Abstract
In extracorporeal life support (ECLS), there are two main types of oxygenators in clinical use for neonates: polymethylpentene (PMP) hollow fiber and polypropylene (PP) hollow fiber. A retrospective study was performed on neonates ( n = 44) who had undergone ECLS for noncardiac indications from 2009 to 2015. Between the two groups (PMP n = 21, PP n = 23), the PP oxygenators failed 91% of the time, whereas the PMP oxygenators failed 43% of the time ( p < 0.05). Analysis suggests PMP oxygenators are less prone to failure than PP oxygenators, and they require fewer number of oxygenator changes during a neonatal ECLS.
Keywords: extracorporeal membrane oxygenation, neonatal, oxygenator, complications
Introduction
Neonatal extracorporeal membrane oxygenation (ECMO) is a lifesaving therapy that is often utilized to stabilize critically ill neonates at level IV neonatal intensive care units (NICUs) across the world. ECMO is an invasive procedure that provides complete support of either lung function, in venovenous (VV) ECMO, or complete support of both heart and lung functions, in venoarterial (VA) ECMO. The critical component in the ECMO circuit is the membrane oxygenator (MO) serving to both add oxygen (O 2 ) and remove carbon dioxide (CO 2 ) from the blood.
There are three commercially available types of MOs: silicone sheet membranes, polypropylene (PP) hollow fiber, and polymethylpentene (PMP) hollow fiber. Silicone membrane oxygenators were used for many years and have since given way to the newer PP and PMP oxygenators. In 2015 there was 1 silicone oxygenator used, 200 PP oxygenators used, and 1,224 PMP oxygenators in the United States. 1 Silicone oxygenators were constructed with a thin sheet of silicone wrapped around a polycarbonate core, which allowed for separation between the blood and gas phases. These, however, were prone to tear, resulting in minor bleeding and in the worst case sheet rupture. 2
PP MOs are composed of microporous, hollow fibers that separate the patient's blood and circuit gas while flowing through the oxygenator. O 2 and CO 2 diffuse down their respective concentration gradients through the micropores with minimal direct contact between blood and gas. 3 The literature describes the problem of plasma leakage with PP MOs. This occurs when blood plasma enters the gas portion of the MO through the micropores leading to oxygenator failure, which is known as “foam out.” 4
This problem leads to the development of MOs composed of PMP hollow fibers, which has nearly completely eliminated plasma leakage. 5 6 Whereas PP MOs have micropores that over time allow plasma and blood proteins to enter the gas portion of the MO, PMP MOs have a thin, tight membrane that provides complete physical separation between the blood and gas phases, nearly eliminating plasma leakage. 7 The majority of circuit complications that occur while on ECMO involve the MO. 7 Of those incidents, the primary reasons for changing the MO include oxygenator failure or oxygenator clot burden. 8
Currently, there are several excellent studies in the literature examining membrane oxygenators. Two of the most influential compare silicone MOs and PMP MOs in adults and neonates. 9 10 Additionally, the first direct comparison between PP and PMP MOs was reported by Robak et al, which showed a decrease in the number of oxygenator changes and a trend toward lower mortality in adult patients with acute respiratory distress syndrome (ARDS) treated with ECMO using a PMP oxygenator. 11 To our knowledge, there has been no direct comparison between PP MOs and PMP MOs in the neonatal population.
As most ECMO centers are changing to PMP MOs for their noncardiac neonatal ECMO runs, it is critical to examine objective data on the rates of oxygenator complications for these two types of hollow fiber MOs. The goal of our study was to determine whether there was a difference in complication rates between PP MOs and PMP MOs at our center.
