Abstract
The effects of obesity on venoarterial extracorporeal membrane oxygenation (VA-ECMO) outcomes in pediatric population are unknown. We performed retrospective analysis of 41 children (age 2–18 years) undergoing VA-ECMO. The percentage difference between actual body weight and lean body weight, referred to as Δmass, was calculated. Ratios of Δmass to ECMO flow were calculated at 4 and 24 hours. In patients with Δmass:flow ≥ 0.1 at 4 hours, higher 24-hour lactates (20.0 vs. 14.5 mg/dL; p = 0.002) and inotrope scores (17.3 vs. 11.2; p = 0.015) were observed. However, elevated Δmass:flow was not associated with mortality, and in-hospital mortality rates between groups were similar (53 vs. 45%; p = 0.647). In obese pediatric patients requiring VA-ECMO, increased flow is necessary to avoid complications of hypoperfusion and related complications.
Keywords: VA-ECMO, obesity, children
Introduction
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) is a life-saving therapy for patients in cardiogenic shock. According to the 2012 Extracorporeal Life Support Organization (ELSO) registry report, VA-ECMO remains the primary modality of support for pediatric patients with refractory cardiac failure despite increased ventricular assist device (VAD) usage.1 Peripheral ECMO flows are limited by the size of the arterial and venous cannulas used.2 Optimum flows are needed to achieve adequate end-organ perfusion, but limb ischemia from inadequate distal flow is a well-documented complication that significantly increases morbidity and mortality.3 4 5 In children with particularly small vasculature, achieving adequate flow using peripheral cannulation presents a unique challenge. Effective cannulation strategies involving distal perfusion catheters have been developed to limit the incidence of limb ischemia.6 7 8 9 Central cannulation can achieve adequate systemic flows; however, it carries inherent disadvantages of a chest incision.10 11 12 The “chimney technique” involving cannulation via an end-to-side graft has also been proposed to reduce limb ischemic rates with varying results.13 14 However, these are difficult to implement in an urgent cannulation in the pediatric population and remain restricted to select cases.
Childhood obesity is approaching epidemic proportions in the United States, affecting up to 15 to 20% of adolescents between ages 5 and 14 years.15 In the healthy population, obesity is a known risk factor for future cardiac disease and mortality. Paradoxically, obesity in some critically ill adult populations, including ECMO, has not been correlated with mortality, and may demonstrate some protective effects.16 17 18 However, studies of obesity in critically ill children are lacking.
The effects of obesity on patient outcomes in the pediatric ECMO population may not align with trends in the adult population, given the differences in vasculature size available for cannulation and indications for ECMO, such as congenital heart disease (requiring multiple catheterization procedures resulting in access issues). Whereas neonates routinely are cannulated through the neck and adults have more developed peripheral vasculature, appropriate cannulation methods for children are still unclear. Adipose tissue is vascular, and the cardiac output required to support obese patients is correspondingly higher.19 20 However, obese children have arterial and venous vessels the same size as lean children of the same age (the “small on the inside” phenomenon). This creates a discrepancy between flow requirements and the ability to accommodate appropriately sized peripheral cannulas to achieve adequate flows. Furthermore, body mass index (BMI) has been shown to be a less ideal measure of increased body fat, particularly in children. Pharmacokinetic studies in children have led to increasing use of other lean body weight formula over BMI. Despite these issues, ECMO guidelines remain unclear on the ideal flow rates or cannulation strategies to achieve adequate flow in obese children, and whether lean body weight or actual body weight (ABW) should be used to calculate target ECMO flows.
With these uncertainties in mind, we sought to evaluate the outcomes of VA-ECMO in our pediatric population, with a particular focus on the various measures of obesity. The purpose of this study was to determine the impact of obesity and flow rates on end-organ perfusion and mortality during VA-ECMO in the noninfant pediatric population.
Methods
Study Population and Inclusion Criteria
This study was a retrospective review of noninfant pediatric VA-ECMO patients treated at our center from January 2004 to May 2015. Analysis was limited to children of ages 2 to 18 years and with weight < 60 kg. Patients who were cannulated emergently for cardiac arrest (extracorporeal cardiopulmonary resuscitation) were excluded from the analysis due to the confounding ischemic effects associated with the arrest period. In addition, patients who received isolated venovenous ECMO were excluded from the analysis.
