Abstract
Background
Full-thickness knee cartilage defects greater than 4 cm2 are best treated with autologous chondrocyte implantation (ACI). Since the articular cartilage surrounding the site of implantation does not always have the normal thickness desirable for successful engraftment, there may be benefit in combining ACI with osteochondral autograft transfer, which provides immediate restoration of condylar contour and mechanical function.
Case presentation
A 19 year-old male who sustained a traumatic anterolateral femoral condyle osteochondral fracture underwent arthroscopic knee surgery three months after injury to harvest healthy cartilage to be sent to the Japan Tissue Engineering Co., Ltd. (J-TEC) for cartilage culture. The patient was re-admitted after four weeks to undergo a procedure using the Osteochondral Autograft Transfer System (OATS®) and the J-TEC autologous cultured cartilage (JACC®) system. Three 4.75-mm osteochondral cylindrical cores were harvested from non-weight-bearing areas of the knee and were transplanted to the lateral periphery of the lateral femoral condyle defect. The cultured cartilage was implanted to the remaining defect with a periosteal cover harvested from the anterolateral ridge of the lateral femoral condyle. Continuous passive range of motion exercises and gait retraining were immediately initiated, with strict no weight-bearing precaution on the operated limb. Partial weight-bearing was allowed four weeks after surgery, which was progressed to full weight-bearing after another two weeks.
Conclusion
ACI must be viewed as a complementary procedure to osteochondral transplantation and this hybrid technique appears to be a promising surgical approach and treatment option for large cartilage lesions, especially in the younger population.
Keywords: Osteochondral autograft transfer, Autologous chondrocyte implantation, Osteochondral lesions
1. Introduction
Focal cartilage defects in the knee can cause substantial patient morbidity in the entire continuum of the disease. In the acute setting, these lesions can be a source of severe and persistent pain, as well as mechanical symptoms and functional impairment [1], [2]. They can also predispose patients to chronic knee problems such as osteoarthritis especially if left untreated [1], [2], [3], [4]. These defects, especially in adults, have limited intrinsic healing potential secondary to the poor regenerative capacity and avascular nature of hyaline cartilage [1], [5], [6].
There is a high prevalence of these defects in the young adult population [2], [4]. Due to their young age and unabated demand for high mobility, these patients do not respond optimally to total joint replacement. Biological repair is the most valuable option to address the needs of this population [4]. It is encouraging that these biological treatment modalities for chondral lesions have shown good results in young patients [2]. Techniques that involve the mechanical removal of the diseased tissue have shown to provide only temporary relief from painful symptoms [7].
Bone marrow stimulation techniques, such as drilling, abrasion arthroplasty, and microfracture, attempt to form repair tissue in the cartilage defect [6], [7]. However, these techniques primarily induce the formation of fibrocartilage, which lacks the histological and biomechanical integrity of hyaline cartilage. This makes the repair tissue formed less durable and susceptible to break down in the long term [6], [7].
Two techniques that have been shown to restore hyaline cartilage are osteochondral transplantation and autologous chondrocyte implantation (ACI). While microfracture is traditionally thought to produce fibrocartilage, osteochondral transplantation and ACI are thought to produce more hyaline-like tissue [3].
In osteochondral autograft transfer or mosaicplasty, plugs of the patient's own healthy cartilage and bone are harvested from non-weight-bearing portions of the femoral condyle and then transferred to pre-drilled holes at the weight-bearing area with a cartilage defect [1], [3], [8]. It replaces chondral defects with normal hyaline articular cartilage [1]. This technique has been used for full-thickness defects up to 2 cm in diameter or 4 cm2 [8]. Several systems have been developed to facilitate this technique, such as the classic mosaicplasty (MO) and the Osteochondral Autograft Transfer System (OATS®; Arthrex, Florida, USA). The main limitation of this technique is defect size as it is best suited for defects 1–4 cm2 [1], [8]. Much larger lesions, up to 8–9 cm2, can be filled with multiple plugs with a risk of significant donor site morbidity [1].
For such large lesions, autologous chondrocyte implantation (ACI) is the more appropriate indication. This technique was introduced in 1994 by Matts Brittberg and Lars Peterson [9] and has become a recognized method to treat full-thickness or International Cartilage Repair Society (ICRS) Grades 3 to 4 cartilage defects in the knee joint [6]. It was the first biological approach using cell transplantation with the aim of providing hyaline-type repair tissue to the defect area [5]. It is a two-stage process in which a biopsy of the patient's articular cartilage is obtained in the first stage, and following ex vivo expansion, cells are implanted into the defect during the second stage [1].
