Abstract
Canine oral papillomavirus (CPV1, also known as COPV), the most common cause of non-neoplastic papillomas, has not been shown to cause squamous cell carcinomas (SCC). Furthermore, malignant transformation of benign papillomas to SCC has only been reported in a single group of dogs with severe combined immunodeficiency infected with CPV2. Here, we report a series of 7 dogs with benign CPV1-associated papillomas with histologic evidence of CPV1 causing malignant transformation to carcinoma in situ and ultimately SCC. Expression of p53 and p16 proteins in CPV1-infected cells within the benign papillomas and lesions that progressed into SCC also supported an association between papillomavirus and malignant transformation. Moreover, our retrospective analysis indicated that while there have been increased numbers of viral papillomas with malignant transformation, the number of annually diagnosed canine viral papillomas has remained constant over the past decade in our laboratory. We speculate that either an altered host immunity from increased usage of immunosuppressive drugs or changing environmental factors, e.g. increase exposure to UV radiation, may cause an increased oncogenic potential of this “low-risk” virus. This study aims to raise awareness of the malignant potential of CPV1 and to encourage further investigations into the cause of this suspected change in its oncogenic potential.
Abbreviations: IHC, immunohistochemistry; ISH, in situ hybridization
Keywords: Canine oral papillomavirus, Benign lesions, Malignant transformation, Squamous cell carcinoma
Graphical abstract
1. Introduction
Papillomaviruses (PV) have been detected in a wide variety of animals as well as in humans and are associated with numerous clinical presentations, ranging from asymptomatic infection to development of benign proliferative lesions or overt malignancies. To date, 20 canine papillomavirus types (CPVs) have been reported and grouped into three genera. Canine oral papillomavirus (CPV1 or COPV) and CPV6 belong to the genus Lambdapapillomavirus [1]. CPVs 2, 7, 13, 17, and 19 are in the genus Taupapillomavirus, and all other known CPVs belong to the genus Chipapillomavirus [2], [3], [4], [5].
Infections with CPVs, regardless of type, have historically been associated with the development of non-neoplastic solitary or multicentric epithelial proliferative plaques and papillomas [2]. Benign lesions associated with CPVs usually do not elicit any serious health consequences. Even lesions of oral papillomatosis tend to spontaneously regress, similar to those induced by low-risk papillomaviruses in humans [6], [7], [8]. In the past, CPV infections in dogs have rarely been associated with the development of malignant neoplasms and reports of detecting CPVs in malignant epithelial lesions are few [9], [10], [11], [12], [13], [14]. Progression of benign CPV-induced papillomas to SCC has only been well documented in a group of dogs with X-linked severe combined immunodeficiency (XSCID) that received bone marrow transplants [9]. More than 70% of the XSCID dogs in this study were infected with CPV2, a Taupapillomavirus. Four of these dogs had viral papillomas that progressed to invasive SCCs, and three of the four dogs eventually developed metastatic SCCs. Since then, there have been numerous reports identifying novel canine Tau-and Chipapillomaviruses as the cause of squamous cell carcinomas in dogs [10], [11], [12], [13], [14], [15], [16], few of which arose within pigmented plaques, but none that were associated with benign viral papillomas. CPV1 has never been associated with transformation of viral papillomas to SCC in dogs [3].
In humans tumorigenesis of high-risk human papillomavirus (HPV) infection occurs due to inactivation of p53 and Rb tumor suppressor genes by the E6 and E7 viral oncogenes, resulting in loss of cell-cycle control, impaired cell differentiation, increased mutations, and chromosomal instability [17], [18]. HPV E6 protein binds to both p53 and E6-associated protein ligase (E6AP), causing ubiquitinylation and the subsequent degradation of p53. Because of the commonly observed loss of p53 expression in different types of HPV positive SCCs, expression of p53 in SCC has been suggested as a surrogate marker for the absence of HPV infection (19). However, co-expression of p53 and p16INK4A has been reported in 15–30% of HPV positive SCCs at various sites (20, 21). Overexpression of p16 has been demonstrated in a high percentage of cervical and head and neck cancers as a result of functional inactivation of RB by the HPV E7 protein [22], [23]. p16 expression measured by IHC correlates with the presence of HPV DNA; therefore, p16 expression has been used as a surrogate biomarker of oncogenic HPV infection indicating HPV-mediated tumorigenesis [24]. In contrast to humans, it has been concluded that p16 immunolabeling of canine SCCs is not associated with canine papillomavirus infection [25]. An early study reported a single case of SCC with co-localization of p53 and CPV1 protein and nucleic acid, and detection of p53 in 35% of benign viral papillomas leading to speculation that progression of CPV1 papillomas to SCCs may occur [26]. However, most recent studies have concluded that CPV1 is not a significant cause of canine SCCs [25], [27], [28].
Over the last decade we recognized in our biopsy service an increase of benign CPV-associated lesions in dogs that underwent malignant transformation. Therefore, a retrospective analysis of cases of CPV-associated lesions submitted as diagnostic cases to the Michigan State University Veterinary Diagnostic Laboratory (MSU VDL) was conducted to 1) examine the incidence of malignant transformation in CPV-associated proliferative lesions and 2) determine the CPV type associated with such lesions. While we identified three different types of CPVs that were associated with benign proliferative epithelial lesions that had regions of malignant transformation to SCC, the most commonly identified virus was CPV1, thus challenging the current understanding of the oncogenic potential of this virus.
2. Materials and methods
A Boolean search of the electronic archive of canine necropsy and biopsy reports generated at the MSU VDL from 2006 to 2016 was performed using the keywords ‘papilloma’, ‘pigmented plaque’, ‘papillomavirus’, ‘dysplasia’, ‘atypia’, ‘malignant transformation’, or ‘squamous cell carcinoma’. Reports from identified cases were screened for histologic descriptions that included characteristics of CPV-associated benign lesions along with regions of dysplasia and/or overt malignant transformation. For final inclusion in the retrospective analysis, 5 µm hematoxylin and eosin-stained (H&E) sections of formalin-fixed, paraffin-embedded (FFPE) tissues were generated from all suspected cases and reviewed independently by three board certified pathologists (TT, DGS, MK) to confirm dysplasia and/or malignant transformation. Additional information compiled for each identified case included the date of initial diagnosis, location of the lesion, age, breed, and sex of the affected dog.
Benign lesions identified in the search included squamous papillomas of the oropharyngeal mucosa and skin, and pigmented plaques in the haired skin. For papillomas, histologic evidence of papillomavirus induction of the lesion included a prominent stratum granulosum within the proliferative epithelium lining papillary fronds; scattered koilocytes within the stratum spinosum, granulosum, and/or corneum; and/or presence of eosinophilic to amphophilic intranuclear inclusions. Dysplasia was defined as regions of loss of polarity, marked anisocytosis and anisokaryosis, asynchronous keratinization, and mitoses throughout the thickness of the proliferative epithelium. Evidence of overt malignant transformation was defined by neoplastic cells with similar cellular indices of dysplasia focally or multifocally extending through the basement membrane of the epithelium and forming trabeculae and islands of neoplastic cells invading the subepithelial stroma.