Methods
Demographics
A retrospective chart review was performed for all ECMO cases performed at Kentucky Children's Hospital from 2009 to 2015 ( Fig. 1 ). Neonatal patients were defined as those placed on ECMO within the first 30 days of life. Those placed on ECMO after that age were classified as pediatric patients. All neonatal patients met institutional criteria for ECMO: (1) gestational age > 34 weeks, (2) weight > 1,800 g, (3) ventilator time < 14 days, (4) no lethal congenital anomalies, and (5) no grade 3 or 4 intraventricular hemorrhage (IVH). Pediatric patients were excluded, as were patients placed on ECMO for congenital heart disease requiring operative repair. Cardiac ECMO patients were excluded because the underlying physiology of these cases, anticoagulation parameters, and cannulation sites dramatically differ from neonates placed on support for pulmonary reasons. We felt that inclusion of cardiac cases would introduce too many variables for which we could not account and were thus excluded. A total of 44 neonatal patients met inclusion criteria and underwent data collection and analysis. Variables collected included type of oxygenator, gestational age, 1-minute Apgar, 5-minute Apgar, and birth weight.
Fig. 1.
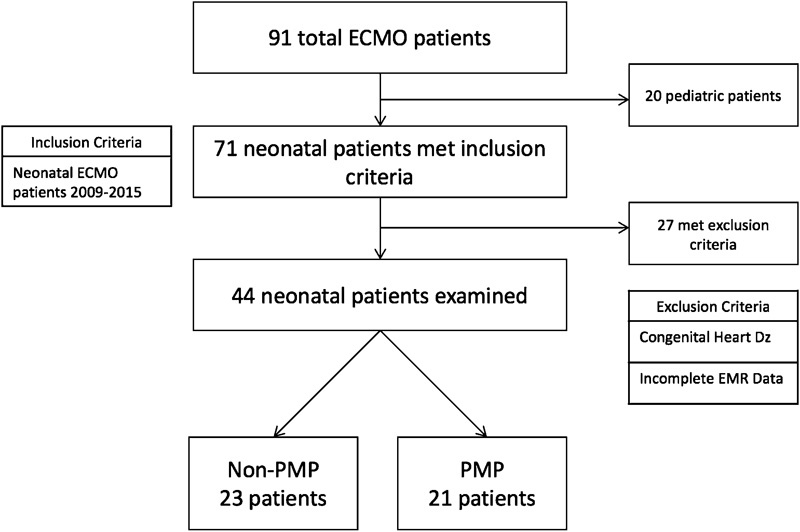
ECMO patient population 2009–2015. Primary inclusion criteria were neonatal patients requiring ECMO from 2009 to 2015. Patients were excluded if their indication for ECMO was congenital heart disease or if their medical records were incomplete. ECMO, extracorporeal membrane oxygenation; EMR, electronic medical record; PMP, polymethylpentene.
ECMO Data
The type of ECMO (VV vs. VA), indications for ECMO, age at cannulation, cannula size, length of ECMO run, type of MO, number of MO changes, days of use per MO, day of change of MO, daily heparin rates, daily ACT ranges, daily morning platelet level, number of platelet transfusions, number of packed red blood cell (PRBC) transfusions, number of fresh frozen plasma (FFP) transfusions, number of cryoprecipitate (Cryo) transfusions, number of significant IVH events, infection while on ECMO, and death were all examined in the retrospective review. Standard statistical analyses were then applied to these data: continuous variables were analyzed with two-tailed t -test; categorical and ordinal variables were examined with chi-square analysis.
ECMO Circuit
ECMO circuits at Kentucky Children's Hospital were built with either a BIOLINE-coated QUADROX-D MO (MAQUET Holding B.V. & Co. Rastatt, Germany) with PMP fibers or a MiniMax (Medtronic Operational Headquarters, Minneapolis, Minnesota, United States) MO with PP fibers.
Circuits were identical to other components. The arterial and venous circuit limbs were a combined 10 ft in length with ¼-in tubing from Medtronic, and 5 ft of ⅜-in S-95-E tubing from Medtronic was used for the pump boot. Each system had an arterial-venous bridge. The ¼-in tubing holds a volume of 10 mL/foot of blood, whereas the ⅜-in tubing holds 20 mL/foot of blood. This 5 ft of ⅜-in tubing ads an additional 50 mL to our circuit volume (200 mL), as compared with a circuit of all ¼-in tubing (150 mL). The priming volume for the Quadrox-D was 250 mL and the MiniMax was 149 mL. The total volumes for each type of circuit were Quadrox 450 mL and MiniMax 349 mL. Each circuit was primed with packed red cells obtained from the blood bank. This difference in volume allows the roller head pump to run at lower number of revolutions per minute, which reduce wear and tear on the tubing while maintaining an equal volume of blood flow to the patient.
