Abstract
Podoplanin (PDPN) is a type I transmembrane heavily glycosylated sialoglycoprotein that is expressed in normal tissues such as pulmonary type I alveolar cells, renal podocytes, and lymphatic endothelial cells. PDPN overexpression in cancerous tissue is associated with hematogenous metastasis through interactions with the C-type lectin-like receptor 2 (CLEC-2). Previously, we have reported the development of a mouse monoclonal antibody (mAb), PMab-38 (IgG1, kappa) against dog PDPN (dPDPN). PMab-38 was found to strongly react with canine squamous cell carcinomas (SCCs) and melanomas; however, it showed no reaction with lymphatic endothelial cells. Recently, we have developed and produced the mouse–canine mAb of subclass B, P38B that showed antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity against Chinese hamster ovary (CHO)/dPDPN cells. In the present study, we investigated the antitumor activity using mouse xenograft model. To induce ADCC activity by P38B, canine mononuclear cells were injected surrounding the tumors in a xenograft model. It was demonstrated that P38B exerted antitumor activity against the mouse xenograft model using CHO/dPDPN. These results suggest that P38B is useful for antibody therapy against dPDPN-expressing canine SCCs and melanomas.
Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; CDC, complement-dependent cytotoxicity; CHO, Chinese hamster ovary; CLEC-2, C-type lectin-like receptor 2; dPDPN, dog podoplanin; mAb, monoclonal antibody; PBS, phosphate-buffered saline; PDPN, podoplanin; SCC, squamous cell carcinomas
Keywords: Mouse-canine chimeric antibody, Dog podoplanin, dPDPN, Monoclonal antibody
Highlights
-
•
Dog PDPN is expressed in canine squamous cell carcinomas and melanomas.
-
•
A mouse-canine mAb of canine subclass B, P38B against dog PDPN was produced.
-
•
P38B exerted antitumor activities via ADCC and CDC.
-
•
P38B could be useful for antibody therapy against dPDPN-expressing canine tumors.
1. Introduction
Podoplanin (PDPN) is expressed in normal tissue, including pulmonary type I alveolar cells, renal podocytes, chondrocytes, myofibroblasts, mesothelial cells, and lymphatic endothelial cells [1]. PDPN overexpression is also observed in different types of tumors, including mesotheliomas [2], [3], squamous cell carcinomas (SCC) [4], testicular tumors [5], and glioblastomas [6]. Recent clinical studies have provided evidence for the association between increased PDPN expression and poor disease prognosis [7]. This observation indicated that the establishment of anti-PDPN monoclonal antibodies (mAbs) is critical for developing novel therapeutic strategies against cancer development and metastatic progression [8].
Dog PDPN (dPDPN) was previously reported in the literature as gp40 [9]. We have recently developed two mAbs PMab-38 (mouse IgG1, kappa) [10] and PMab-48 (mouse IgG1, kappa) [11]. It was shown that PMab-38 recognized dPDPN of renal epithelial cells weakly but showed no reaction with lymphatic endothelial cells [10]. Conversely, PMab-48 reacted with renal epithelial cells as well as with lymphatic endothelial cells [11]. Tyr67 and Glu68 of dPDPN were determined as the critical epitopes of PMab-38 [12], whereas Asp29, Asp30, Ile31, Ile32, and Pro33 of dPDPN were essential for the recognition of PMab-48 [13]. Immunohistochemistry demonstrated that PMab-38 reacted with 83% of canine SCCs (15/18 cases) [14] and 90% of melanomas (9/10 cases) [15]. Recently, we have developed a mouse–canine chimeric antibody (P38B) that was derived from PMab-38 [16]. P38B demonstrated antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) against CHO/dPDPN cells, indicating that P38B is applicable for antibody-based therapy for canine cancers. In the present study, we investigated antitumor activity against mouse xenograft models using CHO/dPDPN and CHO-K1.
