Abstract
In 2008, a quadrivalent human papillomavirus (HPV) vaccine (genotypes 6, 11, 16, 18) became available in New Zealand. This study investigated whether the proportion of cervical intraepithelial neoplasia grade 2 (CIN2) lesions associated with HPV genotypes 16 and 18 changed over time in young women recruited to a prospective CIN2 observational management trial (PRINCess) between 2013 and 2016. Partial HPV genotyping (16, 18, or other high risk HPV) was undertaken on n = 392 women under 25 years (mean age 21.8, range 17–24) with biopsy-diagnosed CIN2. High risk HPV genotypes were detected in 96% of women with CIN2 lesions. Between 2013 and 2016, the proportion of women whose liquid-based cytology samples were HPV 16 or 18 positive decreased from 43% to 13%. HPV vaccination status was known for 78% of women. Between 2013 and 2016, the proportion of HPV 16/18 positivity did not significantly change in HPV-vaccinated women, but decreased from 66% to 17% in unvaccinated women. The reducing proportion of HPV 16/18-related CIN2 in our cohort of young New Zealand women may be attributable to the introduction of a national HPV vaccination program. The substantial decrease in HPV 16/18 positivity observed in unvaccinated women is likely to be due to a herd effect.
Keywords: Cervical intraepithelial neoplasia grade 2, High risk human papillomavirus genotype, Human papillomavirus vaccine, Observational Management, Young women
Highlights
-
•
392 women under 25 years with CIN2 lesions were recruited between 2013 and 2016.
-
•
HPV16/18 positivity decreased from 43% of women in 2013–13% in 2016.
-
•
In vaccinated women, HPV16/18 positivity decreased from 17% in 2013 to 9% in 2016.
-
•
In unvaccinated women, HPV16/18 positivity decreased from 66% in 2013 to 17% in 2016.
-
•
Decreasing HPV16/18-related CIN2 may be due to HPV vaccination and herd effect.
1. Introduction
The human papillomavirus (HPV) is the main cause of cervical cell abnormalities and cervical cancer [1], [2], [3], [4]. HPV infections are common in young women, with reported rates between 28% and 46% in women under the age of 25 [5], [6]. HPV infections are usually associated with low grade cervical cell changes and in most women the immune response causes regression of these abnormalities without treatment. In some women, HPV infection persists and this can be associated with the development of high grade abnormalities. Persistent high grade cervical abnormalities can progress over time to invasive cervical cancer [7].
In New Zealand (NZ), the National Cervical Screening Programme (NCSP) guidelines state that all women who have ever had sexual intercourse should be offered a three-yearly cervical smear test from age 20 to age 69 [8]. If this is the first ever smear, or more than 5 years have elapsed since the previous smear, a second smear is recommended one year after the first, with three-yearly smears thereafter. The incidence of cervical cancer in NZ is currently 5.4 per 100,000 women [9].
The distribution of HPV genotypes in cervical specimens collected from women in NZ who had been diagnosed with high grade cervical cell abnormalities (between 2009 and 2011) [10] or invasive cervical cancer (between 2004 and 2010) [11] has been investigated. Across all ages (20–69 years), the most common HPV types associated with high grade cervical intraepithelial neoplasia (CIN grades 2 and 3) were HPV16 (51%), 52 (19%), 31 (17%), 33 (13%), and 18 (12%). However, there was a trend for higher rates of HPV16 and 18 infection compared with other HPV genotypes in women aged 20–29 years. The most commonly detected HPV genotypes associated with invasive cervical cancer were HPV16 (51%), 18 (21%), 31 (4%), 45 (3%) and 52 (3%).
The efficacy of an HPV vaccine for preventing cervical dysplasia has been demonstrated through randomized controlled trials [12], [13] and was first licensed in 2006. In late 2008, a 3-dose quadrivalent HPV vaccine (containing HPV virus-like particles of types 6, 11, 16, and 18) was offered to young NZ women born in 1990 and 1991. In terms of cervical cancer risk, HPV 16 and 18 genotypes are high risk types, [14] while HPV 6 and 11 genotypes are low risk types but are responsible for ~90% of anogenital warts [14], [15]. In 2009, the HPV vaccine program was extended to girls and young women born from 1992 onwards. As part of the immunization program, the HPV immunization was available fully subsidized for girls and young women from 9 years old to their 20th birthday. HPV vaccination coverage in NZ has increased from 38% (for all three HPV doses) for the cohort born in 1990 to 66% (for all three HPV doses) for the cohort born in 2002 [16]. At the beginning of 2017, a 2-dose nonavalent HPV vaccine which, in addition to the types included in the quadrivalent vaccine, contains HPV virus-like particles of types 31, 33, 45, 52, and 58 was introduced in NZ. The nonavalent vaccine is available fully subsidized in NZ for everyone, male or female, aged 9–26.
Following the introduction of the quadrivalent HPV vaccine in 2008, we were interested to investigate whether there was any evidence of a change in the proportion of HPV 16 and 18 positive lesions in young women with high grade lesions.