Vascular access was obtained via the right jugular vein for VV patients and the right jugular and right carotid artery for VA. VV ECMO was performed using a double-lumen cannula (OriGen BIOMEDICAL, Austin, Texas, United States). VA patients used two single lumen Pediatric Venous Cannulae (Medtronic, Minneapolis, Minnesota, United States).
The pump was a Sorin S5 (SORIN GROUP USA, Inc., Arvada, Colorado, United States) roller head, and a Better Bladder (Circulatory Technology Inc., Oyster Bay, New York, United States) inline reservoir was used as the circuit bladder.
Oxygenator Selection
PP oxygenators were used on patients nearly exclusively during 2009 through 2011. PMP oxygenators entered use at our institution in 2011 and both were used from 2011 to 2014. After 2014, all neonatal circuits were built with a PMP MO. Oxygenator selection was made by the on-call neonatal ECMO physician. Our hospital maintains two contracts, with Medtronic and MAQUET, to supply oxygenators for ECMO and cardiopulmonary bypass circuits. The MiniMax Plus oxygenator was selected for patients who were expected to have a shorter neonatal ECMO run, such as patients with classic persistent pulmonary hypertension of the newborn (PPHN). When the ECMO physician anticipated a more complicated or prolonged ECMO run, a Maquet Quadrox-D oxygenator was selected. At no point during our ECMO runs did the flow through the oxygenators drop below the minimum flow ratings for each oxygenator: Quadrox-D 0.5 to 7 L/min and MiniMax 0.5 to 2.3 L/min. The roller head pump revolutions per minutes range between 28 and 33 rpm, which generate flows ranging from 728 to 858 mL/min, well above the minimum flow ratings for the oxygenators. As the adult Quadrox-D was in a neonatal population, a shunt was placed in the circuit postoxygenator to allow diversion of high-flow volumes from reaching the patient. Postoxygenators were monitored with an inline flow sensor. Oxygenators were changed when the neonatal ECMO attending physician and perfusionist agreed that there was an increase in fibrin stranding or clot burden that posed a risk to the patient.
ECMO Goals
All patients were placed on resting ventilator settings (synchronized intermittent mechanical ventilation with pressure control plus pressure support: fraction of inspired oxygen 21–30%, peak inspiratory pressure 20 cmH 2 O, positive end-expiratory pressure 10 cmH 2 O, pressure support 10 cmH 2 O, rate 10, inspiratory time 1 second) once on ECMO. VA ECMO targeted flows of 120 to 150 mL/kg/min, S V O 2 goals of 70 to 80%, P a O 2 60 to 200 mm Hg, and P a CO 2 35 to 45 mm Hg. VV ECMO targeted flows of 80 to 120 mL/kg/min, P a O 2 > 40 mm Hg, P a CO 2 40 to 50 mm Hg, S p O 2 > 80%, and S v O 2 > 70%. Coagulation goals were prothrombin time of 20 seconds, partial thromboplastin time > 100 seconds, fibrinogen > 150,000, platelets > 100,000, and plasma-free Hb < 20 mg/dL. Unfractionated heparin drip was initiated within 2 hours of ECMO or when the activated clotting time (ACT) fell below 380 seconds. ACT goals during ECMO were 200 to 240 seconds and the heparin drip was adjusted every hour to maintain that range. During the course of this study, anti-Xa levels were not routinely measured.
Transfusion Criteria
Judicious use of blood products guided the transfusion criteria for all neonatal ECMO patients. Hematocrit goals were targeted to be > 35%, and transfusion of 10 mL/kg/dose were administered over 2 hours. Our platelet goal was 100,000/µL if stable, and if patients were actively bleeding, that goal was increased to 125,000/µL. Platelet transfusions were given at 10 mL/kg/dose and directly to the patient if possible. In the rare instance that platelets were unable to be given directly to the patient, they were administered to the circuit postoxygenator. If a patient required more than four transfusions of platelets in a 24-hour period, a diagnosis of circuit disseminated intravascular coagulation (DIC) was made, the circuit was carefully checked for clots, and the circuit was changed if needed. The decision to administer FFP or Cryo was based on the fibrinogen. When marginally low fibrinogen levels were seen (100–150 mg/dL), FFP was used to correct the deficiency at 10 mL/kg/dose. When fibrinogen was low (< 100 mg/dL) or extremely low (< 50 mg/dL), Cryo was administered at either 5 mL/kg/dose of 10 mL/kg/dose, respectively.
