1. Introduction
Neuromyelitis optica (NMO) is an autoimmune inflammatory central nervous system disorder typically associated with longitudinally extensive transverse myelitis (LETM) and optic neuritis. The serum autoantibody against aquaporin-4 (anti-AQP4 antibody) is a highly specific disease marker and is the basis for establishing the concept of NMO spectrum disorder (NMOSD). Isolated involvement of the spinal cord, optic nerve, brainstem, or even cerebrum can be seen in NMOSD [1]. Here we report two patients with late-onset NMOSD who developed isolated cerebral white matter lesions (WMLs) as an initial disease manifestation.
2. Case reports
2.1. Case 1
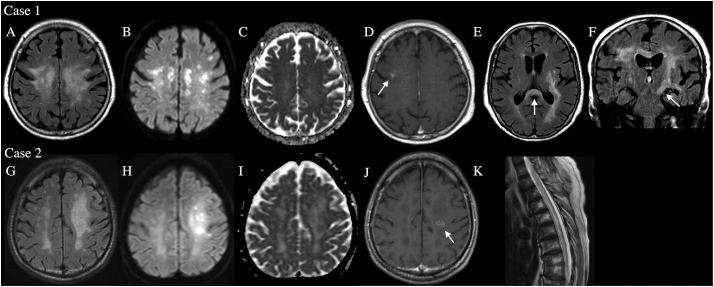
A 79-year-old woman acutely developed dysarthria following an upper respiratory infection. Thereafter, disturbed consciousness, bulbar symptoms, and right hemiplegia emerged. She had a history of breast and gastric cancers and had experienced dry mouth for 10 years. Magnetic resonance imaging (MRI) revealed bilateral supratentorial WMLs (Fig. 1A, B, C, D). The splenium and left corticospinal tract were involved (Fig. 1E, F), but the spinal cord and optic nerves were not. A cerebrospinal fluid (CSF) study showed pleocytosis (7 cells/μL, normally <5) with elevated protein concentration (63.8 mg/dL, normally <43), positive oligoclonal bands (OCBs), and elevated myelin basic protein (1160 pg/mL, normally <102). Polymerase chain reaction did not detect herpes simplex virus DNA. Serology showed anti-SS-A/-B antibodies (>240 and 19.2 U/mL respectively, normally <7), but antibodies against glutamic acid decarboxylase, thyroid peroxidase (TPO), thyroglobulin (Tg), and acetylcholine receptor were absent. Whole-body computed tomography (CT) was negative for malignancy, but lip biopsy revealed chronic sialadenitis compatible with Sjögren's syndrome. The inflammatory CSF findings and coexisting autoimmune disorder suggested autoimmune encephalitis. The patient was treated with high-dose intravenous methylprednisolone (HIMP; 1000 mg/day for three days, twice) followed by oral prednisolone (40 mg/day for a week, then gradually tapered to 15 mg/day) and plasma exchange (2.25 liters every other day for three exchanges). Her neurologic symptoms gradually improved. She could walk with assistance and eat by herself, but cognitive dysfunctions, including emotional lability and moria (Witzelsucht), persisted. She was discharged home 78 days after admission. Afterwards, a cell-based assay (CBA) confirmed anti-AQP4 antibody (titer, 1:4096) in serum frozen before treatment. Antibody against myelin oligodendrocyte glycoprotein (MOG) was negative in serum and CSF. She had not relapsed for 1.5 years with oral prednisolone treatment (15 mg/day).
Fig. 1.
MRI findings. Case 1: A fluid attenuated inversion recovery (FLAIR) image (A) displays an extensive bilateral white matter lesions (WMLs). The WMLs partially show high signals in diffusion-weighted image (DWI) (B), although they are unremarkable in apparent diffusion coefficient (ADC) maps (C). A part of the WMLs is enhanced with gadolinium contrast (an arrow, D). FLAIR images show lesions (arrows) in the splenium (E) and left corticospinal tract (F). Case 2: FLAIR image of a tumefactive extensive WML in the left hemisphere (G). The lesion has cores (which show high signals in DWI [H]) with surrounding vasogenic edema (which shows high intensity in ADC maps [I]). One of the cores is enhanced with gadolinium contrast (an arrow, J). Longitudinally extensive transverse myelitis was evident at the recurrence (K).
2.2. Case 2
An 84-year-old man developed right hemiplegia over a week. Altered consciousness, visual loss, sensory disturbance, or urinary retention were absent. MRI revealed a supratentorial left WML (Fig. 1G, H, I, J) without spinal cord or optic nerve involvement. CSF contained a normal cell count (1 cell/μL) with slightly elevated protein concentration (54.2 mg/dL). OCBs were absent. Serum was negative for antinuclear, anti-SS-A/-B, TPO, and Tg antibodies and rheumatoid factor. Whole-body CT and gallium scintigraphy showed no malignancy. No clinical diagnosis was made, but the patient improved without treatment to walking with a cane and was discharged home 69 days after admission. However, the gait disturbance returned 1 year after the initial onset. MRI revealed LETM (Fig. 1K). An enzyme immunoassay detected anti-AQP4 antibody (>75 U/mL, normally <5.0) in serum. Serum banked at initial presentation was also positive for anti-AQP4 antibody by CBA (titer, 1:4096). Anti-MOG antibody was negative in serum and CSF. He was treated with two courses of HIMP and oral prednisolone (50 mg/day for a week, then gradually tapered to 15 mg/day) but developed dysphagia and quadriplegia and died of aspiration pneumonia 55 days after the second admission.
