within normal range (Table 3, see supplement).
4. Discussion
4.1. Summary of major finding
This study demonstrated that the use of mace was effective and safe alternative herbal treatment to improve symptoms and health-related quality of life in MUI patients. Jackson et al. conduct a systemic review on complementary and alternative therapies for urinary symptoms and reported a range of remedies (saw palmetto, flax seeds, ginseng, barley, cinnamon and moabi and other herbs, juices, and substances) used to treat urinary symptoms.23
4.2. Interpretation
4.2.1. Primary outcome
PGI-I, and PGI-S use: Patient-reported outcomes (PROs) (including symptoms, functional status, and perceived quality of life) are increasingly used alongside clinical measures to monitor the course of UI and its treatment outcomes as perceived and reported by patients, compliment clinical evidence, and judgment of efficacy and effectiveness.24 Hence, in this study patient-reported outcome, PGI-I and PGI-S were used to evaluate the efficacy of mace plus PFMT.
In the control group, PFMT was advised; hence, 16.66% patients reported cure. Recently, the multicenter BE-DRI trial performed by the UITN showed that behavioral therapy incorporating pelvic floor muscle training in addition to medicine was slightly better than medicine alone in improving urinary symptoms in patients with urge urinary incontinence. This is consistent with our study. William et al. adopted the use of pelvic floor therapies for mixed incontinence but did not detect improvements in UI despite improvements in pelvic floor function. Thus, the literature supports the concept that optimal nonsurgical management of urge symptoms should incorporate both medicine and pelvic floor muscle therapies.8
4.2.2. Safety assessment
In this study, no side effects were reported clinically, and biochemical parameters were within the normal limit in both groups. Hence, in the present study, the mace drug was safe clinically. Further, mace is proven for hepatoprotective,25 antioxidant,26 and immunomodulatory properties.27
4.2.3. Secondary outcome parameters
Previous study advocated for the inclusion of quality-of-life measures in clinical trials to complement clinical findings.17 At baseline, no relevant differences regarding women's QoL were found between the mace and control groups. The decrease in the total score of UDI-6, IIQ-7, and ICIQ-SF and increase in the total score PISQ-12 showed that women's urinary distress and QoL were improved after treatment in both groups. Improvement in women's QoL at the third and fourth months from baseline reached significance (P < 0.001) in both groups. However, the improvement was much better in the mace group than in the control group. The authors of the previous study concluded that “women with mixed incontinence were more likely to report a greater overall quality of life impact compared to those with stress incontinence”.28 Further, they suggested that women who have an MUI are at high risk for occurrence of functional limitations because of urinary symptoms regardless of co-morbid factors.28 A study showed that women with pelvic floor dysfunction had significantly lower PISQ-12 scores, similar finding was observed in the present study. Therefore, there needs a treatment for sexual dysfunction in addition to treatment options for PFD alone.29
Mace was useful in improving MUI symptoms, as it was able to tone up the muscles and reduced the laxity of the anterior vagina wall3 as it contain tannins.12 A study showed that Myristicin constitutes “4%–6% of nutmeg and mace essential oil and is responsible for most of its pharmacological effects”.25 A precise mechanism of action of mace is unknown; yet, it has been hypothesized probably the effect of mace is because of psychotropic, anti-inflammatory,25 and anti-depressive properties.30 Dhingra and Sharma (2006) in their study reported the extract of M. fragrans has antidepressant-like effect in mice and the potency was comparable to imipramine and fluoxetine. “The antidepressant-like effect of the extract seems to be mediated by interaction with the adrenergic, dopaminergic and serotonergic systems”. The previous study has proven that M. fragrans has a similar effect like imipramine. It is suggested that mace most likely affected on the CNS as imipramine, “thought to block the reuptake of serotonin and norepinephrine in Onuf's nucleus in the sacral spinal cord, thereby activating pudendal motor neurons that increase the urethral striated muscle tone and the force of sphincter contraction”.30 In summary for MUI patient, three months treatment with mace plus PFMT yielded a better outcome with regard to its effectiveness, safety, and improvement in women's QoL than Placebo plus PFMT.
4.3. Strength
This study is the first of its kind, randomized, single-blind, placebo-controlled design, clinically and biochemically safe, compliance of the participants was good with only 10% drop out and intent-to-treat analysis. This study also included the use of several widely used validated instruments for the evaluation of HRQoL (change in the short form of IIQ-7, PISQ-12, and ICIQ-SF). In the present study, patient reported outcomes were used as clinician's assessment.