2. Materials and methods
Participants were n = 392 consenting women with cervical intraepithelial neoplasia grade 2 (CIN2) enrolled in the PRINCess trial. PRINCess is a multicentre prospective trial of CIN2 observational management in women under 25 years [17]. A total of n = 613 young women who had biopsy-diagnosed CIN2 following a referral to colposcopy for an abnormal smear with no previous high grade abnormality were recruited to the PRINCess trial through large colposcopy units in New Zealand and Australia between 2010 and 2016. Fifty-eight percent (613/1053) of all eligible young women with CIN2 lesions identified through the participating colposcopy units agreed to undertake observational management in place of immediate treatment and consented to participate in the PRINCess trial. As routine partial HPV genotyping only began in 2013, only data from NZ women recruited between 2013 and 2016 with HPV genotyping has been analysed (n = 392). These women (mean age 21.8, range 17–24) had biopsy-diagnosed CIN2 following a referral to colposcopy for an abnormal smear with no previous documented high grade abnormality.
Partial HPV genotyping (HPV 16, HPV 18, other high risk HPV, or high risk HPV not detected) was undertaken on liquid-based cytology samples taken either at the same time as their CIN2 biopsy (n = 309) or at their first follow up visit 6 months later (n = 83). Depending on the usual practice at each recruitment center either SurePath (Becton Dickinson, Franklin Lakes, NJ, USA) or ThinPrep (Hologic, Malborough, MA, USA) liquid-based cytology (LBC) collection systems were used. Depending on the usual practice at each recruitment center either a Roche Cobas X 480 polymerase chain reaction (PCR) (Roche, Pleasanton, CA, USA) or Abbott Real-Time PCR (Abbott Molecular, Inc., Des Plaines, IL, USA) DNA amplification system was used for HPV detection.
Lesions were grouped into four HPV genotype groups; (1) HPV 16 or 18 positive; (2) HPV 16 or 18 plus other high risk HPV positive, (3) positive for any high risk HPV genotype except HPV 16 or 18 (other HRHPV), or (4) high risk HPV not detected. The relative proportion of each group was determined each year to investigate change over time using a non-parametric test for trend across ordered groups [18] as implemented in STATA (nptrend StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP). HPV vaccination status was known for 78% (304/392) women. The impact of quadrivalent HPV vaccination (at least one dose) on the relative proportion of HPV16/18 positive (+/- other HRHPV positive) CIN2 lesions each year was also investigated using nptrend analysis. Significance level was set at alpha =0.05. A logistic regression model was used to investigate predictors of the presence of HPV16/18 genotypes. Covariates included in the regression model were year of CIN2 diagnosis, HPV vaccination status (yes, no, or not recorded), smoker (yes or no), size of the lesion (small lesion <0.5 cm vs medium/large lesion ≥0.5 cm), colposcopic impression of the grade of the lesion as assessed by the colposcopist (normal/low grade vs high grade), grade of referral cytology abnormality (low grade vs high grade), and grade of cytology abnormality at the visit where CIN2 was diagnosed (normal/low grade vs high grade).
PRINCess has clinical trial registration (ANZCTR trial number ACTRN12611000547943), was approved on 14/04/10 by the Multi-region Ethics Committee (Ethics reference: MEC/09/07/079), and has site-specific local authorization. The PRINCess protocol is available at http://www.otago.ac.nz/christchurch/otago073807.pdf.
3. Results
The overall group of n = 392 women included ~100 young women with newly diagnosed CIN2 lesions recruited each year of analysis except 2016 when fewer women were recruited (n = 78) due to recruitment ceasing in October that year. Over time there was an increase in the proportion of women with CIN2 who had been referred with high grade cytology (i.e., atypical squamous cells – cannot exclude HSIL [ASC-H] or greater) (nptrend z = 2.29, p > |z| = 0.022). Fifty-four percent of the women with CIN2 recruited in 2013 were referred with high grade cytology. By 2016, the proportion of women referred with high grade cytology increased to 69%. See Supplementary figure.
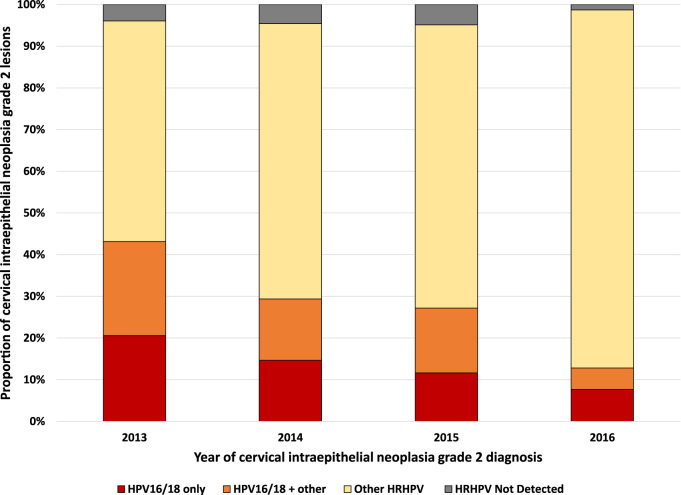
HRHPV genotypes were detected in the cytology of 96% (377/392) of those women. The proportion of women where HRHPV genotypes were not detected was 4–5% between 2013 and 2015, but decreased to 1% by 2016. Overall, 52% (59/114) of HPV16/18 positive women were also positive for other HRHPV genotypes. Table 1 summarizes the HPV genotypes associated with CIN2 lesions in the overall study population and in each year of study.