ECMO Protocol
An outline of UCLA's pediatric ECMO protocol is given in Fig. 1. Potential ECMO candidates are first screened for a feasible “exit strategy”—either VAD as bridge to cardiac transplantation or ECMO as bridge to recovery. If the child is not a candidate for either exit strategy, ECMO is not offered as an option. Critically ill children requiring possible ECMO support are identified in the intensive care unit. These patients undergo an ECMO workup including peripheral arterial and venous duplex studies documenting vessel size and patency, blood type and cross-match, and cannula selection. Children in whom femoral duplex demonstrated inadequate vessel size are candidates for central cannulation. Prior sternotomy is a contraindication to emergent central ECMO. Full-flow extracorporeal support—defined as ECMO flow greater than 2 L/min/m2—is targeted during the first 24 hours of ECMO, after which support is weaned with echocardiographic monitoring. Our institutional policy is to continue ECMO for 7 days for postcardiotomy failure and for 14 days for respiratory failure; support is withdrawn if there are no signs of myocardial recovery and an exit strategy is not feasible.
Fig. 1.
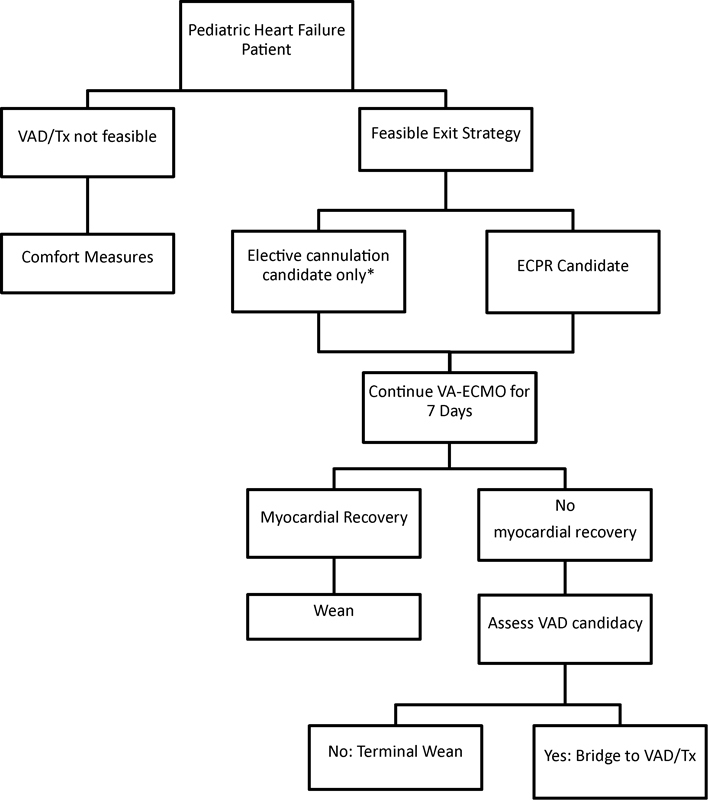
Pediatric ECMO protocol at UCLA. ECPR, extracorporeal cardiopulmonary resuscitation; Tx, cardiac transplantation; VA-ECMO, venoarterial extracorporeal membrane oxygenation; VAD, ventricular assist device. *ECMO candidates are electively cannulated for short-term LVAD support (Centrimag) if they have had a previous sternotomy or femoral duplex reveals inadequate vessel size.
Cannulation Strategies
Minimum cannula sizes for each patient were chosen based on the patient's raw weight. If peripheral duplex studies revealed inadequate peripheral vasculature, central cannulation strategies using right atrial and aortic cannulation were employed. Arterial cannula sizes in this population ranged from 12 to 22 Fr, while venous cannulas range from 14 to 24 Fr. In peripheral ECMO, a distal perfusion cannula was placed only if there were absent palpable pulses or Doppler signals in the distal extremity. Pulse oximetry or invasive monitoring was not used in the early part of our experience. The small size of the distal perfusion cannula often resulted in clotting or dislodgement and therefore was not routinely employed in all patients.
