Abstract
Introduction
Cartilage regeneration is a promising therapy for restoring joint function in patients with cartilage defects. The limited availability of autologous chondrocytes or chondrogenic progenitor cells is an obstacle to its clinical application. We investigated the existence and chondrogenic potential of synovial membrane-derived multilineage-differentiating stress-enduring (Muse)-like cells as an alternative cell source for cartilage regeneration.
Methods
Cells positive for stage-specific embryonic antigen-3 (SSEA-3), a marker of Muse cells, were isolated from the synovial membranes of 6 of 8 patients (median age, 53.5 years; range 36–72 years) by fluorescence-activated cell sorting. SSEA-3-positive cells were cultured in methylcellulose to examine their ability to form Muse clusters that are similar to the embryoid bodies formed by human embryonic stem cells. Muse clusters were expanded and chondrogenic potential of M-cluster-derived MSCs examined using a pellet culture system. Chondrogenic differentiation was evaluated by proteoglycan, safranin O, toluidine blue and type II collagen staining. To evaluate the practicality of the procedure for isolating Muse-like cells, we compared chondrogenic potential of M-cluster derived MSCs with expanded cells derived from the clusters formed by unsorted synovial cells.
Results
Synovial membranes contained SSEA-3-positive cells that after isolation exhibited Muse-like characteristics such as forming clusters that expressed NANOG, OCT3/4, and SOX2. In the pellet culture system, cell pellets created from the M-cluster-derived MSCs exhibited an increase in wet weight, which implied an increase in extracellular matrix production, displayed metachromasia with toluidine blue and safranin O staining and were aggrecan-positive and type II collagen-positive by immunostaining. Unsorted synovial cells also formed clusters in methylcellulose culture, and the expanded cell population derived from them exhibited chondrogenic potential. The histological and immunohistochemical appearance of chondrogenic pellet created from unsorted synovial cell-derived cells were comparable with that from M-cluster-derived MSCs.
Conclusions
Muse-like cells can be isolated from the human synovial membrane, even from older patients, and therefore may provide a source of multipotent cells for regenerative medicine. In addition, the cluster-forming cell population within synovial cells also has excellent chondrogenic potential. These cells may provide a more practical option for cartilage regeneration.
Keywords: Cartilage, Regenerative medicine, Chondrogenic potential, Multilineage-differentiating stress-enduring cells, Stage-specific embryonic antigens-3
Abbreviations: APC, allophycocyanin; BSA, bovine serum albumin; DAB, 3,3′-diaminobenzidine; DAPI, 4′,6-diamidino-2-phenylindole; FBS, fetal bovine serum; FITC, fluorescein isothiocyanate; HRP, horseradish peroxidase; Ig, immunoglobulin; M-cluster, cluster cultured from synovial–Muse cells; MC, methylcellulose; αMEM, alpha-minimum essential medium; MSC, mesenchymal stem cell; Muse, multilineage-differentiating stress-enduring cells; PBS, phosphate-buffered saline; PE, phycoerythrin; SSEA-3, stage-specific embryonic antigen-3; SY-cluster, cluster cultured from unsorted synovial cells
Highlights
-
•
Muse-like cells can be obtained from synovial membrane, even from older patients.
-
•
Synovial Muse-cluster derived MSCs have chondrogenic potential.
-
•
Expanded cluster forming cells in synovial membrane also exhibit chondrogenic potential and can be more readily available cell source than Muse-cluster derived MSCs.
1. Introduction
Hyaline cartilage regeneration is the ultimate goal in the care of patients with a cartilage defect including osteoarthritis. Hyaline cartilage regeneration induced by chondrocyte sheet transplantation has been confirmed in animal models of cartilage defects [1], [2], [3], [4]. Based on these results, we have conducted a clinical study using autologous knee chondrocyte sheets in patients with cartilage defects in the knee joint, including osteoarthritis patients. Examination at the 1-year follow-up demonstrated hyaline cartilage regeneration at the transplanted site, and the outcomes of this clinical study will be reported elsewhere.
Generally, autologous chondrocytes obtained from patients are used in cartilage regenerative medicine. In such procedures, healthy cartilage tissue is sacrificed from healthy nonweight-bearing sites, which can be scarce in the patient's joint. This restraint makes a second treatment by regenerative therapy impossible. In addition, adult human chondrocytes have low proliferative capability, and they easily dedifferentiate and lose their chondrogenic characteristics during cultivation ex vivo. The limited availability of good-quality chondrocytes in a sufficient number is one of the major obstacles to the clinical implementation of cartilage regenerative medicine.
The ideal solution to this chondrogenic cell shortage is to identify easily accessible autologous cells that have both high proliferative capacity and chondrogenic potential. The existence of nontumorigenic endogenous pluripotent-like stem cells, or multilineage-differentiating stress-enduring (Muse) cells, in bone marrow, skin, and adipose tissue has been reported recently [5], [6], [7], [8]. Muse cells were originally identified as cells that are resistant to long-term trypsin incubation and are known as cells that are double positive for the pluripotent surface marker, stage-specific embryonic antigen-3 (SSEA-3), and CD105, and that have the capacity for self-renewal and triploblastic differentiation from a single cell [6]. The applicability of Muse cells for regenerative treatments has been suggested in disease models of liver damage, stroke, skin ulcers related to diabetes mellitus, and osteochondral defects [9], [10], [11], [12].
This is the first study to identify SSEA-3 and CD105 double positive cells isolated from patient-derived synovial membrane, which we designate as Muse-like cells and characterize the chondrogenic differentiation potential of their expanded cell, M-cluster-derived MSCs. Because patients who experience joint problems sometimes undergo arthroscopic examination, which allows for the collection of synovial membranes, we reasoned that membranes collected from these patients may be a candidate autologous cell source for cartilage regenerative medicine. We investigated whether M-cluster-derived MSCs have chondrogenic potential and their practicality for use in regenerative medicine.
2. Methods
2.1. Ethics statement and synovial membrane preparation
Experiments using human synovial membranes were performed with the approval and guidance of the Clinical Research Review Committee of Tokai University School of Medicine (approval number: 09R-070, 10I-52, 13I-33). Debrided synovial tissues were obtained from the knee joints of patients during surgery for total knee arthroplasty, high tibial osteotomy, or treatment of meniscus tear. The patients’ median age was 53.5 years, their age range was 36–72 years, and five were male and three were female. Written informed consent was obtained from all patients.
2.2. Immunohistochemistry for SSEA-3
Synovial membranes were fixed with 10% formalin solution and paraffin embedded, and 10-μm thick sections were prepared. For staining, the sections were deparaffinized and rehydrated, and incubated with 0.3% H2O2 in methanol for 30 min at room temperature to block endogenous peroxidase activity. The samples were washed three times with phosphate-buffered saline (PBS) for 10 min and incubated with blocking buffer (20% Block Ace; DS Pharma Biomedical, Osaka, Japan), 5% bovine serum albumin (BSA; Sigma–Aldrich, St Louis, MO, USA), and 0.3% Triton X-100 in PBS) for 30 min at room temperature. The slides were then incubated with anti-SSEA-3 antibody (1:100, rat-anti-SSEA-3 antibody; MAB4303, Merck Millipore, Tokyo, Japan) diluted with antibody dilution buffer overnight at 4 °C. After the overnight incubation, the samples were washed three times with PBS for 10 min each at room temperature and incubated with secondary antibody (1:200, horseradish peroxidase (HRP)-conjugated goat anti-rat immunoglobulin M (IgM); Jackson ImmunoResearch, West Grove, PA, USA) in dilution buffer for 2 h at room temperature. The samples were washed three times as before, incubated with 3,3′-diaminobenzidine (DAB; Wako Pure Chemical Industries, Osaka, Japan) solution for 20 min at room temperature, incubated with 0.01% H2O2 in DAB solution for 30 min at room temperature, and finally washed with distilled water. Counterstaining was performed with Mayer's hematoxylin dehydrate (Wako Pure Chemical Industries).