Results
Demographics
It is important to note that one patient had their oxygenator changed from a PP to a PMP during their run. For the purposes of this analysis, the time they spent on each MO was treated as a separate run. As such this increased the number of patients from 43 to 44. Our patient population of 44 patients was homogenous in terms of gestational age, birth weight, Apgar scores, type of ECMO, and age at cannulation. However, the groups differed significantly on the type of MO used and indication for EMCO ( Table 1 ). Approximately 80% of the patients placed on ECMO for meconium aspiration syndrome (MAS) ( n = 15) received a PP MO, whereas the remaining 20% received a PMP MO ( p < 0.01). Additionally, in the congenital diaphragmatic hernia (CDH) population, a PMP MO was used in 88% of cases and a PP MO in 12% of cases ( p < 0.05).
Table 1. Patient demographics.
| Category | PP ( n = 23) | PMP ( n = 21) | p -Value |
|---|---|---|---|
| Gestational age | 38.5 ± 1.4 | 38.6 ± 1.4 | 0.80 |
| Birth weight (g) | 3405 ± 437 | 3226 ± 556 | 0.24 |
| 1-min Apgar | 5 ± 3 | 5 ± 3 | 0.62 |
| 5-min Apgar | 7 ± 2 | 6 ± 2 | 0.40 |
| PPHN (18) | 7/18 (39%) | 11/18 (61%) | 0.14 |
| MAS (15) | 12/15 (80%) | 3/15 (20%) | < 0.05 |
| CDH (8) | 1/8 (13%) | 7/8 (88%) | < 0.05 |
| Sepsis (2) | 2/2 (100%) | 0/0 (0%) | – |
| Other (1) | 1/1 (100%) | 0/0 (0%) | – |
| Age at Cannulation | 2.1 ± 2.3 | 1.1 ± 1 | 0.09 |
| VA | 7/24 (30%) | 9/21 (43%) | 0.34 |
| VV | 17/24 (70%) | 12/21 (57%) |
Abbreviations: CDH, congenital diaphragmatic hernia; MAS, meconium aspiration syndrome; PMP, polymethylpentene; PP, polypropylene; PPHN, persistent pulmonary hypertension of the newborn; VA, venoarterial; VV, venovenous.
Note: One patient was converted from VV to VA ECMO due to clinical deterioration. The differences between the PP and PMP groups were not significant, other than for the indication for ECMO. The majority of MAS cases received a PP MO and nearly all of the CDH population received a PMP MO. The total number of individual CDH patients was seven for the study period; however, as one patient had their oxygenator changed from a PP to a PMP, the two periods of the run were treated as two separate patients for the analysis.
ECMO Run Length
Between our two MO populations, there was no difference in the number of days spent on ECMO. PP cases lasted between 1 and 16 days and PMP cases between 2 and 9 days. The lifespan of the MOs did not differ significantly either ( Table 2 ). PP MOs functioned between 0 and 9 days and PMP MOs between 0 and 14 days.
Table 2. Comparison of PP and PMP oxygenators.
| Variable | PP ( n = 23) | PMP ( n = 21) | p -Value |
|---|---|---|---|
| Days on ECMO | 6.2 ± 3.5 | 5.8 ± 2.2 | 0.59 |
| Days per oxygenator | 3.5 ± 2.3 | 3.8 ± 3.0 | 0.65 |
| Oxygenators changed | 21/23 (91%) | 9/21 (43%) | < 0.01 |
| No MO change | 8 | 12 | n/a |
| 1 MO change | 9 | 8 | n/a |
| > 1 MO change | 6 | 1 | n/a |
| # Oxygenators changes | 1.2 ± 1.3 | 0.5 ± 0.5 | < 0.05 |
| Day of change | 4.7 ± 3.9 | 2 ± 0.7 | < 0.05 |
Abbreviations: ECMO, extracorporeal membrane oxygenation; MO, membrane oxygenator; n/a, not applicable; PMP, polymethylpentene; PP, polypropylene.
