Abstract
Interactions between social cognition and symptoms of schizophrenia have been investigated, but mostly component by component. Here we tested the assumption that two categories of deficits exist depending on clinical profiles, one corresponding to a defect in social cognition – “under-social cognition” – and one corresponding to excessive attributions leading to social cognitive impairments – “over-social cognition”. To conduct the investigation, we performed a Hierarchical Clustering Analysis using positive and negative symptoms in seventy patients with schizophrenia and we compared the clusters obtained to a group of healthy controls on social cognitive measures. We distinguished two social cognitive profiles based on prevailing symptoms for emotion processes and Theory of Mind. Actually, patients with negative symptoms showed lower performances in emotion recognition task than both those with positive symptoms and controls. Concerning Theory of Mind, patients with positive symptoms had a significant tendency to make over interpretative errors than both patients with negative symptoms and controls. For other processes assessed, further explorations are needed. Actually, concerning social perception and knowledge both patients' groups presented significant impairments compared to controls. Assessment of attribution bias showed that patients in the positive group presented a significant hostility bias and a higher intentionality score compared to healthy controls. These results favor the existence of different categories of impairments depending more on the clinical characteristics of patients than on nosographical categories, but further investigations are now necessary to specify these profiles. It nevertheless showed the importance of assessing symptoms in relationship with cognitive functioning.
Keywords: Social cognition, Schizophrenia, Symptoms, Emotion, Theory of mind, Attribution style
1. Introduction
Social cognitive impairments constitute a core feature of schizophrenia (Green et al., 2008) and strongly predict functioning in daily life (Couture et al., 2006; Fett et al., 2011; Schmidt et al., 2011). There is now a consensus that social cognition is a multidimensional construct including four main domains which are impaired in schizophrenia (Pinkham, 2014): (1) emotion processing, i.e. the ability to identify and recognize emotions through facial expressions, gestures and tone of voice; (2) social perception and knowledge which can be defined as the decoding and interpretation of social cues, by taking the social context into account and being aware of social rules, roles, and goals, (3) Theory of Mind (ToM), which refers to the ability to represent human mental states and to make inferences about others' intentions, beliefs, desires and knowledge; and (4) attributional style, which refers to the manner in which individuals interpret, explain or make sense of the positive and negative social events encountered in life.
Social cognitive abilities contribute to real life outcome (Fett et al., 2011) and appear to be related to the symptoms of schizophrenia. Interactions between cognition and symptoms have been studied since the 90s and several authors have stressed the impact of cognitive disorders on symptoms production – notably positive symptoms (Frith, 1992; Frith et al., 2000). Research on social cognition is more recent. Nonetheless, the links between social cognition and positive symptoms – hallucinations and delusions notably – are inconsistent (Nelson et al., 2007; Shean and Meyer, 2009), even if significant associations have been observed between positive symptoms and both impairment of facial emotion recognition (Hall et al., 2004; Kohler et al., 2010) and ToM (Bora et al., 2009; Montag et al., 2011). Other studies have shown a positive correlation between attributional biases, including hostile attributional biases, and paranoid delusions (Green and Leitman, 2008). Concerning negative symptoms, most research shows significant associations with poor ToM performances (Brüne, 2005; Sprong et al., 2007) while no relationship has been demonstrated with attributional style. Moreover, it appears that negative symptoms – and more specifically anhedonia or affective flattening – also seem correlated with emotional processes (Sergi et al., 2007). Taken together, the results suggest that the negative dimension of schizophrenia is closely related to specific processes of social cognition, although both remain independent constructs (Piskulic and Addington, 2011). Social cognitive impairments in schizophrenia, and especially ToM impairments, are thus directly associated with the severity of negative symptoms (Lincoln et al., 2011).
A hypothesis postulates that different symptom dimensions could be related to distinct ToM alterations. Some studies indeed suggest that the latter might be related either to excessive (positive symptoms) or defective (negative symptoms) attribution of mental states to others (Montag et al., 2011). The assumption of deficits due to over attribution of knowledge to others was described as “hyper-theory of mind” (Abu-Akel and Bailey, 2000; Ahmad Abu-Akel, 1999). According to this hypothesis, ToM impairments in schizophrenia may depend on symptoms and form a continuum from impaired understanding of others' mental states or difficulties in applying this understanding – patients with negative symptoms – to a hyper-ToM that leads patients with positive symptoms to over attribute knowledge and mental states to their interlocutors (A. Abu-Akel, 2003; Frith, 2004).
Thus, although social cognitive deficit profiles have been studied according to symptoms in schizophrenia, to date, no study have looked at this question taking into account all the components of social cognition together. Furthermore, although there is general agreement among experts that social cognition is a multidimensional construct (Mancuso et al., 2011; van Hooren et al., 2008), boundaries between social cognitive processes are porous and there is considerable overlap between them. That is why we make the supposition here that “hypo/under” to “hyper/over” could be applied to the entire field of social cognition. We assume that two categories of deficits exist depending on the clinical profile of patients and notably on positive and negative symptoms, one corresponding to a defect in social cognition – “under-social cognition” – and one corresponding to excessive attributions “over-social cognition”. According to this assumption, two types of social cognitive profiles may be distinguished: in patients with schizophrenia with prominent negative symptoms, we forecast they would have significant deficits in tasks measuring emotion recognition and social perception, impairment of ToM related to defective mentalization, and a relatively preserved attributional style. This profile is called “under-social cognition”. Conversely, we predicted that patients with positive symptoms would mainly present difficulties in social perception tasks related to a tendency to over interpret contextual cues, ToM impairments related to their excessive attribution of mental states to others – “hyper-ToM”, and attributional bias. These patients correspond to the “over-social cognition” profile.
The purpose of our study was to explore the relationships between positive and negative symptoms and social cognitive performances in patients with schizophrenia using the categorical hypothesis, explaining social cognitive impairment on the one hand by a deficit of social cognitive abilities, and on the other, by over attributions conducting to social cognitive impairments. To conduct the investigation, we first compared a group of patients with schizophrenia to a group of healthy controls using tests for assessing social cognition. We used a social cognitive battery called ClaCoS, regrouping both validated assessments traditionally used to measure social cognitive competences and new tools. The ClaCoS work was conducted in France based on the SCOPE study (Social Cognition Psychometric hypothesis, NIMH Initiative) in the US and will be published soon. After checking the sensitivity of the tests measuring social cognitive impairments, we performed a Hierarchical Clustering Analysis using positive and negative symptoms in our population of patients with schizophrenia and then compared the two clusters obtained to the healthy group on each social cognitive measure.