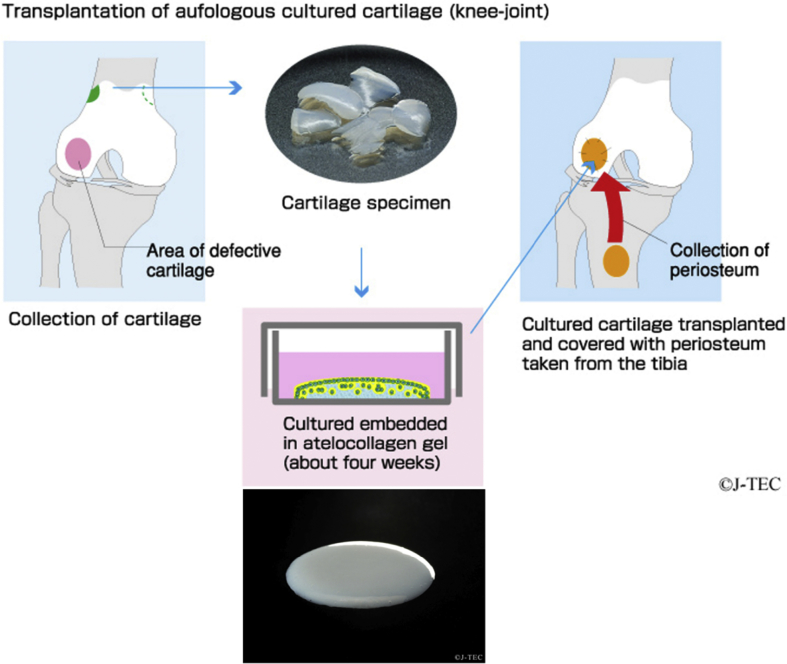
Japan's first cultured cartilage method was developed by Ochi [10]. and commercialized by the Japan Tissue Engineering Co., Ltd. (J-TEC; Aichi, Japan). In this method (Fig. 1), an orthopedic surgeon carries out minimally invasive arthroscopic surgery to collect a small amount of cartilage from the knee. This specimen is sent to J-TEC and isolated chondrocytes are mixed with atelocollagen and shaped into a three-dimensional form. After the gelation at 37 °C, the cultured cartilage is cultivated for about four weeks. During the cultivation, chondrocytes proliferate and produce extracellular matrices, and eventually reach a state closely resembling the properties of the original cartilage. After pre-shipment inspection, J-TEC packages the product and sends it back to the medical institution ready for transplantation.
Fig. 1.
Transplantation process of J-TEC autologous cultured cartilage (JACC). Permission to use this figure was obtained from Japan Tissue Engineering Co., Ltd.
Although cartilage repair tissue after ACI consists mainly of cartilage-like tissue mimicking the macroscopic, microscopic, and biomechanical features of healthy hyaline cartilage [5], evidence shows that most type II collagen was found to be present 30–60 months after treatment [11], suggesting that cartilage repair tissue produced following ACI treatment takes some years to mature [3], [11]. This is where the value of OATS comes in. What it lacks in size-coverage, it makes up for in delivering an immediate, stable, and biomechanically sound cartilage repair [7].
Using a hybrid method that combines both techniques therefore theoretically provides a more effective treatment of large focal osteochondral defects. The osteochondral cores will provide immediate restoration of condylar contour and mechanical function [7] while allowing the implanted chondrocytes in the remaining areas to mature through the years.
2. Case presentation
A 19-year-old male presented at the Emergency Department of our institution after figuring in a motorvehicular accident where his motorcycle crashed against an incoming truck in the opposite lane as he was trying to overtake. After the impact, he got thrown off and hit the ground, sustaining multiple injuries including an open fracture of his right patella. Sagittal cuts of magnetic resonance imaging (MRI) of the right knee showed a large osteochondral fracture of the anterolateral femoral condyle (Fig. 2). Three months following emergency surgeries, he underwent the first stage of ACI. Arthroscopic evaluation of the knee joint was performed and revealed osteochondral lesions on the lateral femoral condyle (ICRS 4 on the anterolateral aspect, measuring 20 × 20 mm). The free osteochondral fragments were removed. The rest of the findings were unremarkable. Approximately 0.5 g of healthy cartilage was harvested from less weight bearing regions of the lateral femoral condyle (Fig. 3). The specimen was sent to J-TEC for cartilage culture. Four weeks after harvest and the start of culture, the patient was readmitted to the hospital for the second stage of the procedure.
