Abstract
STUDY QUESTION
Do the determinants of insulin sensitivity/resistance differ in women with and without polycystic ovary syndrome (PCOS)?
SUMMARY ANSWER
Peri-muscular thigh adipose tissue is uniquely associated with insulin sensitivity/resistance in women with PCOS, whereas adiponectin and thigh subcutaneous adipose are the main correlates of insulin sensitivity/resistance in women without PCOS.
WHAT IS KNOWN ALREADY
In subject populations without PCOS, insulin sensitivity/resistance is determined by body fat distribution and circulating concentrations of hormones and pro-inflammatory mediators. Specifically, visceral (intra-abdominal) adipose tissue mass is adversely associated with insulin sensitivity, whereas thigh subcutaneous adipose appears protective against metabolic disease. Adiponectin is an insulin-sensitizing hormone produced by healthy subcutaneous adipose that may mediate the protective effect of thigh subcutaneous adipose. Testosterone, which is elevated in PCOS, may have an adverse effect on insulin sensitivity/resistance.
STUDY DESIGN, SIZE, DURATION
Cross-sectional study of 30 women with PCOS and 38 women without PCOS; data were collected between 2007 and 2011.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Participants were group-matched for obesity, as reflected in BMI (Mean ± SD; PCOS: 31.8 ± 6.0 kg/m2; without PCOS: 31.5 ± 5.0 kg/m2). The whole-body insulin sensitivity index (WBISI) was assessed using a mixed-meal tolerance test; Homeostasis Model Assessment-Insulin resistance (HOMA-IR) was determined from fasting insulin and glucose values. Adipose tissue distribution was determined by computed tomography (CT) scan. Partial correlation analysis, adjusting for total fat mass, was used to identify correlates of WBISI and HOMA-IR within each group of women from measures of body composition, body fat distribution, reproductive-endocrine hormones and adipokines/cytokines. Stepwise multiple linear regression analysis was used to identify the variables that best predicted WBISI and HOMA-IR.
MAIN RESULTS AND THE ROLE OF CHANCE
Among women with PCOS, both WBISI and HOMA-IR were best predicted by peri-muscular adipose tissue cross-sectional area. Among women without PCOS, both WBISI and HOMA-IR were best predicted by adiponectin and thigh subcutaneous adipose tissue.
LIMITATIONS, REASONS FOR CAUTION
Small sample size, group matching for BMI and age, and the use of surrogate measures of insulin sensitivity/resistance.
WIDER IMPLICATIONS OF THE FINDINGS
Because insulin resistance is the root cause of obesity and comorbidities in PCOS, determining its cause could lead to potential therapies. Present results suggest that peri-muscular adipose tissue may play a unique role in determining insulin sensitivity/resistance in women with PCOS. Interventions such as restriction of dietary carbohydrates that have been shown to selectively reduce fatty infiltration of skeletal muscle may decrease the risk for type 2 diabetes in women with PCOS.
STUDY FUNDING/COMPETING INTEREST(S)
The study was supported by National Institutes of Health grants R01HD054960, R01DK67538, P30DK56336, P60DK079626, M014RR00032 and UL1RR025777. The authors have no conflicts of interest.
TRIAL REGISTRATION NUMBER
Keywords: polycystic ovary syndrome, insulin sensitivity, fat distribution, abdominal adipose tissue, adiponectin, testosterone, skeletal muscle
Introduction
Polycystic ovary syndrome (PCOS), in addition to being a common reproductive-endocrine disorder, is characterized by obesity (Gambineri et al., 2002; Azziz et al., 2004) and insulin resistance (Diamanti-Kandarakis and Dunaif, 2012). As such, women with PCOS are at elevated risk for type 2 diabetes (Salley et al., 2007) and cardiovascular disease (El Hayek et al., 2016). Further, insulin resistance may be one of the root causes of both the obesity and the dysregulation of the Hypothalamic–Pituitary–Gonadal axis in PCOS. The etiology of insulin resistance in PCOS is complex, and it may emanate from several genetic (Diamanti-Kandarakis and Dunaif, 2012) and non-genetic sources. Identification of these sources may lead to means for minimizing insulin resistance and associated disease risk.
Abdominal obesity has been suggested as contributing to insulin resistance in PCOS (Carmina et al., 2007), possibly through inflammation. Subclinical inflammation has been reported in PCOS (Tarkun et al., 2006; Vgontzas et al., 2006), but remains controversial (Duleba and Dokras, 2012). Further, whether intra-abdominal adipose tissue (IAAT), the metabolically active compartment, is elevated in PCOS is not clear. Few studies have actually measured IAAT using high-quality imaging techniques. Two of these studies found no difference in IAAT between women with and without PCOS (Barber et al., 2008; Manneras-Holm et al., 2011). The third, among lean women, found that those with PCOS had significantly less IAAT (Dolfing et al., 2011). Associations of IAAT and inflammation with insulin resistance in PCOS have not been carefully examined.
Dysfunctional subcutaneous adipose tissue (SAT), as reflected in larger adipocytes and lower circulating adiponectin, has been noted in PCOS (Carmina et al., 2008; Manneras-Holm et al., 2011), and may contribute to insulin resistance. Both lower adiponectin and diversion of lipid to ‘ectopic’ depots such as liver and skeletal muscle (Goodpaster et al., 2000, Shulman, 2014) may contribute to insulin resistance in PCOS, but have not been extensively investigated.