2.3. Isolation of synovial cells
Synovial membranes were rinsed with saline, finely minced, and digested with 5 mg/mL collagenase type I (Worthington Biochemical Corp., Lakewood, NJ, USA) in growth medium (alpha-minimum essential medium (αMEM; Sigma–Aldrich, Tokyo, Japan) containing 10% fetal bovine serum (FBS; AusGeneX, Oxenford, Australia) and 1 × kanamycin (Thermo Fisher Scientific, Kanagawa, Japan) at 37 °C. Dispersed synovial cells were collected by centrifugation, washed twice with PBS (Thermo Fisher Scientific), and seeded at 0.5–2.0 × 104 cells/cm2 in culture flasks with growth medium. Nonadherent cells were removed by medium replacement. Adherent cells were cultured until they reached subconfluence and were then dissociated using TripLE Express (Thermo Fisher Scientific). Aliquots of the obtained cells were suspended in Cellbanker 1 (Zenoaq, Fukushima, Japan) and cryopreserved for later use.
2.4. Fluorescence-activated cell sorting and surface marker expression analysis
Cryopreserved synovial cells were thawed, seeded in growth medium in culture flasks, and expanded until they reached confluence. Synovial cells were passaged once or twice, depending on the number of cells obtained, and then sorted to isolate SSEA-3-positive cells. To isolate cells that were double positive for SSEA-3 and CD105, synovial cells were dissociated from culture dishes using TripLE Express and suspended in FACS Buffer (PBS containing 0.2% BSA and 1 mM ethylenediaminetetraacetic acid), incubated with mouse phycoerythrin-conjugated anti-CD105 antibody (1:20; Beckman Coulter, Tokyo, Japan) and rat anti-SSEA-3 IgM antibody (1:50; Merc Millipore), and then incubated with fluorescein isothiocyanate (FITC)-conjugated anti-rat IgM (Jackson ImmunoResearch) as described previously [6], [13]. Synovial cells were incubated with FITC-conjugated anti-rat IgM and PE-conjugated IgG (Beckman coulter), and they were used to set sorting fluorescent thresholds. Cells double positive for CD105 and SSEA-3 were sorted using a FACSVantage (BD Biosciences, Tokyo, Japan). A portion of the synovial cells was reserved before immunostaining as unsorted synovial cells to compare the efficiency of isolation with that of Muse cells for use in the preparation of chondrogenic cells.
2.5. Cluster formation in methylcellulose medium
Cluster formation by SSEA-3 and CD105 double-positive cells and unsorted synovial cells was induced by culturing the cells in methylcellulose (MC) using MethoCult H4230 (MC medium supplemented with FBS, BSA, and 2-mercaptoethanol; StemCell Technologies, Vancouver, Canada) using a slight modification of a method described previously [13]. Muse cells or unsorted synovial cells were suspended at a concentration of 1000–10000 cells/mL in MethoCult H4230 and seeded in 35-mm culture dishes. After 7–10 days of MC culture, the number of clusters with a diameter larger than 25 μm formed from synovial Muse cells (M-clusters) or unsorted synovial cells (SY-clusters) were counted under a microscope. The cluster-forming rate was calculated as the number of clusters per number of cells plated in each dish and was shown as the mean ± standard deviation calculated from three technical replicates.
2.6. Immunocytochemistry of clusters
The expression of pluripotent markers by M− and SY-clusters was assessed using a slight modification of a previously described method [6]. Clusters were collected from MC cultures, resuspended in αMEM, and washed three times with PBS. Clusters were suspended in PBS at a concentration of 500–1000 clusters/mL, and 0.1 mL of the cluster suspension (50–100 clusters/slide) was centrifuged onto the surface of a glass slide using a Cytospin™ 4 cytocentrifuge (Thermo Fisher Scientific) at 500 rpm for 5 min. The slides were dried with cold air and fixed with 4% (w/vol) paraformaldehyde in 0.1 M phosphate buffer. Before immunostaining, clusters were permeabilized with 0.5% Triton X-100 in 0.02 M PBS for 5 min at room temperature and incubated with blocking solution (5% normal goat serum in 0.02 M PBS) for 30 min at room temperature. The blocking solution was discarded, and the slides were incubated with the following primary antibodies: polyclonal rabbit anti-NANOG antibody (1:500; Merck Millipore), polyclonal rabbit anti-OCT3/4 antibody (1:100; Santa Cruz Biotechnology, Dallas, TX, USA), and polyclonal rabbit anti-SOX2 antibody (1:500; Abcam, Cambridge, UK) in antibody diluent (1% normal goat serum and 0.1% Triton X-100 in 0.02 M PBS) at 4 °C overnight. Rabbit IgG (1:250; Agilent Technologies, Santa Clara, CA, USA) was used as the isotype control. Alexa Fluor488-conjugated goat anti-rabbit IgG secondary antibody (1:1000; Thermo Fisher Scientific) was used for detection. Nuclei were detected using ProLong® Diamond Antifade Mountant with 4′,6-diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific). Images were acquired with a BZ-X7000 fluorescence microscope (Keyence, Osaka, Japan).
2.7. In vitro chondrogenesis
Chondrogenic differentiation of cells in pellet culture was assessed as described previously with slight modifications [14], [15]. M− and SY-clusters were collected from MC medium, suspended in αMEM medium, washed, and seeded in growth medium in culture flasks to obtain a sufficient number of cells for culture. Cells were expanded for 1–2 weeks, depending on the number of clusters produced, and harvested using TripLE Express. Two-hundred fifty thousand cells obtained from the expanded M or SY-clusters were placed in a 15 mL polypropylene tube (BD Biosciences) in 400 μL of chondrogenic medium (high-glucose Dulbecco's modified Eagle medium; Gibco, Thermo Fisher Scientific) supplemented with 100 μg/mL sodium pyruvate (Wako Pure Chemical Industries), 100 nM dexamethasone (Sigma–Aldrich), 40 μg/mL l-proline (Sigma–Aldrich), 50 μg/mL ascorbate-2-phosphate (Nissin Pharmaceutical Co., Ltd., Yamagata, Japan), 1/100 (vol/vol) ITS + Premix (Corning, Corning, NY, USA), 100 ng/mL recombinant human BMP2/BMP6 heterodimer (R&D Systems, Minneapolis, MN, USA), and 10 ng/mL transforming growth factor-β1 (PeproTech, Rocky Hill, NJ, USA). The cells were centrifuged at 600×g for 10 min. The tubes were left standing in an incubator at 37 °C with 5% CO2 for 20–21 days or 28 days, during which the medium was changed every 3–4 days. The cell pellets were weighed every week and harvested at 21 days or 28 days for histological examination.