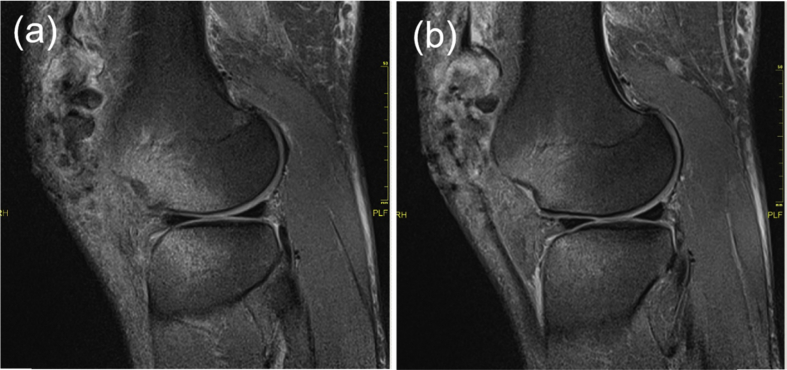
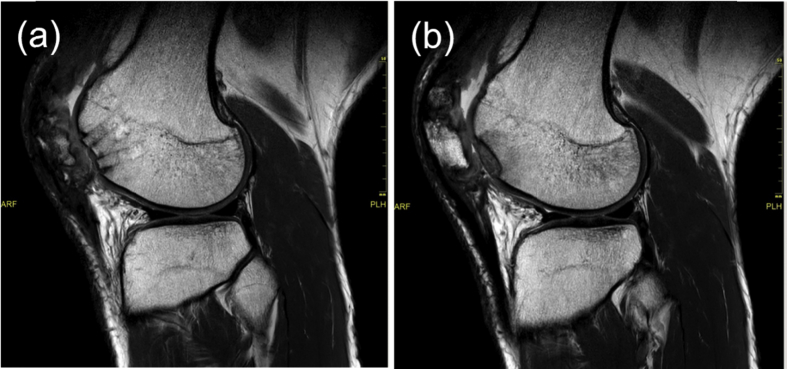
Fig. 2.
Preoperative MRI. (a) Large osteochondral defect along the lateral femoral condyle ridge. (b) Chondral defect and bone bruise in the lateral femoral condyle.
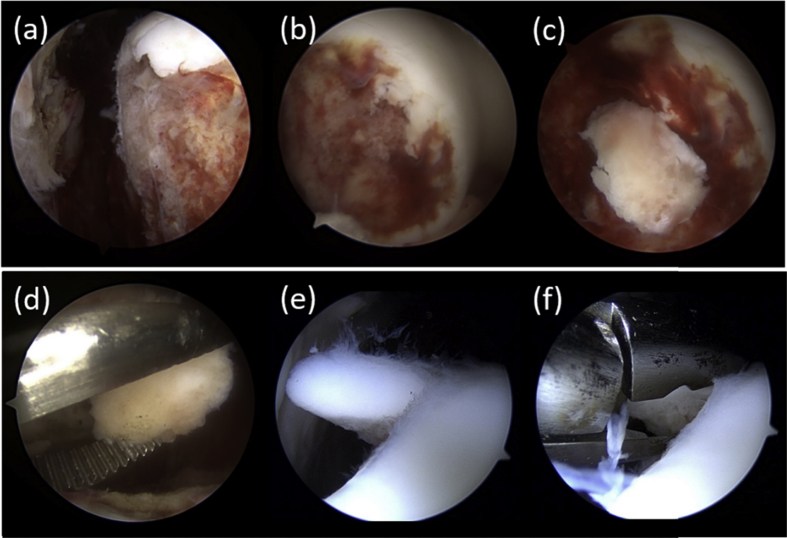
Fig. 3.
(a) Osteochondral defect along the peripheral ridge of lateral femoral condyle. (b) Exposed subchondral bone due to the osteochondral defect. (c) Free osteochondral fragments. (d) Removal of free osteochondral fragments. (e) Shaved unloaded normal articular cartilage. (f) Harvest of normal cartilage tissue.
