Highlights
-
•
Appendiceal neurofibromas (AN) in Neurofibromatosis type (NF) 1 are rare.
-
•
AN in NF1 with Low-grade appendiceal mucinous neoplasms (LAMNs) are extremely rare.
-
•
AN and LAMNs have potential for malignant transformation.
-
•
Surgical resection is the standard treatment for patients with AN and LAMNs.
-
•
However, appropriate surgical procedure remains controversial.
Abbreviations: NF1, neurofibromatosis type 1; LAMN, low-grade appendiceal mucinous neoplasm; CT, computed tomography; MPNST, malignant peripheral nerve sheath tumor
Keywords: Neurofibromatosis type 1, von Recklinghausen’s disease, Appendiceal neurofibroma, Low-grade appendiceal mucinous neoplasm
Abstract
Introduction
Neurofibromas are a characteristic of the autosomal dominant disorder Neurofibromatosis type 1 (NF1), also known as von Recklinghausen’s disease. Appendiceal neurofibromas are extremely rare, and low-grade appendiceal mucinous neoplasms (LAMNs) have not previously been reported in NF1.
Presentation of case
A 62-year-old man with NF1 was scheduled for elective surgical treatment of an asymptomatic, enlarged and diffusely thickened appendix that remained after curative antimicrobial treatment of acute appendicitis. Laboratory analysis revealed all normal. Colonoscopy showed thickened appendiceal mucosa projecting into the cecum. A sample of the mucosa was found to be pathologically benign. The patient was preoperatively diagnosed with treated acute appendicitis with chronic appendiceal inflammation versus appendiceal neoplasms. Laparoscopic cecectomy was performed. Multiple neurofibromas were observed in the muscle layer, submucosa, and mucosa of the appendix on histopathological examination. Immunohistochemical examination showed positive staining for S-100. Pathologically, the patient was diagnosed with appendiceal neurofibroma consistent with NF1 with LAMNs. His postoperative course was unremarkable. He was discharged on post-operative day 3 and remained in good health 7 month after surgery.
Conclusions
Appendiceal neurofibromas are often preoperatively diagnosed as appendicitis. Appendiceal neurofibromas should be considered in patients with NF1 who are suspected of having appendicitis.
1. Introduction
Neurofibromatosis type 1 (NF1), also known as von Recklinghausen’s disease, is an inherited autosomal dominant neurocutaneous syndrome. NF1 is a multisystemic disorder that can affect any organ in the body. The most typical clinical presentations are neurofibromas and café-au-lait spots [1]. Neurofibromas are found in the gastrointestinal tract in 11% of patients with NF1 [2]. However, appendiceal neurofibromas in NF1 are extremely rare. Furthermore, low-grade appendiceal mucinous neoplasms (LAMNs), characterized by low-grade cytologic atypia and absence of destructive invasion [3], have not been reported in NF1.We report herein a case of appendiceal neurofibromas in NF1 with LAMNs. The present work has been reported in accordance with the SCARE criteria [4].