2. Materials and methods
2.1. Cell lines
Chinese hamster ovary (CHO)-K1 cell-line was obtained from the American Type Culture Collection (ATCC; Manassas, VA). In our previous studies, we have inserted dPDPN with a N-terminal PA tag and a C-terminal RAP tag-MAP tag (PA-dPDPN-RAP-MAP) in a pCAG-Ble vector (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) [10]. The PA tag [17], RAP tag [18], and MAP tag [19] comprises 12 amino acids each—GVAMPGAEDDVV, DMVNPGLEDRIE, and GDGMVPPGIEDK, respectively. CHO-K1 cells were transfected with pCAG-Ble/PA-dPDPN-RAP-MAP using Gene Pulser Xcell electroporation system (Bio-Rad Laboratories Inc., Berkeley, CA) resulting in the cell-line CHO/dPDPN. CHO-K1 and CHO/dPDPN were cultured in RPMI 1640 medium (Nacalai Tesque, Inc., Kyoto, Japan) supplemented with 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific Inc., Waltham, MA), 100 units/mL of penicillin, 100 μg/mL of streptomycin, and 25 μg/mL of amphotericin B (Nacalai Tesque, Inc.) at 37 °C in a humidified atmosphere of 5% CO2 and 95% air.
2.2. Antibodies
The mouse anti-dPDPN mAb PMab-38 was developed as previously described [10]. To generate the mouse–canine (subclass B) chimeric antibody P38B, the appropriate VH and VL cDNAs of mouse PMab-38 and the CH and CL of canine IgG subclass B were subcloned into pCAG-Ble and pCAG-Neo vectors (FUJIFILM Wako Pure Chemical Corporation), respectively [16]. Expression vectors were transfected into ExpiCHO-S cells using the ExpiFectamine CHO Transfection kit (Thermo Fisher Scientific Inc.) to express P38B antibody, which was further purified using Protein G-Sepharose (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA).
2.3. Antitumor activity of P38B
Female BALB/c nude mice (5-week-old) were purchased from Charles River (Kanagawa, Japan) and used in experiments when they were 7 weeks old. CHO/dPDPN and CHO-K1 cells (0.3 mL of 1.33 × 108 /mL in RPMI) were mixed with 0.5 mL of BD Matrigel Matrix Growth Factor Reduced (BD Biosciences, San Jose, CA, USA). A 100-μL suspension (containing 5 × 106 cells) was injected subcutaneously into the left flanks of nude mice. After day 1, 100 μg of P38B or dog IgG (Jackson ImmunoResearch Inc., PA, USA) in 100 μL PBS were injected into the peritoneal cavity of each mouse. Additional antibodies were injected on day 8 and day 15. Canine mononuclear cells (5 x 105 cells), which were obtained from Hokkaido University, were injected surrounding the tumors on day 1, day 8, and day 15. The tumor diameter and tumor volume were determined as previously described [20]. The mice were euthanized 17 days after cell implantation. All data were expressed as the mean ± SEM. Statistical analysis was performed using the Tukey–Kramer test. P < 0.05 was considered statistically significant.
3. Results and discussion
We have previously reported the development of the mouse mAb, PMab-38 against dPDPN [10]. The strong reaction of PMab-38 specifically with canine SCCs [15] and melanomas [16] indicated that PMab-38 possesses cancer-specificity. We have previously established cancer-specific mAbs against human PDPN, such as LpMab-2 [8] and LpMab-23 [21]. These cancer-specific mAbs may prove advantageous for targeting cancer cells without adverse events.
To date, few studies have been performed on the subclasses (A, B, C, D) of canine IgGs [22], [23]. However, it was demonstrated that the canine subclasses A and D appear to be effector-function negative, whereas subclasses B and C bind to canine Fc gamma receptors and are positive for ADCC similar to human IgG1 and IgG3, respectively [22]. Furthermore, subclasses B and C can induce CDC. An anti-canine CD20 mAb (1E4) was established to treat canine B-cell lymphoma and produced mouse–canine chimeric antibodies [23]. It was also shown that 1E4-cIgGB (subclass B) and 1E4-cIgGC (subclass C) led to significant depletion of B-cell levels in healthy beagle dogs. Moreover, we produced P38B, a mouse–canine chimeric antibody of canine subclass B, from PMab-38 [16]. In that study, we clearly demonstrated that P38B possesses ADCC and CDC against CHO/dPDPN cells.