Finally, the elevated androgens in PCOS may contribute to insulin resistance. Studies in humans (Elbers et al., 1999; Brochu et al., 2008; Comerford et al., 2012; Mario et al., 2012) and animal models (Holmang et al., 1990) have shown that androgens can directly impair insulin action, as well as adversely affect body composition and fat distribution in ways that may promote insulin resistance (Fontana et al., 2007).
The purpose of this study was to examine associations of fat distribution, inflammation and testosterone with insulin sensitivity/resistance in women with and without PCOS. We tested the specific hypothesis that among premenopausal women without PCOS, the favorable accumulation of peripheral SAT, as reflected in circulating adiponectin, would have beneficial effects on insulin sensitivity/resistance. In contrast, we hypothesize that women with PCOS have dysfunctional peripheral SAT, and as a result, ectopic adipose in skeletal muscle and circulating testosterone become the main determinants of insulin sensitivity/resistance.
Materials and Methods
Study participants
This cross-sectional/correlational study used baseline data from two previous studies (Goree et al., 2011; Gower et al., 2013) conducted in PCOS (Gower et al., 2013) and overweight, but otherwise healthy women (Goree et al., 2011). Data from 38 non-PCOS and 30 PCOS premenopausal women were used. The criteria for the diagnosis of PCOS were consistent with the NIH 1990 criteria and included: (i) hyperandrogenism and/or hyperandrogenemia, (ii) oligo-ovulation and (iii) the exclusion of any existing disorders such as Cushing's syndrome, hyperprolactinemia or congenital (non-classic) adrenal hyperplasia. Inclusion requirements were PCOS diagnosis (PCOS group only), BMI >25 kg/m2 (non-PCOS group only) and ≤45 kg/m2 (both groups), body weight <136 kg, age 21–49 years, non-diabetic and no weight change >2.3 kg over the past 6 months. Exclusion criteria included PCOS diagnosis (non-PCOS group only), regular exercise >2 hours per week, pregnancy, current breastfeeding, any disorders of glucose or lipid metabolism, use of medication that could affect body composition or glucose metabolism (i.e. oral contraceptives, cholesterol and blood pressure medications), current tobacco use, illegal drug use in last 6 months, history of hypoglycemia, major food allergies or dislikes and a medical history that contra-indicated inclusion. In addition, none of the women with PCOS were receiving medical treatment for the condition or had received medications to mediate the disorder for the previous 3 months. Participants were evaluated for glucose tolerance using a 2-h oral glucose tolerance test. Two women without PCOS had 2-h glucose >140 mg/dl; nine women with PCOS had 2-h glucose >140 mg/dl.
Ethical approval
The protocol was approved by the Institutional Review Board at University of Alabama, Birmingham (UAB) and informed consent was obtained prior to testing.
Protocol
Comprehensive metabolic testing (described below) was conducted in UAB's Clinical Research Unit and the Core Laboratory of the Center for Clinical and Translational Sciences, Nutrition Obesity Research Center and Diabetes Research Center.
Body composition
Total body fat and lean mass were measured by dual-energy X-ray absorptiometry (DXA) using a Lunar Prodigy densitometer (GE Medical Systems, Madison, WI, USA, software version 12.3) for the non-PCOS group, and a Lunar iDXA densitometer (GE Medical Systems, enCORE 2002 software, version 6.10.029) for the PCOS group. The two instruments did not differ regarding their quantification of fat mass and lean mass (Morrison et al., 2016). Participants were required to wear light clothing, remove all metal objects and lie supine with arms at their sides during the scan. IAAT, subcutaneous abdominal adipose tissue (SAAT), thigh muscle, thigh SAT, thigh peri-muscular adipose tissue (PMAT) and thigh intermuscular adipose tissue (IMAT) were measured by CT scanning. Five millimeter axial scans at the level of the umbilicus (approximately the L4–L5 intervertebral space) and the mid-thigh were taken. Scans were later analyzed for cross-sectional area (cm2) of adipose tissue and muscle tissue using SliceOmatic image analysis software (version 4.3: Tomovision, Montreal, Canada). The abdominal scan was used to analyze IAAT and SAAT. Thigh IMAT and PMAT were separated from thigh SAT by manually drawing a line along the fascia lata surrounding the thigh muscle. Subsequently, IMAT was partitioned from PMAT by manually drawing a line around the muscle itself to capture adipose tissue located directly between and within muscle groups (Goodpaster, 2002). All scans were evaluated by the same image analyst (A.M.G.).
Insulin sensitivity
The whole-body insulin sensitivity index (WBISI) was derived by obtaining frequent blood samples after consumption of a standardized liquid meal (liquid meal tolerance test; LMTT). Although the euglycemic clamp is considered as the gold standard for assessing insulin sensitivity, an oral test is more physiologically relevant as it engages gastrointestinal hormones, and captures insulin action on both skeletal muscle and liver. The mixed meal has advantages over oral glucose in that it includes fats and proteins that may affect glucose metabolism (Maki et al., 2009). Insulin sensitivity assessed using liquid mixed meal has been validated against the minimal model (Maki et al., 2011). Participants fasted 12 h prior to the test. Women without PCOS were tested in the follicular stage of the menstrual cycle. To perform the test, a flexible intravenous catheter was placed in the antecubital space of one arm. At time ‘zero’, a liquid mixed-macronutrient meal was provided [Carnation Instant Breakfast (Nestle, Minneapolis, MN, USA) and whole milk]. The liquid meal was calculated to provide 7 kcal/kg body weight as 24% fat, 58.6% carbohydrate and 17.4% protein. Subjects were required to consume the meal within 5 min. Blood was drawn at −15 and −5 min before meal ingestion, which began at time zero. Subsequent samples were drawn every 5 min from time zero to 30 min, every 10 min from 30 to 180 min, and at 210 and 240 min. Sera were stored at −85°C.