The cell pellets were fixed in 4% paraformaldehyde in PBS for 20 min and embedded in Tissue-Tek O.C.T. Compound (Sakura Finetech, Tokyo, Japan) and frozen at −80 °C. Frozen sections were cut at 10-μm thickness at −15 °C on a cryostat and mounted onto glass slides, air dried, and fixed with 4% paraformaldehyde in 0.01 M phosphate buffer for 30 min at room temperature. For histological examination, sections were stained with hematoxylin and eosin, safranin O, and toluidine blue according to standard protocols. For immunohistochemical analysis, sections were incubated with blocking solution (0.3% Triton X-100 in BlockAid Blocking Solution (Thermo Fisher Scientific)) for 45 min at room temperature, after which the blocking solution was discarded and the slides were incubated with the following primary antibodies: goat anti-human aggrecan (1:10; Human Mesenchymal Stem Cell Functional Identification Kit, R&D Systems), and goat anti-human collagen 1 (1:200; SouthernBiotech, Birmingham, AL, USA) in blocking solution at 4 °C overnight. Alexa Fluor546-conjugated donkey anti-goat antibody (1:400; Thermo Fisher Scientific) was used as secondary antibody for detection. Nuclei were detected using ProLong® Diamond Antifade Mountant with DAPI (Thermo Fisher Scientific). For type II collagen detection, sections were incubated with 0.4% pepsin (DAKO, Glostrup, Denmark) at 37 °C for 30 min and washed in distilled water, followed by incubation in 0.3% hydrogen peroxide/methanol solution at RT for 15 min. After washing with PBS, sections were incubated with a diluted primary anti-human type II collagen antibody (1:100; F-57: Daiichi Fine Chemical, Toyama, Japan) overnight at 4 °C, followed by incubation with the ImmPRESS Reagent Anti Mouse Ig (Vector Laboratories, Burlingame CA) at room temperature. Finally, the sections were stained with DAKO Liquid DAB substrate chromogen system (DAKO) and counterstained with hematoxylin. Images were acquired with a BZ-X7000 fluorescence microscope (Keyence).
2.8. Surface marker expression
SY-cluster-derived cells were analyzed using flow cytometry at the same time as they were used for in vitro chondrogenesis. Expanded SY-cluster-derived cells were harvested using TripLE Express, suspended in FACS Buffer, and immunostained with the following antibodies: CD31–FITC (clone: 5.6E), CD45–FITC (clone: J.33), and CD105–PE (clone:1G2) from Beckman Coulter; CD81–allophycocyanin (APC) (clone: JS-81), CD90–APC (clone: 5E10), CD49a–PE (clone: SR84), CD106–FITC (clone: 51-10C9), CD44–FITC (Clone: G44-26), CD34–PE (clone: 563), and CD271–PE (clone: C40-1457) from BD Biosciences; CD146–PE (clone: F4-35H7 (S-Endo 1)) from BioCytex (Marseille, France); and STRO-1–FITC from BioLegend (San Diego, CA, USA). SSEA-3 was identified by staining as described above. Fluorochrome-labeled anti-mouse IgG1 antibody (clone: 679.1Mc7, Beckman Coulter) was used as a negative control. Stained cells were analyzed using a FACSVerse (BD Biosciences), and data were analyzed using FlowJo software (Tomy Digital Biology, Tokyo, Japan).
3. Results
3.1. Existence of SSEA-3-positive cells in the human synovial membrane
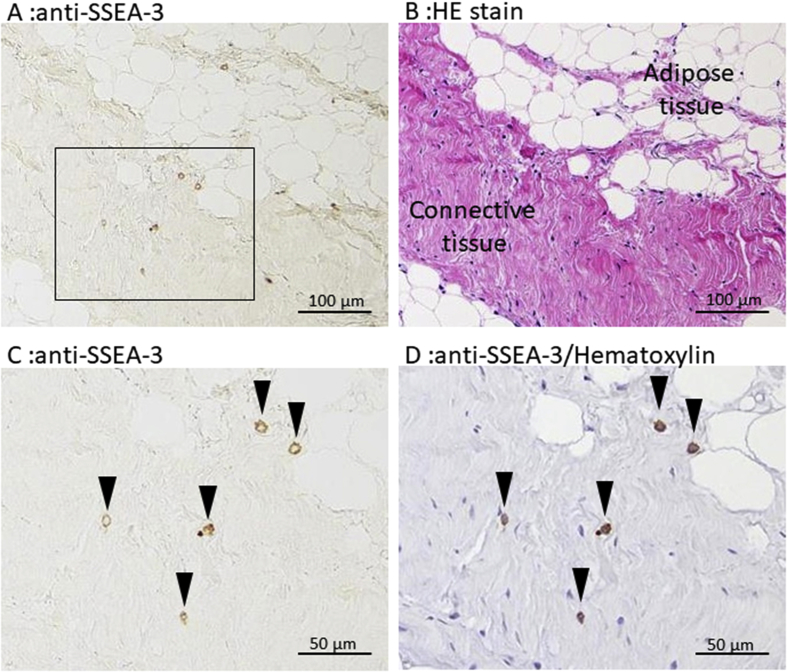
To identify the presence of Muse cells, sections of human synovial membrane were examined by immunostaining using anti-SSEA-3 antibody (Fig. 1). Similar to previous reports for adipose tissue [7] and the dermis [6], synovial membranes obtained from the knee joint of patients contained SSEA-3-positive cells. SSEA-3-positive cells were found in both the connective tissue and adipose tissue areas of the synovial membrane. They were scattered in the connective tissue and did not appear to be related to particular histological structures such as vessels.
Fig. 1.
SSEA-3-positive cells in the synovial membrane. A Representative immunostaining of synovium sections stained with anti-SSEA-3 antibody. B Serial section of A stained with hematoxylin and eosin. SSEA-3-positive cells were found in both adipose tissue and connective tissue. C Magnified image of the area enclosed in the box in A. Arrowheads indicate SSEA-3-positive cells. D Counterstaining of image C with Mayer's hematoxylin.
3.2. Identification of muse-like cells among synovial cells
Muse cells, which exhibit self-renewal capacity and the ability to differentiate triploblastic lineage-cells from a single cell, are known to be enriched by cell sorting using anti-SSEA-3 antibody. To isolate SSEA-3- and CD105-double-positive (Muse-like) cells by fluorescence-activated cell sorting, synovial membrane tissues were dispersed by enzymatic digestion and the adherent cells were used.
Adherent synovial membrane-derived cells exhibited spindle-shaped fibroblast-like features (Fig. 2a). These synovial cells were cultivated until confluent and were then used to Muse-like cell preparation. Cell sorting for SSEA-3-positive cells was performed as described previously [6]. Almost all synovial cells that were expanded ex vivo expressed CD105 antigen (Fig. 2b). Table 1 provides a summary of the Muse-like cells isolated from the samples from 8 patients aged from 36 to 72 years (five males and three females). The percentage of SSEA-3-positive cells among the synovial membrane-derived cells varied between patients and ranged from 0.05% to 8.78% (mean ± SD: 2.4% ± 2.8%). We found no relationship between the percentage of SSEA-3-positive cells and patient age. Passage 1 cells tended to contain a higher percentage of SSEA-3-positive cells than did passage 2 cells, which suggests that a longer culture before sorting decreases the percentage of SSEA-3-positive cells. Therefore, we did not expand the synovial cells past passage 2 to scale up Muse-like cell preparation. Samples from two patients contained a particularly low percentage of SSEA-3-positive cells; therefore, cell sorting and cluster formation was not performed.