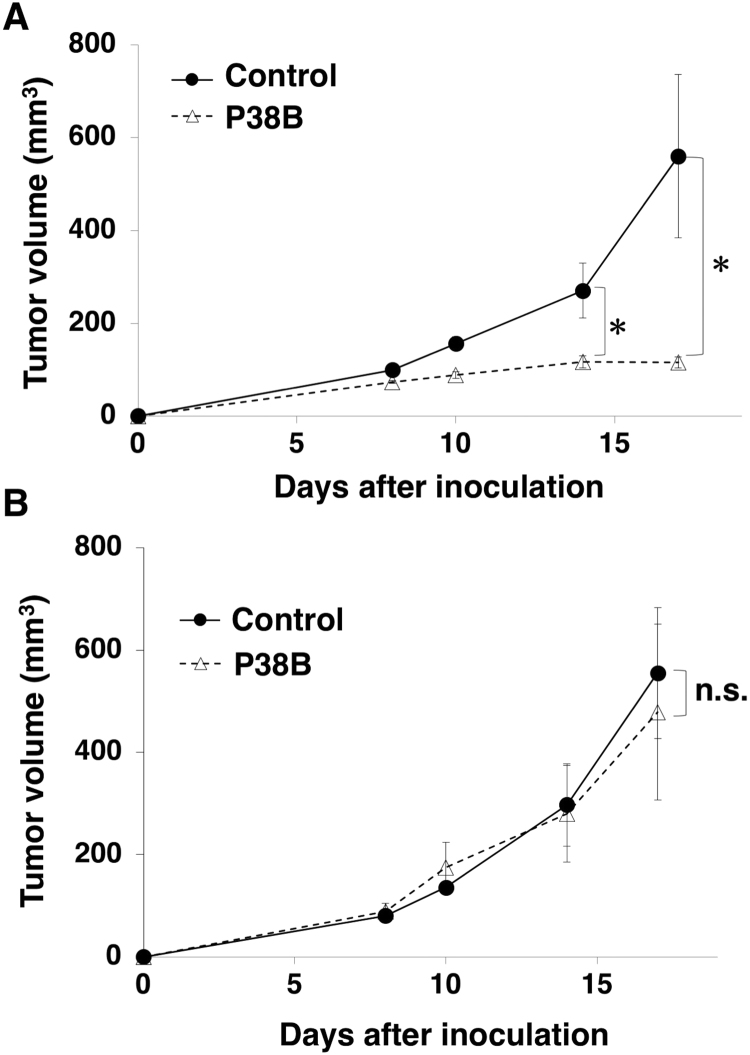
To study the antitumor activity of P38B on cell growth in vivo, CHO/dPDPN cells or CHO-K1 cells were subcutaneously implanted into the flanks of nude mice. P38B and control dog IgG were injected three times (on day 1, 8, and 15 after cell injections) into the peritoneal cavity of mice. Canine mononuclear cells were injected three times (on day 1, 8, and 15) surrounding the xenograft. Tumor formation was observed in mice from the control and P38B-treated groups in CHO/dPDPN xenograft models (Fig. 1A) and CHO-K1 xenograft models (Fig. 1B). However, P38B significantly reduced tumor development of CHO/dPDPN xenograft compared with control dog IgG on day 14 and day 17 (Fig. 1A). Conversely, P38B did not reduce tumor development of CHO-K1 xenograft (Fig. 1B). This observation indicated that P38B is specific against dPDPN.
Fig. 1.
Antitumor activity of P38B against CHO/dPDPN and CHO-K1. (A) Tumor volume of CHO/dPDPN xenografts. CHO/ dPDPN cells were injected subcutaneously into female nude mice. The indicated antibodies (100 μg/day; 5 mg/kg) were administered intraperitoneally on days 1, 8, and 15 after cell inoculation. The tumor volume was measured at the indicated time points. The values are presented as mean ± SEM. (B) CHO-K1 cells were injected subcutaneously into female nude mice. The indicated antibodies (100 μg/day; 5 mg/kg) were administered intraperitoneally, and canine mononuclear cells were injected around the tumors on day 1, day 8, and day 15. The tumor volume was measured at the indicated time points. The values are presented as mean ± SEM. An asterisk indicates statistical significance (* P < 0.05, Tukey-Kramer's test). n.s.: not significant.