Patient Characterization
The following perioperative patient characteristics were retrospectively analyzed from our institutional database: age, ABW, height, BMI, body surface area, indication for ECMO support, cannulation site, 4-hour ECMO flow, and 24-hour ECMO flow. Indications for ECMO support included myocarditis, allograft rejection after cardiac transplantation, dilated cardiomyopathy, congenital heart defects, postcardiotomy (defined as ECMO within 7 days of cardiac operation), and cardiogenic shock following acute respiratory distress syndrome.
In addition, lean body mass (LBM) was calculated for each child using the formula described by Peters et al21:
LBM = (W 0.6469 × H 0.7236) × 0.0817.
Based on this, the percentage difference between LBM and ABW, hereby referred to as Δmass, was calculated as follows:
Finally, the ratios of Δmass to ECMO flow (L/min) were calculated at 4 hours and 24 hours, normalizing the increase in body fat by the amount of perfusion achieved. Increased Δmass:flow represents a mismatch between body fat relative to the ECMO flows provided. Patients were then stratified into two cohorts based on the ratio of their Δmass to their 4-hour ECMO flow: Δmass:flow < 0.10 (high perfusion group) and Δmass:flow ≥ 0.10 (low perfusion group). This cutoff point represents greater than 10% mismatch between increased body mass and ECMO flow:
Primary and Secondary End Points
Primary outcome measures included 12- and 24-hour laboratories (serum creatinine and lactate), and in-hospital mortality. Secondary outcomes measures included 24-hour arterial blood gas values (pH and base excess), average daily vasoactive inotrope score (VIS) during ECMO, complications of ECMO (cerebrovascular accident [CVA], sepsis, bleeding, limb ischemia), duration of ECMO, and mortality during ECMO support.
VIS was calculated using the following equations from Gaies et al22:
VIS = dopamine (µg/kg/min) + dobutamine (µg/kg/min) + 100 × epinephrine (µg/kg/min) + 10 × milrinone (µg/kg/min) + 100 × norepinephrine (µg/kg/min) + 10,000 × vasopressin (units/kg/min).
Statistical Analysis
Baseline characteristics of the study population were expressed as mean ± standard deviation or median (interquartile range) for continuous variables and frequency (percent of study population) for categorical variables. Pearson product-moment correlation procedure was performed between measures of size and perfusion (4-hour flow, 24-hour flow, ABW, LBM, Δmass, Δmass/flow) and continuous outcome variables that were queried. For comparisons between the high and low perfusion groups, the Kruskal–Wallis rank test was used for continuous variables, while the χ 2 test was used for categorical variables. Logistic regression was used to model ECMO mortality. All statistical analyses were performed using SPSS 19 software (IBM, Armonk, New York, United States). A p-value < 0.05 was considered statistical significant.
Results
During the study period, 41 patients were identified who fit inclusion/exclusion criteria. Baseline characteristics of the study population are listed in Table 1. The most common indication for VA-ECMO support was dilated cardiomyopathy, followed by myocarditis and support postcardiotomy. In total, 30 children were cannulated peripherally, while 12 children were cannulated centrally. In three peripheral ECMO children, an extra peripheral venous drainage cannula was placed after documented signs of poor end-organ perfusion 24 to 72 hours after initiation of support.
Table 1. Preoperative and operative demographics.
| Overall (N = 41) | |
|---|---|
| Demographics | |
| Age (y) | 10.8 ± 4.3 |
| Height (cm) | 129 ± 24.1 |
| Weight (kg) | 36.1 ± 16.4 |
| BMI (kg/m2) | 20.5 ± 4.1 |
| BSA (m2) | 1.1 ± 0.4 |
| Diagnosis | |
| Myocarditis | 8 (19.5%) |
| Heart transplant rejection | 6 (14.6%) |
| Dilated cardiomyopathy | 12 (29.3%) |
| Congenital heart defect | 5 (12.2%) |
| Postcardiotomy | 7 (17.1%) |
| ARDS—cardiogenic shock | 3 (7.3%) |
| ECMO cannulation | |
| Central | 11 (26.8%) |
| Peripheral | 30 (73.2%) |
Abbreviations: ARDS, acute respiratory distress syndrome; BMI, body mass index; BSA, body surface area; ECMO, extracorporeal membrane oxygenation.
Note: Values presented as mean ± standard deviation or number (percent of study population).
