Abstract
Rhabdomyomas are histologically benign tumors known to be associated with tuberous sclerosis. The natural history predicts the majority of tumors to be asymptomatic and regress within the first year of life. We describe a neonate presenting on day 1 of life with cardiovascular collapse secondary to a massive rhabdomyoma. Surgical resection was excluded due to the extensive nature of the lesion and oral sirolimus, a mammalian target of rapamycin inhibitor, was commenced to promote tumor regression. The patient developed intractable arrhythmias requiring extracorporeal life support during therapy.
Keywords: rhabdomyoma, extracorporeal life support, mTOR inhibitors
Introduction
A term infant, born in a district general hospital, with a birth weight of 3.5 kg presented within the first hour of life with respiratory distress and cardiovascular collapse, requiring immediate ventilatory support. A chest X-ray demonstrated marked cardiomegaly. There was no family history of note and antenatal ultrasound screening in the second trimester had not detected any abnormalities. The infant was transferred to our tertiary cardiac center for cardiological assessment and cardiac intensive care.
Initial assessment by two-dimensional echocardiography confirmed normal cardiac situs, with normal atrioventricular and ventriculoarterial (VA) connections. There was evidence of a large homogenous, echogenic mass originating from the myocardium of the left ventricular posterior wall (LVPW), extending anteriorly around the right ventricle (RV) and right atrium ( Fig. 1A – C ). Multiple smaller echogenic masses were noted throughout the myocardium in both the RV and left ventricle. Initial electrocardiograms demonstrated sinus rhythm with marked ST depression in leads II, III, AVF, and T wave inversion throughout the left lateral leads. There was no evidence of a pre-excitation. The origin of the right coronary artery was delineated echocardiographically with antegrade flow; however, antegrade flow in the left coronary artery (LCA) was difficult to demonstrate. The infant underwent computed tomography (CT) cardiac angiography to assess the extent of the mass and in addition confirmed the origin of the LCA. The CT confirmed the echocardiographic findings of the location of the mass and demonstrated the LCA originating from the left coronary sinus. Contrast filled the LCA, but the artery was stretched over the surface of the mass ( Fig. 2A , B ).
Fig. 1.
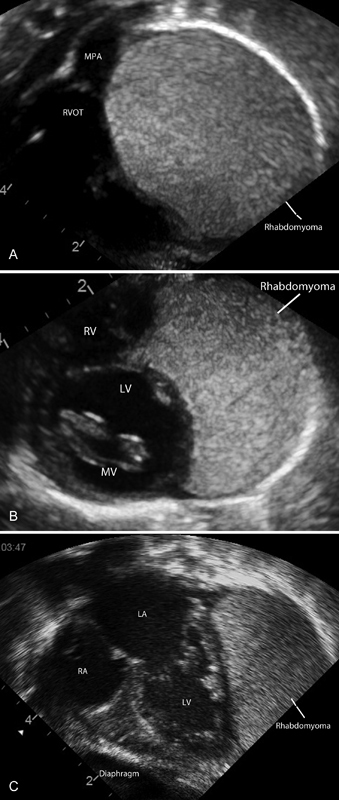
( A ) Short axis subcostal view and right ventricular outflow tract. Rhabdomyoma extending anteriorly around the right ventricle. ( B ) Subcostal short axis view at the level of the mitral valve. Rhabdomyoma surrounding the left ventricle. ( C ) Subcostal apical four chambers. Rhabdomyoma arising from the posterior wall of the left ventricle. Multiple small rhabdomyomas in the left ventricle and mitral valve. LA, left atrium; LV, left ventricle; RA, right atrium; RVOT, right ventricular outflow tract.
Fig. 2.