Note: There was no difference in duration of ECMO between the PP and PMP oxygenators. Days per oxygenator represents number of days each oxygenator was able to be used, and the day of change is the day of the ECMO run when the change, if any, occurred. The major differences between the two types were the number of patients who required oxygenator changes, the total number of changes, and the day of the ECMO run on which the MO change occurred.
Oxygenator Changes
There were significant differences between the number of patients requiring a MO change with 21 out of 23 PP patients requiring a new MO, whereas 9 of 21 PMP MO patients needed a new MO ( p < 0.01) ( Table 2 ). Patients who received a PP MO required at least one change per ECMO run (an average of 1.2 changes per run), with a range of 0 to 5 oxygenator changes per run. PMP MOs required significantly fewer changes (an average of 0.5 changes per run), with no patient requiring more than two changes ( p < 0.05). When examining the days of failure, PMP MOs tended to fail earlier than PP MOs. PMP failures occurred on day 2 whereas PP failures occurred on day 4.7 ( p < 0.05). Despite the differences in failure rates and day of failure, the lifespan of the two oxygenators did not differ significantly (PP 3.5 days, PMP 3.8 days).
Anticoagulation and Blood Products
Assessment of anticoagulation parameters between the PP and PMP groups revealed that PP MOs required significantly higher rates of heparin infusion than PMP MOs (PP 33 U/kg/h vs. PMP 21 U/kg/h, p < 0.05) ( Table 3 ). Despite the increased usage of heparin, ACT ranges for both groups remained similar (PP 179–305, PMP 185–299, p > 0.05) as did morning platelet level.
Table 3. Coagulation factors.
| Variable | PP ( n = 23) | PMP ( n = 21) | p -Value |
|---|---|---|---|
| Average daily heparin rates (U/kg/h) | 33 ± 15 | 21 ± 14 | < 0.05 |
| Min ACT (s) | 179 ± 16 | 185 ± 20 | 0.28 |
| Max ACT (s) | 305 ± 40 | 299 ± 43 | 0.65 |
| Morning platelet level (×1,000) | 104 ± 25 | 99 ± 25 | 0.51 |
Abbreviations: ACT, activated clotting time; PMP, polymethylpentene; PP, polypropylene.
Note: Unfractioned heparin was initiated at an initial dose of 20 to 30 U/kg/h within 2 hours of initiation of ECMO or once ACTs drop below 380 seconds. ACTs were assessed every hour and heparin was titrated to maintain ACTs between 200 and 240 seconds. Platelet levels were assessed q8 to q12h.
The use of blood products between the two groups differed markedly as well. Though the administration of PRBCs and FFP was the same, the amount of Cryo and platelet transfusions was significantly different ( Fig. 2 ). Patients with PMP MOs received an average of 1.2 units of platelets per day and 0.3 unit of Cryo per day, whereas PP MOs received an average of 0.8 unit of platelets per day and 0.1 unit of Cryo per day ( p < 0.05).
Fig. 2.
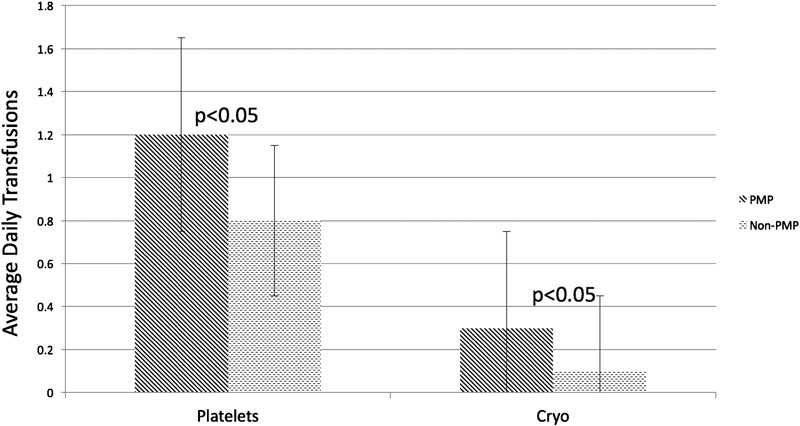
Average daily transfusions between polypropylene and polymethylpentene MOs. Analyzing transfusions as a continuous variable showed that PMP oxygenators required significantly more transfusions of platelets and cryoprecipitate than PP oxygenators, regardless of type of ECMO, type of pump, or cannula size.