Table 2 lists the correlation coefficients between indications of size and perfusion and categorical outcome variables. The ratio of Δmass to 4-hour ECMO flow showed significant correlations with 12-hour lactate, 24-hour lactate, and average VIS, demonstrating a need for increased perfusion with an increased Δmass. Correlations were also observed between 4-hour flow and 24-hour lactate. Several other markers of isolated body mass or flow were not associated with these markers, suggesting that mismatch between ECMO flow and relatively increased body fat is key. In addition, the ratio of Δmass to 24-hour ECMO flow did not show significant correlations with lactate or inotrope use, suggesting that adequate early perfusion is of prime importance.
Table 2. Pearson correlations for labs and inotrope score.
| Weight (kg) | Lean body weight | ΔMass (%) | 4-h flow (L/min) | 24-h flow (L/min) | ΔMass:flow (4 h) | ΔMass:flow (24 h) | ||
|---|---|---|---|---|---|---|---|---|
| 24-h arterial blood gas | pH | −0.116 | −0.102 | −0.150 | −0.061 | 0.063 | −0.157 | −0.126 |
| (0.470) | (0.526) | (0.350) | (0.749) | (0.705) | (0.415) | (0.452) | ||
| Base excess | −0.120 | −0.121 | −0.085 | 0.124 | 0.059 | −0.180 | −0.048 | |
| (0.456) | (0.450) | (0.596) | (0.513) | (0.725) | (0.351) | (0.773) | ||
| 12-h labs | Creatinine (mg/dL) | 0.298 | 0.265 | 0.316 | 0.260 | −0.041 | 0.057 | 0.406a b |
| (0.092) | (0.136) | (0.074) | (0.200) | (0.831) | (0.783) | (0.026) | ||
| Lactate (mg/dL) | −0.091 | −0.128 | 0.189 | −0.332 | −0.031 | 0.527a | 0.184 | |
| (0.665) | (0.542) | (0.366) | (0.153) | (0.890) | (0.017) | (0.412) | ||
| 24-h labs | Creatinine (mg/dL) | 0.221 | 0.200 | 0.262 | 0.169 | 0.015 | 0.174 | 0.272 |
| (0.176) | (0.221) | (0.108) | (0.389) | (0.932) | (0.386) | (0.109) | ||
| Lactate (mg/dL) | −0.056 | −0.058 | 0.024 | −0.475a | −0.248 | 0.707a | 0.222 | |
| (0.751) | (0.742) | (0.890) | (0.019) | (0.172) | (<0.001) | (0.221) | ||
| Average daily inotrope scores | Vasoactive inotrope score | −0.079 | −0.095 | 0.121 | −0.165 | 0.035 | 0.377a | 0.082 |
| (0.624) | (0.556) | (0.450) | (0.385) | (0.834) | (0.044) | (0.623) |
Note: Values presented as a Pearson correlation coefficient (p-value).
Statistical significance at p < 0.05.
Although significant, this finding is not clinically relevant as the flow ratio is recorded far after the 12-hour laboratories have been measured.
Outcome variables by perfusion group are listed in Table 3. Both 24-hour lactate and VIS were significantly greater in the low perfusion group, further demonstrating a mismatch of ECMO flow and metabolic demand in these children. The incidence of in-hospital mortality or postoperative complications including CVA, sepsis, bleeding, and limb ischemia was nondifferent between groups. Of the 41 children in the current study, there were 11 deaths which occurred while on ECMO support. Of these 11, 10 patients died after ECMO support was withdrawn per protocol, while 1 patient died due to circuit complications. On univariate logistic regression modeling, none of the perioperative variables entered were statistically significant predictors of ECMO mortality, and a multivariable model was not pursued (Table 4). Mortality in most cases followed withdrawal of support per our institution's protocol, and was not found to be related to obesity.
Table 3. Perioperative outcomes by group.