Table 1.
Human papillomavirus genotypes associated with cervical intraepithelial neoplasia grade 2 lesions overall and across time.
| Year of CIN2 diagnosis | HPV16/18 only | HPV16/18 + other HRHPV | Other HRHPV | HRHPV not detected |
|---|---|---|---|---|
| All years (n = 392) | 55 (14%) | 59 (15%) | 263 (67%) | 15 (4%) |
| 2013 (n = 102) | 21 (21%) | 23 (23%) | 54 (53%) | 4 (4%) |
| 2014 (n = 109) | 16 (15%) | 16 (15%) | 72 (66%) | 5 (5%) |
| 2015 (n = 103) | 12 (12%) | 16 (16%) | 70 (68%) | 5 (5%) |
| 2016 (n = 78) | 6 (8%) | 4 (5%) | 67 (86%) | 1 (1%) |
HPV = human papillomavirus.
CIN2 = cervical intraepithelial neoplasia grade 2.
Fig. 1 shows the changing relative proportions of HRHPV genotype positivity associated with CIN2 lesions in our cohort between 2013 and 2016. In 2013, 43% of women were HPV16/18 positive (±other HRHPV positivity) but this proportion dropped to 13% in 2016. Trend analysis (excluding women with no HRHPV detected) revealed a significant decrease in the proportion of women who were HPV16/18 positive (±other HRHPV) compared with those who were HPV16/18 negative between 2013 and 2016 (nptrend z = 4.35, p > |z| < 0.001).
Fig. 1.
Cervical intraepithelial neoplasia grade 2 lesions by high risk human papillomavirus genotype positivity in young women (2013–2016).
The proportion of women positive for other HRHPV genotypes (±HPV16 or 18 positivity) increased from 75% to 91% over the same time period. Trend analysis (excluding women with no HRHPV detected) revealed a significant increase in the proportion of women who were positive for other HRHPV genotypes (±HPV16/18) compared with HPV16/18 positive (negative for other HRHPV genotypes) between 2013 and 2016 (nptrend z = 2.59, p > |z| = 0.010).
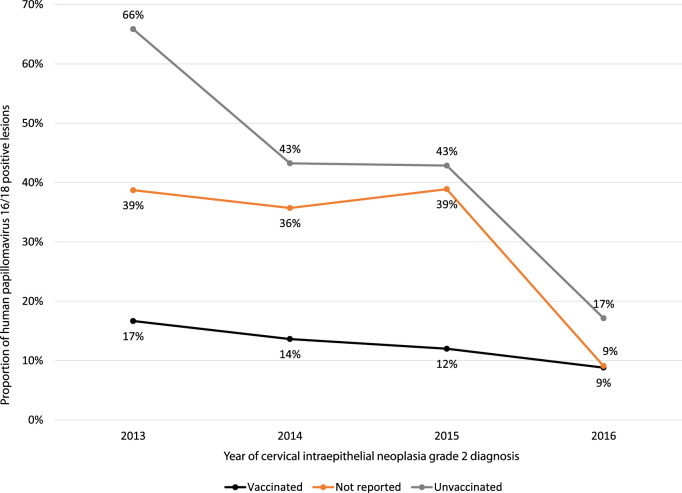
HPV vaccination status was reported for 78% (304/392) women. See Table 2. Where vaccination status was reported, n = 156 (51%) reported having had at least one dose of the quadrivalent HPV vaccine. Of these, 79% (123/156) reported having had all three doses. Thirteen percent of women reported having had 2 or fewer doses and 8% did not recall how many doses they had received. There was no linear trend for change over time in the proportion of women with CIN2 lesions who had had at least one dose of the HPV vaccine (nptrend z = -0.89, p > |z| = 0.38). Trend analysis (excluding women with no HRHPV detected) revealed no significant decrease over time in the proportion of HPV-vaccinated women who were HPV16/18 positive (±other HRHPV) compared with those who were HPV16/18 negative (17% were HPV16/18 positive in 2013 vs 9% in 2016, nptrend z = 0.99, p > |z| = 0.32). In contrast, there was a decrease over time in the proportion of unvaccinated women who were HPV16/18 positive (+/- other HRHPV) (66% were HPV16/18 positive in 2013 vs 17% in 2016, nptrend z = 4.49, p > |z| < 0.001). See Fig. 2.
Table 2.