Patients with Congenital Diaphragmatic Hernia
As patients with congenital diaphragmatic hernia (CDH) represent a special population within the neonatal ECMO cohort, a subanalysis of oxygenator failure rates with those patients removed showed a much larger difference between PP and PMP MOs ( Table 4 ). There were a total of seven patients diagnosed with CDH. One CDH patient had the oxygenator changed from a PP to a PMP oxygenator midway during the run. For the analysis, the time on each oxygenator was treated as separate run. This increased the number of infants who received a PMP oxygenator to 7 from 6. Given the significant physiologic changes that occurred during an operative procedure on ECMO and that more CDH patients received PMP oxygenators, we felt that their removal could provide a clearer picture into the difference between the two MO groups. Removal of the CDH patients from failure rate analysis showed a decrease in the failure rates of PMP MOs from 43 to 26%. The absence of these patients did not affect the difference of the PP MOs, as no CDH patient received a PP MO during this time. With the CDH patients removed, the PMP MO group failed in 26% of cases as opposed to the PP MO group that failed in 91% of cases ( p < 0.01). Interestingly, there was no significant difference between the days of failure of the MO, with failure still occurring around day 2 of the ECMO run.
Table 4. Comparison of PMP MO to PP MO with CDH patients removed.
| Variable | PP ( n = 23) | PMP ( n = 21) | PMP-CDH ( n = 14) | p -Values |
|---|---|---|---|---|
| Days on ECMO | 6.2 ± 3.5 | 5.8 ± 2.2 | 5 ± 2 | 0.2 |
| Days per oxygenator | 3.5 ± 2.3 | 3.8 ± 3.0 | 4 ± 3.2 | 0.45 |
| Oxygenator changed | 21/23 (91%) | 9/21 (43%) | 4/14 (28%) | <0.01 |
| # Oxygenators changes | 1.2 ± 1.3 | 0.5 ± 0.5 | 0.3 ± 0.6 | <0.05 |
| Day of change | 4.7 ± 3.9 | 2 ± 0.7 | 1.8 ± 0.7 | 0.45 |
Abbreviations: CDH, congenital diaphragmatic hernia; ECMO, extracorporeal membrane oxygenation; MO, membrane oxygenator; PMP, polymethylpentene; PP, polypropylene.
Note: All CDH patients were placed on PMP MOs based on physician preference. A total of seven CDH patients underwent surgical repair while on ECMO during the time span of the study. All seven were successfully weaned from ECMO and six of the seven survived to discharge. One patient had their oxygenator changed midway during the run from a PP to a PMP. This analysis attempts reflect this fact.
Discussion
Our study sought to determine whether the composition of hollow fiber membrane oxygenators affected the run time and complication rates in neonates undergoing noncardiac ECMO. Our run durations were identical between PP and PMP oxygenators. This is surprising given PMP MOs were used primarily on patients who were expected to be on EMCO for longer-time periods, such as CDH patients for whom the average run time is 10 days ( Tables 1 , 2 ). 12 PP MOs were used primarily on patients with expected shorter run times, such as MAS whose average run time is 5 to 6 days. 12 Typically, the longer the ECMO run, the higher circuit complication rate. However, as our two groups had similar run times, the drastic difference in failure rates cannot be attributed to run time. Our data indicate that PMP MOs required fewer oxygenator changes during a neonatal ECMO run ( Table 2 ). These results are similar to previous studies conducted in adult patients, which showed a decrease in need for oxygenator changes in patient's whose ECMO circuits contained a PMP MO. 11 These data support our finding that PMP oxygenators are more robust and less prone to failure.