2. Case presentation
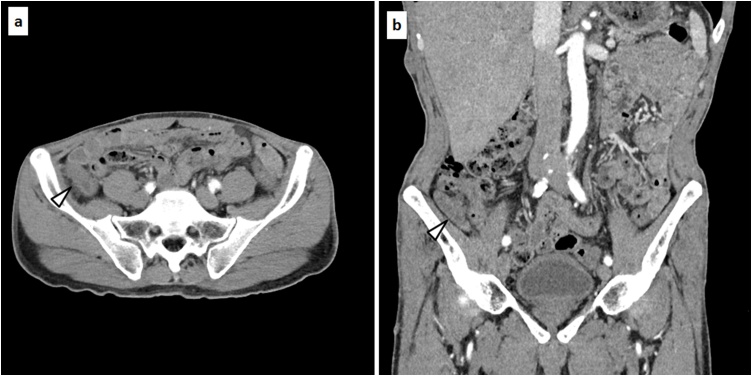
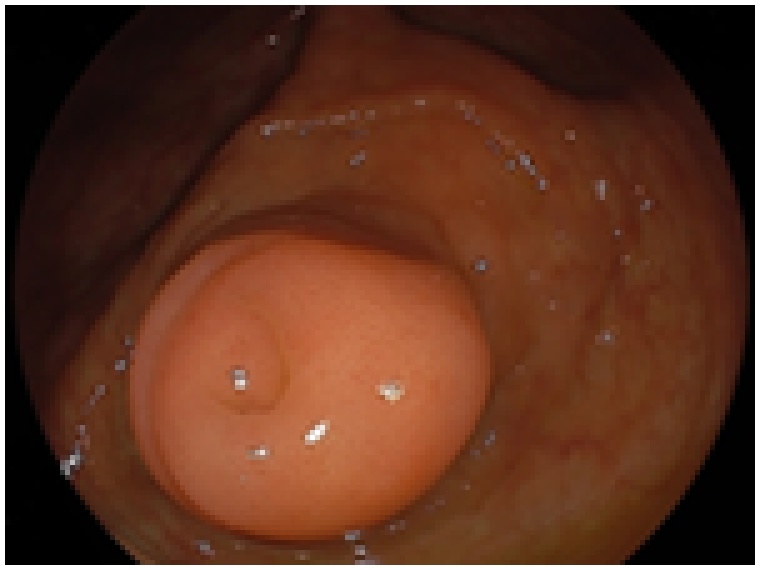
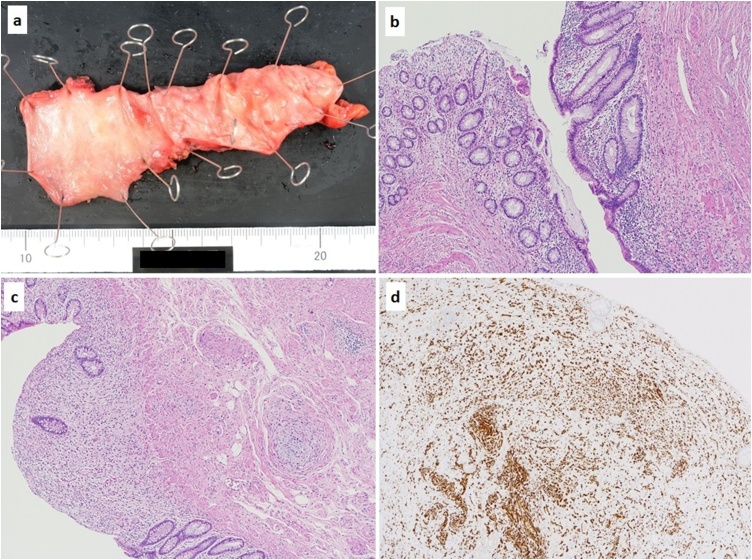
A 62-year-old man with NF1 was scheduled for elective surgical treatment of an asymptomatic, enlarged and diffusely thickened appendix that remained after curative antimicrobial treatment of acute appendicitis because he hoped for an antimicrobial treatment 2 months ago. Physical examination revealed multiple neurofibromas and café-au-lait spots on the skin. He had no other co-morbidities other than having NF1. Laboratory analysis revealed hemoglobin, 15.9 g/dL; white blood cell count, 5.33 × 103/μL; platelets, 17.9 × 104/μL; serum total protein, 7.9 g/dL; serum albumin, 4.8 g/dL; total bilirubin, 0.8 mg/dL; aspartate aminotransferase, 27 IU/L; alanine aminotransferase, 21 IU/L; and lactic acid dehydrogenase, 173 IU/L; C-reactive protein, 0.073 mg/dL. The serum levels of tumor markers were normal, including carcinoembryonic antigen, 3.2 ng/ml and carbohydrate antigen 19–9, 7.1 U/mL. Contrast-enhanced computed tomography (CT) demonstrated an enlarged and diffusely thickened appendix (Fig. 1a, b). Colonoscopy showed thickened appendiceal mucosa projecting to the cecum without evidence of abscess or jellylike liquid (Fig. 2). A sample of the appendiceal mucosa was obtained by colonoscopy and pathologically revealed to be benign. The patient was preoperatively diagnosed with treated acute appendicitis with chronic appendiceal inflammation versus appendiceal neoplasms. Laparoscopic cecectomy was performed. The resected specimen revealed a thick, enlarged and fibrotic appendiceal wall. There was no evidence of appendiceal rupture or serosal mucin extravasation (Fig. 3a). Histopathological examination showed a single layer of atypical mucinous epithelial cells lining the appendix (Fig. 3b). Multiple neurofibromas were observed in the muscle layer, submucosa and mucosa of the appendix (Fig. 3c). Micro plexiform neurofibromas were observed in the neuroplexus of the appendix. Immunohistochemical examination showed positive staining for S-100 (Fig. 3d). Pathologically, the patient was diagnosed with appendiceal neurofibroma of NF1 with LAMNs. His postoperative course was unremarkable. He was discharged on post-operative day 3 and remained in good heath 7 months after surgery.