Proportion women who had had the quadrivalent human papillomavirus vaccination overall and by year.
| Year of CIN2 diagnosis | HPV vaccinated | HPV vaccination status unknown | Not HPV vaccinated |
|---|---|---|---|
| All years (n = 392) | 156 (40%) | 88 (22%) | 148 (38%) |
| 2013 (n = 102) | 30 (29%) | 31 (30%) | 41 (40%) |
| 2014 (n = 109) | 44 (40%) | 28 (26%) | 37 (34%) |
| 2015 (n = 103) | 50 (49%) | 18 (17%) | 35 (34%) |
| 2016 (n = 78) | 32 (41%) | 11 (14%) | 35 (45%) |
Fig. 2.
Proportion of young women with cervical intraepithelial neoplasia grade 2 lesions associated with human papillomavirus 16 or 18 (2013–2016) grouped by quadrivalent human papillomavirus vaccination status.
A logistic regression model revealed that, compared to the unvaccinated group, the vaccinated group had a decreased probability of being HPV16/18 positive (OR 0.11 [95% CI 0.04–0.30], z = -4.40, p <0.001). Compared with the unvaccinated group, the group with unknown vaccination status also had a decreased probability of being HPV16/18 positive (OR 0.40 [95% CI 0.17–0.96], z = -2.05, p =0.04). Later year of CIN2 diagnosis was associated with a decreased probability of being HPV16/18 positive (OR 0.51 [95% CI 0.37–0.72], z = -3.95, p < 0.001). However, there was no significant interaction between vaccination status and year of CIN2 diagnosis. Compared with a normal or low grade colposcopic opinion, high grade colposcopic opinion was associated with an increased probability of being HPV16/18 positive (OR 1.83 [95% CI 1.05–3.2], z = 2.13, p < 0.04). Other covariates (i.e., smoking status, grade of referral cytology, grade of cytology at the time of CIN2 biopsy, and lesion size) were not predictive of HPV16/18 positivity.
4. Discussion
4.1. Main findings
We observed a rapid decline in the proportion of CIN2 lesions which are associated with HPV16 or 18 genotypes in our cohort of young NZ women between 2013 and 2016.
Vaccinated women had the low rates of HPV 16/18 positive lesions across the entire time period, however, a substantial decrease in HPV 16/18 positivity was observed in unvaccinated women.
4.2. Strengths and limitations
The study is of importance because it is the first documentation of a change in HPV-type in NZ women with cervical abnormalities since the vaccination program commenced.
The women in this study were a subset of women enrolled in the PRINCess study. PRINCess was designed to determine the safety and outcome for women with CIN2 undergoing observational management and the data presented in this paper represents the incidental observation of HPV genotype changes over time. As participation in PRINCess required a considerable time commitment including up to four biopsies over two years, the recruitment rate (58%) was considered reasonable. However, as this is not a population-based study, the observations are open to bias. In addition, as women with lesions diagnosed as CIN3 were excluded from the study, our observations cannot be extrapolated to all young NZ women with high grade histological abnormalities.
Vaccination status was recorded by the colposcopist and not verified, some women were unable to recall whether they were vaccinated or not, and in some cases vaccination status was not recorded. The majority (79%) of woman who reported being HPV-vaccinated also reported having had all three doses. The proportion of women who reported incomplete vaccination was relatively small and the collected information was deemed too imprecise to accurately stratify results by number of doses.
PRINCess participants were recruited from all large colposcopy centres around NZ, the number of women recruited per year was reasonably constant, and the proportion of screened women recruited also reasonably constant (~180–200 women annually 2013–2016). While the proportion of HPV16/18-related CIN2 lesions in this cohort decreased rapidly over time, other factors such as smoking and age did not change.
In order to increase the number of women with reported vaccination status available for analysis, we decided to include n = 83 women who had not had HRHPV genotyping until their first follow-up appointment (6 months after CIN2 diagnosis). These additional women were all HRHPV positive and, importantly, the proportion of women each year that were HPV16/18 (±other HRHPV) positivity was not substantially different if these women were excluded from analysis (44% vs 43% in 2013, 30% vs 29% in 2014, 27% vs 27% in 2015, and 13% vs 13% in 2016).
4.3. Interpretation
The rapid decline in HPV 16 and 18 associated CIN2 lesions, may be attributable to the introduction of the quadrivalent HPV vaccination in NZ in 2008. Reports from other countries have indicated relative reductions in vaccine HPV genotype infections in sexually-active young women in the years after HPV vaccine introduction compared with before vaccine introduction [19], [20], [21], [22], [23], [24], [25], [26], [27], [28] and in vaccinated compared with unvaccinated young women [29], [30]. Relative reductions in high grade cytological and histological abnormalities have also been reported in young vaccine-eligible women in the years after HPV vaccine introduction compared with before vaccine introduction [31], [32], [33], [34], [35], [36] and in vaccinated compared with unvaccinated young women [29], [37], [38], [39], [40], [41], [42]. Interestingly, we observed an increase in the proportion of women with CIN2 who had been referred with high grade cytology over time. This may be due to the effect of decreasing HPV 16/18. That is, low grade cytology which is HPV 16/18 negative may be more likely to also be low grade histologically.