2. Methods
The study was carried out in accordance with the Declaration of Helsinki and was approved by the local Ethics Committee (CPP Lyon – Sud Est IV, No. 15/041; ANSM, No. 2015-A00580–49). Written informed consent to take part in the study was received from all participants. The control subjects were paid 30 euros for their participation.
2.1. Participants
Seventy patients with schizophrenia aged from 18 to 45 years old were enrolled in this multisite study conducted in three hospitals in France, Le Vinatier in Lyon, Sainte-Anne in Paris and Bretonneau in Tours.
To be eligible, patients had to be diagnosed with schizophrenia as confirmed by the specific clinical interview for DSM-5, and had to be on a stable medication regimen for a minimum of one month. All patients were treated with typical or atypical antipsychotics. They were from three different psychosocial rehabilitation units, they are all outpatients, mainly stabilized, and without florid symptoms. Each patient was evaluated by an experienced psychiatrist with the Positive and Negative Syndrome Scale (PANSS, Kay et al., 1987; French version by Lépine et al., 1989). The demographical and clinical characteristics of the patients are shown in Table 1.
Table 1.
| Patients with schizophrenia |
Healthy controls |
||
|---|---|---|---|
| N = 70 |
N = 50 |
||
| Mean (SD) | Mean (SD) | ||
| Age (years) | 31.9 (8.3) | 28.4 (7.3) | |
| Gender (M/F) | 52/18 | 34/16 | |
| Education (years) | 12.2 (2.4) | 13.6 (1.7) | |
| PANSS total | 71.5 (14.1) | – | |
| Positive subscore | 14.3 (4.8) | – | |
| Negative subscore | 20.7 (6) | – | |
| General subscore | 36.5 (7.6) | – | |
Fifty healthy controls were recruited via community advertisements. They were screened for the absence of psychopathology using the MINI (International Neuropsychiatric Interview, Lecrubier et al., 1997). Exclusion criteria for both groups included: (i) presence or history of pervasive developmental disorders or intellectual deficiency, (ii) presence or history of neurological disorders affecting the brain function, (iii) presence of severe visual or hearing impairment interfering with assessment, (iv) absence of French language proficiency or important reading difficulties, (v) presence of substance abuse in the past month (tobacco excluded).
2.2. Social cognitive measures
All participants were tested in a silent room by an experienced neuropsychologist. They were proposed a complete assessment, taking approximately 2 h, using the ClaCoS battery (publication in preparation), including the tests presented in Fig. 1.
Fig. 1.
Tests constituting the ClaCoS battery.
In this study, we only focused on the four major components of social cognition assessed with the following tests:
-
(i)
Emotion processing – Facial Emotion Recognition Test (TREF, Gaudelus et al., 2015)
The TREF assesses the ability to correctly recognize six basic and universal emotions (happiness, anger, sadness, fear, disgust and contempt). The test includes 54 photos. Facial expressions are represented with color photographs of six models of different sex and ages. Each photo is displayed for 10 s but response time is not limited. Each emotion is presented with nine levels of intensity from 20 to 100%. This assessment provides an overall percentage of emotion recognition (global score), and for each emotion (score per emotion) and detection thresholds (global and per emotion), that means the level of intensity from which emotion is recognized with certainty. In this study, we specifically analyzed the global and per emotion accuracy scores and the intensity level scores.
-
(ii)
Social perception and knowledge – PerSo (GDR 3557, in preparation)
The PerSo measures competence in the perception of social situations depicted in 4 pictures taken from the material “ColorCards – Social behavior”. For the participant, the first instruction is to describe all the elements contained in the picture (objects, their size, shape or color) in 1 min 30 to ensure that difficulties in interpretation are not due to attentional or perceptual deficits. This task provides a global “fluency score” (one point assigned per element reported). Participants then have to freely explain the social situation (i.e. without cuing). Three components are expected: the context, the main characters and the interaction between them. For each picture, this “non-cued interpretation score” is rated up to 3 with one point being given per element reported. If an element is missing, an indexed question is proposed. The “cued interpretation score” is also rated up to 3 and a “total interpretation score” is then given out of 6. Finally, the participants are asked a question concerning a social convention depicted in the card. This “social knowledge score”, rated for each picture up to 2, evaluates the capacity of a participant to extract a social rule or convention from the picture.
-
(iii)
Theory of Mind (ToM)/mental states attribution – Movie for the Assessment of Social Cognition (MASC test, Dziobek et al., 2006; French version of Martinez et al., 2017)
The MASC test is a video-based tool for measuring ToM abilities. It is a 15-min movie specifically meant to analyze affective and cognitive ToM components and the impairment profiles of the participants presenting mentalizing deficits to over interpretative skills. The movie features four people meeting on a Saturday evening. It includes 45 sequences and at the end of each sequence, the subject has to answer a question referring to the actors' mental states – emotions or feelings, thoughts and intentions – by choosing between four possible answers: the correct answer: “ToM”; the undermentalization response: “less ToM”; a literal answer, with no mentalization: “no ToM”; or the over interpretative response: “excessive ToM”. A total score of correct answers is rated: (all right “ToM” response), and three error scores: a “less ToM” score, a “no ToM” score and an “excessive ToM” score.
-
(iv)
Attributional style – Ambiguous Intentions and Hostility Questionnaire (AIHQ, Combs et al., 2007; French version by Angelard A.)
The AIHQ measures hostile social-cognitive bias. The original version of the AIHQ consists of 15 negative daily situations that differ in terms of intentionality – accidental, intentional or ambiguous situations. In the ClaCoS battery we only use the five ambiguous situations. The subject is asked to read each situation and imagine the scenario happening to him/her. Then, he has to answer several questions to measure three biases: hostility bias (HB), attribution of responsibility score and aggression bias (AB). The hostility score (HB) is rated by the assessor for each ambiguous situation – from 1 “not hostile at all” to 5 “very hostile” – according to the answer to the question “what do you think was the real reason why the person acted that way?”. The attribution of responsibility score is the average of the participant's rates on the following three Likert scales: (1) whether the person acted on purpose – from 1 “absolutely not” to 6 “absolutely on purpose” (intentionality score – IS); (2) how angry it would make the subject feel – from 1 “not angry at all” to 5 “very angry” (anger score – AS); and (3) how much they would blame the other person – from 1 “not at all” to 5 “very much” (blame score – BS). Finally, the aggression score (AB) is rated by the assessor – from 1 “not aggressive at all” to 5 “very aggressive” for each ambiguous situation – according to the participant's proposition to the question “What would you do about it?”