Fig. 1.
Contrast-enhanced CT showed an enlarged and diffusely thickened appendix (appendix: thick white arrow head).
Fig. 2.
Colonoscopy showed a thickened mucosa of appendix projecting to the cecum without outflow of abscess or jellylike liquid.
Fig. 3.
The resected specimen showed the appendiceal wall was fibrotic, enlargement, and thickness. There was no evidence of appendiceal rupture or serosal mucin extravasation (a). Histopathological examination showed atypical mucinous epithelial cells were lining by a single layer in the appendix (b). Multiple neurofibromas were revealed in the muscle layer, submucosa and mucosa of the appendix (c). Immunohistochemistry examination showed positive for S-100 (d).
3. Discussion
NF1, also known as von Recklinghausen’s disease, is the most common autosomal dominant single-gene neurodevelopmental disorder, with an incidence of 1:2700 [5]. Gastrointestinal involvement has been reported in 10–25% of patients with NF1. Gastrointestinal neurofibromas usually occur in groups of multiple lesions, though solitary lesions have been reported. Lesions can be found, in order of frequency, in the jejunum, stomach, ileum, duodenum and colon. However, appendiceal neurofibromas in NF1 are extremely rare. To the best of our knowledge, only 7 cases [[6], [7], [8], [9], [10], [11], [12]] have been reported in the English literature (Table 1). Of these, 4 cases were diagnosed preoperatively as appendicitis.
Table 1.
Reported cases of appendiceal neurofibromas.
| No. | Author | Year | Age | Gender | Main symptom | Preoperative diagnosis | Surgeical procedure | Size (cm) |
|---|---|---|---|---|---|---|---|---|
| 1 | Merck and Kindblom [8] | 1975 | 24 | M | Abdominal pain | Appendicitis | Appendectomy | NA |
| 2 | Olsen [9] | 1987 | 24 | M | Abdominal pain | NA | Appendectomy | 7 × 3 |
| 3 | Samuel et al. [10] | 1997 | 19 | M | Abdominal pain | Appendicitis | Appendectomy | 3 × 7×8 |
| 4 | Rosenberg et al. [11] | 2006 | 33 | F | Asymptom | Finding unexpectedly in cesarean section | Appendectomy | 12 |
| 5 | Agaimy et al. [12] | 2010 | 45 | M | NA | NA | Appendectomy | 0.3 |
| 6 | Guo et al. [6] | 2014 | 62 | F | Abdominal pain | A giant thick-walled tubular mass | Right hemicolectomy | 9 × 7 |
| 7 | Ozaki et al. [7] | 2015 | 51 | M | Abdominal pain | Appendicitis | Appendectomy | 3.5 × 2.5 × 2.5 |
| 8 | Present case | 2018 | 62 | F | Asymptom | Cured appendicitis | Cecectomy | 1.7 × 7 |
NA: not available.
Gastrointestinal neurofibromas are often asymptomatic. However, when the lesions grow in size, they may present as constipation, abdominal pain, palpable abdominal masses or bowel obstruction [13]. Neurofibromas are benign neoplasms consisting of the cells and tissues that cover nerves. However, there is a risk of malignant transformation, particularly in individuals over 40 years old [6]. Patients with NF1 have an 8–12% lifetime risk of developing malignant peripheral nerve sheath tumors (MPNSTs), a term designated by the World Health Organization in 2002 to replace the previous terms “malignant schwannoma”, “malignant neurilemmoma”, “neurogenic sarcoma”, and “neurofibrosarcoma” [14]. MPNSTs have a poor prognosis [15]. Neurofibromas were recently found to be precursor lesions for MPNSTs [15]. Therefore, rapid enlargement of neurofibromas should alert practitioners to the possibility of malignant transformation [6].