Over a similar time period to our study, the NZ National Cervical Screening Programme (NCSP) reported a 6% decrease in the number of screening smears in women aged 20–24 years (2012–2016) [43], [44]. However, over the same period, there has been a 21% drop in high grade CIN2 and CIN3 histologies reported in women aged 20–24 years [43], [44]. If our figures are extrapolated, the reduction in high grade histologies observed in NZ is consistent with the hypothesis that vaccination is associated with reduced prevalence of HPV16/18-related high grade abnormalities. From our data we are unable to determine whether the prevalence of non-HPV16/18 disease has changed and population studies are required to determine whether the prevalence of high risk HPV genotypes is changing in the overall population.
Perhaps the most interesting observation from our study is the decreasing proportion of HPV16/18-related lesions in unvaccinated women over the study period. While these numbers are small and caution is needed in extrapolating to the overall population, this may reflect a reduction in HPV16/18 associated CIN2 lesions due to a reduction in the prevalence of HPV16/18 infections in the sexually active population or ‘herd effect’. Evidence of the herd effect leading to decreased HPV16/18 prevalence in unvaccinated young women has previously been reported in the Australia [19], [45] and Scotland [28]. However, both Australia and Scotland have consistently had higher HPV vaccination rates than NZ (e.g., vaccination rates for women born in 1994 of 73% in Australia and 86% in Scotland compared with 54% in NZ) [16], [42], [46]. With a HPV vaccination rate around 50% for women aged 13–17 years, [47], [48] the United States has more similar vaccination rates to NZ. A US population study observed a decrease in the proportion of HPV16/18 positive high grade lesions from 54% to 28% between 2008 and 2012 in vaccinated women (18–39 years), but observed no decrease in HPV16/18 positive lesions in unvaccinated women or women with unknown vaccination status [49], [50]. However, a more recent US study, has observed a decrease in the prevalence of vaccine-type HPV from 19.5% in 2009–2010 to 9.7% in 2013–2014 (prevalence ratio 0.44, 95% CI 0.22–0.91) in a general population of unvaccinated women aged 18–26 years [35]. Vaccine-type HPV prevalence did not change and remained low in young vaccinated US women over the same time period [35], [47]. Inclusion of boys and young men in vaccination programs should further enhance the herd effect. The HPV vaccination program was extended to boys and young men in Australia in 2013 and at the beginning of 2017 in NZ.
While vaccinated women had the lowest rates of HPV16/18 positive lesions across the entire time period, a significant proportion of vaccinated women were nonetheless HPV16/18 positive. This could be due to a number of factors including incorrect self-reporting of vaccination status or incomplete vaccination (i.e., receiving fewer than the recommended 3 doses). However, the most likely important contributing factor is that when the vaccine was introduced in late 2008 it was offered only to those born in 1990 and 1991 (i.e., 17 and 18 year olds). The following year, the vaccine was offered to women born from 1992 onwards (i.e., 17 years or younger). The mean age of vaccine uptake has dropped over time but in those initial years the mean age of vaccination was around 17 years old meaning that a proportion of those women may have already been exposed to HPV16/18 prior to vaccination. While we do not have data on the age that our participants were vaccinated, 50% of our cohort of vaccinated women were born in 1992 or earlier meaning that it is likely that they were 17 or 18 when they were vaccinated.
If the epidemiology of HPV-related abnormalities is changing in the young vaccine-eligible population, it is important to consider if this has implications with regard to the natural history of cervical disease. Other research has shown non-HPV16/18 lesions are less likely to progress to CIN3 or greater [51]. We look forward to ongoing population-based studies of HPV prevalence and further studies that correlate HPV-type to the natural history of disease and clinical outcomes.
5. Conclusion
We have observed evidence of a reducing proportion of HPV16/18-related CIN2 in our cohort of NZ women under the age of 25. We attribute this to the national HPV vaccination program which was introduced in NZ in 2008. It is notable that while vaccinated women had the lowest rates of HPV16/18 positive lesions across the entire time period, a decrease in HPV16/18 positivity was observed in unvaccinated women which may be due to a herd effect.
Disclosure of interests
The authors report no conflicts of interest.
Ethical approval
The PRINCess trial was approved by the New Zealand Multi-region Ethics Committee on 14/04/2010 (Ethics reference: MEC/09/07/079), has site-specific local authorization, and clinical trial registration (ANZCTR registration number 12611000547943 http://www.anzctr.org.au/).
Contribution to authorship
Author CI was involved in data curation, methodology, formal analysis, and writing the original draft of the paper. Author JW was involved in data curation, methodology, formal analysis, and review and editing of the paper. Authors PS and BS were involved in the conceptualization, methodology, funding acquisition, formal analysis, and review and editing of the paper. Author DH was involved with data curation, project administration, and review and editing of the paper. Authors MW, MH, BL, PF, ND, SP, JF, CB, and LE were involved in conceptualisation, methodology, and review and editing of the paper. Author RV was involved in the methodology and review and editing of the paper. Author LS was involved in the conceptualization, methodology, formal analysis, and review and editing of the paper.