2.3. Statistical analysis
Statistical analysis was performed with R software (R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/). Groups were matched on demographic characteristics and independence was assessed with the chi-square test (sex), Fisher's exact-test (level of education) and Student's t-test (age). Normality and variance homogeneity were checked using the Shapiro-Wilk and Bartlett-test, respectively. Comparisons of two groups were carried out with Student's t-test (parametric) or Wilcoxon's rank-sum test (non-parametric). Next, an unsupervised Hierarchical Cluster Analysis of the PANSS items was performed and clusters were combined using Ward's method. Finally, to compare clusters, continuous variables were compared using ANOVA with post-hoc Tukey test, or Kruskal-Wallis with Dunn test for parametric and nonparametric data respectively (Benjamini-Hochberg multiple testing correction). Two-sided p values < .05 were considered statistically significant.
3. Results
3.1. Comparative results of social cognitive assessments in patients with schizophrenia and controls
First, we assessed performances in ClaCoS tests in a group of fifty patients with schizophrenia compared to a group of fifty healthy controls matched for age (patients: m = 30.9, sd = 7.8 vs controls: m28.4, sd = 7.3; t(98) = −1.7; p = .1, n.s.) and gender (patients: 38 males/12 females vs controls: 34 males/16 females; khi2(1) = 0.45; p = .5, n.s.). Unfortunately, the groups were not matched for education level (patients: m = 13.1, sd = 1.9 vs controls: m = 13.6, sd = 1.7; p = .001, Fisher's exact test and Phi = 0.52).
In accordance with our expectations and the literature, results highlighted significant differences between patients and controls for each social cognitive assessment proposed (Table 2).
Table 2.
| Patients with schizophrenia |
Controls |
Results |
p Value | Effect size (Cohen's d) | ||
|---|---|---|---|---|---|---|
| N = 50 |
N = 50 |
T test or Wilcoxon test | ||||
| Mean (SD) | Mean (SD) | |||||
| TREF - emotion processes | ||||||
| % of correct recognition - total Scorea | 67.7 (8.5) | 72.5 (7.8) | 3 | <0.01 | 0.60 | |
| Joy | 88.9 (11.7) | 89.6 (10.1) | 1273.5 | ns | 0.03 | |
| Anger | 66.0 (20.0) | 71.8 (17.7) | 1450 | ns | 0.28 | |
| Sadness | 73.6 (20.8) | 76.0 (17.9) | 1330.5 | ns | 0.11 | |
| Feara | 78.9 (15.8) | 85.1 (13.6) | 1549.5 | <0.05 | 0.43 | |
| Disgust | 55.6 (18.7) | 61.1 (13.1) | 1448.5 | ns | 0.28 | |
| Contempta | 41.8 (20.9) | 51.8 (21.8) | 1578 | <0.05 | 0.47 | |
| Intensity level - total Scorea | 51.5 (7.9) | 47.0 (9.0) | −2.6 | <0.05 | 0.53 | |
| Joy | 32.1 (11.8) | 30.2 (10.2) | 1110 | ns | 0.17 | |
| Anger | 53.4 (17.8) | 47.3 (19.4) | 974.5 | ns | 0.36 | |
| Sadness | 47.4 (19.0) | 46.1 (16.6) | 1160.5 | ns | 0.09 | |
| Fear | 43.0 (17.2) | 36.6 (14.6) | 969 | ns | 0.37 | |
| Disgust | 61.6 (16.8) | 56.3 (12.9) | 1026.5 | ns | 0.28 | |
| Contempta | 71.8 (19.3) | 62.6 (17.5) | 807.5 | <0.01 | 0.62 | |
| PerSo - social perception & knowledge | ||||||
| Fluency scorea | 58.9 (22.5) | 94.9 (30.8) | 6.7 | <0.01 | 1.41 | |
| Interpretation - total scorea | 17.7 (3.6) | 21.1 (2.2) | 1974.5 | <0.01 | 1.16 | |
| Non indexeda | 8.1 (2.2) | 9.9 (1.6) | 1827 | <0.01 | 0.88 | |
| Indexeda | 9.6 (1.6) | 11.2 (0.9) | 2040 | <0.01 | 1.35 | |
| Social knowledge scorea | 3.5 (2.0) | 5.9 (1.6) | 2028 | <0.01 | 1.29 | |
| MASC - theory of mind | ||||||
| Total scorea | 27.6 (4.2) | 32.7 (3.0) | 6.9 | <0.01 | 1.46 | |
| Error types | ||||||
| Excessive ToM | 5.4 (2.8) | 5.1 (2.5) | 1147 | ns | 0.14 | |
| Less ToMa | 8.8 (3.2) | 5.7 (2.5) | −5.5 | <0.01 | 1.10 | |
| No ToMa | 3.2 (2.2) | 1.6 (1.2) | 674 | <0.01 | 0.89 | |
| AIHQ - attributional style | ||||||
| Hostility bias - HB | 1.8 (0.7) | 1.7 (0.7) | 1052.5 | ns | 0.25 | |
| Attribution of responsability scorea | 2.7 (0.8) | 2.3 (0.7) | −2.7 | <0.01 | 0.54 | |
| Intentionality score-ISa | 2.9 (1.0) | 2.5 (0.8) | −2.4 | <0.05 | 0.48 | |
| Anger score - ASa | 2.4 (0.8) | 2.0 (0.6) | 830 | <0.01 | 0.58 | |
| Blame score - BSa | 2.8 (0.9) | 2.4 (0.8) | −2.3 | <0.05 | 0.46 | |
| Agression bias – AB | 1.7 (0.5) | 1.7 (0.4) | 1018.5 | ns | 0.30 | |
TREF: facial emotion recognition test; PerSo: perception and social knowledge test; MASC: movie for the assessment of social cognition; AIHQ: ambiguous intentions and hostility questionnaire.
Processes with significant differences between the two groups.
3.2. Links between social cognition and symptom profiles in schizophrenia
To study the links between social cognitive profiles and symptoms, we regrouped the 70 patients with schizophrenia. Globally in our population, symptoms assessed with the PANSS were moderate with, for the majority of patients, a slightly higher negative symptom score compared to positive symptoms (Table 1).
Based on this general clinical profile, we did not divide our population into two groups depending only on total negative and positive subscale scores, but classified them using Hierarchical Cluster Analysis (HCA) on the positive and negative PANSS scores. HCA makes it possible to partition the population into homogeneous clusters (low within-variability) that are different from one and other (high between-variability) (Ramsay and Silverman, 2005). The number of resulting groups and the sample of each group have been derived from the data themselves and not user determined. This kind of analysis has already been used in studies focusing on symptoms in schizophrenia (Correll et al., 2011).