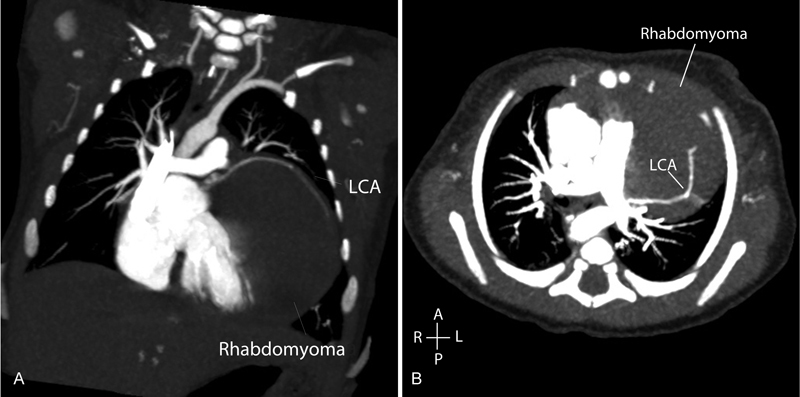
( A ) Coronal slice, cardiac CT demonstrating large rhabdomyoma with LCA extending over the surface of the mass. ( B ) Transverse slice, cardiac CT demonstrating large rhabdomyoma extending anterior to posterior, LCA filled by contrast. CT, computed tomography; LCA, left coronary artery.
Genetic analysis was not initially available, but renal ultrasonography demonstrated normal anatomy with no evidence of cysts or angiomyolipomas, Wood's lamp examination was negative; however, a cortical tuber was detected by cranial ultrasonography. A provisional diagnosis of a massive rhabdomyoma, secondary to tuberous sclerosis (TS) was made. The diagnosis was made based on the appearance of the dominant mass, the smaller masses observed in the interventricular septum/mitral valve apparatus and evidence of a cortical tuber.
Due to tumor size and position, and the requirement for ongoing cardiovascular support, the patient was commenced on oral sirolimus, a mammalian target of rapamycin (m-TOR) inhibitor, in a bid to regress the tumor mass, with response expected over the following 10 days. Side effects of sirolimus are reported to include dyslipidemia, deranged liver function, and immunosuppression, and these parameters were monitored throughout the treatment and prophylaxis with cotrimoxazole was used.
After 48 hours of treatment, the infant developed fulminant ventricular ectopy with associated systemic hypotension. Amiodarone was initially commenced in a bid to suppress the ectopy and sirolimus levels were closely monitored throughout. After 24 hours, intravenous β blockers were commenced but also failed to suppress the ectopy.
By day 5 of sirolimus therapy, and despite antiarrhythmic therapy, the ventricular ectopy became incessant, resulting in persistent hypotension, poor systemic perfusion with increasing lactate, and poor oxygen delivery. A decision was made to place the infant on VA extracorporeal life support (ECLS), as a bridge to tumor regression and recovery. Bridge to cardiac transplantation was discussed but declined by the parents. Within 2 hours of commencing ECLS, the infant developed periods of recurrent and sustained ventricular tachycardia ( Fig. 3 ).
Fig. 3.
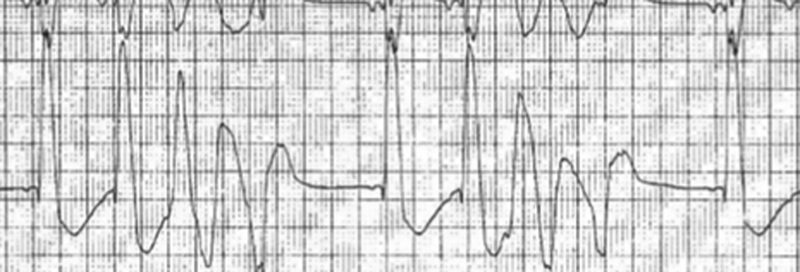
Rhythm strip demonstrating periods of a broad complex tachycardia representing ventricular tachycardia.
Despite maximum medical management, the infant developed fulminant sepsis and necrotizing enterocolitis, which failed to respond to conventional antibiotic therapy. Following appropriate multidisciplinary discussions and with the parents, the patient was reoriented to comfort care as further treatment was not seen in favor of survival. A postmortem examination confirmed the ante mortem findings and genetic analysis confirmed a TS complex 2 (TSC2) mutation, suggesting mosaicism and a likely de novo mutation. Histological and pathological studies confirmed the cardiac rhabdomyoma.