Funding
This work was supported by grants from the Health Research Council, New Zealand (HRC 13/143) and Cancer Society of New Zealand (CS 12/30). Neither sponsor had any input into the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pvr.2018.10.010.
Appendix A. Supplementary material
Supplementary material Supplementary Figure. Proportion of young women with cervical intraepithelial neoplasia grade 2 lesions referred with high or low grade cytology (2013–2016).
References
- 1.Schiffman M.H., Bauer H.M., Hoover R.N., Glass A.G., Cadell D.M., Rush B.B. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J. Natl. Cancer Inst. 1993;85(12):958–964. doi: 10.1093/jnci/85.12.958. [DOI] [PubMed] [Google Scholar]
- 2.Schiffman M.H., Castle P. Epidemiologic studies of a necessary causal risk factor: human papillomavirus infection and cervical neoplasia. J. Natl. Cancer Inst. 2003;95(6):E2. doi: 10.1093/jnci/95.6.e2. [DOI] [PubMed] [Google Scholar]
- 3.Kjaer S.K., van den Brule A.J., Bock J.E., Poll P.A., Engholm G., Sherman M.E. Human papillomavirus--the most significant risk determinant of cervical intraepithelial neoplasia. Int J. Cancer. 1996;65(5):601–606. doi: 10.1002/(SICI)1097-0215(19960301)65:5<601::AID-IJC8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Walboomers J.M., Jacobs M.V., Manos M.M., Bosch F.X., Kummer J.A., Shah K.V. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Bauer H.M., Ting Y., Greer C.E., Chambers J.C., Tashiro C.J., Chimera J. Genital human papillomavirus infection in female university students as determined by a PCR-based method. JAMA. 1991;265(4):472–477. [PubMed] [Google Scholar]
- 6.Burk R.D., Ho G.Y., Beardsley L., Lempa M., Peters M., Bierman R. Sexual behavior and partner characteristics are the predominant risk factors for genital human papillomavirus infection in young women. J. Infect. Dis. 1996;174(4):679–689. doi: 10.1093/infdis/174.4.679. [DOI] [PubMed] [Google Scholar]
- 7.McCredie M.R., Sharples K.J., Paul C., Baranyai J., Medley G., Jones R.W. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9(5):425–434. doi: 10.1016/S1470-2045(08)70103-7. [DOI] [PubMed] [Google Scholar]
- 8.Guidelines for Cervical Screening in New Zealand - Incorporating the Management of Women with Abnormal Cervical Smears, ed. H. Lewis, G. Fentiman, and P. Bethwaite, Wellington, New Zealand National Screening Unit, Ministry of Health, 2008. Available from: 〈www.nsu.govt.nz/files/NCSP/NCSP_Guidelines_ALL_small(1).pdf〉.
- 9.M. Smith, L. Rumlee, K. Canfell, National Cervical Screening Programme Annual Report 2015, New Zealand National Screening Unit, 2018.
- 10.Simonella L.M., Lewis H., Smith M., Neal H., Bromhead C., Canfell K. Type-specific oncogenic human papillomavirus infection in high grade cervical disease in New Zealand. BMC Infect. Dis. 2013;13:114. doi: 10.1186/1471-2334-13-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sykes P., Gopala K., Tan A.L., Kenwright D., Petrich S., Molijn A. Type distribution of human papillomavirus among adult women diagnosed with invasive cervical cancer (stage 1b or higher) in New Zealand. BMC Infect. Dis. 2014;14:374. doi: 10.1186/1471-2334-14-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. Vol. 356 Future II Study Group. 1915-27. 19, 2007. Available from: 〈http://www.ncbi.nlm.nih.gov/pubmed/17494925〉. [DOI] [PubMed]
- 13.Paavonen J., Naud P., Salmeron J., Wheeler C.M., Chow S.N., Apter D. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 14.Munoz N., Bosch F.X., de Sanjose S., Herrero R., Castellsague X., Shah K.V. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003;348(6):518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 15.Garland S.M., Steben M., Sings H.L., James M., Lu S., Railkar R. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J. Infect. Dis. 2009;199(6):805–814. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- 16.Final Dose HPV Immmunisation Coverage All DHBs: girls born between 1990 and 2002 [cited 2018 14 June], 2016. Available from: 〈http://www.health.govt.nz/our-work/preventative-health-wellness/immunisation/hpv-immunisation-programme〉.