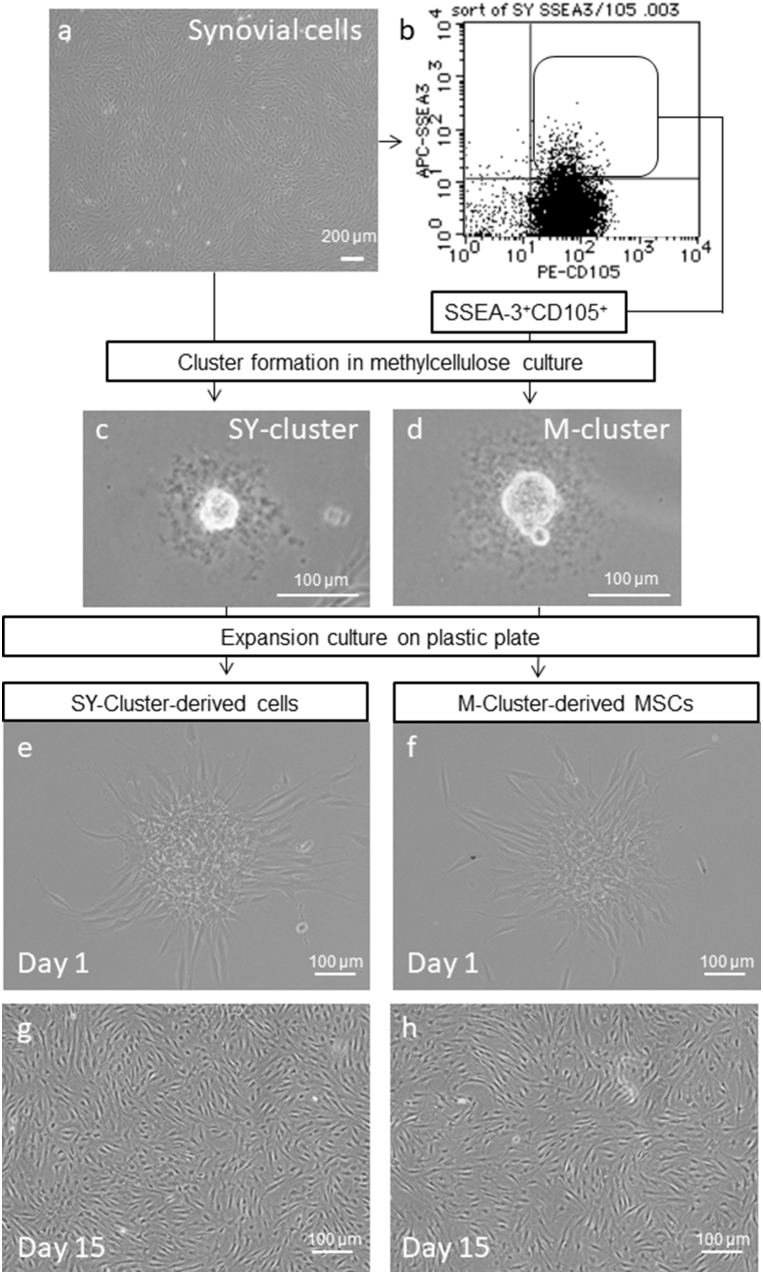
Fig. 2.
Procedures to prepare M-cluster-derived MSCs and SY-cluster-derived cells. Clusters formed from the unsorted cell population were named SY-clusters and those from SSEA-3 and CD105 double-positive populations were named M-clusters. Photographs show typical phase–contrast microscopic appearance. a Synovial cells cultured in a plastic dish. b Representative dot plot of SSEA-3 and CD105 double-positive cell sorting. c, d A cluster formed in MC medium from unsorted synovial cells (c) and double-positive cells (d). e–h Expansion cultures on day 1 (e, f) and day 15 (g, h) of SY-cluster-derived cells (e, g) or M-cluster-derived MSCs (f, h). Clusters were collected from MC and seeded on plastic dishes. Both cluster-forming cells showed good proliferative capacity.
Table 1.
Summary of Muse-like cell isolation from synovium and cluster formation in MC culture.
| Patient | Age | Gender | Passage | SSEA-3+ CD105+ cells |
SY-cluster forming cells in synovial cells (%)c | ||
|---|---|---|---|---|---|---|---|
| (%)a | M-cluster forming rate (%)b | Estimation of M-cluster forming rate in unsorted synovial cells (%) | |||||
| 1 | 56 | F | P1 | 8.78 | 12.3 ± 0.35 | 1.08 | NT |
| 2 | 42 | M | P1 | 2.1 | 14 ± 0.6 | 0.29 | NT |
| 3 | 59 | F | P1 | 1.86 | 20.1 ± 1.1 | 0.37 | NT |
| 4 | 50 | M | P1 | 3.67 | 4.34 ± 0.11 | 0.16 | NT |
| 5 | 71 | M | P2 | 1.2 | 4.74 ± 0.80 | 0.06 | 6.80 ± 0.37 |
| 6 | 36 | M | P2 | 1 | 8.02 ± 2.05 | 0.08 | 3.31 ± 0.55 |
| 7 | 51 | M | P2 | 0.76 | NT | NT | 20.2 ± 1.30 |
| 8 | 72 | F | P2 | 0.05 | NT | NT | 19.6 ± 4.19 |
NT: Not tested.
The percentage of SSEA-3+ CD105+ cells based on FACS sorting.
The percentage of cluster forming cells in SSEA-3+ CD105+.sorted cells obtained from cluster formation in MC culture.
Cluster forming rate in unsorted synovial cells obtained from cluster formation in MC culture.
Muse cells have been shown to form clusters similar to the embryoid bodies formed by human embryonic stem cells when cultured in suspension culture [5], [6], [7], [8]. To evaluate the characteristics of the SSEA-3-positive cells isolated from synovial cells, they were cultured in MC according to the protocol for M-cluster formation described by Kuroda et al. [13] and the clusters were analyzed. After 7–10 days of MC culture, Muse-like cells obtained from all patients formed embryoid body-like clusters (M-clusters) (Fig. 2d). Among the Muse-like cells from six patients, 4%–20% of SSEA-3-positive cells formed M-clusters in MC culture (average: 10.6% ± 6.1%). The estimated content of M-cluster forming cells for unsorted synovial cells ranged from 0.1 to 1.1%, as estimated by the multiplication of the percentage of SSEA-3 positive cells and the corresponding percentage of M-cluster forming cells (Table 1).
To compare the efficiency of isolation of Muse-like cells, unsorted synovial cells, which were thought to contain Muse-like cells, were also subjected to MC culture.
Among the unsorted synovial cells, 3%–20% of cells formed clusters in MC culture (SY-cluster) (Fig. 2c, Table 1). The estimated number of M-cluster forming cells in unsorted synovial cells was lower than that of SY-clusters, suggesting that the SSEA-3-positive cells were once damaged by laser irradiation during FACS isolation and their cluster forming rate was decreased compared to unsorted synovial cells. Alternatively, SY-clusters might consist of not only Muse-like cell-derived clusters but also clusters formed from SSEA-3-negative cells. We next evaluated the characteristics of these clusters further.