PCR was used to detect the presence of papillomavirus within FFPE tissues from cases that met the search criteria and had defined morphologic characteristics of malignant transformation. For PCR, total genomic DNA was extracted from shavings from the FFPE tissue blocks, and CPV DNA was amplified using two consensus primer sets targeting the L1 gene [29], [30] and a set of multiple degenerate primers targeting the E1 gene [31]. The amplified DNA was then sequenced and compared to sequences reported in GenBank.
To assess papillomavirus antigen and viral nucleic acid distribution within selected lesions, immunohistochemistry (IHC) and in situ hybridization (ISH) were performed on all cases. In addition, immunohistochemistry for p16 and p53 was used to further investigate the spatial association between papillomavirus and malignant transformation. For PV IHC, 5 µm sections of FFPE tissues were labeled with an automated staining system (Benchmark, Ventana Medical Systems Inc, Tucson, AZ) using a monoclonal antibody that has been shown to cross-react with multiple human and nonhuman PVs (Lifespan Biosciences, Seattle, WA) [32]. Immunohistochemistry for p16 and p53 was performed as previously described using a mouse anti-human p16 monoclonal antibody (BD Biosciences, San Jose, CA, USA) and a rabbit polyclonal anti-human p53 antibody (Signet Laboratories, Dedham,MA), respectively [27], [33]. ISH for papillomavirus DNA was performed on 5 µm sections of FFPE tissues using an automated RiboMap in situ hybridization reagent system (Ventana Medical Systems), as previously described [34].
3. Results
Out of a total of 110,374 canine cases that had been submitted from January 2006 to October 2016, 930 cases of squamous papilloma and 16 cases of pigmented plaques were identified in the initial electronic search. Of the 930 squamous papillomas, 349 papillomas had typical characteristics of CPV infection including acanthosis with prominent keratohyalin granules, koilocytosis, and the presence of intranuclear viral inclusions. Thirteen of these 349 viral papillomas had foci of epidermal dysplasia and/or cellular atypia and 11 viral papillomas underwent overt malignant transformation. Two of the 16 cases of pigmented viral plaques had histologic evidence of malignant transformation. The overall frequency of malignant transformation of CPV-associated lesions was 3.6% (13/365). CPV was identified by PCR in 69% (9/13) of those cases with overt malignant transformation including 7 of the 11 viral papillomas and both pigmented plaques. We were unable to identify viral DNA in the remaining 4 cases. The 9 cases of malignant transformation with confirmed CPV infection were diagnosed in 2006, 2009, 2010, 2011, 2013 (2 cases), 2015, and 2016 (2 cases). Of these 9 dogs, 4 were spayed females, 4 were neutered males, and one was an intact male. The mean age at the time of diagnosis was 8.5 years (range: 14 months to 16 years), and 3 of the animals were less than 5 years old. Four of these dogs had no known underlying health conditions. One dog was reported to have a concurrent benign melanocytic neoplasm, one had a history of a completely excised trichoepithelioma as well as a completely excised SCC, one had a metastatic hemangiosarcoma, one had a history of severe demodecosis, and one had a concurrent periodontitis with oral papillomas.
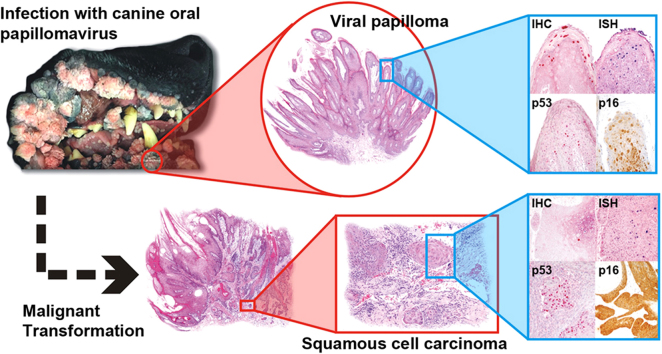
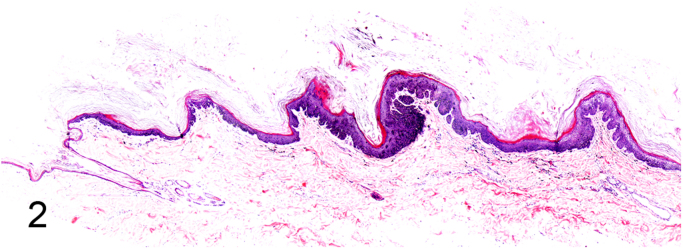
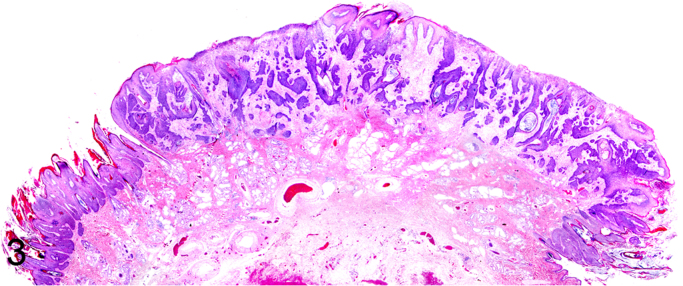
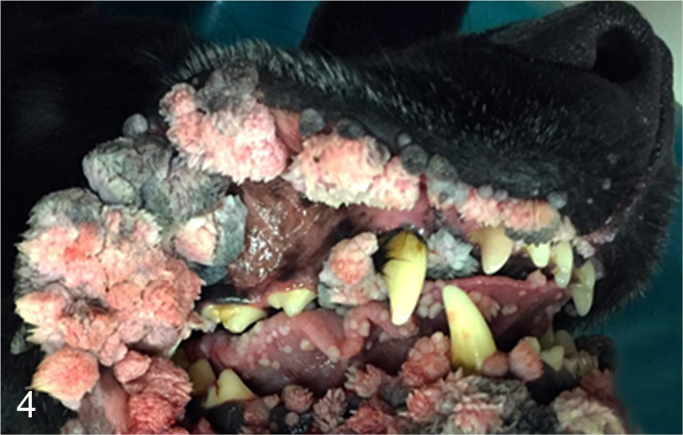
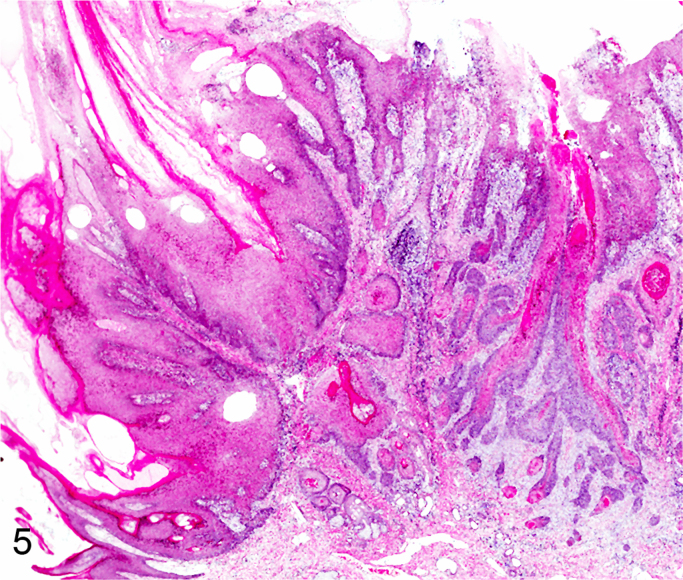
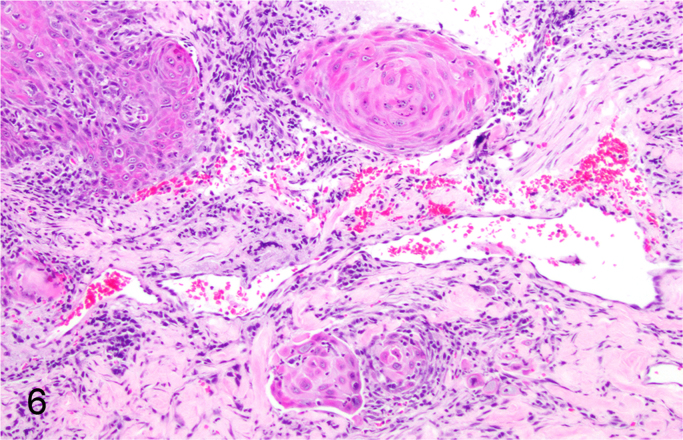
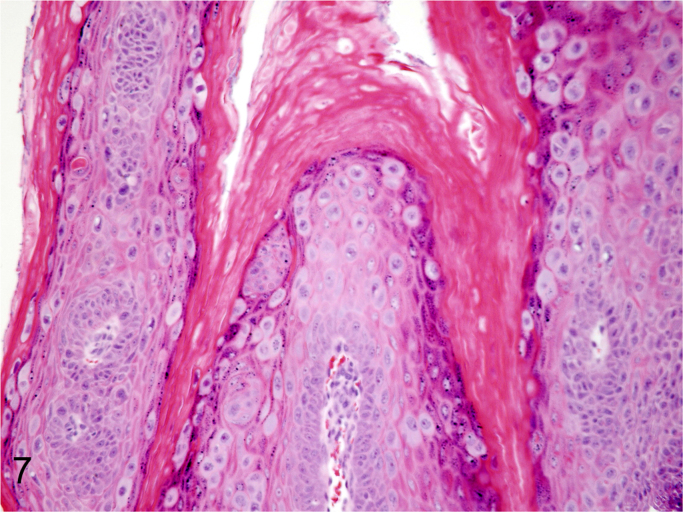
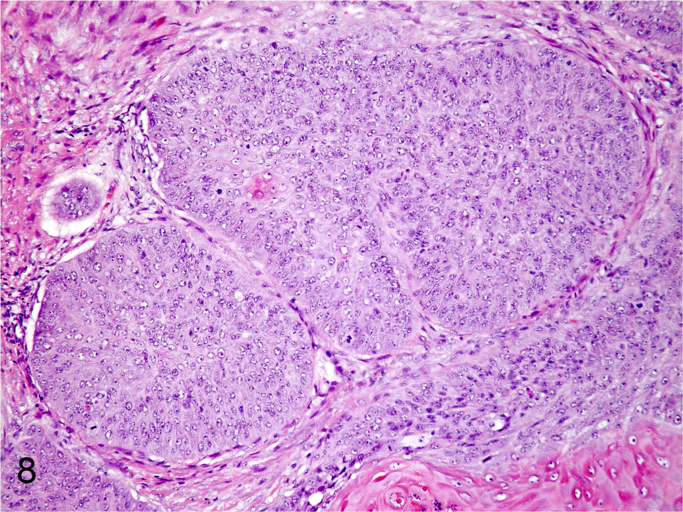