To our knowledge this is the first direct head-to-head comparison for heparin usage between PMP and PP MOs in neonates. Previous studies have compared PMP MOs to silicone MOs in adult patients. 13 These studies noted a decrease in the need for anticoagulation with PMP MOs due to decreased surface area in the PMP MO as compared with silicone MOs (1.9 m 2 PMP vs. 9 m 2 silicone MOs). 14 This reasoning did not hold true for our study as the PP MO used had a surface area of 0.8 m 2 as compared with the PMP MO that had a surface area of 1.8 m 2 . Our patients who received a PMP oxygenator required significantly less heparin than those who received a PP oxygenator ( Table 3 ). This perhaps indicates that MO surface area does not play a major role in anticoagulation with these two MOs. The decreased use of heparin in the PMP MOs was not completely surprising as all had a BIOLINE coating, which combines albumin and heparin to imitate human endothelial tissue and thus decrease the interaction of clotting factors with the plastic tubing resulting in less activation of the coagulation cascade.
PMP oxygenators had a higher average daily transfusion number for platelets and Cryo than their PP counterparts ( Fig. 2 ) most likely because of the nearly exclusive use of PMP oxygenators in CDH patients ( Table 1 ). However, when examining blood product administration data with the CDH population removed, the PMP MOs continued to have higher transfusion requirements than the PP MOs. The reasons for this remain unknown at this time. Given the extensive use of PMP oxygenators, this finding is worthy of further study. 1
In our PMP group CDH patients accounted for 33% of the ECMO runs (7 out of 21). Removal of these patients from the analysis resulted in decreased failure rates for the PMP group from 43 to 26% ( Table 4 ). The reason for this decrease is most likely based on the standard of care for CDH patients in our institution. Our center places CDH patients on ECMO and repairs the defect on day 2 or 3 of the run. During this time these patients have their heparin drips decreased and are given aminocaproic acid to help control postsurgical bleeding. This has hindered our ability to appropriately anticoagulate these patients in the immediate postoperative period. The duration of time these infants were on decreased heparin drips was negligible and did not affect the statistical difference between average daily heparin doses PMP and PP oxygenators or oxygenator failure rates. We feel that the postoperative inflammation from CDH repair is the major contributing factor for thrombosis and failure of the PMP MO in the postoperative ECMO setting.
Our study has several limitations, the first being that it was a retrospective analysis over a period of 6 years. During that 6-year time, the understanding of ECMO physiology advanced rapidly and practice guidelines were updated; therefore, our patients may not be a pure homogenous group regarding management practices. Additionally, biomaterials and mechanical technology evolved and improvements were made to our technology during the study period adding further unaccounted for variables into our results. As the development and release of PMP MOs occurred, we were biased toward the PMP MOs and tended to place sicker infants on an ECMO circuit with this type of membrane. This could skew our analysis as well. One would expect the MO with fewer changes to have a longer lifespan; however, our results failed to show a difference in lifespan. Surprisingly, there was no difference in length of EMCO between the two groups, despite the physician's initial clinical assessment of expected duration of the ECMO run. The second limitation is that our center exclusively used roller pumps in our neonatal patients and one criticism of these pumps is the increased damage to RBCs and thus increased risk of MO failure in neonates as opposed to centrifugal pumps. Recent studies indicate that roller head circuits do not have increased complications as compared with centrifugal circuits. 14 However, we are not able to comment on the use of PP versus PMP MOs in centrifugal circuits. Finally, this is a single-center study that has a limited number of patients and lacks the rigorous statistical power that could come from a large, multicenter, randomized trial.
Despite the aforementioned limitations in our study, we feel that it provides a good evaluation of PMP MOs as compared with PP MOs. Though there are many factors that could affect the generalizability of our results, including how centers and physicians choose the components of their ECMO circuits, a true randomized control trial is not practical. Our study attempts to provide guidance to neonatal ECMO physicians in membrane oxygenator selection. Given our results, we feel that PMP MOs technology is superior to PP MOs and should be used whenever possible in neonatal patients.
Acknowledgment
The authors would like to thank Charles McLendon, CCP of Perfusion Concepts Inc., the Chief of Perfusion Services at the University of Kentucky Medical Center for his help and assistance with this project.