| Demographics | ΔMass:flow < 0.10 (N = 22) | ΔMass:flow ≥ 0.10 (N = 19) | p-Value |
|---|---|---|---|
| Age (y) | 12.4 (6.4–14.0) | 10.0 (9.1–13.4) | 0.628 |
| Height (cm) | 133 (117–159) | 121 (104–139) | 0.069 |
| BMI (percentile) | 0.59 (0.28–0.90) | 0.92 (0.84–0.97) | 0.004a |
| 4-h flow (L/min) | 3.1 (2.2–3.9) | 2.3 (2.0–2.7) | 0.017a |
| 24-h flow (L/min) | 2.5 (2.1–3.2) | 2.5 (2.0–2.9) | 0.566 |
| ECMO cannulation | |||
| Central | 6 (27%) | 5 (26%) | 0.945 |
| Peripheral | 16 (73%) | 14 (74%) | |
| 12-h labs | |||
| Creatinine (mg/dL) | 0.8 (0.5–1.5) | 1.0 (0.7–1.4) | 0.547 |
| Lactate (mg/dL) | 25.5 (15.8–34.8) | 42.0 (15.0–107) | 0.257 |
| 24-h labs | |||
| Creatinine (mg/dL) | 0.6 (0.4–1.5) | 1.0 (0.8–1.6) | 0.098 |
| Lactate (mg/dL) | 14.5 (9.3–17.0) | 20.0 (11.0–84.0) | 0.022a |
| ABG at 24 h | |||
| pH | 7.45 (7.42–7.49) | 7.45 (7.38–7.50) | 0.824 |
| Base excess | 3.0 (0.8–5.8) | 1.2 (−1.0 to 7.0) | 0.610 |
| Vasoactive inotrope score | 11.2 (6.1–14.5) | 17.3 (11.5–31.7) | 0.015a |
| Composite complications | 6 (27%) | 6 (32%) | 0.763 |
| Cerebrovascular accident | 3 (14%) | 2 (11%) | 0.762 |
| Sepsis | 3 (14%) | 2 (11%) | 0.762 |
| Bleeding | 1 (5%) | 1 (5%) | 0.915 |
| Limb ischemia | 2 (9%) | 1 (5%) | 0.639 |
| Other | 0 (0%) | 1 (5%) | 0.276 |
| Duration of ECMO (d) | 6.0 (5.0–10.5) | 5.0 (3.0–8.0) | 0.133 |
| Outcome | |||
| Wean | 11 (50%) | 9 (47%) | 0.275 |
| VAD | 4 (18%) | 1 (5%) | |
| Transplant | 1 (5%) | 4 (21%) | |
| Death | 6 (27%) | 5 (26%) | |
| In-Hospital mortality | 10 (45%) | 10 (53%) | 0.647 |
Abbreviations: ABG, arterial blood gas; BMI, body mass index; ECMO, extracorporeal membrane oxygenation.
Note: Values presented as median (interquartile range) or number (percent of study population).
Statistical significance at p < 0.05.
Table 4. Univariate logistic regression modeling for ECMO mortality.
| Risk factors | ECMO mortality (N = 11) | ||
|---|---|---|---|
| Odds ratio | 95% CI | p-Value | |
| Age (y) | 0.99 | (0.85–1.16) | 0.897 |
| BMI (kg/m2) | 0.92 | (0.76–1.10) | 0.343 |
| ΔMass | 0.02 | (0.00–82.2) | 0.359 |
| 4-h ECMO flow (L/min) | 0.49 | (0.16–1.49) | 0.211 |
| 24-h ECMO flow (L/min) | 2.39 | (0.80–7.10) | 0.117 |
| ΔMass:ECMO flow ≥ 0.1 | 0.95 | (0.24–3.81) | 0.945 |
| Diagnosis | |||
| Myocarditis | Ref | – | 0.527 |
| Heart transplant rejection | 1.40 | (0.07–28.1) | 0.826 |
| Dilated cardiomyopathy | 3.50 | (0.31–39.2) | 0.309 |
| ARDS—cardiogenic shock | 14.0 | (0.58–339) | 0.105 |
| Postcardiac surgery | 1.17 | (0.06–22.9) | 0.919 |
| Congenital heart defect | 4.67 | (0.30–73.4) | 0.273 |
Abbreviations: ARDS, acute respiratory distress syndrome; BMI, body mass index; ECMO, extracorporeal membrane oxygenation.
Discussion
The effect of obesity on outcomes after pediatric VA-ECMO is unknown. Obese patients require a higher cardiac output to meet necessary metabolic demands; compounded with the small vasculature in growing children, achieving necessary flow rates through peripheral cannulation may be difficult. The current study was undertaken to determine the impact of obesity on end-organ perfusion and outcomes in the pediatric VA-ECMO population at our center.