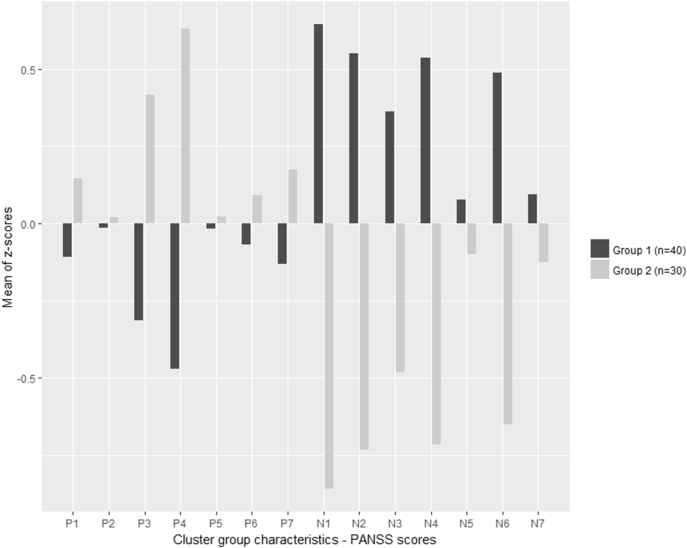
We obtained two clusters (Fig. 2) with our population: one composed of patients presenting high scores for the positive items of the PANSS (P1, P3, P4 and P7) and low scores for the negative items (N1, N2, N3, N4 and N6). This group was named the “positive group” (N = 30). The second cluster named the “negative group” (N = 40) contained patients with low scores for the positive items of the PANSS (P3 and P4) and high scores for the negative items (N1, N2, N3, N4 and N6).
Fig. 2.
Cluster group characteristics according to the PANSS items.
We then compared performances of the patients included in the “positive group” and the “negative group” to healthy controls on each of the tests assessing social cognition.
-
(i)
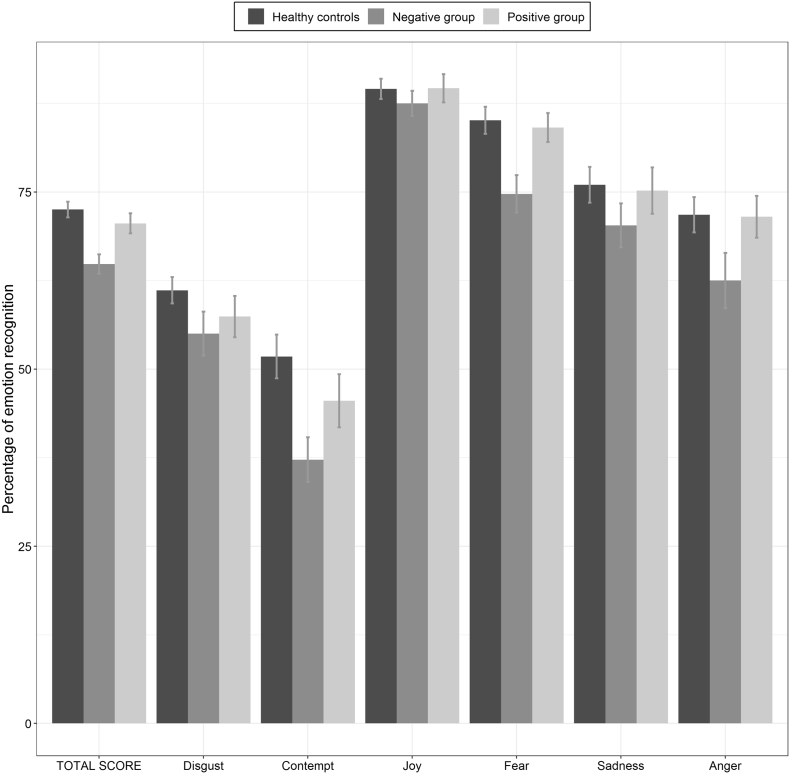
Emotion recognition assessed with the TREF showed significant group effects for both the global emotion recognition score (F(2,117) = 10.52; p < .001, ηp2 = 0.152) and the global intensity level score (F(2,117) = 7.46; p = .001, ηp2 = 0.113). The post-hoc analyses using Tukey or Dunn indicated that the “negative group” had lower global emotion recognition scores (m = 64.8, sd = 8.2) than both the “positive group” (m = 70.6, sd = 7.7) and healthy controls (m = 72.5, sd = 7.82). No difference between the “positive group” and healthy controls has been observed (Fig. 3). Concerning the intensity level score, patients in the “negative group” had a significantly higher detection threshold than healthy controls (m = 53.9, sd = 8.2 vs. m = 49.6, sd = 7.3), but no difference has been noted with patients in the “positive group” (m = 49.6, sd = 7.29).
-
(ii)
Concerning social perception assessed with the PerSo test, significant group effects have been observed for all the measures: fluency score (F(2,73.92) = 25.9; p < .001, ηp2 = 0.334), total interpretation score (H(2) = 30.25; p < .001, ηp2 = 0.228), and social knowledge score (H(2) = 33.33; p < .001, ηp2 = 0.248). Unfortunately, post-hoc analyses did not highlight any significant differences between the “positive” and “negative” groups of patients. Both groups, positive one (m = 59.17, sd = 20.93) and negative one (m = 57.4, sd = 22.43) used significantly less elements than controls (m = 94.9, sd = 30.79) to describe the pictures, there interpretations of the social situations were less correct (respectively m = 18.27, sd = 4.05 for “positive group” and m = 17.23, sd = 3.44 for “negative group”) than those of the healthy controls (m = 21.1, sd = 2.23), and they had more difficulties to infer the social knowledge depicted in the images (respectively m = 4.2, sd = 2.23 for “positive group” and m = 3.3, sd = 1.87) than controls (m = 5.88, sd = 1.57).
