Abstract
Objective
The prognosis of adrenoleukodystrophy (ALD)with neurological involvement is generally dismal; however, allogeneic stem cell transplantation (SCT) is recognized as effective to stabilize or improve the clinical symptoms of ALD. Herein, we report the clinical outcomes of patients with ALD who consecutively underwent allogeneic stem cell transplantation with reduced intensity conditioning at our institution.
Patients
Sixteen patients with ALD, who were symptomatic (n = 14) or presymptomatic (n = 2), received SCT from 2010 to 2016. The stem cell source was cord blood (n = 14), or bone marrow from a human leukocyte antigen identical sibling (n = 2). The conditioning regimen prior to transplantation was reduced intensity and consisted of fludarabine (125 mg/m2), melphalan (140 mg/m2) and low dose total body irradiation (TBI) of 4Gy (n = 15) or 3Gy (n = 1).
Results
Primary engraftment was obtained in 11 patients, and 4 of the 5 patients who lost the primary graft received a second cord blood transplantation and were engrafted. Five years overall and event-free survival were 90.9% and 61.1% respectively, with a median of 45 months (range 16–91). Loes score stabilized or improved by 18 months after transplantation except for patients with internal capsule involvement.
Conclusion
Allogeneic SCT with reduced intensity conditioning for patients with ALD was safely performed without major transplant-related complications even in symptomatic patients and neurological symptoms were stabilized after SCT in patients without internal capsule involvement.
Keywords: Adrenoleukodystrophy, Allogeneic stem cell transplantation, Loes score, Very long chain fatty acid
Abbreviations: ALD, adrenoleukodystrophy; SCT, stem cell transplantation; MAC, myeloablative conditioning; RIC, reduced intensity conditioning; MRI, magnetic resonance imaging; VLCFA, very long chain fatty acid; GVHD, graft-versus host disease; FLU, fludarabine; MEL, melphalan; ATG, anti-thymocyte globulin; MTX, methotrexate; CSA, cyclosporine A; BMT, bone marrow transplantation; HLA, human leukocyte antigen; CB, cord blood; BM, bone marrow; HHV-6, human herpesvirus-6; CMV, cytomegalovirus; EBV, Epstein-Barr virus; FISH, fluorescent in situ hybridization; IQ, intelligence quotient; DQ, developmental quotient; OS, overall survival; EFS, event free survival; CY, cyclophosphamide; IC, internal capsule; Gd, Gadolinium
1. Introduction
Adrenoleukodystrophy (ALD) is a neurodegenerative disorder with progressive neurological symptoms and clinical outcome is usually dismal in terms of neurological function and survival [1]. Various treatments were not effective to stabilize or improve the neurological symptoms [2,3]. However, allogeneic stem cell transplantation (SCT) for ALD was initiated in 1984 and was reported promising in stabilizing clinical symptoms [4]. Since then, allogeneic SCT has been recognized as the only effective treatment modality for ALD patients, although it depends on neurological status at SCT [5]. Originally, SCT for ALD patients was done with myeloablative conditioning (MAC) [4,5] consisting of busulfan (BU) or cyclophosphamide (CY), but recently a reduced intensity conditioning (RIC) regimen was introduced to ALD patients undergoing allogeneic SCT [[6], [7], [8]] in a small patient cohort. However, transplant outcomes such as engraftment, transplant-related complications or overall survival are not well documented. Herein, we describe the transplant outcomes of allogeneic SCT with RIC regimen for patients with ALD and analyze the safety and efficacy of transplantation.
2. Patients and methods
2.1. Patients
Sixteen patients with ALD underwent SCT from 2009 to 2016 at our institution. The diagnosis of ALD was confirmed by ABCD1 gene mutation and brain magnetic resonance imaging (MRI) findings, as well as elevated very long chain fatty acid (VLCFA) at C24:0/C22:0, and C26:0/C22:0. Informed consent for SCT was obtained from guardians and this study received approval from the institutional review board of the Japanese Red Cross Nagoya First Hospital.
2.2. Transplantation
2.2.1. Conditioning regimen and graft-versus host disease prophylaxis (GVHD)
The conditioning regimen prior to transplantation was of reduced intensity and consisted of fludarabine (FLU) at 25 mg/m2/day from day −7 to day −3, melphalan (MEL) at 70 mg/m2/day from day −4 and −3, and total body irradiation (TBI) at 4 Gy (n = 15) or 3 Gy (n = 1) on day −1. GVHD prophylaxis was done with short- term methotrexate (MTX) and cyclosporine A for bone marrow transplantation (BMT) from human leukocyte antigen (HLA) identical siblings, otherwise MTX and tacrolimus were given. MTX was given at 15 mg/m2 on day 1 and 10 mg/m2 on day 3, 6, 11. The trough level of cyclosporine A was 100–200 ng/ml, and that of tacrolimus was 7–12 ng/ml.