Surgical resection is the standard treatment for appendiceal neurofibromas and is aimed at improving symptoms, preventing complications and avoiding the risk of malignant transformation [16]. However, the best surgical procedure (appendectomy alone, cecectomy, ileocecal resection, or right hemicolectomy with or without regional lymph node dissection) for patients with appendiceal neurofibromas remains controversial. In the present case, the patient underwent laparoscopic cecectomy rather than laparoscopic appendectomy due to obtain the negative resection margin because he was suspected to have appendiceal neoplasm during surgery, for example LAMNs. It is difficult to diagnosis appendiceal neoplasms preoperatively. The patient would have had to undergo a two-stage ileocecal resection with or without regional lymph node dissection for a pathological diagnosis of mucinous adenocarcinoma or positive resection margin of the LAMNs. The most appropriate surgical procedure for appendiceal neurofibromas might be cecectomy, as the root of the appendix has the potential for malignant transformation and progression to MPNSTs. The surgical indication for patients without symptoms remains controversial. Many patients diagnosed with appendicitis preoperatively undergo appendectomy alone, even if they were pathologically diagnosed with appendiceal neurofibromas. Other groups have supported a two-stage ileocecal resection with regional lymph node dissection if patients are pathologically diagnosed with a malignant tumor or positive resection margins.
LAMNs are characterized by low-grade cytologic atypia and the absence of destructive invasion. These tumors have the potential for peritoneal spread giving rise to pseudomyxoma peritonei [17]. Thus, LAMNs are regarded as low-grade adenocarcinomas according to the 2010 World Health Organization classification [18]. To the best of our knowledge, this is the first report of appendiceal neurofibromas in NF1 with LAMNs in the English literature.
Surgical resection is the standard treatment for patients with LAMNs. However, the most appropriate surgical procedure for patients with LAMNs remains controversial. Generally, tumor involvement of a surgical margin is an indication for additional surgery. However, Arnason et al. reported that in patients with appendiceal LAMNs without discharge of mucin or exposure of the appendiceal serosa, involvement of the appendectomy margin by either neoplastic epithelium or acellular mucin was not associated with disease recurrence or peritoneal dissemination [19]. Therefore, in the present case, additional treatment for LAMNs was not necessary.
There are no guidelines for the optimal management strategy of appendiceal neurofibromas and LAMNs. Thus, the most appropriate surgical procedure for patients with appendiceal neurofibromas or LAMNs is unknown. At the very least, it is important to obtain negative resection margins. A larger number of long-term follow-up patients with appendiceal neurofibromas and LAMNs are required to establish optimal, evidence-based treatment.
4. Conclusions
We report herein a rare case of appendiceal neurofibroma with LAMN in NF1 patient. Patients with NF1 may have gastrointestinal tract involvement such as appendiceal neurofibromas. These cases are often preoperatively diagnosed as appendicitis. Therefore, it is necessary to suspect appendiceal neurofibromas in NF1 patient who have symptoms of appendicitis.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding source
The authors declare that this study was not funded externally.
Ethical approval
The study such as this case report was exempted from ethical approval by the Institutional Review Board of Hiroshima City Asa Citizens Hospital.
Consent
When obtaining informed consent for surgical procedures, general consent for publication and presentation was obtained from the patient.
Authors’ contributions
TK drafted the manuscript. TK and KO reviewed and edited the manuscript. TK, KO, TK, MY, NT, MK, AN, YA, MS, and MM participated in the care of the patients. MK and HM provided the histopathological examination and diagnosis. TK, JH, MF, HM, and NH participated in critical revision of the manuscript. All authors read and approved the final manuscript.
Registration of research studies
This is case report not research study.
Guarantor
Koichi Oishi.
Provenance and peer review
Not commissioned, externally peer reviewed.
Acknowledgement
We thank JAM Post (http://www.jamp.com/index.cfm) for the English language editing.