3.3. Evaluation of the pluripotent marker expression of M− and SY-cluster-forming cells
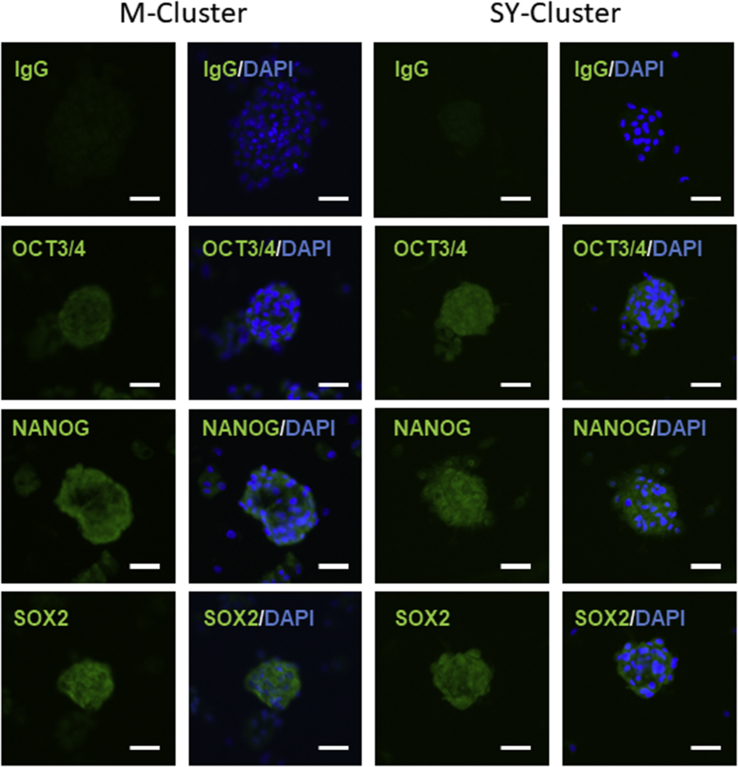
Clusters formed from bone marrow, dermis, and adipose tissue derived Muse cells can express NANOG, SOX2, and OCT3/4 and exhibit triploblastic differentiation [5], [6], [7], [8]. Immunostaining was used to examine the expression of pluripotent markers in M- and SY-clusters. M- and SY-clusters were collected from MC cultures and attached to glass slides. Immunohistochemical staining for NANOG, SOX2, and OCT3/4 revealed that both synovial membrane-derived M- and SY-clusters expressed these markers (Fig. 3). Because of the overlapped cells on the slides, the outlines of specific green signals for markers were unclear; however, colocalization of brighter green signals and DAPI signals indicated that the markers are located in the nucleus.
Fig. 3.
Expression of stemness markers by M- and SY-clusters. Immunostaining of clusters formed from Muse-like cells and unsorted synovial cells. Clusters were positive for NANOG, OCT3/4, and SOX2. Scale bars = 50 μm. DAPI was used as a counterstain.
3.4. Comparison of chondrogenic potency of M− and SY-cluster-derived cells
One of our aims was to assess Muse-like cells as a potential cell source for cartilage regeneration, which means that a sufficient number of cells must be obtained from the cell preparation procedures. Because of the shortage of M-cluster-forming cells, they needed to be expanded to obtain a sufficient number of cells for performing in vitro chondrogenic differentiation. The higher ratio of SY-cluster forming cells in synovial cells and they can be prepared without damaging by FACS sorting suggested that unsorted synovial cells may also be a potential cell source. To compare the chondrogenic potency of cells derived from M- and SY-clusters, both type of clusters were expanded for the same culture period. Both the M- and SY-cluster-forming cells proliferated actively and this generated M-cluster-derived MSCs and SY-cluster-derived cells, respectively (Fig. 2e -h).
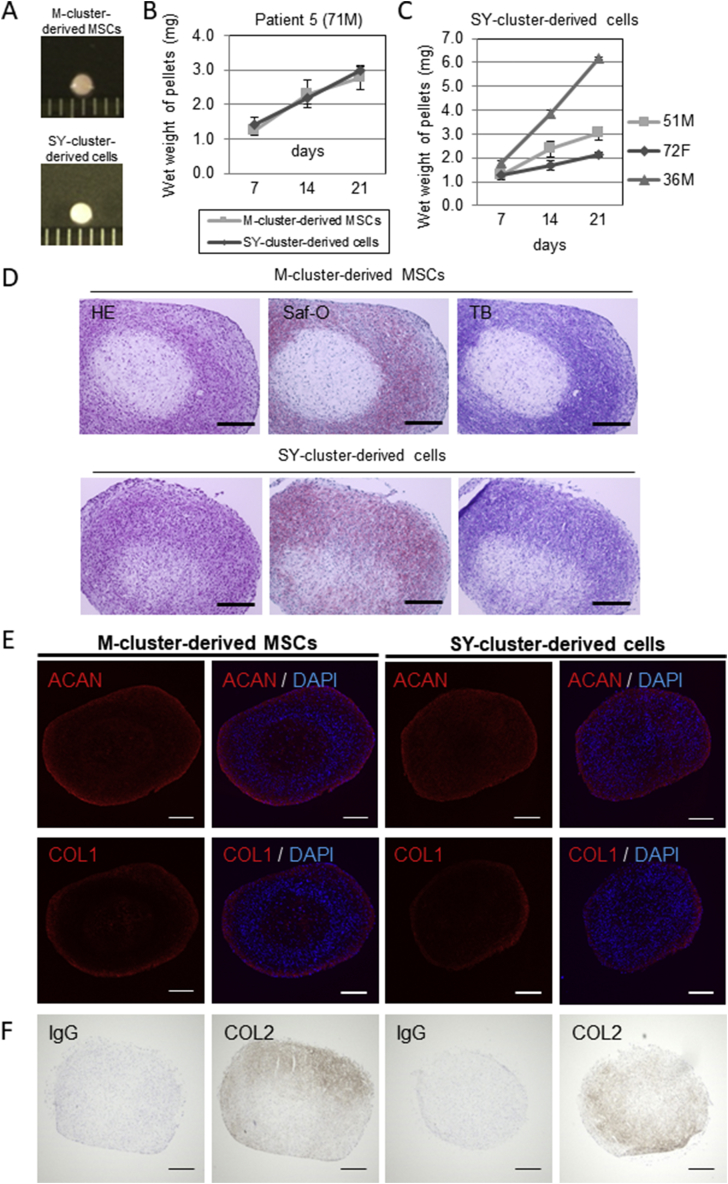
In vitro chondrogenesis was examined using M-cluster-derived MSCs and SY-cluster-derived cells from same patient (Fig. 4A). Both cell pellets created from M-cluster-derived MSCs and SY-cluster-derived cells exhibited an increase in wet weight with culture time (Fig. 4B), which implied an increase in extracellular matrix production [16]. Cell pellets created from the SY-cluster-derived cell from other patients also exhibited an increase in wet weight with culture time (Fig. 4C). Chondrogenic differentiation of pellets was confirmed by safranin O staining, toluidine blue staining, and immunostaining of aggrecan at 21 days (Fig. 4D and E) and type II collagen at day 28 days (Fig. 4F) after chondrogenic induction. Both M-cluster-derived MSCs and SY-cluster-derived cells generated pellets that displayed metachromasia with toluidine blue and safranin O staining and were aggrecan-positive and type II collagen -positive by immunostaining. Type I collagen staining was also detected, especially in the surface region of pellets (Fig. 4E).