The WBISI was calculated using a formula based on the insulin and glucose values over the course of the meal test (Matsuda and DeFronzo, 1999). In addition, Homeostasis Model Assessment-Insulin resistance (HOMA-IR) was calculated using a formula based on fasting glucose and fasting insulin: Fasting Glucose (mg/dl) × Fasting Insulin (µU/ml)/405 (Matthews et al., 1985). HOMA-IR was included as a measure of insulin resistance because it is simple to obtain, and allows for comparison with data from studies where the more demanding meal tolerance test is not feasible.
Analysis of glucose and hormones
Glucose was measured in 3 µl sera by using the glucose oxidase method on a Stanbio SIRRUS analyzer (Stanbio Laboratory, Boerne, TX, USA). This analysis had an inter-assay coefficient of variation (CV) of 3.1% and an intra-assay CV of 1.2%. Insulin was assayed in 50-µl aliquots by using immunofluorescence on a TOSOH AIA-II analyzer (TOSOH Corp, South San Francisco, CA, USA). This analysis had an inter-assay CV of 4.4% and an intra-assay CV of 1.5%. Estradiol and total testosterone were determined by immunofluorescence using the TOSOH with the following CV and sensitivities: estradiol: inter-assay CV 5.4%, intra-assay CV 6.0%, minimum sensitivity 25 pg/ml; testosterone: intra-assay CV 2.4%, inter-assay CV 2.7%, minimum sensitivity 10 ng/dl. Sex hormone binding globulin (SHBG) was determined using an immunoradiometric assay (Siemens Corp., Los Angeles, CA, USA); inter-assay CV 8.0%, intra-assay CV 4.0% and minimum sensitivity 0.68 ng/dl. Free testosterone (pmol/l) was calculated from serum concentrations of total testosterone and SHBG using the method of Sodergard, Backstrom and Shanbhag (Sodergard et al., 1982). This method is based on the concentration of albumin, the binding capacity of SHBG and the association constants of testosterone for SHBG and albumin, as determined in a sample of normal men and women. Adiponectin was determined in duplicate by a radioimmunoassay (Millipore, St. Charles, MO, USA), which utilizes a polyclonal antibody directed against the oligomeric forms of the hormone (i.e. it will not detect monomeric or globular adiponectin). Adiponectin CV and sensitivity: inter-assay CV 10.9%, intra-assay CV 4.8%, minimum sensitivity 1.29 μg/ml. Serum leptin was measured in duplicate 100-ml aliquots using RIA reagents from Millipore. Leptin CV and sensitivity: mean inter-assay CV 7.9%, mean intra-assay CV 9.1%, minimum sensitivity 0.29 ng/ml.
Analysis of markers of inflammation
Circulating inflammation markers were assessed by immunoassay. High sensitivity C-reactive protein (hsCRP) was assessed by turbidometric methodology using a SIRRUS analyzer (Boerne, TX, USA) with reagents obtained from Pointe Scientific (Canton, MI, USA); tumor necrosis factor-alpha (TNF-α) using electrochemiluminscence (Meso Scale Discovery, Rockville, MD, USA); and interleukin (IL-6) using electrochemiluminscence (Meso Scale Discovery). Mean inter-assay CVs are 2.1%, 5.5% and 9.7%, respectively. Mean intra-assay CVs are 7.49%, 7.61% and 6.68%, respectively. Minimum detectable concentrations for each assay are 0.05 mg/l, 0.51 pg/ml and 0.25 pg/ml, respectively.
Statistical analyses
This was a hypothesis generating study, with the intent of using the results to generate sample size for future studies with the effect size we provide. As such, power or sample size analyses were not included. Analyses were performed with SAS version 9 (Cary, NC, USA). Data were log-transformed as necessary to correct for skewness (HOMA-IR, WBISI, thigh muscle, IAAT, IMAT, CRP, IL-6, adiponectin, estradiol, testosterone, free testosterone). ANOVA was used to compare baseline characteristics between women with and without PCOS. ANCOVA was used to compare groups after adjusting for total fat mass and in the case of IMAT and PMAT, also for muscle cross-sectional area. Pearson partial correlation analyses, controlling for total body fat mass, were employed to examine the relationships of WBISI and HOMA-IR with measures of body composition, body fat distribution, markers of inflammation and serum hormone concentrations within each group of women. Because analyses indicated a unique association of PMAT with WBISI and HOMA-IR in women with PCOS, we also examined whether PMAT was associated with thigh SAT (Pearson partial correlation adjusted for fat mass and thigh muscle) within each group of women.