-
(iii)
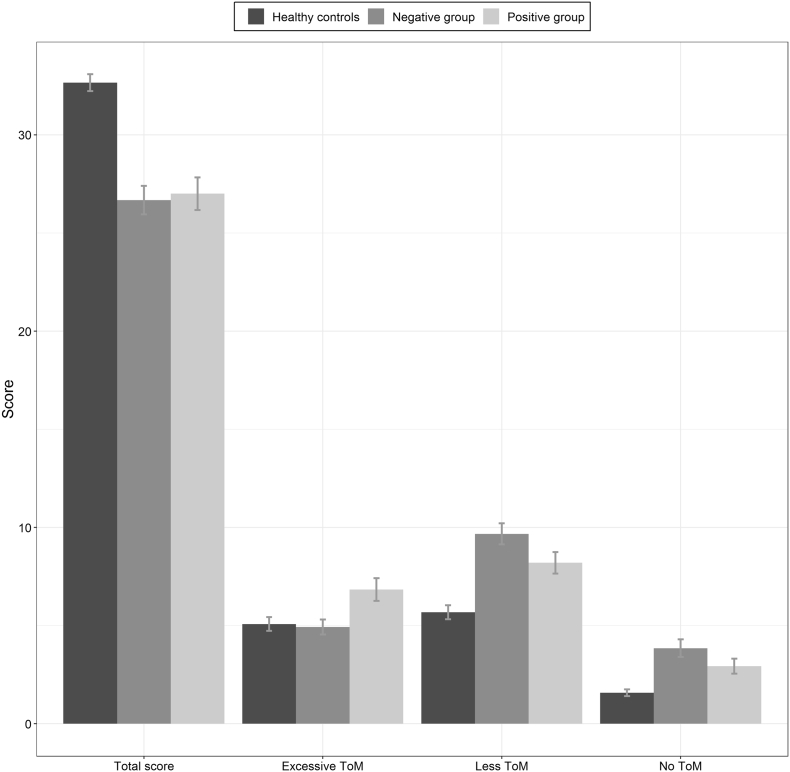
With the MASC test used to assess Theory of Mind, a significant group effect has been highlighted for the total score (F(2,62.24) = 34.9; p < .001, ηp2 = 0.346) and post-hoc analysis showed than both “positive group” (m = 27, sd = 4.56) and “negative group” (m = 26.68, sd = 4.62) had significant lower scores than controls (m = 32.66, sd = 3.03). Groups effects have been also observed for each error type – (H(2) = 7.68; p < .05, ηp2 = 0.064) for over-interpretative “Exc ToM” errors, (H(2) = 31.2; p < .001, ηp2 = 0.234) for undermentalisation “Less ToM” errors, and (H(2) = 23.93; p < .001, ηp2 = 0.185) for literal “no ToM” answers – and post-hoc analysis showed that patients in the “positive group” (m = 6.83, sd = 3.17) made significantly more over-interpretative errors than both patients in the “negative group” (m = 4.92, sd = 2.42) and controls (m = 5.08, sd = 2.5). For other error types both patients' groups (respectively for “Less ToM” errors m = 8.2, sd = 3 for “positive group” and m = 9.68, sd = 3.36 for “negative group”, and for “no ToM” error m = 2.93, sd = 2.08 for “positive” and m = 3.85, sd = 2.86 for “negative”) made more errors than controls (m = 5.68, sd = 2.49 for “Less ToM” errors and m=1.58, sd=1.2 for “no ToM” errors) (Fig. 4).
-
(iv)
For attributional bias assessed with the AIHQ, a trend effect was observed for the hostility score (HB) (H(2) = 5.48, p = .064, ηp2 = 0.047) and for the intentionality score (IS) (F(2,115) = 3.05, p = .051, ηp2 = 0.050). In order to investigate these trends we compared results of the three groups of participants. We found for each two scores a significantly pairwise difference between “positive group” (HB: m = 2.01, sd = 0.72; IS: m = 2.98, sd = 1.09) and healthy controls (HB: m = 1.65, sd = 0.66; IS: m = 2.47, sd = 0.81).
Fig. 3.
Percentage of correct answers on the TREF assessing emotion recognition.
Fig. 4.
Scores for the MASC test assessing Theory of Mind.
4. Discussion
This study explored the assumption that categories of social cognitive impairments exist in schizophrenia based on the prevalence of positive or negative symptoms, one in which abilities are under-developed, and one in which they are excessive, leading to over attributions. Since we used a new French battery of tests including classical tests but also new ones to assess social cognition, we will first present our findings concerning the comparison of patients with schizophrenia and healthy controls. We will then introduce the results of the comparison of patients with positive or negative symptoms categorized using the HAC to healthy controls. Finally, we will discuss the relationships between symptoms and social cognitive profiles in light of the international literature.
Firstly, we observed that patients with schizophrenia presented significant impairments compared to healthy controls for each social cognitive component assessed. They presented significant deficits in emotion processing, notably in recognizing “fear” and “contempt” compared to controls. Patients also presented impairments in the task evaluating social perception and knowledge. They reported significantly less elements of the social situation depicted, and interpreted the context of the scene, the main characters, and the interaction between them, less well than the healthy controls. They also showed a smaller capacity to extract social rules of convention from the pictures. Concerning ToM, we observed significant impairment in attributing mental states to characters depicted in a movie in patients with schizophrenia. Given that the MASC offers the possibility to dissociate overmentalizing from undermentalizing errors, analysis of the error types provided more details. Reduced ToM performances in schizophrenia are related to increase undermentalizing errors. This reflects reduced ToM or, in some cases, absence of ToM related to the inability to correctly identify the characters' attitudes and behaviors. This pattern of results has been already extensively described in this population, with a positive correlation between undermentalizing and negative symptoms (Montag et al., 2011). Finally, regarding attributional style, we observed a significant “attribution of responsibility bias” in people with schizophrenia compared to the control group. Considering that the score is composed of the average of the participant's rates on three scales, it is possible to further interpret the bias of attribution. Patients with schizophrenia exhibited lower performances than controls on the three scales: “intentionality” – patients tended to judge that the person in the daily situation acted more on purpose – “anger” – they tended to feel angrier than controls in the situations – and “blame” – people with schizophrenia tended to blame the person more than the controls. All these results are consistent with those of the international literature. Many reviews and meta-analyses report that individuals with schizophrenia exhibit large deficits in social cognitive processes (Green et al., 2015; Penn et al., 2008; Pinkham, 2014; Savla et al., 2013). Moreover, the results suggest that the tests used in our study are sensitive to social cognitive impairments of patients with schizophrenia even if a few biases limit the impact of our data. The two populations were indeed not perfectly matched: the patients with schizophrenia had a significantly lower education level than the healthy controls; thus the performances observed could be partly related to educational level. Nonetheless, all the patients had an education level exceeding 9 years of study meaning that they all presented normal intellectual efficiency. In addition, social cognitive impairments are quite independent of measures of intelligence, even if neurocognitive functioning seems to be a necessary but not sufficient condition for good social cognition (Fanning et al., 2012). In future studies, we will need to demonstrate that the measures used to assess social cognition are relevant for distinguishing people with social cognitive impairments from those without, by controlling the level of education.