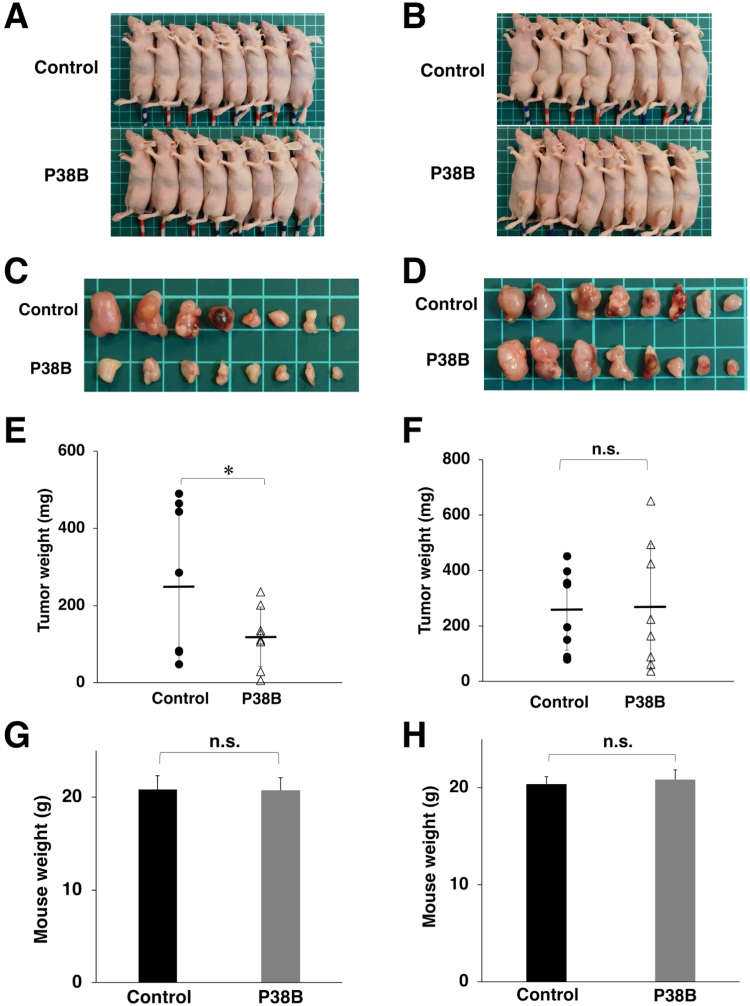
CHO/dPDPN and CHO-K1 xenograft mice models on day 17 are shown in Fig. 2A and B, respectively. The resected tumors of CHO/dPDPN and CHO-K1 xenografts are depicted in Fig. 2C and D, respectively. The tumor weight of mice in P38B-treated was significantly lower than in the control dog IgG group in CHO/dPDPN xenograft models (Fig. 2E) although there was no difference in CHO-K1 xenograft models (Fig. 2F). However, body weight was not significantly different among the two groups in the CHO/dPDPN xenograft models (Fig. 2G) and the CHO-K1 xenograft models (Fig. 2H).
Fig. 2.
Evaluation of antitumor activity of P38B against CHO/dPDPN and CHO-K1. (A) CHO/dPDPN xenograft mice models on day 17. (B) CHO-K1 xenograft mice models on day 17. (C) Resected tumors of CHO/dPDPN xenografts. (D) Resected tumors of CHO-K1 xenografts. (E) Tumor weight of CHO/dPDPN xenografts (day 17). (F) Tumor weight of CHO-K1 xenografts (day 17). (G) Body weight of CHO/dPDPN xenografts (day 17). (H) Body weight of CHO-K1 xenografts (day 17). The values are presented as mean ± SEM. An asterisk indicates statistical significance (* P < 0.05, Tukey–Kramer's test). n.s.: not significant.
In conclusion, P38B is applicable for antibody therapy against canine cancers expressing dPDPN. Further studies on antitumor activities against endogenous dPDPN-expressing tumors are necessary to obtain a more detailed understanding of antibody therapy against canine cancers.
Acknowledgments
We thank Akiko Harakawa, Takuro Nakamura, Miyuki Yanaka, Kayo Hisamatsu, Saori Handa, and Yoshimi Nakamura for their excellent technical assistance.