2.1. Operative technique
The patient was placed in supine position under general anesthesia and femoral nerve block. No tourniquet was used for the procedure. After routine asepsis, antisepsis, and sterile draping, an anterolateral skin incision over the right knee was made, carried down to the underlying fascia. A lateral parapatellar approach was used to expose the knee joint. The defect on the lateral femoral condyle was reassessed and was found to be 20 × 20 mm in size (Fig. 4-a, b). The fibrocartilage as well as all layers of remaining fibrocartilage over the defect were all removed using a bone curette and a high-speed burr, sparing the subchondral bone (Fig. 4-c). Two 4.75 mm-diameter osteochondral cylindrical cores or plugs were harvested from the intercondylar notch and one above the sulcus terminalis using OATS®. The plugs were transplanted to the lateral periphery of the defect (Fig. 4-d). Four JuggerKnot® (Zimmer Biomet, Indiana, USA) 1.2 mm suture anchors with needles were placed in the corners of the remaining defect. A template of the remaining area of the defect was made. The cultured cartilage was shaped using the template and was placed over the defect (Fig. 4-e). A sleeve of periosteum was harvested from the anterolateral ridge of the lateral femoral condyle and was sutured to area of the defect as a flap, with the cambium layer down over the cultured cartilage. The periphery of the implantation was reinforced with nylon 6-0 sutures (Fig. 4-f). The procedure was completed by copious irrigation and wound closure.
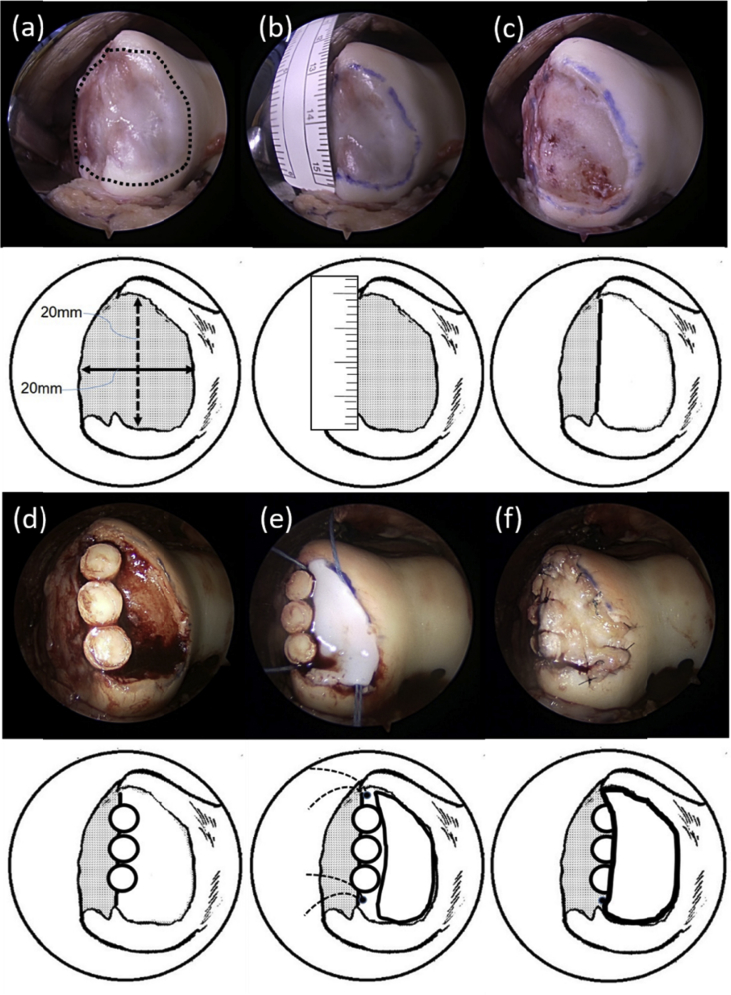
Fig. 4.
(a) Osteochondral defect on the anterolateral femoral condyle, measuring 20 × 20 mm. (b) Fibrocartilage tissue covering the osteochondral defect. (c) Refreshed osteochondral defect site. (d) Transplantation of osteochondral plugs on the peripheral ridge of lateral femoral condyle. (e) Placement of suture anchors and transplantation of autologous cultured cartilage. (f) Periosteum coverage on the transplanted site.