While social cognitive impairments have been widely documented, less is known about how these impairments taken together can be affected by positive and negative symptoms in schizophrenia. Our study explored these relationships and highlighted some specificity of social cognitive profiles according to prevailing symptoms. Some of these results are in line with those previously highlighted in the international literature.
Patients with negative symptoms presented lower performances in emotion recognition tasks than both patients with positive symptoms and healthy controls. This positive association between emotion recognition deficits and severity of negative symptoms has been already confirmed by several studies, reviews and meta-analyses (Chan et al., 2010; Hoekert et al., 2007; Kohler et al., 2010) in facial but also prosodic modalities.
About social perception and knowledge both patients' groups presented significant impairments compared to controls. These components have received less research attention than other dimensions of social cognition, and even fewer studies address the relationship between this component and symptomatology. In a study focusing on nonverbal social perception, Toomey et al. (2002) hypothesized that a correlation existed between negative symptoms of schizophrenia and reduced nonverbal social perception due to the greater association observed between negative symptoms and impaired cognition. However their results did not confirm the assumption. Nonetheless, they observed that nonverbal social perception was significantly correlated with the PANSS item “conceptual disorganization”. In our study, we unfortunately did not analyze the relationship with specific symptoms. Actually we found than both patients' groups were significantly less performant than controls in the task assessing social perception and knowledge, but we were not able to find a difference between patients with positive symptoms and those with negative ones. Some studies however suggested that impaired social perception may be influenced by abnormalities in early aspects of visual processing (Sergi et al., 2006) associated with particular abnormalities in brain functioning in schizophrenia (Bjorkquist and Herbener, 2013). According to these studies, these dysfunctions might be not influenced by symptoms. Nevertheless, qualitatively, we observed differences in the pattern of errors between patients with positive symptoms and those with negative ones. While patients with negative symptoms had difficulties interpreting social situations, patients with positive symptoms obtained low scores because of a tendency to propose wrong interpretations concerning social situations depicted. The PerSo test however did not permit to quantitatively measure these differences.
Concerning ToM, patients with positive symptoms had a significant tendency to make more over-interpretative errors than both patients with negative symptoms and controls. The possibility that there may be different forms of impairments depending on symptoms has been already mentioned in the literature (A. Abu-Akel, 2003; Frith, 2004). Our results partly confirmed those previously obtained with the same task (Fretland et al., 2015; Montag et al., 2011). Authors using the MASC test showed that negative symptoms were associated with a lack of a mental state concept and patients with such symptoms performed more “undermentalizing” errors, while in patients with positive symptoms, more “overmentalizing” errors were observed. In the present study, we highlighted a significant impairment of people with schizophrenia compared with healthy controls in the total score of the MASC. Moreover, we found that patients with positive symptoms made more “overmentalizing” errors than both patients with negative symptoms and controls. Nevertheless we did not observe any significant difference between patients of the positive group and those of the negative one on undermentalizing errors. Actually both groups had a tendency to make more errors than controls. These results a thus partly in favor of the assumption of a continuum, from a deficit in the understanding of others' mental states or difficulties in applying this understanding to a hyper-ToM that leads patients with positive symptoms to over-attribute knowledge and mental states to their interlocutors.
Finally, we expected a strong link between positive symptoms and attributional biases based on the literature reporting exaggeration of some biases in persecutory delusions (Freeman and Garety, 2014). In our study, only trends have been highlighted. Comparisons between the three groups showed that patients in the positive group presented a significant hostility bias and a higher intentionality score compared to healthy controls. Unfortunately, we did not find any difference between patients with negative symptoms and those with positive ones. There could be two explanations for this. Firstly, the results of the different studies on this topic are controversial and the relationship between persecutory delusions and attributional biases does not seem to be symptom-specific (Fraguas et al., 2008). Secondly, the present study only concerned globally stabilized patients, since they were recruited in psychosocial rehabilitation units. They only had mild positive symptoms and their scores on the specific PANSS item “delusions” were low. Our mixed results could be explained by this selection bias.
In the present study, we proposed the assumption that categories of social cognitive impairments exist in schizophrenia, depending on the clinical profiles of patients and notably on positive and negative symptoms, one corresponding to a defect in social cognition – “under-social cognition” – and one corresponding to excessive interpretation – “over-social cognition”. Concerning the two social cognitive profiles that we described based on dominant symptoms; our results are only partly consistent with our predictions. This categorical assumption that social cognition may be underdeveloped in some cases and “hyper”-developed in others has been previously proposed by Crespi and Badcock (2008) with the purpose of explaining autism and psychosis. The authors proposed that a large set of phenotypic traits exhibit diametrically opposite phenotypes in autism versus in schizophrenia, notably in the field of social cognition, and they hypothesized that these processes are underdeveloped in autistic-spectrum conditions and over-developed in the psychotic spectrum. According to our results, this dichotomy between “under” and “over” could also be applied to patients with schizophrenia depending on their symptoms. However this observation seemed also depend of the social cognitive processes assessed. Actually, the assumption of under/over social cognitive impairments appeared to be definitively true for emotion processes but need further explorations for other processes composing social cognition. Our results however showed the importance of assessing symptoms in relationship with cognitive functioning. Actually the profiles of impairments of social cognitive processes seemed partly depending on clinical characteristics of patients rather than on nosographical categories. This observation is particularly important in the field of cognitive remediation where trainings or therapies targeting specific impairments according to patients' profiles are expected to meet the needs of the population.
Finally, in the present study, we did not assess the disorganization. However, according to other studies and meta-analyses, patients with symptoms of disorganization are significantly more impaired in ToM tasks than other subgroups (Sprong et al., 2007), and this dimension appears to be associated with failure to infer others' intentions, as well as impairments in causal attributions (Sarfati et al., 1999; Sarfati et al., 1997). Moreover, some authors suggested that disorganization could mediate relationships between neurocognition and both social cognition and metacognitive processes (Minor et al., 2015; Minor and Lysaker, 2014). Future works should assess both the concomitant impact of disorganization and the role of some specific symptoms on profiles of social cognitive impairment in schizophrenia.
Ethical approval
The study was carried out in accordance with the Declaration of Helsinki and was approved by the local Ethics Committee (CPP Lyon – Sud Est IV, No. 15/041; ANSM, No. 2015-A00580-49). Written informed consent to take part in the study was received from all participants.
Funding sources
This work was supported by Le Vinatier Hospital and Lyon 2 University [CSLV07].