We further characterized those 9 dogs with malignant transformation of benign epithelial lesions for which CPV infection had been confirmed. Two of these 9 dogs had multiple plaque-like lesions (Fig. 1) of marked epidermal hyperplasia (Fig. 2), and areas of basal pigmentation over the body that were contiguous with dysplastic foci that transitioned into regions of Bowenoid in situ carcinoma and invasive SCC (Fig. 3). The other 7 dogs had papillomas with intranuclear viral inclusions in keratinocytes within the stratum spinosum and stratum corneum. Four of these 7 dogs had multiple viral papillomas over the lips and oropharyngeal mucosa (Fig. 4) at the base of which were focal or multifocal regions in which neoplastic cells with cellular indices of malignancy invaded through the basement membrane (Fig. 5) and formed islands within the subepithelial stroma (Fig. 6). Two of 7 dogs had solitary viral papillomas with prominent koilocytes over the foot and bulbar conjunctiva (Fig. 7) that transitioned to SCC (Fig. 8). One dog had an inverted viral papilloma over the vulva that transitioned to full thickness dysplasia and Bowenoid in situ carcinoma in the adjacent epidermis. Sequencing of the amplified PCR products from all 7 viral papillomas with malignant transformation demonstrated 100% similarity of the intralesional viruses with CPV1. Sequence analysis of the amplified PCR product from the 2 cases of pigmented plaques with malignant transformation demonstrated a 100% similarity to CPV3 and CPV16, respectively. Papillomaviral protein and nucleic acid were identified within both benign CPV1 papillomas (Fig. 9, Fig. 10) and malignantly transformed SCCs (Fig. 11, Fig. 12) using immunohistochemistry (Fig. 9, Fig. 11) and in situ hybridization (Fig. 10, Fig. 12). Moreover, a spatial association between papillomavirus infection and malignant transformation was demonstrated by immunohistochemical expression of p53 and p16. Similar to the presence of papillomavirus, both benign papillomas (Fig. 13, Fig. 14) and malignantly transformed SCCs (Fig. 15, Fig. 16), had immunoreactivity for p53 (Fig. 13, Fig. 15) and p16 (Fig. 14, Fig. 16). Strong nuclear labeling for p53 (Fig. 13), as well as nuclear and cytoplasmic labeling for p16 (Fig. 14) were detected in benign papillomas, with labeling being particularly prominent in proliferative cells with papillomaviral cytopathology (koilocytes). Moreover, diffuse p53-nuclear labeling (Fig. 15) and strong immunoreactivity for p16 (Fig. 16) were detected in all 7 dogs within regions of transformation to invasive SCCs. Dual labeling using IHC for p16 or p53 and ISH for CPV demonstrated CPV DNA within p16 and p53 positive cells, respectively.
Fig. 1.
Gross appearance of canine pigmented viral plaques caused by CPV16.
Fig. 2.
Subgross feature of canine pigmented viral plaque caused by CPV16 (Hematoxylin and Eosin).
Fig. 3.
Subgross histopathology exhibiting malignant transformation of CPV16-associated canine pigmented viral plaque to epidermal dysplasia, Bowenoid carcinoma, and squamous cell carcinoma (Hematoxylin and Eosin).
Fig. 4.
Clinical appearance of multiple canine oral viral papillomas caused by CPV1.
Fig. 5.
Progression of CPV1-induced viral papilloma to epidermal dysplasia, Bowenoid carcinoma, and squamous cell carcinoma (Hematoxylin and Eosin).
Fig. 6.
Malignant transformation of canine oral CPV1-induced papilloma as evident by basement membrane invasion of neoplastic cells (Hematoxylin and Eosin).
Fig. 7.
Koilocytes and intranuclear viral inclusions of CPV1-induced papilloma with prominent hyperkeratosis and keratohyalin granules are histologically characteristic for papillomavirus infection (Hematoxylin and Eosin).
Fig. 8.
Invasive nests of CPV1-associated SCC (Hematoxylin and Eosin).
Fig. 9.