After identifying the variables that were significantly associated with WBISI and HOMA-IR, stepwise multiple linear regression analysis was used to identify the independent variables that best predicted WBISI and HOMA-IR within each group. The variables that were entered were total body fat, thigh muscle cross-sectional area, thigh subcutaneous adipose tissue, IAAT, PMAT, free testosterone and adiponectin.
Results
Participant characteristics
All women without PCOS were overweight or obese. Within the PCOS group, five participants were normal weight (BMI ≤24.9) and the remaining (n = 25) participants were overweight/obese. All participants were premenopausal (aged 21–49 years) and were African American (54.6%) or European American (45.1%). Participant characteristics are shown in Table I by group. Not all outcomes were available for all participants; in the PCOS group, sample size ranged from 24 to 30, and in the women without PCOS, sample size ranged from 35 to 38. Significant between-group differences were noted for TNF-α, total testosterone and free testosterone.
Table I.
Participant characteristics by group.
| Polycystic ovary syndrome | ||||
|---|---|---|---|---|
| Yes | No | |||
| n = 30 | n = 38 | P | ||
| Mean (SD) | Mean (SD) | P | Adjusted for total body fat | |
| Age (years) | 31.6 ± 5.7 | 34.8 ± 8.7 | 0.078 | |
| Racea | 57% (non-white) | 53% (non-white) | 0.645 | |
| BMI (kg/m2) | 31.8 ± 5.7 | 31.5 ± 4.9 | 0.795 | |
| Lean body mass (kg) | 46.6 ± 6.2 | 45.3 ± 6.3 | 0.387 | 0.082 |
| Fat mass (kg) | 36.6 ± 11.7 | 39.6 ± 10.3 | 0.288 | |
| Intra-abdominal adipose tissue (cm2) | 63.6 ± 48.3 | 68.0 ± 36.5 | 0.211 | 0.339 |
| Subcutaneous abdominal adipose tissue (cm2) | 412.2 ± 132 | 433.9 ± 143 | 0.534 | 0.555 |
| Thigh muscle (cm2) | 275.7 ± 43 | 276.4 ± 44 | 0.959 | 0.724 |
| Thigh subcutaneous adipose tissue (cm2) | 317 ± 102 | 329 ± 92 | 0.652 | 0.613 |
| Thigh intermuscular adipose tissueb (cm2) | 12.1 ± 6.9 | 14.9 ± 6.9 | 0.086 | 0.164 |
| Thigh peri-muscular adipose tissueb (cm2) | 22.1 ± 8.4 | 21.6 ± 7.4 | 0.831 | 0.250 |
| Adiponectin (µg/ml) | 10.0 ± 5.1 | 8.8 ± 4.9 | 0.322 | 0.354 |
| Leptin (ng/ml) | 37.4 ± 18.8 | 40.8 ± 16.3 | 0.427 | 0.964 |
| C-reactive protein (mg/dl) | 5.0 ± 5.4 | 5.0 ± 6.9 | 0.403 | 0.167 |
| Tumor necrosis factor-α (pg/ml) | 5.4 ± 2.3 | 7.8 ± 1.5 | <0.001** | <0.001*** |
| Interleukin-6 (pg/ml) | 1.8 ± 0.9 | 2.2 ± 1.6 | 0.273 | 0.479 |
| Estradiol (pg/ml) | 77.6 ± 54.3 | 77.0 ± 58.1 | 0.768 | 0.840 |
| Total testosterone (ng/dl) | 53.7 ± 28.3 | 29.3 ± 18.0 | <0.001*** | <0.001*** |
| Free testosterone (ng/dl) | 3.0–11± 1.8–11 | 1.6–11 ± 1.2–11 | <0.001*** | <0.001*** |
| Fasting insulin (µU/ml) | 8.7 ± 6.6 | 10.3 ± 5.1 | 0.063 | 0.112 |
| Fasting glucose (mg/dl) | 96.0 ± 9.0 | 94.8 ± 9.7 | 0.580 | 0.453 |
| Sex hormone binding globulin (nmol/l) | 49.3 ± 21.0 | 68.1 ± 51.4 | 0.156 | 0.140 |
| Whole-body insulin sensitivity index | 7.4 ± 6.4 | 6.0 ± 4.2 | 0.263 | 0.506 |
| Homeostasis Model Assessment-Insulin resistance (HOMA-IR) | 2.1 ± 1.7 | 2.4 ± 1.3 | 0.094 | 0.171 |
aChi-square analysis.
bFurther controlled for thigh muscle cross-sectional area.
**P < 0.01, ***P < 0.001.
Correlations of insulin sensitivity/resistance with measures of body composition/fat distribution, hormones and markers of inflammation
Results from partial correlation analysis for WBISI and HOMA-IR are shown in Tables II and III by group. Among women with PCOS, WBISI was inversely associated with PMAT, and positively associated with thigh SAT; the inverse association with free testosterone approached significance (P = 0.056). HOMA-IR was positively associated with PMAT and free testosterone, and inversely with thigh SAT. Among women without PCOS, WBISI was positively associated with adiponectin and thigh SAT, and inversely associated with free testosterone. HOMA-IR was inversely associated with thigh SAT and adiponectin, and positively associated with free testosterone. In women with PCOS, thigh SAT was inversely associated with PMAT (−0.587, P< 0.01; adjusted for total body fat and thigh muscle); this association was not observed in women without PCOS (0.003, P = 0.986).