3. Discussion
Our elderly patients initially presented with isolated cerebral WMLs. Leukoencephalopathies and WMLs occur frequently in elderly patients, and a correct diagnosis is often difficult; ischemia, infections, inflammations, tumors, toxins or drugs, and metabolic derangements are differential diagnoses. Genetic disorders can also cause WMLs [2]. On the other hand, NMO usually occurs in younger patients (in their 30s or 40s) [[3], [4], [5], [6], [7]], even when brain symptoms are the initial presentation (the mean age of onset: 24 years) [[3], [4], [5]]. Although NMO has been reported in elderly patients, myelitis is the initial presentation in most late-onset cases [[5], [6], [7]]. The oldest patient being 88-year-old also developed myelitis [8]. Therefore, NMOSD with cerebral WMLs might be overlooked in elderly patients. NMOSD can coexist with other autoimmune diseases such as systemic lupus erythematosus, Sjögren's syndrome (as in Case 1), or myasthenia gravis [1]. Coexisting autoimmune diseases and characteristic MRI findings can aid in diagnosing late-onset NMOSD initially presenting with isolated cerebral WMLs.
The hypothalamic and periventricular brain regions have high AQP4 expression and are often involved in NMOSD, but extensive WML and long corticospinal tract lesions are also typical of NMOSD [1]. The WMLs are often tumefactive (longest diameter > 3 cm) or have long spindle-like or radial-shaped signal changes that follow white matter tracts. The lesions are likely related to vasogenic edema [9]. AQP4 is not highly expressed in the corticospinal tracts and deep cerebral white matter, and why these regions are involved in NMOSD is unclear. Relatively long axons may be particularly vulnerable, as the optic nerve and spinal cord are susceptible. MOG-antibody positive encephalitis may develop with acute disseminated encephalomyelitis-like WMLs [10], but in our cases both serum and CSF were negative for anti-MOG antibodies.
In conclusion, cerebral WMLs can be the initial manifestation of NMOSD even in elderly patients. We should consider NMOSD as a differential diagnosis for acute leukoencephalopathy, because NMOSD can be treated immunologically to prevent severe neurologic sequelae and does not require invasive diagnostic procedures such as brain biopsy.
Acknowledgments
Acknowledgements
Authors T.O. and T.I. thank Dr. Hiroaki Takahashi (Department of Diagnostic and Interventional Radiology, University of Tsukuba Hospital, Ibaraki, Japan) for helping us prepare the manuscript.
Ethical standards
Written informed consent was obtained from the patients' families.
Conflicts of interest
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Wingerchuk D.M., Banwell B., Bennett J.L. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koralnik I.J., Schellingerhout D., Frosch M.P. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 14-2004. A 66-year-old man with progressive neurologic deficits. N Engl J Med. 2004;350(18):1882–1893. doi: 10.1056/NEJMcpc030038. [DOI] [PubMed] [Google Scholar]
- 3.Min J.H., Waters P., Vincent A. Symptomatic brain involvement as the initial manifestation of neuromyelitis optica. J Clin Neurosci. 2013;20(7):938–942. doi: 10.1016/j.jocn.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Kim S.H., Kim W., Li X.F. Clinical spectrum of CNS aquaporin-4 autoimmunity. Neurology. 2012;78(15):1179–1185. doi: 10.1212/WNL.0b013e31824f8069. [DOI] [PubMed] [Google Scholar]
- 5.Nagaishi A., Takagi M., Umemura A. Clinical features of neuromyelitis optica in a large Japanese cohort: comparison between phenotypes. J Neurol Neurosurg Psychiatry. 2011;82(12):1360–1364. doi: 10.1136/jnnp-2011-300403. [DOI] [PubMed] [Google Scholar]
- 6.Seok J.M., Cho H.J., Ahn S.W. Clinical characteristics of late-onset neuromyelitis optica spectrum disorder: a multicenter retrospective study in Korea. Mult Scler. 2017;23(13):1748–1756. doi: 10.1177/1352458516685416. [DOI] [PubMed] [Google Scholar]
- 7.Collongues N., Marignier R., Jacob A. Characterization of neuromyelitis optica and neuromyelitis optica spectrum disorder patients with a late onset. Mult Scler. 2013;20(8):1086–1094. doi: 10.1177/1352458513515085. [DOI] [PubMed] [Google Scholar]
- 8.Krumbholz M., Hofstadt-Van Oy U., Anfstwurm K. Very late-onset neuromyelitis optica spectrum disorder beyond the age of 75. J Neurol. 2015;262(5):1379–1384. doi: 10.1007/s00415-015-7766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim W., Park M.S., Lee S.H. Characteristic brain magnetic resonance imaging abnormalities in central nervous system aquaporin-4 autoimmunity. Mult Scler. 2010;16(10):1229–1236. doi: 10.1177/1352458510376640. [DOI] [PubMed] [Google Scholar]
- 10.Jarius S., Paul F., Aktas O. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation. 2018;15(1):134. doi: 10.1186/s12974-018-1144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]