Fig. 4.
Chondrogenic differentiation of M-cluster-derived MSCs and SY-cluster-derived cells. A Representative gross appearance of pellets cultured with a 1-mm scaled ruler. B Increase in the wet weight of pellets created from M-cluster-derived MSCs and SY-cluster-derived cells obtained from one patient. C Wet weight of pellets created from SY-clusters -derived cells from 3 individual patients. Values are the mean and SD of three pellets created from each source from each patient. D Representative histological staining with hematoxylin and eosin (HE), safranin O (Saf-O), and toluidine blue (TB). E-F Immunohistochemical examination of chondrogenic differentiation of pellets at d21(e) or d28(f). Scale bars in d, e and f = 300 μm.
The increase in the weight of pellets and their histological and immunohistochemical appearance did not seem to differ between M-cluster-derived MSCs and SY-cluster-derived cells.
3.5. Phenotypic characterization of SY-cluster-derived cells
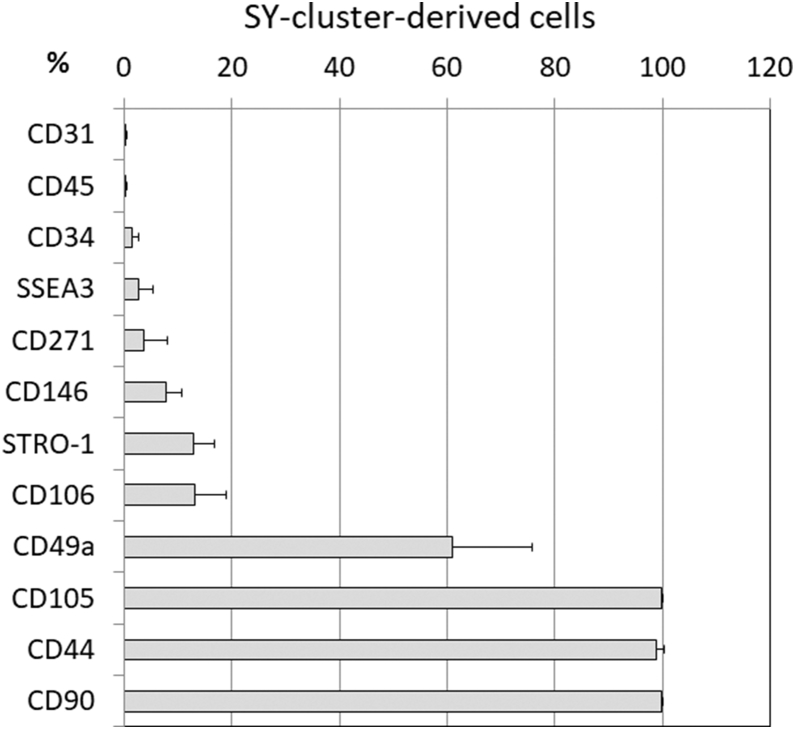
As a cell source for cartilage regeneration, the SY-cluster-derived cell population that is obtained from cluster formation of synovial cells in semisolid culture without SSEA-3-sorting may be a practical option. To characterize the expanded SY-cluster-derived cells, the expression of surface markers was analyzed using flow cytometry at the same time as their chondrogenic potential was evaluated. We could not perform this analysis using M-cluster-derived cells because of their limited availability. Expanded SY-cluster-derived cells did not express the hematopoietic marker CD45 or the endothelial marker CD31, but they did express mesenchymal stem cell (MSC) markers CD90, CD105, and CD44. A small subset of cells expressed the hematopoietic stem cell marker CD34, the low-affinity nerve growth factor receptor CD271, CD146 (M-CAM), CD106 (V-CAM-1), another MSC marker STRO-1, and SSEA-3 (Fig. 5).
Fig. 5.
Phenotypic characterization of SY-cluster-derived cells. Expression of cell surface markers was analyzed by flow cytometry. Values are the mean and SD of the percentage expression of each cell surface marker obtained from SY-cluster-derived cells from four patients.
4. Discussion
Muse cells have been described as stress-enduring cluster-forming cells with multipotency. In this report, we investigated the existence of Muse-like cells in the synovial membrane. Kuroda et al. reported that the percentage of Muse cells was 1.3% ± 0.1% in naive human fibroblasts and 1.1% ± 0.1% in human mesenchymal stromal cells [6]. They also reported that the percentage of Muse cells in the adherent fraction of bone marrow aspirate was 0.3% ± 0.08% for first passage, 0.5% ± 0.04% for second passage, and 0.9% ± 0.1% for fifth passage cells. They also confirmed the SSEA-3 and CD105 double-positive phenotype as a marker of Muse cells and reported that more than half of the double-positive cells obtained from human bone marrow-derived MSCs and human fibroblasts formed M-clusters in suspension culture. Ogura et al. found a frequency of 3.8% ± 0.9% SSEA-3-positive cells in purchased adipose MSCs and 8.8% ± 1.3% in freshly isolated subcutaneous adipose MSCs. They also reported that 30–40% of SSEA-3-positive adipose-Muse cells formed M-clusters in a single suspension culture but that no cluster formation was observed in SSEA-3-negative adipose tissue-derived MSCs [7]. Heneidi et al. also reported Muse cells in adipose tissue and that an adipose tissue-derived Muse cell population could be prepared under highly stressed conditions [5]. This population contained about 90% SSEA-3- and CD105-positive cells.
In this report, we used the SSEA-3- and CD105-double-positive phenotype to identify Muse-like cells obtained from synovial membrane-derived cells. We confirmed that the synovial-derived adherent cells passaged once or twice also contained SSEA-3 and CD105 double-positive cells at an average frequency of 2.4% ± 2.8% (range 0.05–8.78%). Although we did not examine the multilineage differentiation and self-renewal capacity of the Muse-like cells, 0.3% ± 0.4% of these cells obtained from the synovial cell population exhibited Muse-like characteristics as shown by their SSEA-3 positivity and their formation of clusters in MC culture that express NANOG, SOX2 and OCT3/4. Therefore, the frequency of Muse-like cells in the synovial membrane might be similar to that in other tissues, human fibroblasts, and bone marrow but may be less than that of adipose tissue. We also found that the synovial membranes obtained from older osteoarthritis patients contained Muse-like cells. In general, although the somatic stem cell number declines with age [17], [18], [19], our results suggest that synovial Muse-like cells may be a potential autologous cell source for cartilage regeneration and regenerative therapy in older patients. Considering that the low yield of Muse-like cells may be caused by cell damage accompanying FACS sorting, an alternative procedure for preparation of SSEA-3-positive cells may improve the yield of Muse-like cells. The superior effect of intra-articular injection of bone marrow-derived Muse cells compared to non-Muse cells was previously reported in an osteochondral defect model [12]. Instead of Muse cells obtained from bone marrow aspirates, synovial membrane derived Muse-cells is another promising option for the treatment of osteochondral defects, whereas unsorted SY-cluster derived cells may not be suitable.