Table II.
Association of body composition and fat distribution with insulin sensitivity and insulin resistance; results shown are Pearson partial correlations adjusted for total body fat.
| Non-PCOS: Log WBISI | PCOS: Log WBISI | Non-PCOS: Log HOMA-IR | PCOS: Log HOMA-IR | |
|---|---|---|---|---|
| Lean mass | −0.028 | −0.022 | 0.069 | 0.078 |
| SAAT | −0.203 | −0.315 | 0.232 | 0.247 |
| IAAT | −0.325 | −0.348 | 0.274 | 0.437* |
| Thigh muscle | −0.008 | −0.307 | 0.161 | 0.373 |
| Thigh SAT | 0.705*** | 0.503** | −0.592*** | −0.542** |
| Thigh IMATa | 0.070 | −0.288 | −0.031 | 0.295 |
| Thigh PMATa | 0.045 | −0.428* | 0.100 | 0.522** |
PCOS, polycystic ovary syndrome; WBISI, whole-body insulin sensitivity index; HOMA-IR, homeostatic model assessment-insulin resistance; SAAT, subcutaneous abdominal adipose tissue; IAAT, intra-abdominal adipose tissue; thigh SAT, thigh subcutaneous adipose tissue; thigh IMAT, thigh intermuscular adipose tissue; thigh PMAT, thigh peri-muscular adipose tissue.
aFurther adjusted for thigh muscle cross-sectional area.
*P < 0.05, **P< 0.01, ***P < 0.001.
Table III.
Association of hormones and markers of inflammation with insulin sensitivity and insulin resistance; results shown are Pearson partial correlations adjusted for total body fat.
| Non-PCOS: Log WBISI | PCOS: Log WBISI | Non-PCOS: Log HOMA-IR | PCOS: Log HOMA-IR | |
|---|---|---|---|---|
| Log CRP | −0.130 | 0.053 | 0.217 | −0.007 |
| Log IL-6 | 0.016 | −0.225 | 0.115 | 0.082 |
| TNF-α | 0.048 | −0.105 | 0.142 | 0.101 |
| Leptin | −0.131 | 0.189 | 0.266 | −0.272 |
| Log adiponectin | 0.603*** | 0.264 | −0.594*** | −0.341 |
| Log estradiol | −0.181 | 0.151 | 0.230 | −0.098 |
| Log testosterone | −0.286 | −0.216 | 0.230 | 0.263 |
| Log free testost. | −0.538** | −0.371a | 0.404* | 0.492* |
CRP, C-reactive protein; IL-6, Interleukin-6; TNF-α, tumor necrosis factor-alpha.
aP = 0.056.
*P< 0.05, **P < 0.01, ***P < 0.001.
Variables that best predicted insulin sensitivity/resistance
Stepwise multiple linear regression analysis among women with PCOS indicated that both WBISI and HOMA-IR were best predicted by PMAT (partial R2 of 0.40 and 0.49, respectively; P < 0.001; data not shown), with a small amount of residual variance (8%) in HOMA-IR explained by IAAT (P < 0.05). Free testosterone entered the model for HOMA-IR (partial R2 of 0.08) but was not significant (P= 0.051). Among women without PCOS, both WBISI and HOMA-IR were best predicted by adiponectin, total body fat and thigh SAT (P ≤ 0.001 for all). In addition, free testosterone explained 5% of the variance in WBISI (P < 0.01).
Discussion
Insulin resistance is the root cause of obesity and comorbidities in PCOS. Determining the cause of insulin resistance could lead to potential therapies. The main finding of this study was that PMAT, an ectopic adipose depot adjacent to skeletal muscle, may play a unique role in determining insulin sensitivity/resistance in women with PCOS. In contrast, the protective effect of thigh SAT on insulin sensitivity/resistance, dominant in women without PCOS, is less apparent in those with PCOS.
In this study, thigh PMAT was the strongest determinant of insulin sensitivity/resistance in the PCOS group (Fig. 1). Few studies have examined the association of all three thigh fat compartments (subcutaneous, intermuscular and peri-muscular) with insulin sensitivity/resistance. While there is general consensus regarding the relatively positive influence of thigh SAT, findings related to the relationship of thigh IMAT and PMAT are less clear (Goodpaster et al., 2000; Goss and Gower, 2012). In a heterogeneous sample of men and women, both thigh IMAT and PMAT were inversely related to insulin sensitivity; however, these associations were not significant after adjusting for total body fat (Goodpaster et al., 2000). Our group previously reported that thigh IMAT, but not PMAT, was inversely associated with insulin sensitivity in a sample of healthy postmenopausal women, a relationship that remained significant after adjusting for total body fat (Goss and Gower, 2012). Discrepancies between study findings may be the result of variation in sample population or measurement techniques. The mechanisms through which thigh PMAT may interfere with insulin action are not clear; however, it has been hypothesized that reduced blood flow in and around the thigh muscle, increased local concentrations of free fatty acids or pro-inflammatory cytokines, as well as a alterations in insulin diffusion capability, may impair insulin action (Ragheb et al., 2009). To our knowledge, this is the first study to examine thigh adipose tissue sub-compartments in relation to insulin sensitivity/resistance in premenopausal women with PCOS.
Figure 1.