- 17.Sykes P., Innes C., Harker D., Whitehead M., van der Griend R., Lawton B. Observational management of CIN 2 in young women: a prospective multicenter trial. J. Low. Genit. Tract. Dis. 2016;20(4):343–347. doi: 10.1097/LGT.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 18.Cuzick J. A Wilcoxon-type test for trend. Stat. Med. 1985;4(1):87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 19.Tabrizi S.N., Brotherton J.M., Kaldor J.M., Skinner S.R., Cummins E., Liu B. Fall in human papillomavirus prevalence following a national vaccination program. J. Infect. Dis. 2012;206(11):1645–1651. doi: 10.1093/infdis/jis590. [DOI] [PubMed] [Google Scholar]
- 20.Mesher D., Soldan K., Howell-Jones R., Panwar K., Manyenga P., Jit M. Reduction in HPV 16/18 prevalence in sexually active young women following the introduction of HPV immunisation in England. Vaccine. 2013;32(1):26–32. doi: 10.1016/j.vaccine.2013.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cummings T., Zimet G.D., Brown D., Tu W., Yang Z., Fortenberry J.D. Reduction of HPV infections through vaccination among at-risk urban adolescents. Vaccine. 2012;30(37):5496–5499. doi: 10.1016/j.vaccine.2012.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahn J.A., Brown D.R., Ding L., Widdice L.E., Shew M.L., Glynn S. Vaccine-type human papillomavirus and evidence of herd protection after vaccine introduction. Pediatrics. 2012;130(2):e249–e256. doi: 10.1542/peds.2011-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markowitz L.E., Hariri S., Lin C., Dunne E.F., Steinau M., McQuillan G. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J. Infect. Dis. 2013;208(3):385–393. doi: 10.1093/infdis/jit192. [DOI] [PubMed] [Google Scholar]
- 24.Kavanagh K., Pollock K.G., Potts A., Love J., Cuschieri K., Cubie H. Introduction and sustained high coverage of the HPV bivalent vaccine leads to a reduction in prevalence of HPV 16/18 and closely related HPV types. Br. J. Cancer. 2014;110(11):2804–2811. doi: 10.1038/bjc.2014.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soderlund-Strand A., Uhnoo I., Dillner J. Change in population prevalences of human papillomavirus after initiation of vaccination: the high-throughput HPV monitoring study. Cancer Epidemiol. Biomark. Prev. 2014;23(12):2757–2764. doi: 10.1158/1055-9965.EPI-14-0687. [DOI] [PubMed] [Google Scholar]
- 26.Mesher D., Panwar K., Thomas S.L., Beddows S., Soldan K. Continuing reductions in HPV 16/18 in a population with high coverage of bivalent HPV vaccination in England: an ongoing cross-sectional study. BMJ Open. 2016;6(2):e009915. doi: 10.1136/bmjopen-2015-009915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drolet M., Benard E., Boily M.C., Ali H., Baandrup L., Bauer H. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect. Dis. 2015;15(5):565–580. doi: 10.1016/S1473-3099(14)71073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cameron R.L., Kavanagh K., Pan J., Love J., Cuschieri K., Robertson C. Human papillomavirus prevalence and herd immunity after introduction of vaccination program, Scotland, 2009–2013. Emerg. Infect. Dis. 2016;22(1):56–64. doi: 10.3201/eid2201.150736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munro A., Gillespie C., Cotton S., Busby-Earle C., Kavanagh K., Cuschieri K. The impact of human papillomavirus type on colposcopy performance in women offered HPV immunisation in a catch-up vaccine programme: a two-centre observational study. BJOG. 2017;124(9):1394–1401. doi: 10.1111/1471-0528.14563. [DOI] [PubMed] [Google Scholar]
- 30.Bhatia R., Kavanagh K., Cubie H.A., Serrano I., Wennington H., Hopkins M. Use of HPV testing for cervical screening in vaccinated women--Insights from the SHEVa (Scottish HPV Prevalence in Vaccinated Women) study. Int. J. Cancer. 2016;138(12):2922–2931. doi: 10.1002/ijc.30030. [DOI] [PubMed] [Google Scholar]
- 31.Hariri S., Johnson M.L., Bennett N.M., Bauer H.M., Park I.U., Schafer S. Population-based trends in high-grade cervical lesions in the early human papillomavirus vaccine era in the United States. Cancer. 2015;121(16):2775–2781. doi: 10.1002/cncr.29266. [DOI] [PubMed] [Google Scholar]
- 32.Brotherton J.M., Fridman M., May C.L., Chappell G., Saville A.M., Gertig D.M. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet. 2011;377(9783):2085–2092. doi: 10.1016/S0140-6736(11)60551-5. [DOI] [PubMed] [Google Scholar]
- 33.Brotherton J.M., Saville A.M., May C.L., Chappell G., Gertig D.M. Human papillomavirus vaccination is changing the epidemiology of high-grade cervical lesions in Australia. Cancer Causes Control. 2015;26(6):953–954. doi: 10.1007/s10552-015-0568-6. [DOI] [PubMed] [Google Scholar]