Our results show that in the subgroup of children whose ABW far exceeds their lean body weight, inadequate flow is associated with poorer outcomes. Children with a Δmass to flow ratio greater than 0.1 had higher serum lactates and inotrope requirements than those with a ratio less than 0.1. Importantly, correlations were most prevalent when Δmass:flow was examined and not with measures of body mass or flow individually. This suggests that obesity itself is not a negative predictor for ECMO; however, commiserate increases in circuit flows are required to meet the increased end-organ demands of the obese child. Furthermore, the significance of the 4-hour Δmass:flow ratio emphasizes the importance of achieving sufficient early perfusion. Although full-flow support (>2 L/min/m2) was targeted in all cases during the first 24 hours, these results suggest that higher targets may be necessary to meet the increased metabolic demands of the obese child.
Achieving ideal flow rates for this cohort of children remains difficult. It may mechanically be impossible to accommodate the ideal cannula due to the small peripheral vessel size. Furthermore, increasing peripheral cannula size may accommodate higher flows, but increases the risk of distal ischemia. The addition of a distal perfusion cannula in this population is complicated by clotting due to small luminal caliber and low flow. Central cannulation methods allow for improved flow rates. In studies of pediatric patients with refractory septic shock, MacLaren and colleagues have shown improved outcomes when choosing central cannulation over peripheral cannulation to meet the increased flow requirements of septic patients.23 24 Applying this to the obese population, central cannulation may be better able to achieve improved early perfusion. However, central cannulation carries increased risk of bleeding and infection and may not be expedient in the acute setting.10 11 12
Another strategy to improve flow involves placing additional venous drainage cannulas (venovenous-arterial [VVA] ECMO). Limited literature has been published about the use of VVA-ECMO to increase end-organ perfusion.25 26 Placement of additional venous drainage cannulas was performed in three children subsequent to clinical signs of inadequate perfusion after 24 hours. Further research is warranted to identify if prophylactic VVA-ECMO may be indicated in obese children with known vascular issues.
Obesity in the current study was determined by using the difference between LBM and ABW. Because children vary greatly in body size throughout their development, using other indices of body mass such as raw weight or BMI as an indicator for obesity is incorrect as they do not address the percentage of body fat in the obese child. Our study suggests that Δmass:flow < 0.1 may be used as an appropriate early target for these children.
No preoperative variables examined, including obesity, were significantly predictive of mortality. In the 11 deaths observed in our population, 10 occurred with withdrawal of support. In these patients, survival is dependent on myocardial recovery—the lack of which prompted ECMO discontinuation in the time frame dictated by institutional protocol.
Although other groups have analyzed the effect of weight on ECMO outcomes, they have used weight or BMI as a surrogate for obesity, not differentiating between lean body weight and ABW in determining survival outcomes. Our study is the first to examine this correlation directly. Al-Soufi and colleagues analyzed adult VV-ECMO patients from the ELSO database between 2005 and 2011.16 They reported that weight was not associated with mortality, and actually demonstrated a reduced risk for mortality on univariate analysis (odds ratio = 0.69 for those >101 kg).16 In 2004, Morris and colleagues reported on 137 pediatric ECMO patients supported at their center over a 6-year period.27 They did not find weight to be a significant predictor of in-hospital mortality among their postoperative and nonsurgical cohorts. In 2010, Mehta and colleagues reported on 58 pediatric ECMO patients at their center from 1999 to 2008, and also did not see a difference in weight between their surviving and nonsurviving cohort.28 However, none of the aforementioned studies have examined indices of end-organ perfusion, such as lactate concentration, with relation to obesity or body weight, which are more subtle indicators of end-organ perfusion. Although absolute weight may not correlate with worse outcomes, elevated differences between ABW and LBM as seen in obesity can impact outcomes when flows are not concomitantly increased to accommodate the increased demand, as seen in our study.
The limitations of our study include those inherent in the retrospective study design. The small sample size also increases type II error and limits the conclusions that can be drawn. Furthermore, we were unable to control for all patient variance and account for all factors that may have affected postoperative laboratory values or mortality. Mortality in most cases was due to cessation of ECMO support according to institutional protocol and not due to failure of ECMO support. Finally, selection bias inherent in our center's protocol for selecting ECMO candidates may confound results when analyzed among other institutions.