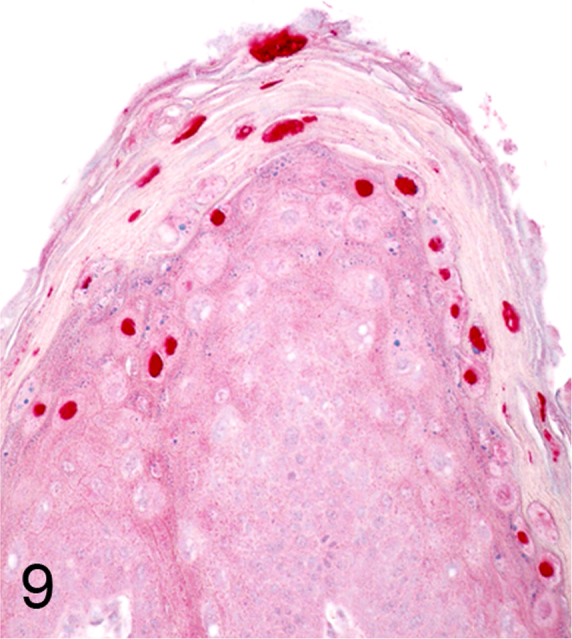
Immunohistochemistry for CPV1 demonstrating intense nuclear labeling within papillomavirus-infected keratinocytes (Alkaline phosphatase, vector-Red chromogen with hematoxylin counterstain).
Fig. 10.
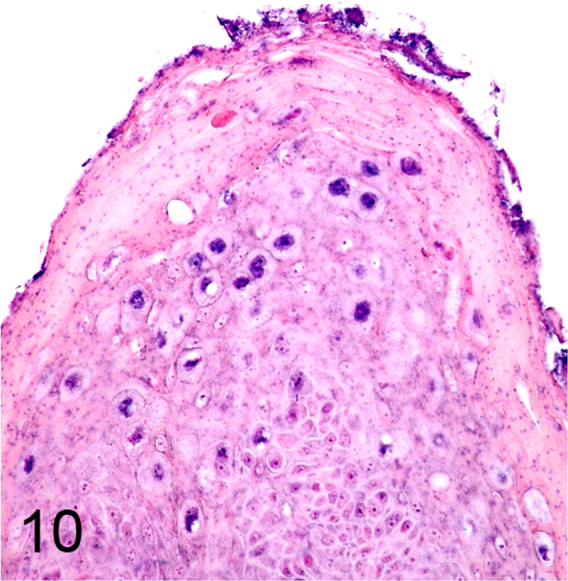
CPV1 DNA detected by in situ hybridization within infected keratinocytes (Alkaline phosphatase, NBT (blue) chromogen with nuclear fast red counterstain).
Fig. 11.
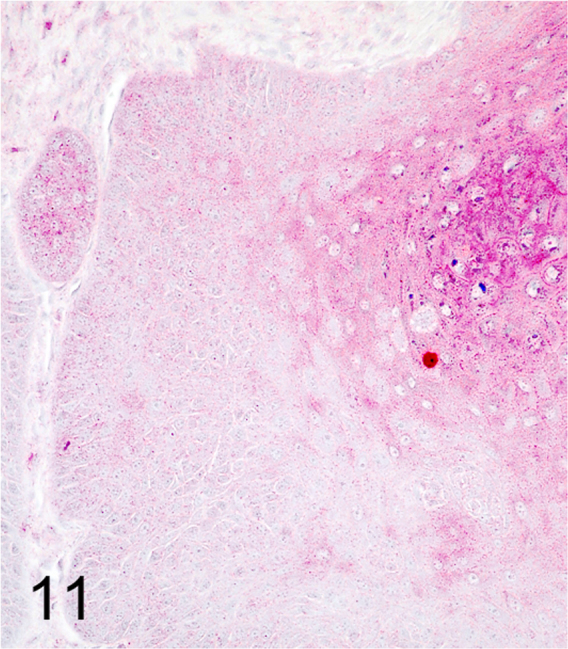
Rare nuclear labeling for CPV1 immunohistochemistry within invasive portion of SCC (Alkaline phosphatase, vector-Red chromogen with hematoxylin counterstain).
Fig. 12.
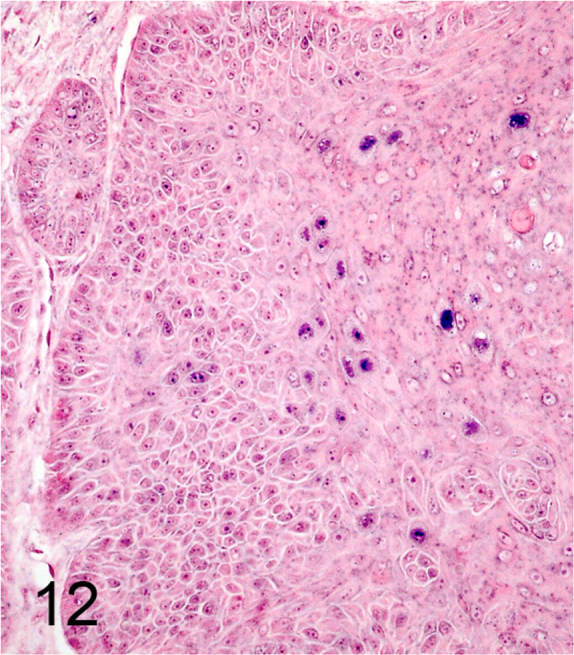
Small amounts of CPV1 DNA detected by in situ hybridization within invasive portion of SCC (Alkaline phosphatase, NBT (blue) chromogen with nuclear fast red counterstain).
Fig. 13.
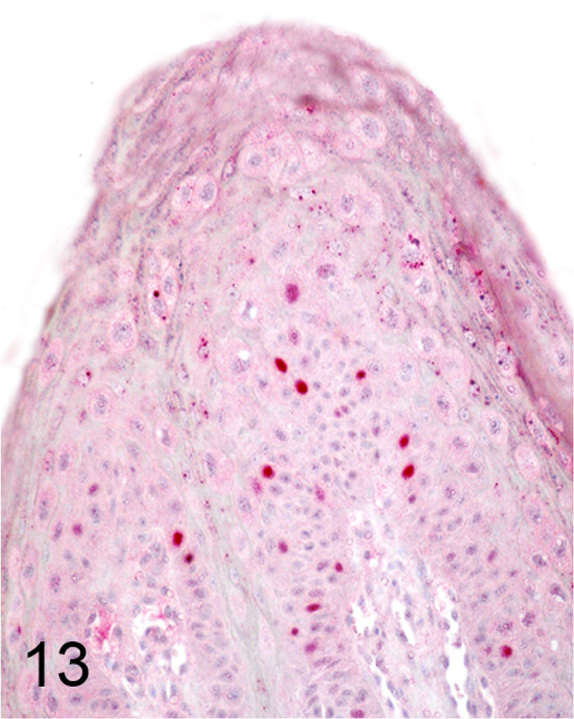
Nuclear labeling for p53 was detected in CPV1-associated benign proliferative keratinocytes (Alkaline phosphatase, vector-Red chromogen with hematoxylin counterstain).
Fig. 14.