4.4. Limitation
Some of the limitations were single-blind and follow-up-assessment took place only once post-intervention. Lack of infrastructure, facility, resources, and man power were the reason for not carrying the double-blind study.
4.5. Future recommendation
Future studies are recommended to find specific molecule responsible for the efficacy of mace in mixed urinary incontinence. Furthermore, studies are needed for the investigation of its effect on serotonergic pathways.
In conclusion, mace plus PFMT for three months was beneficial than PFMT alone in the symptomatic improvement of mixed urinary incontinence and for the improvement of woman's quality of life. Hence, it is suggested that mace represents a safe, effective, and easily available alternative treatment for mixed urinary incontinence. Further studies are recommended to evaluate the exact mechanism of action of mace.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgement
The authors are gratified to the Director and staff of the National Institute of Unani Medicine and Ministry of AYUSH, India for providing funds and their support in endeavoring this dissertation work. The authors are also grateful to the patients for their cooperation and contribution to complete this study.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.imr.2018.10.001.
Appendix I. Supplementary data
The following are the supplementary data to this article:
References
- 1.Gil K.M., Somerville A.M., Cichowski S., Savitski J.L. Distress and quality of life characteristics associated with seeking surgical treatment for stress urinary incontinence. Health Qual Life Outcomes. 2009;7:8. doi: 10.1186/1477-7525-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen S.H. Differential diagnosis of urinary incontinence. Tzu Chi Med J. 2007;19:53–59. [Google Scholar]
- 3.Porena M., Costantini E., Lazzeri M. Mixed incontinence: how best to manage it? Curr Bladder Dysfunct Rep. 2013;8:7–12. doi: 10.1007/s11884-012-0161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brubaker L., Lukacz E.S., Burgio k, Zimmern P.H., Norton P., Leng W. Mixed incontinence: comparing definitions in non-surgical patients. Neurourol Urodyn. 2011;30:45–51. doi: 10.1002/nau.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin C.C., Huang K.H., Wu M.P. Determining when to sling for mixed urinary incontinence? Incont Pelvic Floor Dysfunct. 2009;3:11–16. [Google Scholar]
- 6.Smith P.P., Mc Crery R.J., Appell R.A. Current trends in the evaluation and management of female urinary incontinence. CMAJ. 2006;175:1233–1240. doi: 10.1503/cmaj.060034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomelsky A., Dmochowski R.R. Treatment of mixed urinary incontinence. Cent Eur J Urol. 2011;64:120–126. doi: 10.5173/ceju.2011.03.art2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brubaker L., Moalli P., Richter H.E., AlbodM, Sirls L., Chaif T. Challenges in designing a pragmatic clinical trial: the mixed incontinence – medical or surgical approach (MIMOSA) trial experience. Clin Trials. 2009;6:355–364. doi: 10.1177/1740774509339239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sen S., Chakraborty R. Toward the integration and advancement of herbal medicine: a focus on traditional Indian medicine. Bot Targets Ther. 2015;5:33–44. [Google Scholar]
- 10.Khare C.P. Springer India (P) Ltd; New Delhi: 2007. Indian medicinal plants: an illustrated dictionary; pp. 428–429. [Google Scholar]
- 11.Ghani N. Idarae Kitab us Shifa; YNM; New Delhi: 2001. Khazainul Advia; p. 572. [Google Scholar]
- 12.Assa J.R., Widjanarko S.B., KusnadiJ, Berhimpon S. Antioxidant potential of flesh, seed, mace of nutmeg (Myristica fragrans Houtt) Int J Chem Tech Res. 2014;6:2460–2468. [Google Scholar]
- 13.Asgarpanah J., Kazemivash N. Phytochemistry and pharmacologic properties of Myristica fragrans Houtt.: a review. Afr J Biotechnol. 2012;11:12787–12793. [Google Scholar]