References
- 1.Rycus P. Message to John Daniel; 2016. Re: ELSO Database Question. [Google Scholar]
- 2.Fuhrman B P. Philadelphia, PA: Elsevier Saunders; 2011. Pediatric Critical care. 4th ed. [Google Scholar]
- 3.Wickramasinghe S R, Han B. Designing microporous hollow fibre blood oxygenators. Chem Eng Res Des. 2005;83(03):256–267. [Google Scholar]
- 4.Shimono T, Shomura Y, Hioki I et al. Silicone-coated polypropylene hollow-fiber oxygenator: experimental evaluation and preliminary clinical use. Ann Thorac Surg. 1997;63(06):1730–1736. doi: 10.1016/s0003-4975(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 5.Puis L, Ampe L, Hertleer R. Case report: plasma leakage in a polymethylpentene oxygenator during extracorporeal life support. Perfusion. 2009;24(01):51–52. doi: 10.1177/0267659109106294. [DOI] [PubMed] [Google Scholar]
- 6.Lehle K, Philipp A, Gleich O et al. Efficiency in extracorporeal membrane oxygenation-cellular deposits on polymethylpentene membranes increase resistance to blood flow and reduce gas exchange capacity. ASAIO J. 2008;54(06):612–617. doi: 10.1097/MAT.0b013e318186a807. [DOI] [PubMed] [Google Scholar]
- 7.Talor J, Yee S, Rider A, Kunselman A R, Guan Y, Undar A. Comparison of perfusion quality in hollow-fiber membrane oxygenators for neonatal extracorporeal life support. Artif Organs. 2010;34(04):E110–E116. doi: 10.1111/j.1525-1594.2009.00971.x. [DOI] [PubMed] [Google Scholar]
- 8.Haines N M, Rycus P T, Zwischenberger J B, Bartlett R H, Undar A. Extracorporeal Life Support Registry Report 2008: neonatal and pediatric cardiac cases. ASAIO J. 2009;55(01):111–116. doi: 10.1097/MAT.0b013e318190b6f7. [DOI] [PubMed] [Google Scholar]
- 9.Khoshbin E, Dux A E, Killer H, Sosnowski A W, Firmin R K, Peek G J. A comparison of radiographic signs of pulmonary inflammation during ECMO between silicon and poly-methyl pentene oxygenators. Perfusion. 2007;22(01):15–21. doi: 10.1177/0267659106075950. [DOI] [PubMed] [Google Scholar]
- 10.Khoshbin E, Westrope C, Pooboni S et al. Performance of polymethyl pentene oxygenators for neonatal extracorporeal membrane oxygenation: a comparison with silicone membrane oxygenators. Perfusion. 2005;20(03):129–134. doi: 10.1191/0267659105pf797oa. [DOI] [PubMed] [Google Scholar]
- 11.Robak O, Lakatos P K, Bojic A et al. Influence of different oxygenator types on changing frequency, infection incidence, and mortality in ARDS patients on veno-venous ECMO. Int J Artif Organs. 2014;37(11):839–846. doi: 10.5301/ijao.5000360. [DOI] [PubMed] [Google Scholar]
- 12.Paden M L, Rycus P T, Thiagarajan R R, Registry E; ELSO Registry.Update and outcomes in extracorporeal life support Semin Perinatol 2014380265–70. [DOI] [PubMed] [Google Scholar]
- 13.Khoshbin E, Roberts N, Harvey C et al. Poly-methyl pentene oxygenators have improved gas exchange capability and reduced transfusion requirements in adult extracorporeal membrane oxygenation. ASAIO J. 2005;51(03):281–287. doi: 10.1097/01.mat.0000159741.33681.f1. [DOI] [PubMed] [Google Scholar]
- 14.Barrett C S, Jaggers J J, Cook E F et al. Outcomes of neonates undergoing extracorporeal membrane oxygenation support using centrifugal versus roller blood pumps. Ann Thorac Surg. 2012;94(05):1635–1641. doi: 10.1016/j.athoracsur.2012.06.061. [DOI] [PubMed] [Google Scholar]