The cluster-forming rates of SSEA-3 and CD105 double-positive cells derived from synovial membranes were low compared with those of Muse cells derived from other tissues. Given the definition of Muse cells as cells exhibiting stress-enduring characteristics, the use of more stringent cell preparation processes before cell isolation using SSEA-3 may improve the yield of Muse-like cells [5], [6].
We found that unsorted synovial cells also formed clusters when cultured under the same conditions, and that they also exhibited an OCT3/4-, NANOG-, and SOX2-positive phenotype on immunostaining, which implies multipotency. This cell population is thought to be a mixture of Muse-like cells and non-Muse cells. We found a higher cluster-forming cell number would be obtained from unsorted synovial cells than from sorted Muse-like cells. The estimated number of SSEA-3-positive cells in the unsorted cell cultures (0.05%∼8.78%) was less than that of clusters formed from unsorted synovial cells (3.31%∼20.2%). There is a possibility that the isolation of SSEA-3-positive cell by FACS sorting damaged Muse-like cells and their cluster forming rate decreased. Alternatively, this suggests that SY-clusters were formed to some extent from both Muse-like cells and non-Muse cells. In addition, the cluster-forming ratios of SSEA-3-positive cells and unsorted synovial cells from the same individual were related. These results imply that cells with the ability to form clusters in semisolid culture conditions exist among synovial cells and that this ability is independent of the SSEA-3-positive phenotype.
These cluster-forming cells from unsorted synovial cells also exhibited similar chondrogenic potential to those from sorted cells. We confirmed their chondrogenic differentiation by an increase in wet weight of pellets and an aggrecan-positive and type II collagen-positive phenotype. Because the expression of type II collagen tends to lag behind that of type I collagen [20] and increases continuously during chondrogenic maturation of the cell pellet in culture [16], we reasoned that the 28-day differentiation period might have been needed for detecting type II collagen produced under our immunostaining conditions.
The preparative procedures for clinical application should ensure a balance between chondrogenic potency and cell yield. In this study, we found that almost all expanded SY-cluster-derived cells that can be obtained by simply culturing synovial cells in semisolid condition expressed CD90, CD105, CD44 and exhibited chondrogenic potential. We have reported that adult knee chondrocyte sheets that demonstrated the efficacy in hyaline cartilage regeneration also expressed CD90 and CD44 [21]. Further, our results agree with previous findings that CD90 and CD105 are markers of cells with excellent chondrogenic potential within the synovial tissue [22], [23], [24], [25]. Positivity of CD90, CD44 and CD105 of SY-cluster -derived cells support the idea that the cells can be potential alternative cell source for cartilage regeneration. It is possible that the cell populations in the unsorted synovial cells that exhibited chondrogenic potential could be the same population as the reported synovial-derived chondrogenic cells [26], [27], [28], [29]. SY-cluster-derived cells contained a small population of cells that expressed CD34, CD271, CD146, CD106, or STRO-1. Variation in the ratios of these subsets between patients may be an important factor determining the balance between chondrogenic potential and the risk of unexpected lineage differentiation. SY-cluster-derived cells also contained a few percent of SSEA-3-positive cells, which may be self-renewing Muse-like cells.
We found that the unsorted synovial cells formed clusters that expressed the proteins NANOG, OCT-3/4, and SOX2. Bone marrow-derived MSCs from young rats express stemness markers (Oct 4, Sox 2, and Nanog) and exhibit chondrogenic, osteogenic and adipogenic potential [23]. Wang et al. reported that human ectopic expression of OCT-4 in MSCs from amniotic fluid can upregulate NANOG and SOX-2 expression and that this is associated with an increase in colony-forming ability [30]. Although further study is needed, we believe that these markers expression of SY-clusters might represent the properties of synovial MSCs elicited under semisolid culture conditions. Chondrocytes can proliferate in semisolid culture conditions and require three-dimensional conditions to maintain their chondrogenic properties [31], [32]. Therefore, it seems that the process of cluster formation in semisolid culture places selective pressure on cells with chondrogenic potential.
Our results have indicated that arthroscopically collectable synovial membranes, even from older patients, provide one practical cell source for obtaining autologous Muse-like cells in situations where a small number of the cells is sufficient and multipotency is required. When considered for application in tissue engineering for regenerative medicine, cells with high proliferative and monodifferentiating potential toward the expected lineage are ideal. Multipotency is inseparably linked to the risk of unexpected cell differentiation. Precise procedures and meticulous monitoring of cell differentiation are needed if multipotent cells are to be used in human therapy. In this context, SSEA-3 selection is dispensable for chondrogenic cell preparation from synovial membrane-derived cells. Instead, cluster-forming synovial cells may be applicable as a cell source for cartilage tissue engineering. This would ensure both chondrogenic potency and availability.
5. Conclusions
Muse-like cells can be isolated from the human synovial membrane, even from older patients, and thus may provide a multipotent cell source for regenerative medicine. Additionally, the expanded cells derived from cluster-forming cell within the synovial cells seems to contain Muse-like cells, also has excellent chondrogenic potential and may provide a more practical option for cartilage regeneration.
Conflicts of interest
The authors declare that they have no competing interests.
Funding
Not applicable.
Acknowledgements
We would like to express our gratitude to Yoshihiko Kushida and Mari Dezawa, Department of Stem Cell Biology and Histology, Tohoku University Graduate School of Medicine, for assistance with the histological examination and comments that greatly improved the manuscript. We are grateful to the Education and Research Support Center, Tokai University, for providing expertise that greatly assisted the research.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Eriko Toyoda, Email: etoyoda@tokai-u.jp.
Masato Sato, Email: sato-m@is.icc.u-tokai.ac.jp.
Takumi Takahashi, Email: ttakahashi@tokai-u.jp.
Miki Maehara, Email: m-maehara@tsc.u-tokai.ac.jp.
Yoshihiko Nakamura, Email: kahiko@is.icc.u-tokai.ac.jp.
Genya Mitani, Email: genya@syd.odn.ne.jp.
Tomonori Takagaki, Email: t-tom8@pc4.so-net.ne.jp.
Kosuke Hamahashi, Email: hamako@is.icc.u-tokai.ac.jp.
Masahiko Watanabe, Email: masahiko@is.icc.u-tokai.ac.jp.