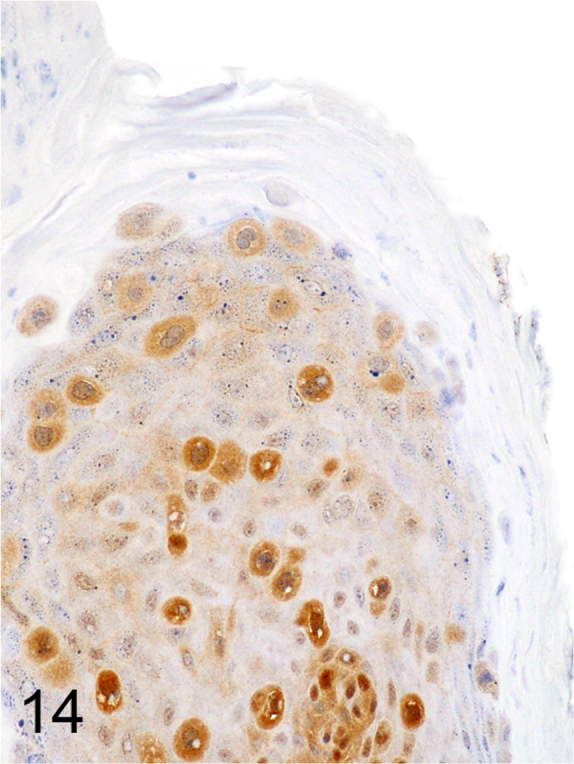
Cytoplasmic and nuclear immunoreactivity for p16 within CPV1-associated benign proliferative keratinocytes (Streptavidin-biotin-peroxidase, DAB chromogen with hematoxylin counterstain).
Fig. 15.
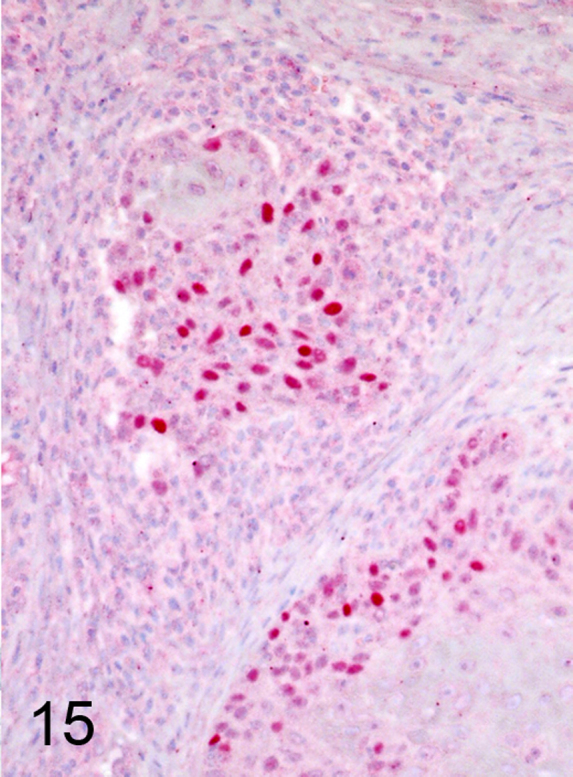
Nuclear labeling for p53 was detected in invasive nests of CPV1-associated SCC (Alkaline phosphatase, vector-Red chromogen with hematoxylin counterstain).
Fig. 16.
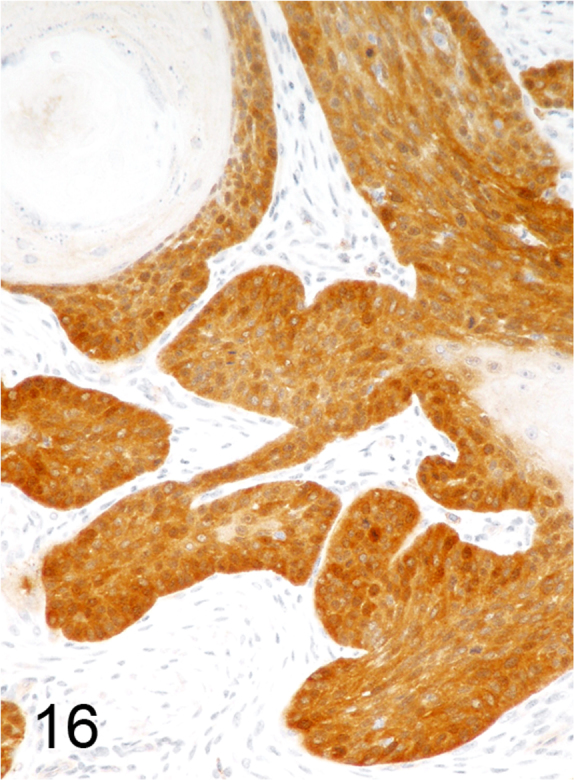
Intense cytoplasmic and nuclear immunoreactivity for p16 within nests and trabeculae of CPV1-associated SCC (Streptavidin-biotin-peroxidase, DAB chromogen with hematoxylin counterstain).
4. Discussion
Our study confirmed that less commonly detected types of CPV, such as CPV3 and CPV16, can be associated with malignant transformation of viral epithelial plaques. Two SCCs in our study that progressed from pigmented plaques were associated with CPV3 and CPV16 infection. CPV3 and CPV16 are both Chipapillomaviruses and have been previously reported within viral plaques, some of which progressed to SCC [10], [14], [35]. To our knowledge, neither of these viruses has been associated with viral papillomas. Based on the current literature, CPV-associated malignant transformation occurs primarily from pigmented plaques, and CPVs associated with such transformation belong to the Tau or Chipapillomaviruses [3].