- 34.Baldur-Felskov B., Munk C., Nielsen T.S., Dehlendorff C., Kirschner B., Junge J. Trends in the incidence of cervical cancer and severe precancerous lesions in Denmark, 1997–2012. Cancer Causes Control. 2015;26(8):1105–1116. doi: 10.1007/s10552-015-0603-7. [DOI] [PubMed] [Google Scholar]
- 35.Berenson A.B., Hirth J.M., Chang M. Change in human papillomavirus prevalence among U.S. women aged 18-59 Years, 2009–2014. Obstet. Gynecol. 2017;130(4):693–701. doi: 10.1097/AOG.0000000000002193. [DOI] [PubMed] [Google Scholar]
- 36.Benard V.B., Castle P.E., Jenison S.A., Hunt W.C., Kim J.J., Cuzick J. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol. 2017;3(6):833–837. doi: 10.1001/jamaoncol.2016.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baldur-Felskov B., Dehlendorff C., Munk C., Kjaer S.K. Early impact of human papillomavirus vaccination on cervical neoplasia--nationwide follow-up of young Danish women. J. Natl. Cancer Inst. 2014;106(3):djt460. doi: 10.1093/jnci/djt460. [DOI] [PubMed] [Google Scholar]
- 38.Crowe E., Pandeya N., Brotherton J.M., Dobson A.J., Kisely S., Lambert S.B. Effectiveness of quadrivalent human papillomavirus vaccine for the prevention of cervical abnormalities: case-control study nested within a population based screening programme in Australia. BMJ. 2014;348:g1458. doi: 10.1136/bmj.g1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollock K.G., Kavanagh K., Potts A., Love J., Cuschieri K., Cubie H. Reduction of low- and high-grade cervical abnormalities associated with high uptake of the HPV bivalent vaccine in Scotland. Br. J. Cancer. 2014;111(9):1824–1830. doi: 10.1038/bjc.2014.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahmud S.M., Kliewer E.V., Lambert P., Bozat-Emre S., Demers A.A. Effectiveness of the quadrivalent human papillomavirus vaccine against cervical dysplasia in Manitoba, Canada. J. Clin. Oncol. 2014;32(5):438–443. doi: 10.1200/JCO.2013.52.4645. [DOI] [PubMed] [Google Scholar]
- 41.Gertig D.M., Brotherton J.M., Budd A.C., Drennan K., Chappell G., Saville A.M. Impact of a population-based HPV vaccination program on cervical abnormalities: a data linkage study. BMC Med. 2013;11:227. doi: 10.1186/1741-7015-11-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cameron R.L., Kavanagh K., Cameron Watt D., Robertson C., Cuschieri K., Ahmed S. The impact of bivalent HPV vaccine on cervical intraepithelial neoplasia by deprivation in Scotland: reducing the gap. J. Epidemiol. Community Health. 2017;71(10):954–960. doi: 10.1136/jech-2017-209113. [DOI] [PubMed] [Google Scholar]
- 43.M. Smith, S. Edwards, K. Canfell, National Cervical Screening Programme Annual Report 2013, New Zealand National Screening Unit, 2016.
- 44.M. Smith, S. Yap, K. Canfell, Monitoring Reports 41-45. 1 January 2014 - 30 June. 2017 National Cervical Screening Programme. Technical Report Numbers 41-45, 2016.
- 45.Tabrizi S.N., Brotherton J.M., Kaldor J.M., Skinner S.R., Liu B., Bateson D. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect. Dis. 2014;14(10):958–966. doi: 10.1016/S1473-3099(14)70841-2. [DOI] [PubMed] [Google Scholar]
- 46.HPV Vaccination Coverage Data [cited 2017 19 May], 2017. Available from: 〈http://www.hpvregister.org.au/research/coverage-data〉.
- 47.Attia A.C., Wolf J., Nunez A.E. On surmounting the barriers to HPV vaccination: we can do better. Ann. Med. 2018:1–17. doi: 10.1080/07853890.2018.1426875. [DOI] [PubMed] [Google Scholar]
- 48.S. Stokley, J. Jeyarajah, D. Yankey, M. Cano, J. Gee, J. Roark, et al., Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014--United States. MMWR Morb Mortal Wkly Rep, 2014. 63(29):620-4. [PMC free article] [PubMed]
- 49.Hariri S., Bennett N.M., Niccolai L.M., Schafer S., Park I.U., Bloch K.C. Reduction in HPV 16/18-associated high grade cervical lesions following HPV vaccine introduction in the United States – 2008–2012. Vaccine. 2015;33(13) doi: 10.1016/j.vaccine.2015.01.084. (1608-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hariri S., Markowitz L.E., Unger E.R. Response to Pendleton et al. regarding reduction in HPV 16/18-associated high grade cervical lesions following HPV vaccine introduction in the United States. Vaccine. 2016;34(2):201. doi: 10.1016/j.vaccine.2015.10.138. [DOI] [PubMed] [Google Scholar]
- 51.Castle P.E., Solomon D., Schiffman M., Wheeler C.M. Human papillomavirus type 16 infections and 2-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities. J. Natl. Cancer Inst. 2005;97(14):1066–1071. doi: 10.1093/jnci/dji186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material Supplementary Figure. Proportion of young women with cervical intraepithelial neoplasia grade 2 lesions referred with high or low grade cytology (2013–2016).