Acknowledgments
Funding
This research was supported in part by AMED under Grant nos. JP18am0101078 (Y.K.), JP18am0301010 (Y.K.), and JP18ae0101028 (Y.K.), and by JSPS KAKENHI Grant nos. 17K07299 (M.K.K.) and 16K10748 (Y.K.).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.11.005.
Appendix A. Transparency document
Supplementary material
References
- 1.Breiteneder-Geleff S., Matsui K., Soleiman A., Meraner P., Poczewski H., Kalt R., Schaffner G., Kerjaschki D. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am. J. Pathol. 1997;151:1141–1152. [PMC free article] [PubMed] [Google Scholar]
- 2.Kimura N., Kimura I. Podoplanin as a marker for mesothelioma. Pathol. Int. 2005;55:83–86. doi: 10.1111/j.1440-1827.2005.01791.x. [DOI] [PubMed] [Google Scholar]
- 3.Abe S., Morita Y., Kaneko M.K., Hanibuchi M., Tsujimoto Y., Goto H., Kakiuchi S., Aono Y., Huang J., Sato S., Kishuku M., Taniguchi Y., Azuma M., Kawazoe K., Sekido Y., Yano S., Akiyama S., Sone S., Minakuchi K., Kato Y., Nishioka Y. A novel targeting therapy of malignant mesothelioma using anti-podoplanin antibody. J. Immunol. 2013;190:6239–6249. doi: 10.4049/jimmunol.1300448. [DOI] [PubMed] [Google Scholar]
- 4.Kato Y., Kaneko M., Sata M., Fujita N., Tsuruo T., Osawa M. Enhanced expression of Aggrus (T1alpha/podoplanin), a platelet-aggregation-inducing factor in lung squamous cell carcinoma. Tumor Biol. 2005;26:195–200. doi: 10.1159/000086952. [DOI] [PubMed] [Google Scholar]
- 5.Kato Y., Sasagawa I., Kaneko M., Osawa M., Fujita N., Tsuruo T. Aggrus: a diagnostic marker that distinguishes seminoma from embryonal carcinoma in testicular germ cell tumors. Oncogene. 2004;23:8552–8556. doi: 10.1038/sj.onc.1207869. [DOI] [PubMed] [Google Scholar]
- 6.Mishima K., Kato Y., Kaneko M.K., Nishikawa R., Hirose T., Matsutani M. Increased expression of podoplanin in malignant astrocytic tumors as a novel molecular marker of malignant progression. Acta Neuropathol. 2006;111:483–488. doi: 10.1007/s00401-006-0063-y. [DOI] [PubMed] [Google Scholar]
- 7.Retzbach E.P., Sheehan S.A., Nevel E., Batra A., Phi T., Kato Y., Baredes S., Fatahzadeh M., Shienbaum A.J., Goldberg G.S. Podoplanin emerges as a functionally relevant oral cancer biomarker and therapeutic target. Oral Oncol. 2018 doi: 10.1016/j.oraloncology.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Kato Y., Kaneko M.K. A cancer-specific monoclonal antibody recognizes the aberrantly glycosylated podoplanin. Sci. Rep. 2014;4:5924. doi: 10.1038/srep05924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmer G., Klenk H.D., Herrler G. Identification of a 40-kDa cell surface sialoglycoprotein with the characteristics of a major influenza C virus receptor in a Madin-Darby canine kidney cell line. J. Biol. Chem. 1995;270:17815–17822. doi: 10.1074/jbc.270.30.17815. [DOI] [PubMed] [Google Scholar]
- 10.Honma R., Kaneko M.K., Ogasawara S., Fujii Y., Konnai S., Takagi M., Kato Y. Specific detection of dog podoplanin expressed in renal glomerulus by a novel monoclonal antibody PMab-38 in immunohistochemistry. Monoclon. Antib. Immunodiagn. Immunother. 2016;35:212–216. doi: 10.1089/mab.2016.0022. [DOI] [PubMed] [Google Scholar]
- 11.Yamada S., Itai S., Kaneko M.K., Kato Y. PMab-48 recognizes dog podoplanin of lymphatic endothelial cells. Monoclon. antibodies Immunodiagn. Immunother. 2018;37:63–66. doi: 10.1089/mab.2017.0053. [DOI] [PubMed] [Google Scholar]