2.2. Postoperative care
The patient was immediately allowed to do continuous passive range of motion exercises the day after surgery. Gait retraining was initiated with strict no weight-bearing precaution on the operated limb. There were no wound complications observed and the stitches were removed 10 days postoperatively. Partial weight-bearing (30% body weight) was allowed four weeks after surgery, and full weight-bearing was permitted from 6 weeks after surgery.
2.3. Outcome and follow-up
Clinical data was collected preoperatively and will be done so at every 3-month point after surgery. The subjective clinical evaluation will be carried out using the Lysholm score and the Knee Injury and Osteoarthritis Outcome Score (KOOS) at each follow up point. One year after the surgery, MRI showed that the three transplanted osteochondral plugs remained in place along the lateral femoral condyle ridge and that the three-dimentional cultured cartilage has well-integrated with the surrounding cartilage of the lateral femoral condyle (Fig. 5).
Fig. 5.
One year postoperative MRI. (a) Three osteochondral plugs remaining in place along the lateral femoral condyle ridge. (b) Well-integrated transplanted autologous cultured cartilage.
3. Discussion
Focal, full-thickness articular and osteochondral defects in the knee cause considerable morbidity to patients and predispose them to chronic knee problems such as osteoarthritis [1], [2], [3], [4], [6]. Arthritis of the knee joint is a major debilitating musculoskeletal condition in modern society, where unicondylar and total knee replacements are the mainstays of patient care for advanced stages [2]. Unfortunately, focal, full-thickness cartilage defects of the knee joint are quite common [3], with numerous studies reporting articular defects in 60–66% of knees undergoing arthroscopy for knee pain [1]. There is a reportedly a large proportion and quite a high prevalence in the young adult population [2], [4]. Current estimates of the prevalence range from 5 to 11% in young patients and up to 60% in older patients [4]. Athletes may be at greater risk [3]. Since these patients do not respond optimally to total joint replacement, biological repair is the most valuable option for them [4].
It is also believed that addressing cartilage defect development is an important target for the prevention of cartilage loss and ultimately for the need of total knee replacement [6]. The currently available surgical options for the treatment of cartilage defects can be divided into transplant procedures and bone marrow stimulation techniques [6], [12]. ACI and autologous osteochondral transplantation (OCT, OATS®, MO) represent the group of transplant procedures. Microfracture, abrasion arthroplasty, and drilling procedures are among the techniques used for bone marrow stimulation. These techniques primarily induce the formation of fibrocartilage, which appears to be inferior when compared with the histological-structural qualities after ACI, the latter resulting in a greater proportion of hyaline-like tissue at the repair site, which may have a beneficial effect on durability [3], [5], [6].
A meta-analysis investigated these various cartilage repair techniques and found that repair tissue after osteochondral transplantation contained a larger amount of hyaline cartilage than was achieved with the other investigated techniques [3]. A histologic analysis also demonstrated a high rate of survivorship of the transferred hyaline cartilage [1]. The existing healthy cartilage of the plugs persists and repair tissue forms around it [3]. Good to excellent clinical results have been obtained in up to 92% of femoral condyle lesions, 87% of tibial lesions, and 79% of trochlear or patellar lesions. This has been replicated in multiple studies with significant improvements both in pain and activity levels in 85% of patients with osteochondral defects of the knee [1]. Since it utilizes the patient's own tissue, the risk of infectious disease transmission possible with allografts is eliminated [1].
Osteochondral transplantation is advocated for full-thickness chondral lesions up to 2 cm in diameter or 4 cm2 [8]. However, it is not appropriate to treat particularly larger cartilage defects. ACI has the advantage of treating these larger lesions (up to 10 cm2) by restoring hyaline-like cartilage [1], [5].
In 2016, Niemeyer and associates laid down precise definitions of appropriate indications for ACI [6]. Their publication aimed to revise the recommendations published in 2004 by the “Clinical Tissue Regeneration” working group of the German Society of Orthopaedics and Trauma using current research and adjusted them to the best currently available evidence (Table 1). The same group has also come up with an algorithm on the appropriate procedure for biological reconstruction of isolated cartilage defects of the knee depending on various factors. The algorithm showed that the appropriate indications for ACI were symptomatic focal cartilage defect (ICRS grade 3 or 4, >3–4 cm2) and large cartilage defect with subchondral bone defect. In the present case, there was an appropriate indication for ACI.
Table 1.