In contrast, canine Lambdapapillomaviruses, CPV1 and CPV6, have only been identified as the cause of benign, non-neoplastic viral papillomas [3]. Recent studies have not associated CPV1 with the development of SCC, and malignant transformation of CPV1-induced papillomas has not been reported. The only progression of benign CPV-induced papillomas to SCC has been reported for a group of XSCID dogs infected with CPV2, a Taupapillomavirus [9]. Numerous studies have investigated the prevalence of CPV1 within canine SCCs. Detection of CPV1 by PCR was first reported in 1998 in 3 dogs with gingival SCCs within a population of 54 dogs with oral or non-oral SCCs [26]. One of the SCCs was also positive by IHC for CPV1, and CPV1 nucleic acid was detected by ISH in nests of neoplastic epithelial cells surrounding keratin pearls, leading the authors to suggest a potential role of CPV1 in SCC oncogenesis. Interestingly, 35% of SCCs expressed p53 by IHC, but 11% of cutaneous viral papillomas also expressed p53 suggesting potential malignant transformation in the absence of microscopic evidence [24]. In 2005, another retrospective study identified the presence of CPV in 9 of 42 (21%) canine SCCs using a broad-range PCR for papillomaviruses [36]. Of these 9 cases, CPV1 was confirmed in only 1 dog and the other 8 cases had different papillomavirus types, which had not been genetically characterized at the time [36]. A more recent study identified CPV1 DNA in 10% of oral SCCs; however, all cases were negative by IHC, and no CPV1 RNA was detected suggesting that CPV1 most likely represented an innocent bystander in SCC oncogenesis, thus leading the authors to conclude that CPV1 was not a suitable animal model for high-risk HPV-induced oral cancer [28]. Another study also concluded that CPV1 does not play a significant role in the development of canine SCCs [25]. The study identified expression of p16 in 17.3% of 52 canine SCCs, but CPV1 DNA was detected in only 5.8% of cases, and there was no association between presence of CPV1 DNA and p16 labeling [25]. Another study reached similar conclusions identifying p16 immunolabeling in 4 of 28 canine SCCs, while not detecting CPV DNA in any of the 28 cases [27]. The same authors did not identify CPV DNA in a series of 28 subungual SCCs [37]. None of these studies reported histologic evidence of progression from benign proliferative CPV1-associated lesions to SCC. In fact, all except one study dismissed CPV1 as a cause of SCC [26]. These data are in strong contrast to our findings, which clearly demonstrate histologic evidence of malignant transformation of CPV1-induced viral papillomas to SCCs. However, it is important to recognize that previous studies that simply dismissed CPV1 as a cause of SCC may have been hampered by both technical difficulties, such as the use of archival material, as well as the disappearance of CPV DNA from lesions after the virus induced irreversible immortalization of infected cells [38]. This explanation would also account for the discrepancies in some of these studies between the detection of viral DNA versus RNA and/or protein. Furthermore, in our study benign papillomatous lesions had intense p16 and p53 immunoreactivity, especially within cells with cytopathic effects. Moreover, even stronger immunoreactivity for p16 and p53 was also detected within the invasive SCCs arising from the benign viral papillomas. While expression of either antigen is no proof of CPV infection, p16 expression has been used as a surrogate biomarker of oncogenic HPV infection indicating HPV-mediated tumorigenesis [19]. Our data in regard to expression of p16 are similar to reports of various types of HPV positive SCCs in humans [19], [20], [21], but are in contrast to previous studies in dogs that identified p16 in subsets of CPV negative SCCs [25], [27] further raising concerns about technical difficulties having prevented detection of CPV in archival material in these previous studies. The expression of p53 in CPV positive lesions and even more the documentation of viral DNA in p53 positive cells are not commonly observed in HPV positive SCCs [20], [21]. There are conflicting data about the effect of various animal papillomaviruses on p53. While in cats with SCCs some studies could not find evidence that feline PVs degrade p53 [39], in vitro studies concluded that binding of feline PV E6 and E7 downregulated p53 by different mechanisms [40]. Interestingly, in horses with bovine PV1 (BPV-1) induced sarcoids, expression of p53 has consistently been reported both in tumors and neoplastic cell lines [41], [42]. Regardless, the number of cases in our study is too small to draw conclusion on the effect of CPV1 on p53, but the findings are intriguing enough to warrant more detailed future investigations.
Papillomavirus could not be detected in 17 of the 24 total papillomas that had histologic features associated with CPV infection and evidence of dysplasia and/or overt progression to invasive SCC; as such, these cases were excluded from further study. While sufficient DNA was extracted from all cases, PCR failed to detect CPV nucleic acid in these samples. Further, IHC and ISH respectively failed to demonstrate viral antigen or DNA. It is unlikely that all 17 cases were caused by a novel papillomavirus that was not detected by our methods. Since these were archival tissues submitted by veterinary practitioners, prolonged formalin fixation may have damaged DNA and lead to failure of IHC in some cases preventing detection. Alternatively, these cases may reflect the “hit and run” hypothesis which suggests that CPV DNA may disappear from the lesion the virus induces after the virus causes irreversible immortalization of infected cells through the action of the E6 and E7 viral proteins [38]. While we excluded the 17 cases with no detectable CPV DNA from the IHC study, the existence of these cases suggests that the potential incidence of malignant transformation of CPV-induced viral papillomas in dogs may be up to 3 times higher as earlier suggested.
Histologic evidence of malignant transition of benign proliferative CPV1-induced papillomas is a strong indicator that CPV1 infection plays a role in lesion development. While it is unlikely that CPV1 alone is capable of mediating malignant transformation of benign non-neoplastic papillomas, it is conceivable that its oncogenic potential is enhanced by other factors or conditions to provoke such transition. When reviewing the annual submission rate of all cases microscopically consistent with viral papillomas with transformation toward a malignant phenotype in comparison to our overall submissions, there is a strong trend toward a 2–3 fold increase of the number of such cases between 2006 and 2014. We speculate that there is an emergence of malignant transformation of CPV1-induced viral papillomas to SCC that could be explained in different ways. The reported increase may simply reflect an increased awareness of such lesions and improved diagnostic techniques. As molecular diagnostic techniques have improved in sensitivity and become more widely available, their use in detecting and reporting CPV infections in diagnostic settings has increased [43]. Moreover, an increased awareness by pathologists and veterinarians regarding the occurrence of malignant transformation of benign CPV-associated lesions may have contributed to the identification of larger numbers of cases. Thus, the increasing number of reports may only reflect a closer estimate of the real incidence of CPV1-induced papillomas with malignant transformation. However, this explanation seems insufficient since microscopic descriptions recognizing such changes are very rare in historical archives for CPV-associated benign lesions with malignant transformation prior to 2010 while the annual number of viral papillomas being submitted to the biopsy service has been constant.
Second, there may have been recent changes in host or virus related factors that cause an increase of the malignant potential of CPV1. An underlying genetic predisposition has been suggested for two Basenji dog siblings that both developed pigmented plaques associated with different papillomaviruses, CPV12 and CPV16, which progressed to SCCs [13]. Another factor may be an altered immune response within the host. The immune system plays a pivotal role in determining the clinical outcome of PV-associated diseases, as demonstrated by the increased persistence and enhanced neoplastic progression in humans with cell-mediated immune deficiencies [44]. In vitro studies revealed that dexamethasone is capable of inducing early viral gene upregulation, enhancing immortalization of HPV16-infected cells, and mediating malignant transformation [45]. Moreover, higher rates of HPV infection and persistence, and higher viral loads due to immunosuppression or disabled host immune systems by PV infections, are all potential explanations for the increased risk of HPV-associated cancers in patients treated with steroid [46]. Virus-induced chronic inflammation may create a microenvironment that favors expression of viral oncogenes [47]. In dogs, malignant transformation of benign CPV2-induced papillomas and metastasis was first recognized in XSCID dogs and occurred even after immune function was restored through transplantation of hematopoietic stem cells [7]. Prednisolone, cyclosporine, and piroxicam are immunosuppressive drugs increasingly used to treat various diseases in dogs, ranging from atopy to cancer. It is feasible that the extensive use of immunosuppressive drugs in modern practice may contribute to an increased incidence of malignant transformation of CPV-associated lesions.
Lastly, some environmental factors such as pollutants, food additives, global warming, or damage to the ozone layer may weaken the immune system or potentially enhance the oncogenic potential of CPVs, by escalating both rates and incidence of persistent infections with sequelae.
5. Conclusion
This retrospective analysis provides the first evidence of malignant transformation of CPV1-induced benign non-neoplastic papillomas to SCCs and expands the number of cases with CPV-associated pigmented plaques progressing to SCC. Our results further support the hypothesis that malignant transformation of viral papillomas versus viral plaques may be caused by different types of CPVs, regardless of the location of the lesions in the skin or mucosa. CPVs have been well recognized as an oncogenic driver for epithelial proliferation, and the overall increased incidence of lesions with malignant transformation in the past decade has caused concern regarding potential factors contributing to their increasing oncogenic potential. We hope that our study increases the awareness of the malignant potential of CPV1 and stipulates further investigations into the cause of this recent change in its oncogenic potential.