Discussion
Rhabdomyomas are histologically benign tumors known to be associated with TS and are the most common cardiac tumor in infancy. 1 The natural history predicts the majority of tumors to be asymptomatic and regress within the first year of life. 2 There are reports pre- and postnatally of an array of electrophysiological disturbances including pre-excitation, heart block, atrial tachycardias, and ventricular rhythms. 3 4 Fetal dysrhythmia has been shown to be associated with a poor neonatal outcome. 5 In a large retrospective study, Miyake et al report in the presence of a rhabdomyoma, a 16% risk of developing a clinically significant arrhythmia. 1 This risk decreased with patient age and natural tumor regression. 1 In patients with pre-excitation, studies have hypothesized that the accessory pathway may be closely related to the tumor or be part of the tumor itself. 3
As rhabdomyomas are benign tumors, intervention is only required in the presence of cardiac obstruction or arrhythmias. Multiple studies have established the efficacy and safety of mTOR inhibitors. 6 Both everolimus and sirolimus have been reported to promote regression of rhabdomyomas in TSC, particularly in the presence of obstructive lesions. 7 8 9 10 11 mTOR inhibitors have an antiproliferative effect and have previously been used in treating hemangiomas, 12 as an immunosuppressant in kidney and cardiac transplant recipients and in treatment of patients with TS with subependymal giant cell astrocytomas and renal angiomyolipomas. 13
mTOR is a serine–threonine protein kinase that regulates protein synthesis, cell growth, differentiation, and migration. 14 In normal circumstances, hamartin and tuberin are activated via biosynthetic processes mediated by the mTOR complex 1 which includes mTOR and raptor (regulatory-associated protein of mTOR). 11 In TS, mutations in the tumor suppressor genes TSC1 (encoding hamartin) and TSC2 (encoding tuberin) give rise to a hyperactivation of the mTOR pathway. 11 mTOR inhibitors cause a dissociation of mTOR from its cofactor raptor which inactivates the mTOR molecule, 11 and therefore, disrupts this pathway. mTOR inhibitors also inhibit lymphocyte and fibroblast proliferation allowing their use as immunosuppressants and antiproliferative agents. 11 mTOR inhibitors have been shown to produce massive, rapid volume reductions in cardiac myomas, with responses seen within days. 9 15
In this case, despite instituting sirolimus to promote tumor regression, the presence of such a large tumor mass resulted in intractable ventricular arrhythmias precipitating a low cardiac output state and systemic hypoperfusion. Although this was a unique and innovative use of ECLS, it is commonly used in the pediatric intensive care setting as a bridge to recovery or bridge to heart transplantation. Unfortunately, ECLS and mTOR inhibitors have recognized side effects which prevented a favorable outcome in this particular case.
To the best of our knowledge, there is no reported case in the literature of a rhabdomyoma causing intractable arrhythmias whereby cardiovascular support by ECLS was necessary. Despite the final outcome in this case, there is increasing evidence that the use of mTOR inhibitors for the regression of rhabdomyomas is feasible, and we would advocate the use of ECLS as a bridge to tumor regression. Despite the benign morphology of rhabdomyomas, these tumors can rarely cause life-threatening complications which require multidisciplinary team input and cardiac intensive care until the tumor regresses.
Acknowledgment
The authors would like to thank GE Medical for providing the CT images.
Funding Statement
Funding None.
Footnotes
Conflict of Interest None.