Authorship
The design of the study has been proposed by EP, ZP, CDA, LBH, IA, EHD, IA and NF. The acquisition has been realized by EP, ZP, CDA, LBH, ICM, BG, CJ, DA, IA, JG, EHD and NF. The analysis has been performed by JP, EP and ZP. EP, ZP, CDA, LBH, ICM, IA, JG, EHD and NF participated in the interpretation of data for the work. EP, ZP and NF drafted the paper, and all the authors approved the final version.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgement
The authors would like to thank all the people involved in the ClaCoS project, and notably Alix Thillay, Shasha Kohlmeyer, Sarah-Lise Farhat, Marie-Cécile Bralet and George Michael. This study was monitored by Veronique Vial whom we wish to thank. We also wish to thank Jeanne Beattie for editing the manuscript.
References
- Abu-Akel A. Impaired theory of mind in schizophrenia. Pragmat. Cogn. 1999;7(2):247–282. [Google Scholar]
- Abu-Akel A. The neurochemical hypothesis of “theory of mind”. Med. Hypotheses. 2003;60(3):382–386. doi: 10.1016/s0306-9877(02)00406-1. [DOI] [PubMed] [Google Scholar]
- Abu-Akel A., Bailey A.L. The possibility of different forms of theory of mind impairment in psychiatric and developmental disorders. Psychol. Med. 2000;30(3):735–738. doi: 10.1017/s0033291799002123. [DOI] [PubMed] [Google Scholar]
- Bjorkquist O.A., Herbener E.S. Social perception in schizophrenia: evidence of temporo-occipital and prefrontal dysfunction. Psychiatry Res. 2013;212(3):175–182. doi: 10.1016/j.pscychresns.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Bora E., Yucel M., Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr. Res. 2009;109(1–3):1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Brüne M. “Theory of mind” in schizophrenia: a review of the literature. Schizophr. Bull. 2005;31(1):21–42. doi: 10.1093/schbul/sbi002. [DOI] [PubMed] [Google Scholar]
- Chan R.C.K., Li H., Cheung E.F.C., Gong Q.-Y. Impaired facial emotion perception in schizophrenia: a meta-analysis. Psychiatry Res. 2010;178(2):381–390. doi: 10.1016/j.psychres.2009.03.035. [DOI] [PubMed] [Google Scholar]
- Combs D.R., Penn D.L., Wicher M., Waldheter E. The Ambiguous Intentions Hostility Questionnaire (AIHQ): a new measure for evaluating attributional biases in paranoia. Cogn. Neuropsychiatry. 2007;12:128–143. doi: 10.1080/13546800600787854. [DOI] [PubMed] [Google Scholar]
- Correll C.U., Cañas F., Larmo I., Levy P., Montes J.-M., Fagiolini A.…Zink M. Individualizing antipsychotic treatment selection in schizophrenia: characteristics of empirically derived patient subgroups. Eur. Psychiatry. 2011;26(1):3–16. doi: 10.1016/S0924-9338(11)71709-6. [DOI] [PubMed] [Google Scholar]
- Couture S.M., Penn D.L., Roberts D.L. The functional significance of social cognition in schizophrenia: a review. Schizophr. Bull. 2006;32(Suppl. 1):S44–S63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi B., Badcock C. Psychosis and autism as diametrical disorders of the social brain. Behav. Brain Sci. 2008;31(3):241–261. doi: 10.1017/S0140525X08004214. discussion 261-320. [DOI] [PubMed] [Google Scholar]
- Dziobek I., Fleck S., Kalbe E., Rogers K., Hassenstab J., Brand M., Kessler J., Woike J.K., Wolf O.T., Convit A. Introducing MASC: a movie for the assessment of social cognition. J. Autism Dev. Disord. 2006;36(5):623–636. doi: 10.1007/s10803-006-0107-0. [DOI] [PubMed] [Google Scholar]
- Fanning J.R., Bell M.D., Fiszdon J.M. Is it possible to have impaired neurocognition but good social cognition in schizophrenia? Schizophr. Res. 2012;135(1–3):68–71. doi: 10.1016/j.schres.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett A.-K.J., Viechtbauer W., Dominguez M.-G., Penn D.L., van Os J., Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci. Biobehav. Rev. 2011;35(3):573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Fraguas D., Mena A., Franco C., Martín-Blas M.M., Nugent K., Rodríguez-Solano J.J. Attributional style, symptomatology and awareness of illness in schizophrenia. Psychiatry Res. 2008;158(3):316–323. doi: 10.1016/j.psychres.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Freeman D., Garety P. Advances in understanding and treating persecutory delusions: a review. Soc. Psychiatry Psychiatr. Epidemiol. 2014;49(8):1179–1189. doi: 10.1007/s00127-014-0928-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fretland R.A., Andersson S., Sundet K., Andreassen O.A., Melle I., Vaskinn A. Theory of mind in schizophrenia: error types and associations with symptoms. Schizophr. Res. 2015;162(1–3):42–46. doi: 10.1016/j.schres.2015.01.024. [DOI] [PubMed] [Google Scholar]
- Frith C.D. Lawrence Erlbaum Associate Publishers; Hove: 1992. Cognitive Neuropsychology of Schizophrenia. [Google Scholar]
- Frith C.D. Schizophrenia and theory of mind. Psychol. Med. 2004;34(3):385–389. doi: 10.1017/s0033291703001326. [DOI] [PubMed] [Google Scholar]
- Frith C.D., Blakemore S., Wolpert D.M. Explaining the symptoms of schizophrenia: abnormalities in the awareness of action. Brain Res. Brain Res. Rev. 2000;31(2–3):357–363. doi: 10.1016/s0165-0173(99)00052-1. [DOI] [PubMed] [Google Scholar]
- Gaudelus B., Virgile J., Peyroux E., Leleu A., Baudouin J.-Y., Franck N. Measuring impairment of facial affects recognition in schizophrenia. Preliminary study of the facial emotions recognition task (TREF) L’Encephale. 2015;41(3):251–259. doi: 10.1016/j.encep.2014.08.013. [DOI] [PubMed] [Google Scholar]
- Green M.F., Leitman D.I. Social cognition in schizophrenia. Schizophr. Bull. 2008;34(4):670–672. doi: 10.1093/schbul/sbn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.F., Penn D.L., Bentall R., Carpenter W.T., Gaebel W., Gur R.C.…Heinssen R. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr. Bull. 2008;34(6):1211–1220. doi: 10.1093/schbul/sbm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.F., Horan W.P., Lee J. Social cognition in schizophrenia. Nat. Rev. Neurosci. 2015;16(10):620–631. doi: 10.1038/nrn4005. [DOI] [PubMed] [Google Scholar]