- 14.Padmaja A.R., Sultana A., Rahman K., Sumana N. Efficacy of Boswelliaserrata L. and Cyperusscariosus L. plus pelvic floor muscle training in stress incontinence in women of reproductive age. Compl Ther Clin Pract. 2014:230–236. doi: 10.1016/j.ctcp.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Samuelsson E.C., Victor F.T.A., Tibblin G., Kurt F. Syarddsudd. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J ObstetGynecol. 1999;180:299–305. doi: 10.1016/s0002-9378(99)70203-6. [DOI] [PubMed] [Google Scholar]
- 16.Abdel-fattah M., Mostafa A., Young D., Ramsay I. Evaluation of transobturator tension-free vaginal tapes in the management of women with mixed urinary incontinence: one-year outcomes. Am J ObstetGynecol. 2011;205 doi: 10.1016/j.ajog.2011.03.018. 150.e1–e6. [DOI] [PubMed] [Google Scholar]
- 17.Wren P.A., Janz N.K., Brubaker L., Fitzgerald M.P., Weber A.M., Laporte F.B. Reliability of health related quality of life measures 1 year after surgical procedures for pelvic floor disorders. Am J Obstet Gynecol. 2005;192:780–788. doi: 10.1016/j.ajog.2004.10.603. [DOI] [PubMed] [Google Scholar]
- 18.Labrie J., Berghmans B.L.C.M., Fischer K., Milani A.L., Wijk I., Smalbraak D.J.C. Surgery versus physiotherapy for stress urinary incontinence. N Engl J Med. 2013;369:1124–1133. doi: 10.1056/NEJMoa1210627. [DOI] [PubMed] [Google Scholar]
- 19.Cam C., Sakalli M., Ay P., Cam M., Karateke A. Validation of the short forms of the Incontinence Impact Questionnaire (IIQ-7) and the Urogenital Distress Inventory (UDI-6) in a Turkish population. Neurourol Urodyn. 2007;26:129–133. doi: 10.1002/nau.20292. [DOI] [PubMed] [Google Scholar]
- 20.Utomo E., Korfage I.J., Wildhagen M.F., Steensma A.B., Bangma C.H., Blok B.F. Validation of the Urogenital Distress Inventory (UDI-6) and Incontinence Impact Questionnaire (IIQ-7) in a Dutch population. Neurourol Urodyn. 2015;34:24–31. doi: 10.1002/nau.22496. [DOI] [PubMed] [Google Scholar]
- 21.Bayrak O., SeckinerI, Erturhan M.S., Erbagci A., Yagci F. The effect of biofeed back therapy on ICIQ-SF Scores and urodynamic parameters in patients with Stress Urinary Incontinence. Nephro Urol Mon. 2011;3:268–271. [Google Scholar]
- 22.Norton P.A., Zinner N.R., yalcin I., Bump R.C. Duloxetine Urinary Incontinence Study Group: duloxetine versus placebo in the treatment of stress urinaryincontinence. Am J Obstet Gynecol. 2002;187:40–48. doi: 10.1067/mob.2002.124840. [DOI] [PubMed] [Google Scholar]
- 23.Jackson C.B., Taubenberger S.P., Botelho E., Journel J., Tennstedt S.L. Complementary and alternative therapies for urinary symptoms: use in a diverse population sample qualitative study. Urol Nurs. 2012;32:149–157. [PMC free article] [PubMed] [Google Scholar]
- 24.Bushnell D.M., Martin M.L., Summers K.H., Svihra J., Lionis C., Donald L. Quality of life of women with urinary incontinence: cross-cultural performance of 15 language versions of the I-QOL. Qual Life Res. 2005;14:1901–1913. doi: 10.1007/s11136-005-5266-5. [DOI] [PubMed] [Google Scholar]
- 25.Latha P.G., Sindhu G., Suja S.R., Geetha B.S., Pushpangadan P., Rajasekharan S. Pharmacology and chemistry of Myristica fragrans Houtt. – a review. J Spices Arom Crops. 2005;14:94–101. [Google Scholar]
- 26.Yadav A.S., Bhatnagar D. Modulatory Effect of spice extracts on iron-induced lipid peroxidation in rat liver. Biofactors. 2007;29:147–157. doi: 10.1002/biof.552029205. [DOI] [PubMed] [Google Scholar]
- 27.Checker R., Chatterjee S., Sharma D., Gupta S., Variyar P., Sharma A. Immunomodulatory and radioprotective effects of lignans derived from fresh nutmeg mace (Myristica fragrans) in mammalian splenocytes. Int Immunopharmacol. 2008;8:661–669. doi: 10.1016/j.intimp.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Frick A.C., Huang A.J., Eeden S.K.V.D., Knight S.K., Creasman J.M., Yang J. Mixed urinary incontinence: greater impact on quality of life. J Urol. 2009;182:596–600. doi: 10.1016/j.juro.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caetano A. Incontinence: physical activity as a supporting preventive approach. Available from: URL: www.intechopen.com. Accessed 08.04.15.
- 30.Dhingra D., Sharma A. Antidepressant-like activity of n-hexane extract of Nutmeg (Myristica fragrans) seeds in mice. J Med Food. 2006;9:84–89. doi: 10.1089/jmf.2006.9.84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.