- 12.Chang Y.W., Yamada S., Kaneko M.K., Kato Y. Epitope mapping of monoclonal antibody PMab-38 against dog podoplanin. Monoclon. Antibodies Immunodiagn. Immunother. 2017;36:291–295. doi: 10.1089/mab.2017.0048. [DOI] [PubMed] [Google Scholar]
- 13.Yamada S., Kaneko M.K., Itai S., Chang Y.W., Nakamura T., Yanaka M., Ogasawara S., Murata T., Uchida H., Tahara H., Harada H., Kato Y. Epitope mapping of monoclonal antibody PMab-48 against dog podoplanin. Monoclon. Antibodies Immunodiagn. Immunother. 2018;37:162–165. doi: 10.1089/mab.2018.0006. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko M.K., Honma R., Ogasawara S., Fujii Y., Nakamura T., Saidoh N., Takagi M., Kagawa Y., Konnai S., Kato Y. PMab-38 recognizes canine podoplanin of squamous cell Carcinomas. Monoclon. Antib. Immunodiagn. Immunother. 2016;35:263–266. doi: 10.1089/mab.2016.0036. [DOI] [PubMed] [Google Scholar]
- 15.Ogasawara S., Honma R., Kaneko M.K., Fujii Y., Kagawa Y., Konnai S., Kato Y. Podoplanin expression in Canine Melanoma. Monoclon. Antib. Immunodiagn. Immunother. 2016;35:304–306. doi: 10.1089/mab.2016.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato Y., Mizuno T., Yamada S., Nakamura T., Itai S., Yanaka M., Sano M., Kaneko M.K. Establishment of P38Bf, a core-fucose-deficient mouse-canine chimeric antibody against dog podoplanin. Monoclon. Antib. Immunodiagn. Immunother. 2018;37:218–223. doi: 10.1089/mab.2018.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujii Y., Kaneko M., Neyazaki M., Nogi T., Kato Y., Takagi J. PA tag: a versatile protein tagging system using a super high affinity antibody against a dodecapeptide derived from human podoplanin. Protein Expr. Purif. 2014;95:240–247. doi: 10.1016/j.pep.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Fujii Y., Kaneko M.K., Ogasawara S., Yamada S., Yanaka M., Nakamura T., Saidoh N., Yoshida K., Honma R., Kato Y. Development of RAP Tag, a novel tagging system for protein detection and purification. Monoclon. Antibodies Immunodiagn. Immunother. 2017;36:68–71. doi: 10.1089/mab.2016.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujii Y., Kaneko M.K., Kato Y. MAP tag: a novel tagging system for protein purification and detection. Monoclon. Antibodies Immunodiagn. Immunother. 2016;35:293–299. doi: 10.1089/mab.2016.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato Y., Kunita A., Abe S., Ogasawara S., Fujii Y., Oki H., Fukayama M., Nishioka Y., Kaneko M.K. The chimeric antibody chLpMab-7 targeting human podoplanin suppresses pulmonary metastasis via ADCC and CDC rather than via its neutralizing activity. Oncotarget. 2015;6:36003–36018. doi: 10.18632/oncotarget.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamada S., Kaneko M.K., Kato Y. LpMab-23: a cancer-specific monoclonal antibody against human Podoplanin. Monoclon. Antib. Immunodiagn. Immunother. 2017;36:72–76. doi: 10.1089/mab.2017.0001. [DOI] [PubMed] [Google Scholar]
- 22.Bergeron L.M., McCandless E.E., Dunham S., Dunkle B., Zhu Y., Shelly J., Lightle S., Gonzales A., Bainbridge G. Comparative functional characterization of canine IgG subclasses. Vet. Immunol. Immunopathol. 2014;157:31–41. doi: 10.1016/j.vetimm.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Rue S.M., Eckelman B.P., Efe J.A., Bloink K., Deveraux Q.L., Lowery D., Nasoff M. Identification of a candidate therapeutic antibody for treatment of canine B-cell lymphoma. Vet. Immunol. Immunopathol. 2015;164:148–159. doi: 10.1016/j.vetimm.2015.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material