References
- 1.Ferner R.E. Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol. 2007;6:340–351. doi: 10.1016/S1474-4422(07)70075-3. [DOI] [PubMed] [Google Scholar]
- 2.Pinsk I., Dukhno O., Ovnat A., Levy I. Gastrointestinal complications of von Recklinghausen’s disease: two case reports and a review of the literature. Scand. J. Gastroenterol. 2003;38:1275–1278. doi: 10.1080/00365520310005712. [DOI] [PubMed] [Google Scholar]
- 3.Umetsu S.E., Shafizadeh N., Kakar S. Grading and staging mucinous neoplasms of the appendix: a case series and review of the literature. Hum. Pathol. 2017;69:81–89. doi: 10.1016/j.humpath.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., the SCARE Group The SCARE statement: consensus-based surgical case reportguidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Stivaros S., Garg S., Tziraki M., Cai Y., Thomas O., Mellor J., Morris A.A., Jim C., Szumanska-Ryt K., Parkes L.M., Haroon H.A., Montaldi D., Webb N., Keane J., Castellanos F.X., Silva A.J., Huson S., Williams S., Gareth Evans D., Emsley R., Green J., SANTA Consortium Randomised controlled trial of simvastatin treatment for autism in young children with neurofibromatosis type 1 (SANTA) Mol. Autism. 2018;22:9–12. doi: 10.1186/s13229-018-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo L., He K., Xu X., Li G., Li Z., Xia Y., Teng X., Teng L. Giant appendiceal neurofibroma in von Recklinghausen’s disease: a case report and literature review. Oncol. Lett. 2014;8:1957–1960. doi: 10.3892/ol.2014.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozaki A., Tsukada M., Watanabe K., Tsubokura M., Kato S., Tanimoto T., Kami M., Ohira H., Kanazawa Y. Perforated appendiceal diverticulitis associated with appendiceal neurofibroma in neurofibromatosis type 1. World J. Gastroenterol. 2015;21:9817–9821. doi: 10.3748/wjg.v21.i33.9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merck C., Kindblom L.G. Neurofibromatosis of the appendix in von Recklinghausen’s disease. A report of a case. Acta Pathol. Microbiol. Scand. A. 1975;6:623–627. doi: 10.1111/j.1699-0463.1975.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 9.Olsen B.S. Giant appendicular neurofibroma. A light and immunohistochemical study. Histopathology. 1987;11:851–855. doi: 10.1111/j.1365-2559.1987.tb01888.x. [DOI] [PubMed] [Google Scholar]
- 10.Samuel I., Jakate S., Ramsey M.M., Saclarides T.J. Abdominal mass in a 19-year-old with neurofibromatosis. Postgrad. Med. J. 1997;73:325–326. doi: 10.1136/pgmj.73.860.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg E., Sheiner E., Holcberg G. Neurofibromatosis type 1 and masses of the appendix: a case report. J. Reprod. Med. 2006;51:578–580. [PubMed] [Google Scholar]
- 12.Agaimy A., Märkl B., Kitz J., Wünsch P.H., Arnholdt H., Füzesi L., Hartmann A., Chetty R. Peripheral nerve sheath tumors of the gastrointestinal tract: a multicenter study of 58 patients including NF1-associated gastric schwannoma and unusual morphologic variants. Virchows Arch. 2010;456:411–422. doi: 10.1007/s00428-010-0886-8. [DOI] [PubMed] [Google Scholar]
- 13.Carter J.E., Laurini J.A. Isolated intestinal neurofibromatous proliferations in the absence of associated systemic syndromes. World J. Gastroenterol. 2008;14:6569–6571. doi: 10.3748/wjg.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan Z., Xu L., Zhao Z., Xu S., Zhang X., Liu T., Zhang S., Yu S. Clinicopathological features and prognosis of malignant peripheral nerve sheath tumor: a retrospective study of 159 cases from 1999 to 2016. Oncotarget. 2017;62:104785–104795. doi: 10.18632/oncotarget.18975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higham C.S., Dombi E., Rogiers A., Bhaumik S., Pans S., Connor S.E.J., Miettinen M., Sciot R., Tirabosco R., Brems H., Baldwin A., Legius E., Widemann B.C., Ferner R.E. The characteristics of 76 atypical neurofibromas as precursors to neurofibromatosis 1 associated malignant peripheral nerve sheath tumors. Neuro Oncol. 2018;(February (2)) doi: 10.1093/neuonc/noy013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakker J.R., Haber M.M., Garcia F.U. Gastrointestinal neurofibromatosis: an unusual cause of gastric outlet obstruction. Am. Surg. 2005;71:100–105. [PubMed] [Google Scholar]
- 17.Rastogi R. Intra-abdominal manifestations of von Recklinghausen’s neurofibromatosis. Saudi J. Gastroenterol. 2008;14:80–82. doi: 10.4103/1319-3767.39623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carr N.J., Sobin L.H. Tumours of the appendix. In: Bosman F.T., Carneiro F., Hruban R.H., Theise N.D., editors. WHO Classification of Tumours of the Digestive System. 4th ed. IARC Press; Lyon, France: 2010. pp. 122–125. [Google Scholar]
- 19.Arnason T., Kamionek M., Yang M., Yantiss R.K., Misdraji J. Significance of proximal margin involvement in low-grade appendiceal mucinous neoplasms. Arch. Pathol. Lab. Med. 2015;139:518–521. doi: 10.5858/arpa.2014-0246-OA. [DOI] [PubMed] [Google Scholar]