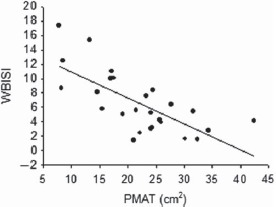
Whole-body insulin sensitivity index (WBISI) is related to peri-muscular adipose tissue (PMAT) in women with polycystic ovary syndrome (simple correlation, r = −0.66, P < 0.001).
Our results also suggest that dysfunctional subcutaneous adipose tissue in PCOS may contribute to insulin resistance. In general, among premenopausal women, a preponderance of subcutaneous adipose in the hip and thigh region is thought to contribute to their relatively high insulin sensitivity and favorable metabolic health (Snijder et al., 2005; Goss and Gower, 2012; Pinnick et al., 2014). In this study, the total amount of thigh SAT did not differ between groups, but only in women without PCOS was thigh SAT independently associated with insulin sensitivity/resistance. Further, only in women with PCOS was PMAT associated with thigh SAT. Taken together, these observations suggest that women with PCOS allocated lipid to PMAT at the expense of thigh SAT, which had an adverse effect on insulin sensitivity/resistance.
The influence of thigh SAT on insulin sensitivity/resistance was also reflected in associations with adiponectin. Adiponectin was the strongest hormonal correlate of both WBISI and HOMA-IR in women without PCOS (Fig. 2). Adiponectin is a marker for functional adipose tissue (Kadowaki et al., 2008; Martella et al., 2014), and when secreted, may play a role in inflammation, glucose metabolism and insulin sensitivity (Diez and Iglesias, 2003). In this study, adiponectin concentrations did not differ between women with and without PCOS, but the relationship of adiponectin to insulin sensitivity/resistance was completely absent in women with PCOS. Research is needed to explore the role of adiponectin in adipose tissue function and processes regulating insulin action in women with PCOS.
Figure 2.
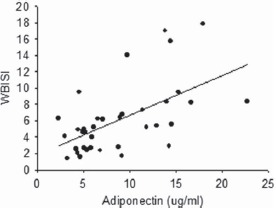
Whole-body insulin sensitivity index (WBISI) is related to adiponectin in women without polycystic ovary syndrome (simple correlation, r = 0.54, P < 0.001).
Free testosterone appeared to explain a small amount of variance in insulin sensitivity/resistance in both groups of women. The most likely explanation for this is an insulin-mediated depression in sex hormone binding globulin. Blockage of androgen synthesis in PCOS has no effect (Dunaif et al., 1990; Diamanti-Kandarakis et al., 1995; Lemieux et al., 1999) or only a minor effect (Elkind-Hirsch et al., 1993; Moghetti et al., 1996; Cagnacci et al., 1999) on insulin sensitivity, and testosterone alone cannot explain insulin resistance in PCOS (Diamanti-Kandarakis and Dunaif, 2012). Taken together, data do not point to a strong role for testosterone in insulin sensitivity/resistance in women.
Our study is only the fourth study using imaging techniques to compare IAAT in women with and without PCOS. We, like two of the other studies (Barber et al., 2008; Manneras-Holm et al., 2011), found no difference between groups. The fourth study, of exclusively lean women, found significantly lower IAAT in women with PCOS (Dolfing et al., 2011). Our data support a minor role at best for IAAT in determining HOMA-IR in women with PCOS. Thus, existing data suggest that the obesity of PCOS may mask a fat distribution characterized by less IAAT, and that IAAT cannot explain the insulin resistance of PCOS.
Our data supported neither higher inflammation in PCOS nor an independent contribution of inflammation to insulin resistance. Although higher systemic inflammation has been reported in PCOS (Gonzalez et al., 2009), this concept is controversial (Duleba and Dokras, 2012), with data possibly being confounded by obesity and/or participant heterogeneity regarding PCOS diagnostic criteria.
Strengths of the study were the strong measures of body composition and body fat distribution. Limitations were the small sample size, difference in range of BMI between the two groups and the use of surrogate measures of insulin sensitivity/resistance rather than the gold standard euglycemic clamp.
To conclude, results indicate that correlates of insulin sensitivity/resistance differ in women with and without PCOS. These differences may provide insight into cause-and-effect in both groups. It is known that a genetic factor in skeletal muscle confers insulin resistance in PCOS, with resultant elevated post-prandial insulin secretion. This combination of inherent insulin resistance and compensatory elevated insulin response, perhaps in concert with elevated testosterone, may have diverse effects on body composition, fat distribution and endocrine factors, leading to preferential deposition of fat within the muscle fascia (PMAT). With this same logic, women without PCOS may preferentially deposit and expand subcutaneous thigh fat in response to adequate levels of both insulin sensitivity and estrogen. However, it is possible that fatty infiltration of skeletal muscle impairs both metabolic and physical function. Our data have shown that an insulin-lowering diet selectively depletes IMAT and improves insulin sensitivity in women with PCOS, even without weight loss (Gower et al., 2013; Goss et al., 2014). Further research with such insulin-lowering diets is needed both to probe mechanisms and to develop diet guidelines for improving the metabolic heath and quality of life of women with PCOS.
Acknowledgements
The authors gratefully acknowledge the assistance of the Core Laboratory and clinic staff of the Nutrition Obesity Research Center, the Diabetes Research Center and the Center for Clinical and Translational Science for all testing and sample analyses.