Conclusion
In summary, the current study demonstrates that in pediatric patients on VA-ECMO, poorer end-organ perfusion is seen in the low perfusion cohort with Δmass:flow ≥ 0.1, or:
Obesity is not a risk factor for mortality in the pediatric VA-ECMO population, but it requires increased ECMO flows to meet the demands of the ABW; higher circuit flows should be targeted for obese children with consideration of alternative cannulation strategies.
Acknowledgment
This work was supported by CTSI grant UL1TR000124UCLA (Clinical and Translational Science Institute).
References
- 1.Paden M L Conrad S A Rycus P T Thiagarajan R R; ELSO Registry. Extracorporeal Life Support Organization Registry Report 2012 ASAIO J 2013593202–210. [DOI] [PubMed] [Google Scholar]
- 2.Ehren H, Frenckner B, Palmér K. In-vitro evaluation of neonatal ECMO cannulae with regard to flow characteristics. Perfusion. 1990;5(1):45–51. doi: 10.1177/026765919000500106. [DOI] [PubMed] [Google Scholar]
- 3.Gander J W, Fisher J C, Reichstein A R. et al. Limb ischemia after common femoral artery cannulation for venoarterial extracorporeal membrane oxygenation: an unresolved problem. J Pediatr Surg. 2010;45(11):2136–2140. doi: 10.1016/j.jpedsurg.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foley P J, Morris R J, Woo E Y. et al. Limb ischemia during femoral cannulation for cardiopulmonary support. J Vasc Surg. 2010;52(4):850–853. doi: 10.1016/j.jvs.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Bisdas T, Beutel G, Warnecke G. et al. Vascular complications in patients undergoing femoral cannulation for extracorporeal membrane oxygenation support. Ann Thorac Surg. 2011;92(2):626–631. doi: 10.1016/j.athoracsur.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 6.Read R, St Cyr J, Tornabene S, Whitman G. Improved cannulation method for extracorporeal membrane oxygenation. Ann Thorac Surg. 1990;50(4):670–671. doi: 10.1016/0003-4975(90)90219-v. [DOI] [PubMed] [Google Scholar]
- 7.Madershahian N, Nagib R, Wippermann J, Strauch J, Wahlers T. A simple technique of distal limb perfusion during prolonged femoro-femoral cannulation. J Card Surg. 2006;21(2):168–169. doi: 10.1111/j.1540-8191.2006.00201.x. [DOI] [PubMed] [Google Scholar]
- 8.Spurlock D J, Toomasian J M, Romano M A, Cooley E, Bartlett R H, Haft J W. A simple technique to prevent limb ischemia during veno-arterial ECMO using the femoral artery: the posterior tibial approach. Perfusion. 2012;27(2):141–145. doi: 10.1177/0267659111430760. [DOI] [PubMed] [Google Scholar]
- 9.Haley M J, Fisher J C, Ruiz-Elizalde A R, Stolar C JH, Morrissey N J, Middlesworth W. Percutaneous distal perfusion of the lower extremity after femoral cannulation for venoarterial extracorporeal membrane oxygenation in a small child. J Pediatr Surg. 2009;44(2):437–440. doi: 10.1016/j.jpedsurg.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saeed D, Stosik H, Islamovic M. et al. Femoro-femoral versus atrio-aortic extracorporeal membrane oxygenation: selecting the ideal cannulation technique. Artif Organs. 2014;38(7):549–555. doi: 10.1111/aor.12245. [DOI] [PubMed] [Google Scholar]
- 11.Kanji H D, Schulze C J, Oreopoulos A, Lehr E J, Wang W, MacArthur R M. Peripheral versus central cannulation for extracorporeal membrane oxygenation: a comparison of limb ischemia and transfusion requirements. Thorac Cardiovasc Surg. 2010;58(8):459–462. doi: 10.1055/s-0030-1250005. [DOI] [PubMed] [Google Scholar]
- 12.Kurkluoglu M, Hynes C F, Alfares F A. et al. Choice of peripheral venoarterial extracorporeal membrane oxygenation cannulation site in patients above 15 kilograms. J Card Surg. 2015;30(5):461–465. doi: 10.1111/jocs.12538. [DOI] [PubMed] [Google Scholar]