References
- 1.Miyake C Y, Del Nido P J, Alexander M E et al. Cardiac tumors and associated arrhythmias in pediatric patients, with observations on surgical therapy for ventricular tachycardia. J Am Coll Cardiol. 2011;58(18):1903–1909. doi: 10.1016/j.jacc.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Smythe J F, Dyck J D, Smallhorn J F, Freedom R M. Natural history of cardiac rhabdomyoma in infancy and childhood. Am J Cardiol. 1990;66(17):1247–1249. doi: 10.1016/0002-9149(90)91109-j. [DOI] [PubMed] [Google Scholar]
- 3.Van Hare G F, Phoon C K, Munkenbeck F, Patel C R, Fink D L, Silverman N H. Electrophysiologic study and radiofrequency ablation in patients with intracardiac tumors and accessory pathways: is the tumor the pathway? J Cardiovasc Electrophysiol. 1996;7(12):1204–1210. doi: 10.1111/j.1540-8167.1996.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 4.Bar-Cohen Y, Silka M J, Sklansky M S. Images in cardiovascular medicine. Neonatal tuberous sclerosis and multiple cardiac arrhythmias. Circulation. 2007;115(15):e395–e397. doi: 10.1161/CIRCULATIONAHA.106.659771. [DOI] [PubMed] [Google Scholar]
- 5.Chao A S, Chao A, Wang T H et al. Outcome of antenatally diagnosed cardiac rhabdomyoma: case series and a meta-analysis. Ultrasound Obstet Gynecol. 2008;31(03):289–295. doi: 10.1002/uog.5264. [DOI] [PubMed] [Google Scholar]
- 6.Goyer I, Dahdah N, Major P. Use of mTOR inhibitor everolimus in three neonates for treatment of tumors associated with tuberous sclerosis complex. Pediatr Neurol. 2015;52(04):450–453. doi: 10.1016/j.pediatrneurol.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Doğan V, Yeşil Ş, Kayalı Ş et al. Regression of symptomatic multiple cardiac rhabdomyomas associated with tuberous sclerosis complex in a newborn receiving everolimus. J Trop Pediatr. 2015;61(01):74–77. doi: 10.1093/tropej/fmu056. [DOI] [PubMed] [Google Scholar]
- 8.Mohamed I, Ethier G, Goyer I, Major P, Dahdah N. Oral everolimus treatment in a preterm infant with multifocal inoperable cardiac rhabdomyoma associated with tuberous sclerosis complex and a structural heart defect. BMJ Case Rep. 2014;2014(14):bcr2014205138. doi: 10.1136/bcr-2014-205138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breathnach C, Pears J, Franklin O, Webb D, McMahon C J. Rapid regression of left ventricular outflow tract rhabdomyoma after sirolimus therapy. Pediatrics. 2014;134(04):e1199–e1202. doi: 10.1542/peds.2013-3293. [DOI] [PubMed] [Google Scholar]
- 10.Demir H A, Ekici F, Yazal Erdem A, Emir S, Tunç B. Everolimus: a challenging drug in the treatment of multifocal inoperable cardiac rhabdomyoma. Pediatrics. 2012;130(01):e243–e247. doi: 10.1542/peds.2011-3476. [DOI] [PubMed] [Google Scholar]
- 11.Tiberio D, Franz D N, Phillips J R. Regression of a cardiac rhabdomyoma in a patient receiving everolimus. Pediatrics. 2011;127(05):e1335–e1337. doi: 10.1542/peds.2010-2910. [DOI] [PubMed] [Google Scholar]
- 12.Kaylani S, Theos A J, Pressey J G. Treatment of infantile hemangiomas with sirolimus in a patient with PHACE syndrome. Pediatr Dermatol. 2013;30(06):e194–e197. doi: 10.1111/pde.12023. [DOI] [PubMed] [Google Scholar]
- 13.Moavero R, Coniglio A, Garaci F, Curatolo P. Is mTOR inhibition a systemic treatment for tuberous sclerosis? Ital J Pediatr. 2013;39:57. doi: 10.1186/1824-7288-39-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 15.Choudhry S, Nguyen H H, Anwar S. Rapid resolution of cardiac rhabdomyomas following everolimus therapy. BMJ Case Rep. 2015 doi: 10.1136/bcr-2015-212946. [DOI] [PMC free article] [PubMed] [Google Scholar]