Indications for ACI.
| Defect stage |
|
| |
| Defect size |
|
| |
| Defect localization | No limitation |
| |
| |
| |
| |
| Age |
|
| |
| |
| Contra-indications |
|
| |
| |
| |
|
ACI; autologous chondrocyte implantation.
Cartilage repair tissue after ACI consists mainly of cartilage-like tissue mimicking the macroscopic, microscopic, and biomechanical features of healthy hyaline cartilage [5]. A study by Roberts and associates [11] assessed the relative proportions of type I and II collagens and IIA procollagen in full depth biopsies of repair tissue in a large sample of patients treated with ACI alone (n = 55) or in combination with mosaicplasty (n = 10). 65% of the biopsies were predominantly fibrocartilage (mostly type I collagen and IIA procollagen), 15% were hyaline cartilage (mostly type II collagen), 17% were of mixed morphology and 3% were fibrous tissue (mostly type I collagen). Most type II collagen was present 30–60 months after treatment, suggesting that cartilage repair tissue produced following ACI treatment takes some years to mature.
Due to this time needed for the repair tissue from ACI to mature to hyaline form, as well as the size-limitation of osteochondral transplantation, a hybrid procedure has been proposed for the individual procedures to augment each other. Sharpe and colleagues hypothesized that the osteochondral cores provide restoration of the condylar contour and mechanical function, while the implantation of chondrocytes will fill the remaining areas with a functional covering and improve chondral integration [7]. The group investigated the use of a hybrid method combining OATS® with ACI for patients with large defects (mean size of 4.84 ± 3.45 cm2). 13 patients underwent surgery by this method and were assessed for up to three years to determine the quality of the repair. Arthroscopic evaluation of the joint surfaces was done after one and three years, revealing that the cores had become well-integrated in all cases. In general, the repair tissue seemed to be closely adherent to native and donor cartilage, although it remained fibrillated at one year, was whiter than the cores, and did not reach the same height as the cores. Clinically, ten of 13 (77%) patients showed an improvement in KSS after 3 years.
While the principles of the aforementioned procedure are essentially the same as those of the procedure described in the current study, there are differences in technique that the authors wish to highlight. While the OATS® part of the procedure remains standard in both studies, the way cartilage is cultured and prepared for implantation differs significantly. The chondrocytes in the study of Sharpe and colleagues [7] were cultured according to the original methods of Brittberg and colleagues in 1994 [9], which is representative of first-generation, conventional ACI [13]. In this procedure, autologous chondrocytes are expanded in a monolayer culture system, and then implanted in suspension into the osteochondral lesion, and covered with a periosteal flap [13], [14]. While excellent clinical results have been reported, this method has raised concerns particularly about re-expression of the chondrocyte phenotype of the monolayer-cultured fibroblastic cells after transplantation, uneven distribution of cells in the defect, and leakage of the chondrocyte suspension [13]. To address these problems, second-generation methods have been developed, involving implantation of cultured chondrocytes via cell-seeded, three-dimensional, bioabsorbable scaffolds [15]. These have been said to preserve the phenotype of the cultured chondrocytes, enable even distribution of cells in the transplanted tissue, and have a lower risk of leakage from the grafted site [13].
J-TEC employs such second-generation methods in preparing tissue-engineered cartilage-like tissue by culturing the autologous chondrocytes in atelocollagen gel for about four weeks. This procedure results in the formation of a gel-like material after culture (Fig. 1), in which the risks of leakage and uneven distribution of chondrocytes are reduced. This is not monolayer-cultured but three-dimensional cultured. The implantation is done as described in the operative technique previously. However, even three-dimensional cultured cartilage cannot reconstruct bony structures. In the present case, osteochondral plugs were implanted in order to reconstruct a part of lateral femoral chondral peripheral ridge. The periosteum flap was sutured, bridging tops of osteochondral plugs and normal articular cartilage of intact lateral femoral condyle. Therefore, normal shape of articular cartilage of lateral femoral condyle was maintained.
4. Conclusions
The significant value of ACI at this time is the treatment of larger cartilage lesions. It must then be viewed as a complementary rather than competitive procedure to osteochondral transplantation. Although still without long-term follow up to demonstrate results, the described hybrid technique appears to be a promising surgical approach and treatment option for large focal osteochondral lesions, especially in the younger population.