- 13.Vander Salm T J. Prevention of lower extremity ischemia during cardiopulmonary bypass via femoral cannulation. Ann Thorac Surg. 1997;63(1):251–252. doi: 10.1016/s0003-4975(96)00772-2. [DOI] [PubMed] [Google Scholar]
- 14.Jackson K W, Timpa J, McIlwain R B. et al. Side-arm grafts for femoral extracorporeal membrane oxygenation cannulation. Ann Thorac Surg. 2012;94(5):e111–e112. doi: 10.1016/j.athoracsur.2012.05.064. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham S A, Kramer M R, Narayan K M. Incidence of childhood obesity in the United States. N Engl J Med. 2014;370(5):403–411. doi: 10.1056/NEJMoa1309753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Soufi S, Buscher H, Nguyen N D, Rycus P, Nair P. Lack of association between body weight and mortality in patients on veno-venous extracorporeal membrane oxygenation. Intensive Care Med. 2013;39(11):1995–2002. doi: 10.1007/s00134-013-3028-3. [DOI] [PubMed] [Google Scholar]
- 17.Hutagalung R, Marques J, Kobylka K. et al. The obesity paradox in surgical intensive care unit patients. Intensive Care Med. 2011;37(11):1793–1799. doi: 10.1007/s00134-011-2321-2. [DOI] [PubMed] [Google Scholar]
- 18.Hogue C W Jr, Stearns J D, Colantuoni E. et al. The impact of obesity on outcomes after critical illness: a meta-analysis. Intensive Care Med. 2009;35(7):1152–1170. doi: 10.1007/s00134-009-1424-5. [DOI] [PubMed] [Google Scholar]
- 19.Christiaens V Lijnen H R Angiogenesis and development of adipose tissue Mol Cell Endocrinol 2010318(1–2):2–9. [DOI] [PubMed] [Google Scholar]
- 20.Hausman G J, Richardson R L. Adipose tissue angiogenesis. J Anim Sci. 2004;82(3):925–934. doi: 10.2527/2004.823925x. [DOI] [PubMed] [Google Scholar]
- 21.Peters A M, Snelling H L, Glass D M, Bird N J. Estimation of lean body mass in children. Br J Anaesth. 2011;106(5):719–723. doi: 10.1093/bja/aer057. [DOI] [PubMed] [Google Scholar]
- 22.Gaies M G, Gurney J G, Yen A H. et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11(2):234–238. doi: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 23.Maclaren G, Butt W, Best D, Donath S, Taylor A. Extracorporeal membrane oxygenation for refractory septic shock in children: one institution's experience. Pediatr Crit Care Med. 2007;8(5):447–451. doi: 10.1097/01.PCC.0000282155.25974.8F. [DOI] [PubMed] [Google Scholar]
- 24.MacLaren G, Butt W, Best D, Donath S. Central extracorporeal membrane oxygenation for refractory pediatric septic shock. Pediatr Crit Care Med. 2011;12(2):133–136. doi: 10.1097/PCC.0b013e3181e2a4a1. [DOI] [PubMed] [Google Scholar]
- 25.Stöhr F, Emmert M Y, Lachat M L. et al. Extracorporeal membrane oxygenation for acute respiratory distress syndrome: is the configuration mode an important predictor for the outcome? Interact Cardiovasc Thorac Surg. 2011;12(5):676–680. doi: 10.1510/icvts.2010.258384. [DOI] [PubMed] [Google Scholar]
- 26.Ius F, Sommer W, Tudorache I. et al. Veno-veno-arterial extracorporeal membrane oxygenation for respiratory failure with severe haemodynamic impairment: technique and early outcomes. Interact Cardiovasc Thorac Surg. 2015;20(6):761–767. doi: 10.1093/icvts/ivv035. [DOI] [PubMed] [Google Scholar]
- 27.Morris M C, Ittenbach R F, Godinez R I. et al. Risk factors for mortality in 137 pediatric cardiac intensive care unit patients managed with extracorporeal membrane oxygenation. Crit Care Med. 2004;32(4):1061–1069. doi: 10.1097/01.ccm.0000119425.04364.cf. [DOI] [PubMed] [Google Scholar]
- 28.Mehta N M, Turner D, Walsh B. et al. Factors associated with survival in pediatric extracorporeal membrane oxygenation—a single-center experience. J Pediatr Surg. 2010;45(10):1995–2003. doi: 10.1016/j.jpedsurg.2010.05.028. [DOI] [PubMed] [Google Scholar]