Authors’ roles
Drs B.A.G. and R.A. conceived of the original project for assessment of women with PCOS. Drs B.A.G. and A.M.G. conceived of the original project for assessment of women without PCOS, and conducted all patient-oriented research and data collection. Drs B.A.G., A.M.G. and S.A.M. conceived of the cross-sectional study design shown here for comparing women with and without PCOS. Dr A.M.G. performed all MRI scan analysis. Drs B.A.G., A.M.G., S.A.M. and D.A.R. conducted statistical analyses. Drs B.A.G., A.M.G., S.A.M. and R.A. were involved in writing the manuscript. Dr B.A.G. is a principal investigator on both studies used for these analyses, and takes ultimate responsibility for the results shown.
Funding
R01HD054960 and R01DK67538; the University of Alabama, Birmingham (UAB) Metabolism/Human Physiology Core Laboratory (Nutrition Obesity Research Center, P30DK56336; Diabetes Research Center, P60DK079626); and the UAB Center for Clinical and Translational Science (M014RR00032, UL1RR025777).
Conflict of interest
The authors have no conflicts of interest.
References
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004;89:2745–2749. [DOI] [PubMed] [Google Scholar]
- Barber TM, Golding SJ, Alvey C, Wass JA, Karpe F, Franks S, McCarthy MI. Global adiposity rather than abnormal regional fat distribution characterizes women with polycystic ovary syndrome. J Clin Endocrinol Metab 2008;93:999–1004. [DOI] [PubMed] [Google Scholar]
- Brochu M, Mathieu ME, Karelis AD, Doucet E, Lavoie ME, Garrel D, Rabasa-Lhoret R. Contribution of the lean body mass to insulin resistance in postmenopausal women with visceral obesity: a Monet study. Obesity (Silver Spring) 2008;16:1085–1093. [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Paoletti AM, Arangino S, Melis GB, Volpe A. Effect of ovarian suppression on glucose metabolism of young lean women with and without ovarian hyperandrogenism. Hum Reprod 1999;14:893–897. [DOI] [PubMed] [Google Scholar]
- Carmina E, Bucchieri S, Esposito A, Del Puente A, Mansueto P, Orio F, Di Fede G, Rini G. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. J Clin Endocrinol Metab 2007;92:2500–2505. [DOI] [PubMed] [Google Scholar]
- Carmina E, Chu M, Moran C, Tortoriello D, Vardhana P, Tena G, Preciado R, Lobo R. Subcutaneous and omental fat expression of adiponectin and leptin in women with polycystic ovary syndrome. Fertil Steril 2008;89:642–648. [DOI] [PubMed] [Google Scholar]
- Comerford KB, Almario RU, Kim K, Karakas SE. Lean mass and insulin resistance in women with polycystic ovary syndrome. Metabolism 2012;61:1256–1260. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 2012;33:981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Mitrakou A, Hennes MM, Platanissiotis D, Kaklas N, Spina J, Georgiadou E, Hoffmann RG, Kissebah AH, Raptis S. Insulin sensitivity and antiandrogenic therapy in women with polycystic ovary syndrome. Metabolism 1995;44:525–531. [DOI] [PubMed] [Google Scholar]
- Diez J, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol 2003;148:293–300. [DOI] [PubMed] [Google Scholar]
- Dolfing JG, Stassen CM, van Haard PM, Wolffenbuttel BH, Schweitzer DH. Comparison of MRI-assessed body fat content between lean women with polycystic ovary syndrome (PCOS) and matched controls: less visceral fat with PCOS. Hum Reprod 2011;26:1495–1500. [DOI] [PubMed] [Google Scholar]
- Duleba AJ, Dokras A. Is PCOS an inflammatory process. Fertil Steril 2012;97:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaif A, Green G, Futterweit W, Dobrjansky A. Suppression of hyperandrogenism does not improve peripheral or hepatic insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab 1990;70:699–704. [DOI] [PubMed] [Google Scholar]
- El Hayek S, Bitar L, Hamdar LH, Mirza FG, Daoud G. Poly cystic ovarian syndrome: an updated overview. Front Physiol 2016;7:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbers JMH, Asschemen H, Seidell JC, Gooren LJG. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am J Physiol 1999;276:E317–E325. [DOI] [PubMed] [Google Scholar]
- Elkind-Hirsch KE, Valdes CT, Malinak LR. Insulin resistance improves in hyperandrogenic women treated with Lupron. Fertil Steril 1993;60:634–641. [DOI] [PubMed] [Google Scholar]
- Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007;56:1010–1013. [DOI] [PubMed] [Google Scholar]
- Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord 2002;26:883–896. [DOI] [PubMed] [Google Scholar]
- Gonzalez F, Rote NS, Minium J, Kirwan JP. Evidence of proatherogenic inflammation in polycystic ovary syndrome. Metabolism 2009;58:954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster BH. Measuring body fat distribution and content in humans. Curr Opin Clin Nutr Metab Care 2002;5:481–487. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 2000;71:885–892. [DOI] [PubMed] [Google Scholar]