Acknowledgments
We thank Thomas Wood for technical assistance in immunohistochemistry and in situ hybridization.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that they have not conflicts of interest to disclose.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pvr.2018.10.007.
Appendix A. Supplementary material
Supplementary material
References
- 1.Bernard H.U., Burk R.D., Chen Z., van Doorslaer K., zur Hausen H., de Villiers E.M. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010;401:70–79. doi: 10.1016/j.virol.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lange C.E., Favrot C. Canine papillomaviruses. Vet. Clin. North. Am. Small. Anim. Pract. 2011;41:1183–1195. doi: 10.1016/j.cvsm.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Gil da Costa R.M., Peleteiro M.C., Pires M.A., DiMaio D. An update on canine, feline and bovine papillomaviruses. Transbound. Emerg. Dis. 2017;64:1371–1379. doi: 10.1111/tbed.12555. [DOI] [PubMed] [Google Scholar]
- 4.Lange C.E., Diallo A., Zewe C., Ferrer L. Novel canine papillomavirus type 18 found in pigmented plaques. Papillomavirus Res. 2016;2:159–163. doi: 10.1016/j.pvr.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tisza M.J., Yuan H., Schlegel R., Buck C.B. Genomic sequence of canine papillomavirus 19. Genome Announc. 2016;4 doi: 10.1128/genomeA.01380-16. (pii: e01380-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholls P.K., Moore P.F., Anderson D.M., Moore R.A., Parry N.R., Gough G.W., Stanley M.A. Regression of canine oral papillomas is associated with infiltration of CD4+ and CD8+ lymphocytes. Virology. 2001;283:31–39. doi: 10.1006/viro.2000.0789. [DOI] [PubMed] [Google Scholar]
- 7.Nicholls P.K., Klaunberg B.A., Moore R.A., Santos E.B., Parry N.R., Gough G.W., Stanley M.A. Naturally occurring, nonregressing canine oral papillomavirus infection: host immunity, virus characterization, and experimental infection. Virology. 1999;265:365–374. doi: 10.1006/viro.1999.0060. [DOI] [PubMed] [Google Scholar]
- 8.Kuntsi-Vaattovaara H., Verstraete F.J., Newsome J.T., Yuan H. Resolution of persistent oral papillomatosis in a dog after treatment with a recombinant canine oral papillomavirus vaccine. Vet. Comp. Oncol. 2003;1:57–63. doi: 10.1046/j.1476-5829.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- 9.Goldschmidt M.H., Kennedy J.S., Kennedy D.R., Yuan H., Holt D.E., Casal M.L., Traas A.M., Mauldin E.A., Moore P.F., Henthorn P.S., Hartnett B.J., Weinberg K.I., Schlegel R., Felsburg P.J. Severe papillomavirus infection progressing to metastatic squamous cell carcinoma in bone marrow-transplanted X-linked SCID dogs. J. Virol. 2006;80:6621–6628. doi: 10.1128/JVI.02571-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobler K., Favrot C., Nespeca G., Ackermann M. Detection of the prototype of a potential novel genus in the family Papillomaviridae in association with canine epidermodysplasia verruciformis. J. Gen. Virol. 2006;87:3551–3557. doi: 10.1099/vir.0.82305-0. [DOI] [PubMed] [Google Scholar]
- 11.Lange C.E., Tobler K., Ackermann M., Panakova L., Thoday K.L., Favrot C. Three novel canine papillomaviruses support taxonomic clade formation. J. Gen. Virol. 2009;90:2615–2621. doi: 10.1099/vir.0.014498-0. [DOI] [PubMed] [Google Scholar]
- 12.Munday J.S., O'Connor K.I., Smits B. Development of multiple pigmented viral plaques and squamous cell carcinomas in a dog infected by a novel papillomavirus. Vet. Dermatol. 2011;22:104–110. doi: 10.1111/j.1365-3164.2010.00913.x. [DOI] [PubMed] [Google Scholar]
- 13.Munday J.S., Tucker R.S., Kiupel M., Harvey C.J. Multiple oral carcinomas associated with a novel papillomavirus in a dog. J. Vet. Diagn. Investig. 2015;27:221–225. doi: 10.1177/1040638714567191. [DOI] [PubMed] [Google Scholar]
- 14.Luff J., Mader M., Britton M., Fass J., Rowland P., Orr C., Schlegel R., Yuan H. Complete genome sequence of canine papillomavirus type 16. Genome, Announc. 2015;(3) doi: 10.1128/genomeA.00404-15. (pii: e00404-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luff J., Rowland P., Mader M., Orr C., Yuan H. Two canine papillomaviruses associated with metastatic squamous cell carcinoma in two related Basenji dogs. Vet. Pathol. 2016;53:1160–1163. doi: 10.1177/0300985816630795. [DOI] [PubMed] [Google Scholar]
- 16.Munday J.S., Dunowska M., Laurie R.E., Hills S. Genomic characterisation of canine papillomavirus type 17, a possible rare cause of canine oral squamous cell carcinoma. Vet. Microbiol. 2016;182:135–140. doi: 10.1016/j.vetmic.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Husain N., Neyaz A. Human papillomavirus associated head and neck squamous cell carcinoma: controversies and new concepts. J. Oral. Biol. Craniofac. Res. 2017;7:198–205. doi: 10.1016/j.jobcr.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherr C.J., McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2:103–112. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 19.Zargar-Shoshtari K., Spiess P.E., Berglund A.E., Sharma P., Powsang J.M., Giuliano A., Magliocco A.M., Dhillon J. Clinical significance of p53 and p16(ink4a) status in a Contemporary North American Penile carcinoma cohort. Clin. Genitourin. Cancer. 2016;4:346–351. doi: 10.1016/j.clgc.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu X.J.D., Liu K.Y.P., Soares R.C., Thomson T., Prisman E., Wu J., Poh C.F. Potential clinical implications of HPV status and expressions of p53 and cyclin D1 among oropharyngeal cancer patients. J. Oral. Pathol. Med. 2018 doi: 10.1111/jop.12779. (Epub) [DOI] [PubMed] [Google Scholar]
- 21.Barzon L., Cappellesso R., Peta E., Militello V., Sinigaglia A., Fassan M., Simonato F., Guzzardo V., Ventura L., Blandamura S., Gardiman M., Palù G., Fassina A. Profiling of expression of human papillomavirus-related cancer miRNAs in penile squamous cell carcinomas. Am. J. Pathol. 2014;184:3376–3383. doi: 10.1016/j.ajpath.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Andl T., Kahn T., Pfuhl A., Nicola T., Erber R., Conradt C., Klein W., Helbig M., Dietz A., Weidauer H., Bosch F.X. Etiological involvement of oncogenic human papillomavirus in tonsillar squamous cell carcinomas lacking retinoblastoma cell cycle control. Cancer Res. 1998;58:5–13. [PubMed] [Google Scholar]
- 23.Tam S.W., Shay J.W., Pagano M. Differential expression and cell cycle regulation of the cyclin-dependent kinase 4 inhibitor p16Ink4. Cancer Res. 1994;54:5816–5820. [PubMed] [Google Scholar]