References
- 1.Ebihara G., Sato M., Yamato M., Mitani G., Kutsuna T., Nagai T. Cartilage repair in transplanted scaffold-free chondrocyte sheets using a minipig model. Biomaterials. 2012;33:3846–3851. doi: 10.1016/j.biomaterials.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 2.Ito S., Sato M., Yamato M., Mitani G., Kutsuna T., Nagai T. Repair of articular cartilage defect with layered chondrocyte sheets and cultured synovial cells. Biomaterials. 2012;33:5278–5286. doi: 10.1016/j.biomaterials.2012.03.073. [DOI] [PubMed] [Google Scholar]
- 3.Kaneshiro N., Sato M., Ishihara M., Mitani G., Sakai H., Mochida J. Bioengineered chondrocyte sheets may be potentially useful for the treatment of partial thickness defects of articular cartilage. Biochem Biophys Res Commun. 2006;349:723–731. doi: 10.1016/j.bbrc.2006.08.096. [DOI] [PubMed] [Google Scholar]
- 4.Takaku Y., Murai K., Ukai T., Ito S., Kokubo M., Satoh M. In vivo cell tracking by bioluminescence imaging after transplantation of bioengineered cell sheets to the knee joint. Biomaterials. 2014;35:2199–2206. doi: 10.1016/j.biomaterials.2013.11.071. [DOI] [PubMed] [Google Scholar]
- 5.Heneidi S., Simerman A.A., Keller E., Singh P., Li X., Dumesic D.A. Awakened by cellular stress: isolation and characterization of a novel population of pluripotent stem cells derived from human adipose tissue. PloS One. 2013;8 doi: 10.1371/journal.pone.0064752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuroda Y., Kitada M., Wakao S., Nishikawa K., Tanimura Y., Makinoshima H. Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci U S A. 2010;107:8639–8643. doi: 10.1073/pnas.0911647107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogura F., Wakao S., Kuroda Y., Tsuchiyama K., Bagheri M., Heneidi S. Human adipose tissue possesses a unique population of pluripotent stem cells with nontumorigenic and low telomerase activities: potential implications in regenerative medicine. Stem Cell Dev. 2013;23:717–728. doi: 10.1089/scd.2013.0473. [DOI] [PubMed] [Google Scholar]
- 8.Wakao S., Kitada M., Kuroda Y., Tsuchiyama K., Bagheri M., Heneidi S. Multilineage-differentiating stress-enduring (Muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts. Proc Natl Acad Sci U S A. 2011;108:9875–9880. doi: 10.1073/pnas.1100816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinoshita K., Kuno S., Ishimine H., Aoi N., Mineda K., Kato H. Therapeutic potential of adipose-derived SSEA-3-positive Muse cells for treating diabetic skin ulcers. Stem Cells Transl Med. 2015;4:146–155. doi: 10.5966/sctm.2014-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iseki M., Kushida Y., Wakao S., Akimoto T., Mizuma M., Motoi F. Human Muse cells, non-tumorigenic pluripotent-like stem cells, have the capacity for liver regeneration by specific homing and replenishment of new hepatocytes in liver fibrosis mouse model. Cell Transplant. 2016;26:821–840. doi: 10.3727/096368916X693662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchida H., Niizuma K., Kushida Y., Wakao S., Tominaga T., Borlongan C.V. Human Muse cells reconstruct neuronal circuitry in subacute lacunar stroke model. Stroke. 2017;48:428–435. doi: 10.1161/STROKEAHA.116.014950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmoud E.E., Kamei N., Shimizu R., Wakao S., Dezawa M., Adachi N. Therapeutic potential of multilineage-differentiating stress-enduring cells for osteochondral repair in a rat model. Stem Cell Int. 2017;2017:8154569. doi: 10.1155/2017/8154569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuroda Y., Wakao S., Kitada M., Murakami T., Nojima M., Dezawa M. Isolation, culture and evaluation of multilineage-differentiating stress-enduring (Muse) cells. Nat Protoc. 2013;8:1391–1415. doi: 10.1038/nprot.2013.076. [DOI] [PubMed] [Google Scholar]
- 14.Mochizuki T., Muneta T., Sakaguchi Y., Nimura A., Yokoyama A., Koga H. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006;54:843–853. doi: 10.1002/art.21651. [DOI] [PubMed] [Google Scholar]
- 15.Sekiya I., Colter D.C., Prockop D.J. BMP-6 enhances chondrogenesis in a subpopulation of human marrow stromal cells. Biochem Biophys Res Commun. 2001;284:411–418. doi: 10.1006/bbrc.2001.4898. [DOI] [PubMed] [Google Scholar]
- 16.Sekiya I., Vuoristo J.T., Larson B.L., Prockop D.J. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci U S A. 2002;99:4397–4402. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caplan A.I. The mesengenic process. Clin Plast Surg. 1994;21:429–435. [PubMed] [Google Scholar]
- 18.Ye X., Liao C., Liu G., Xu Y., Tan J., Song Z. Age-related changes in the regenerative potential of adipose-derived stem cells isolated from the prominent fat pads in human lower eyelids. PloS One. 2016;11 doi: 10.1371/journal.pone.0166590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fibbe W.E., Noort W.A. Mesenchymal stem cells and hematopoietic stem cell transplantation. Ann N Y Acad Sci. 2003;996:235–244. doi: 10.1111/j.1749-6632.2003.tb03252.x. [DOI] [PubMed] [Google Scholar]
- 20.Yoo J.U., Barthel T.S., Nishimura K., Solchaga L., Caplan A.I., Goldberg V.M. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Maehara M., Sato M., Toyoda E., Takahashi T., Okada E., Kotoku T. Characterization of polydactyly-derived chondrocyte sheets versus adult chondrocyte sheets for articular cartilage repair. Inflamm Regen. 2017;37:22. doi: 10.1186/s41232-017-0053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagase T., Muneta T., Ju Y.J., Hara K., Morito T., Koga H. Analysis of the chondrogenic potential of human synovial stem cells according to harvest site and culture parameters in knees with medial compartment osteoarthritis. Arthritis Rheum. 2008;58:1389–1398. doi: 10.1002/art.23418. [DOI] [PubMed] [Google Scholar]
- 23.Asumda F.Z., Chase P.B. Age-related changes in rat bone-marrow mesenchymal stem cell plasticity. BMC Cell Biol. 2011;12:44. doi: 10.1186/1471-2121-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim Y.S., Lee H.J., Yeo J.E., Kim Y.I., Choi Y.J., Koh Y.G. Isolation and characterization of human mesenchymal stem cells derived from synovial fluid in patients with osteochondral lesion of the talus. Am J Sports Med. 2015;43:399–406. doi: 10.1177/0363546514559822. [DOI] [PubMed] [Google Scholar]
- 25.Krawetz R.J., Wu Y.E., Martin L., Rattner J.B., Matyas J.R., Hart D.A. Synovial fluid progenitors expressing CD90+ from normal but not osteoarthritic joints undergo chondrogenic differentiation without micro-mass culture. PloS One. 2012;7 doi: 10.1371/journal.pone.0043616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 27.De Bari C., Dell'Accio F., Tylzanowski P., Luyten F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 28.Sakaguchi Y., Sekiya I., Yagishita K., Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 29.Nimura A., Muneta T., Koga H., Mochizuki T., Suzuki K., Makino H. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis Rheum. 2008;58:501–510. doi: 10.1002/art.23219. [DOI] [PubMed] [Google Scholar]
- 30.Wang K.-H., Kao A.-P., Chang C.-C., Lin T.C., Kuo T.C. Upregulation of Nanog and Sox-2 genes following ectopic expression of Oct-4 in amniotic fluid mesenchymal stem cells. Biotechnol Appl Biochem. 2015;62:591–597. doi: 10.1002/bab.1315. [DOI] [PubMed] [Google Scholar]
- 31.Benya P.D., Shaffer J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 32.Mhanna R., Kashyap A., Palazzolo G., Vallmajo-Martin Q., Becher J., Möller S. Chondrocyte culture in three dimensional alginate sulfate hydrogels promotes proliferation while maintaining expression of chondrogenic markers. Tissue Eng. 2014;20(9–10):1454–1464. doi: 10.1089/ten.tea.2013.0544. [DOI] [PMC free article] [PubMed] [Google Scholar]