- Goree LL, Chandler-Laney P, Ellis AC, Casazza K, Granger WM, Gower BA. Dietary macronutrient composition affects beta cell responsiveness but not insulin sensitivity. Am J Clin Nutr 2011;94:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss AM, Chandler-Laney PC, Ovalle F, Goree LL, Azziz R, Desmond RA, Wright Bates G, Gower BA. Effects of a eucaloric reduced-carbohydrate diet on body composition and fat distribution in women with PCOS. Metabolism 2014;63:1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss AM, Gower BA. Insulin sensitivity is associated with thigh adipose tissue distribution in healthy postmenopausal women. Metabolism 2012;61:1817–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower BA, Chandler-Laney PC, Ovalle F, Goree LL, Azziz R, Desmond RA, Granger WM, Goss AM, Bates GW. Favourable metabolic effects of a eucaloric lower-carbohydrate diet in women with PCOS. Clin Endocrinol (Oxf) 2013;79:550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmang A, Svedberg J, Jennische E, Bjorntorp P. Effects of testosterone on muscle insulin sensitivity and morphology in female rats. Am J Physiol 1990;259:E555–E560. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett 2008;582:74–80. [DOI] [PubMed] [Google Scholar]
- Lemieux S, Lewis GF, Ben-Chetrit A, Steiner G, Greenblatt EM. Correction of hyperandrogenemia by laparoscopic ovarian cautery in women with polycystic ovarian syndrome is not accompanied by improved insulin sensitivity or lipid-lipoprotein levels. J Clin Endocrinol Metab 1999;84:4278–4282. [DOI] [PubMed] [Google Scholar]
- Maki KC, Kelley KM, Lawless AL, Hubacher RL, Schild AL, Dicklin MR, Rains TM. Validation of insulin sensitivity and secretion indices derived from the liquid meal tolerance test. Diabetes Technol Ther 2011;13:661–666. [DOI] [PubMed] [Google Scholar]
- Maki KC, McKenney JM, Farmer MV, Reeves MS, Dicklin MR. Indices of insulin sensitivity and secretion from a standard liquid meal test in subjects with type 2 diabetes, impaired or normal fasting glucose. Nutr J 2009;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manneras-Holm L, Leonhardt H, Kullberg J, Jennische E, Oden A, Holm G, Hellstrom M, Lonn L, Olivecrona G, Stener-Victorin E et al. . Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab 2011;96:E304–E311. [DOI] [PubMed] [Google Scholar]
- Mario FM, do Amarante F, Toscani MK, Spritzer PM. Lean muscle mass in classic or ovulatory PCOS: association with central obesity and insulin resistance. Exp Clin Endocrinol Diabetes 2012;120:511–516. [DOI] [PubMed] [Google Scholar]
- Martella E, Bellotti C, Dozza B, Perrone S, Donati D, Lucarelli E. Secreted adiponectin as a marker to evaluate in vitro the adipogenic differentiation of human mesenchymal stromal cells. Cytotherapy 2014;16:1476–1485. [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- Moghetti P, Tosi F, Castello R, Magnani CM, Negri C, Brun E, Furlani L, Caputo M, Muggeo M. The insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: evidence that androgens impair insulin action in women. J Clin Endocrinol Metab 1996;81:952–960. [DOI] [PubMed] [Google Scholar]
- Morrison SA, Petri RM, Hunter HL, Raju D, Gower B. Comparison of the Lunar Prodigy and iDXA dual-energy X-ray absorptiometers for assessing total and regional body composition. J Clin Densitom 2016;19:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnick KE, Nicholson G, Manolopoulos KN, McQuaid SE, Valet P, Frayn KN, Denton N, Min JL, Zondervan KT, Fleckner J et al. . Distinct developmental profile of lower-body adipose tissue defines resistance against obesity-associated metabolic complications. Diabetes 2014;63:3785–3797. [DOI] [PubMed] [Google Scholar]
- Ragheb R, Shanab GM, Medhat AM, Seoudi DM, Adeli K, Fantus IG. Free fatty acid-induced muscle insulin resistance and glucose uptake dysfunction: evidence for PKC activation and oxidative stress-activated signaling pathways. Biochem Biophys Res Commun 2009;389:211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salley KE, Wickham EP, Cheang KI, Essah PA, Karjane NW, Nestler JE. Glucose intolerance in polycystic ovary syndrome--a position statement of the Androgen Excess Society. J Clin Endocrinol Metab 2007;92:4546–4556. [DOI] [PubMed] [Google Scholar]
- Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med 2014;371:2237–2238. [DOI] [PubMed] [Google Scholar]
- Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB, De Rekeneire N, Kanaya AM, Newman AB, Tylavsky FA et al. . Low subcutaneous thigh fat is a risk factor for unfavorable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia 2005;48:301–308. [DOI] [PubMed] [Google Scholar]
- Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem 1982;16:801–810. [DOI] [PubMed] [Google Scholar]
- Tarkun I, Cetinarslan B, Turemen E, Canturk Z, Biyikli M. Association between circulating tumor necrosis factor-alpha, interleukin-6, and insulin resistance in normal-weight women with polycystic ovary syndrome. Metab Syndr Relat Disord 2006;4:122–128. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Trakada G, Bixler EO, Lin HM, Pejovic S, Zoumakis E, Chrousos GP, Legro RS. Plasma interleukin 6 levels are elevated in polycystic ovary syndrome independently of obesity or sleep apnea. Metabolism 2006;55:1076–1082. [DOI] [PubMed] [Google Scholar]