- 24.Lewis J.S., Thorstad W.L., Chernock R.D., Haughey W.H., Yip J.H., Zhang Q., El-Mofty S.K. p16 Positive oropharyngeal squamous cell carcinoma: an entity with a favorable prognosis regardless of tumor HPV status. Am. J. Surg. Pathol. 2010;34:1088–1096. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabattini S., Savini F., Gallina L., Scagliarini A., Bassi P., Bettini G G. p16 Immunostaining of canine squamous cell carcinomas Is not associated with papillomaviral DNA. PLoS. One. 2016;11:e0159687. doi: 10.1371/journal.pone.0159687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teifke J.P., Löhr C.V., Shirasawa H. Detection of canine oral papillomavirus-DNA in canine oral squamous cell carcinomas and p53 overexpressing skin papillomas of the dog using the polymerase chain reaction and non-radioactive in situ hybridization. Vet. Microbiol. 1998;60:119–130. doi: 10.1016/s0378-1135(98)00151-5. [DOI] [PubMed] [Google Scholar]
- 27.Munday J.S., French A., Harvey C.J. Molecular and immunohistochemical studies do not support a role for papillomaviruses in canine oral squamous cell carcinoma development. Vet. J. 2015;204:223–225. doi: 10.1016/j.tvjl.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Porcellato I., Brachelente C., Guelfi G., Reginato A., Sforna M., Bongiovanni L., Mechelli L. A retrospective investigation on canine papillomavirus 1 (CPV1) in oral oncogenesis reveals dogs are not a suitable animal model for high-risk HPV-induced oral cancer. PLoS. One. 2014;9:e112833. doi: 10.1371/journal.pone.0112833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Resnick R.M., Cornelissen M.T., Wright D.K., Eichinger G.H., Fox H.S., ter Schegget J., Manos M.M. Detection and typing of human papillomavirus in archival cervical cancer specimens by DNA amplification with consensus primers. J. Natl. Cancer Inst. 1990;82:1477–1484. doi: 10.1093/jnci/82.18.1477. [DOI] [PubMed] [Google Scholar]
- 30.Chouhy D., Gorosito M., Sánchez A., Serra E.C., Bergero A., Fernandez Bussy R., Giri A.A. New generic primer system targeting mucosal/genital and cutaneous human papillomaviruses leads to the characterization of HPV 115, a novel Beta-papillomavirus species 3. Virology. 2010;397:205–216. doi: 10.1016/j.virol.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iftner A., Klug S.J., Garbe C., Blum A., Stancu A., Wilczynski S.P., Iftner T. The prevalence of human papillomavirus genotypes in nonmelanoma skin cancers of nonimmunosuppressed individuals identifies high-risk genital types as possible risk factors. Cancer Res. 2003;63:7515–7519. [PubMed] [Google Scholar]
- 32.Knight C.G., Munday J.S., Rosa B.V., Kiupel M. Persistent, widespread papilloma formation on the penis of a horse: a novel presentation of equine papillomavirus type 2 infection. Vet. Dermatol. 2011;22:570–574. doi: 10.1111/j.1365-3164.2011.00987.x. [DOI] [PubMed] [Google Scholar]
- 33.Montiani-Ferreira F., Kiupel M., Muzolon P., Truppel J. Corneal squamous cell carcinoma in a dog: a case report. Vet. Ophthalmol. 2008;11:269–272. doi: 10.1111/j.1463-5224.2008.00622.x. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues A., Gates L., Payne H.R., Kiupel M., Mansell J. Multicentric squamous cell carcinoma in situ associated with papillomavirus in a ferret. Vet. Pathol. 2010;47:964–968. doi: 10.1177/0300985810369899. [DOI] [PubMed] [Google Scholar]
- 35.Luff J.A., Affolter V.K., Yeargan B., Moore P.F. Detection of six novel papillomavirus sequences within canine pigmented plaques. J. Vet. Diagn. Investig. 2012;24:576–580. doi: 10.1177/1040638712443360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaugg N., Nespeca G., Hauser B., Ackermann M., Favrot C. Detection of novel papillomaviruses in canine mucosal, cutaneous and in situ squamous cell carcinomas. Vet. Dermatol. 2005;16:290–298. doi: 10.1111/j.1365-3164.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- 37.Munday J.S., Waropastrakul S., Gibson I., French A.F. Papillomaviral DNA sequences are not amplifiable from canine subungual squamous cell carcinomas. N. Z. Vet. J. 2013;61:234–236. doi: 10.1080/00480169.2012.731718. [DOI] [PubMed] [Google Scholar]
- 38.Moody C.A., Laimins L.A. Human papillomavirus oncoproteins: pathways to transformation. Nat. Rev. Cancer. 2010;10:550–560. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 39.Munday J.S., Aberdein D. Loss of retinoblastoma protein, but notp53, is associated with the presence of papillomaviral DNA in feline viral plaques, Bowenoid in situ carcinomas, and squamous cell carcinomas. Vet. Pathol. 2012;49:538–545. doi: 10.1177/0300985811419534. [DOI] [PubMed] [Google Scholar]
- 40.Altamura G., Corteggio A., Pacini L., Conte A., Pierantoni G.M., Tommasino M., Accardi R., Borzacchiello G. Transforming properties of Felis catus papillomavirus type 2 E6 and E7 putative oncogenes in vitro and their transcriptional activity in feline squamous cell carcinoma in vivo. Virology. 2016;96:1–8. doi: 10.1016/j.virol.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 41.Finlay M., Yuan Z., Morgan I.M., Campo M.S., Nasir L. Equine sarcoids: bovine Papillomavirus type 1 transformed fibroblasts are sensitive to cisplatin and UVB induced apoptosis and show aberrant expression ofp53. Vet. Res. 2012;43(43):81. doi: 10.1186/1297-9716-43-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nixon C., Chambers G., Ellsmore V., Campo M.S., Burr P., Argyle D.J., Reid S.W., Nasir L. Expression of cell cycle associated proteins cyclin A, CDK-2, p27kip1 and p53 in equine sarcoids. Cancer Lett. 2005;221:237–245. doi: 10.1016/j.canlet.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 43.Maes R.K., Langohr I.M., Wise A.G., Smedley R.C., Thaiwong T., Kiupel M. Beyond H&E: integration of nucleic acid-based analyses into diagnostic pathology. Vet. Pathol. 2014;51:238–256. doi: 10.1177/0300985813505878. [DOI] [PubMed] [Google Scholar]
- 44.Stanley M.A., Sterling J.C. Host responses to infection with human papillomavirus. Curr., Probl. Dermatol. 2014;45:58–74. doi: 10.1159/000355964. [DOI] [PubMed] [Google Scholar]
- 45.von Knebel Doeberitz M., Rittmüller C., Aengeneyndt F., Jansen-Dürr P., Spitkovsky D. Reversible repression of papillomavirus oncogene expression in cervical carcinoma cells: consequences for the phenotype and E6-p53 and E7-pRB interactions. J. Virol. 1994;68:2811–2821. doi: 10.1128/jvi.68.5.2811-2821.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maglennon G.A., McIntosh P.B., Doorbar J. Immunosuppression facilitates the reactivation of latent papillomavirus infections. J. Virol. 2014;88:710–716. doi: 10.1128/JVI.02589-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernandes J.V., DE Medeiros Fernandes T.A., DE Azevedo J.C., Cobucci R.N., DE Carvalho M.G., Andrade V.S., Araújo J.M. Link between chronic inflammation and human papillomavirus-induced carcinogenesis (Review) Oncol. Lett. 2015;9:1015–1026. doi: 10.3892/ol.2015.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material