- Hall J., Harris J.M., Sprengelmeyer R., Sprengelmeyer A., Young A.W., Santos I.M.…Lawrie S.M. Social cognition and face processing in schizophrenia. Br. J. Psychiatry J. Ment. Sci. 2004;185:169–170. doi: 10.1192/bjp.185.2.169. [DOI] [PubMed] [Google Scholar]
- Hoekert M., Kahn R.S., Pijnenborg M., Aleman A. Impaired recognition and expression of emotional prosody in schizophrenia: review and meta-analysis. Schizophr. Res. 2007;96(1–3):135–145. doi: 10.1016/j.schres.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kohler C.G., Walker J.B., Martin E.A., Healey K.M., Moberg P.J. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr. Bull. 2010;36(5):1009–1019. doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrubier Y., Sheehan D., Weiller E., Amorim P., Bonora I., Harnett Sheehan K.…Dunbar G. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur. Psychiatry. 1997;12(5):224–231. [Google Scholar]
- Lincoln T.M., Mehl S., Kesting M.-L., Rief W. Negative symptoms and social cognition: identifying targets for psychological interventions. Schizophr. Bull. 2011;37(Suppl. 2):S23–S32. doi: 10.1093/schbul/sbr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso F., Horan W.P., Kern R.S., Green M.F. Social cognition in psychosis: multidimensional structure, clinical correlates, and relationship with functional outcome. Schizophr. Res. 2011;125(2–3):143–151. doi: 10.1016/j.schres.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez G., Alexandre C., Mam-Lam-Fook C., Bendjemaa N., Gaillard R., Garel P., Dziobek I., Amado I., Krebs M.O. Phenotypic continuum between autism and schizophrenia: evidence from the Movie for the Assessment of Social Cognition (MASC) Schizophr. Res. 2017;185:161–166. doi: 10.1016/j.schres.2017.01.012. [DOI] [PubMed] [Google Scholar]
- Minor K.S., Lysaker P.H. Necessary, but not sufficient: links between neurocognition, social cognition, and metacognition in schizophrenia are moderated by disorganized symptoms. Schizophr. Res. 2014;159(1):198–204. doi: 10.1016/j.schres.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Minor K.S., Marggraf M.P., Davis B.J., Luther L., Vohs J.L., Buck K.D., Lysaker P.H. Conceptual disorganization weakens links in cognitive pathways: disentangling neurocognition, social cognition, and metacognition in schizophrenia. Schizophr. Res. 2015;169(1–3):153–158. doi: 10.1016/j.schres.2015.09.026. [DOI] [PubMed] [Google Scholar]
- Montag C., Dziobek I., Richter I.S., Neuhaus K., Lehmann A., Sylla R.…Gallinat J. Different aspects of theory of mind in paranoid schizophrenia: evidence from a video-based assessment. Psychiatry Res. 2011;186(2–3):203–209. doi: 10.1016/j.psychres.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Nelson A.L., Combs D.R., Penn D.L., Basso M.R. Subtypes of social perception deficits in schizophrenia. Schizophr. Res. 2007;94(1–3):139–147. doi: 10.1016/j.schres.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Penn D.L., Sanna L.J., Roberts D.L. Social cognition in schizophrenia: an overview. Schizophr. Bull. 2008;34(3):408–411. doi: 10.1093/schbul/sbn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham A.E. Social cognition in schizophrenia. J. Clin. Psychiatry. 2014;75(Suppl. 2):14–19. doi: 10.4088/JCP.13065su1.04. [DOI] [PubMed] [Google Scholar]
- Piskulic D., Addington J. Social cognition and negative symptoms in psychosis. Psychiatry Res. 2011;188(2):283–285. doi: 10.1016/j.psychres.2011.04.028. [DOI] [PubMed] [Google Scholar]
- Ramsay J., Silverman B.W. Springer; New York: 2005. Functional Data Analysis. [Google Scholar]
- Sarfati Y., Hardy-Bayle M.C., Nadel J., Chevalier J.F., Widlocher D. Attribution of mental states to others by schizophrenic patients. Cogn. Neuropsychiatry. 1997;2(1):1–18. doi: 10.1080/135468097396388. [DOI] [PubMed] [Google Scholar]
- Sarfati Y., Hardy-Baylé M.C., Brunet E., Widlöcher D. Investigating theory of mind in schizophrenia: influence of verbalization in disorganized and non-disorganized patients. Schizophr. Res. 1999;37(2):183–190. doi: 10.1016/s0920-9964(98)00154-6. [DOI] [PubMed] [Google Scholar]
- Savla G.N., Vella L., Armstrong C.C., Penn D.L., Twamley E.W. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophr. Bull. 2013;39(5):979–992. doi: 10.1093/schbul/sbs080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S.J., Mueller D.R., Roder V. Social cognition as a mediator variable between neurocognition and functional outcome in schizophrenia: empirical review and new results by structural equation modeling. Schizophr. Bull. 2011;37(Suppl. 2):S41–S54. doi: 10.1093/schbul/sbr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergi M.J., Rassovsky Y., Nuechterlein K.H., Green M.F. Social perception as a mediator of the influence of early visual processing on functional status in schizophrenia. Am. J. Psychiatry. 2006;163(3):448–454. doi: 10.1176/appi.ajp.163.3.448. [DOI] [PubMed] [Google Scholar]
- Sergi M.J., Rassovsky Y., Widmark C., Reist C., Erhart S., Braff D.L.…Green M.F. Social cognition in schizophrenia: relationships with neurocognition and negative symptoms. Schizophr. Res. 2007;90(1–3):316–324. doi: 10.1016/j.schres.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Shean G., Meyer J. Symptoms of schizophrenia and social cognition. Psychiatry Res. 2009;170(2–3):157–160. doi: 10.1016/j.psychres.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Sprong M., Schothorst P., Vos E., Hox J., van Engeland H. Theory of mind in schizophrenia: meta-analysis. Br. J. Psychiatry J. Ment. Sci. 2007;191:5–13. doi: 10.1192/bjp.bp.107.035899. [DOI] [PubMed] [Google Scholar]
- Toomey R., Schuldberg D., Corrigan P., Green M.F. Nonverbal social perception and symptomatology in schizophrenia. Schizophr. Res. 2002;53(1–2):83–91. doi: 10.1016/s0920-9964(01)00177-3. [DOI] [PubMed] [Google Scholar]
- van Hooren S., Versmissen D., Janssen I., Myin-Germeys I., à Campo J., Mengelers R.…Krabbendam L. Social cognition and neurocognition as independent domains in psychosis. Schizophr. Res. 2008;103(1–3):257–265. doi: 10.1016/j.schres.2008.02.022. [DOI] [PubMed] [Google Scholar]