Conflicts of interest
All authors have no potential conflicts of interest, including financial interests, activities, relationships, and affiliations, to disclose.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Camp C.L., Stuart M.J., Krych A.J. Current concepts of articular cartilage restoration techniques in the knee. Sport Health. 2014;6(3):265–273. doi: 10.1177/1941738113508917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pascual-Garrido C., Daley E., Verma N.N., Cole B.J. A comparison of the outcomes for cartilage defects of the knee treated with biologic resurfacing versus focal metallic implants. Arthroscopy. 2017;33(2):364–373. doi: 10.1016/j.arthro.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 3.DiBartola A.C., Everhart J.S., Magnussen R.A., Carey J.L., Brophy R.H., Schmitt L.C. Correlation between histological outcome and surgical cartilage repair technique in the knee: a meta-analysis. Knee. 2016;23(3):344–349. doi: 10.1016/j.knee.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Vavken P., Samartzis D. Effectiveness of autologous chondrocyte implantation in cartilage repair of the knee: a systematic review of controlled trials. Osteoarthritis Cartilage. 2010;18(6):857–863. doi: 10.1016/j.joca.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Moradi B., Schonit E., Nierhoff C., Hagmann S., Oberle D., Gotterbarm T. First-generation autologous chondrocyte implantation in patients with cartilage defects of the knee: 7 to 14 years' clinical and magnetic resonance imaging follow-up evaluation. Arthroscopy. 2012;28(12):1851–1861. doi: 10.1016/j.arthro.2012.05.883. [DOI] [PubMed] [Google Scholar]
- 6.Niemeyer P., Albrecht D., Andereya S., Angele P., Ateschrang A., Aurich M. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: a guideline by the working group "Clinical Tissue Regeneration" of the German Society of Orthopaedics and Trauma (DGOU) Knee. 2016;23(3):426–435. doi: 10.1016/j.knee.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Sharpe J.R., Ahmed S.U., Fleetcroft J.P., Martin R. The treatment of osteochondral lesions using a combination of autologous chondrocyte implantation and autograft: three-year follow-up. J Bone Joint Surg Br. 2005;87(5):730–735. doi: 10.1302/0301-620X.87B5.14936. [DOI] [PubMed] [Google Scholar]
- 8.Goyal D., Keyhani S., Goyal A., Lee E.H., Hui J.H., Vaziri A.S. Evidence-based status of osteochondral cylinder transfer techniques: a systematic review of level I and II studies. Arthroscopy. 2014;30(4):497–505. doi: 10.1016/j.arthro.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 9.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 10.Ochi M., Uchio Y., Kawasaki K., Wakitani S., Iwasa J. Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defects of the knee. J Bone Joint Surg Br. 2002;84(4):571–578. doi: 10.1302/0301-620x.84b4.11947. [DOI] [PubMed] [Google Scholar]
- 11.Roberts S., Menage J., Sandell L.J., Evans E.H., Richardson J.B. Immunohistochemical study of collagen types I and II and procollagen IIA in human cartilage repair tissue following autologous chondrocyte implantation. Knee. 2009;16(5):398–404. doi: 10.1016/j.knee.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brophy R.H., Zeltser D., Wright R.W., Flanigan D. Anterior cruciate ligament reconstruction and concomitant articular cartilage injury: incidence and treatment. Arthroscopy. 2010;26(1):112–120. doi: 10.1016/j.arthro.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Tadenuma T., Uchio Y., Kumahashi N., Fukuba E., Kitagaki H., Iwasa J. Delayed gadolinium-enhanced MRI of cartilage and T2 mapping for evaluation of reparative cartilage-like tissue after autologous chondrocyte implantation associated with Atelocollagen-based scaffold in the knee. Skeletal Radiol. 2016;45(10):1357–1363. doi: 10.1007/s00256-016-2438-z. [DOI] [PubMed] [Google Scholar]
- 14.Adachi N., Ochi M., Deie M., Nakamae A., Kamei G., Uchio Y. Implantation of tissue-engineered cartilage-like tissue for the treatment for full-thickness cartilage defects of the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1241–1248. doi: 10.1007/s00167-013-2521-0. [DOI] [PubMed] [Google Scholar]
- 15.Harris J.D., Siston R.A., Brophy R.H., Lattermann C., Carey J.L., Flanigan D.C. Failures, re-operations, and complications after autologous chondrocyte implantation--a systematic review. Osteoarthritis Cartilage. 2011;19(7):779–791. doi: 10.1016/j.joca.2011.02.010. [DOI] [PubMed] [Google Scholar]