2.2.2. Donor selection and HLA disparity
Stem cell source was cord blood (CB) from an unrelated donor (n = 14) or BM from an HLA identical sibling (n = 2). In case of SCT from a sibling, the donor was identified as a non-carrier by biochemical analysis of VLCFA and genetic analysis of ABCD1 gene. HLA compatibility for graft-versus host direction at the allelic level for HLA-A, B, C, and DR was 8/8 (n = 5), 7/8 (n = 2), and 6/8 (n = 8), and one patient was serologically identical in 6/6 antigens with a sibling donor. Anti-HLA antibody was weakly positive in two patients, but they were not donor-specific.
2.2.3. Supportive care
Patients were isolated in a laminar air flow room from the beginning of the conditioning regimen until engraftment was confirmed, and sulfamethoxazole trimethoprim, polymyxin B, micafungin, acyclovir, intravenous gamma globulin were given as infection prophylaxis.
For prophylaxis of transplant-related complications such as sinus obstruction syndrome or thrombotic microangiopathy, danaparoid sodium [9,10], ursodeoxycholic acid, eicosapentaenoic acid and tocopherol alpha were given from the beginning of the conditioning regimen to day 60 or stabilization of clinical condition. Virological assessment for human herpesvirus-6 (HHV-6), cytomegalovirus (CMV) and Epstein-Barr virus (EBV) was performed weekly after SCT until discharge.
2.2.4. Chimerism
Chimerism of patient and donor was periodically analyzed after SCT with whole leukocytes. For sex-mismatched pairs, it was analyzed weekly until day 28 after SCT (on day 7, 14, 21, and 28) with fluorescent in situ hybridization (FISH) using sex chromosome, and in sex-matched pairs, it was done on day 14 and 28 by PCR of short tandem repeats. Long-term follow-up of chimeric analysis was done periodically in available cases.
2.2.5. Evaluation of neurological and biochemical outcomes
Pre- and post-transplant development scores were evaluated by the intelligence quotient (IQ) of full-scale IQ and verbal IQ. Performance IQ was evaluated by WISC III before and after SCT. The IQ of patients after SCT was evaluated with full scale IQ or verbal IQ because performance IQ was rarely evaluated in patients with motor dysfunction or visual impairment. The post-transplantation developmental score was assessed with achievement at last follow-up. Developmental quotient (DQ) was assessed in patients less than three years old.
Brain MRI of patients was performed within two months prior to SCT and periodically after SCT. The Loes score of MRI was counted by neurologists specialized in ALD brain MRI according to an original published paper [11]. Serum VLCFA was periodically measured before and after SCT by gas chromatography-mass spectrometry, and transition of VLCFA was evaluated by comparison of the pre-transplant level and that of last follow-up. Neurological status of patients before and after SCT was described according to neurologic function score (NFS) [12].
2.2.6. Statistical analysis
Neutrophil engraftment was defined as the first day of three successive days of an absolute neutrophil count of over 500/mm3. Acute and chronic GVHD were defined according to the published criteria [13,14]. Overall survival (OS) and event free survival (EFS) were calculated by Kaplan-Meier method, and log-rank test was used for comparison. An event was defined as engraftment failure or any death. Cumulative incidence was calculated for the probabilities of neutrophil engraftment, and acute or chronic GVHD. All statistical analyses were performed with EZR (Saitama, Japan) [15].
3. Results
3.1. Patients
The patient characteristics described in Table 1 were collected from the electronic chart at the author's institution, as well as the original institutions from where the patients were referred to our hospital. Median ages at diagnosis and SCT were eight years old (range 3–14) and 10 years old (range 3–14), respectively.
Table 1.
Patient characteristics.
| Case | Age at SCT (y.o.) | Family history | Type of transplant | HLA compatibility for GVH direction at the allelic level | Conditioning regimen | GVHD prophylaxis | VLCFA before SCT |
Loes score |
NFS |
Engraftment (day) |
Outcome (months) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C24:0/C22:0 | C26:0/C22:0 | Before SCT | After SCT (months) | Before SCT | After SCT | 1st SCT | 2nd SCT | ||||||||
| 1 | 11 | − | UR-CBT | 8/8 | FLU+MEL+TBI 4Gy | TAC + MTX | 1.184 | 0.020 | 14 (IC−) | 12 (52) | 1 | 3 | 19 | Alive (91) | |
| 2 | 9 | − | UR-CBT | 6/8 | FLU+MEL+TBI 4Gy | TAC + MTX | 1.166 | 0.018 | 18 (IC−) | 14 (72) | 2 | 8 | 24 | Alive (88) | |
| 3 | 10 | + | ID-SIB-BMT | 6/6 | FLU+MEL+TBI 4Gy | CSA + MTX | 1.270 | 0.048 | 13 (IC+) | 21 (51) | 1 | 19 | 15 | Alive (86) | |
| 4 | 6 | + | UR-CBT | 6/8 | FLU+MEL+TBI 3Gy | TAC + MTX | 1.500 | 0.054 | 16 (IC−) | 14 (58) | 1 | 3 | − | 20 | Alive (76) |
| 5 | 6 | − | ID-SIB-BMT | 8/8 | FLU+MEL+TBI 4Gy | CSA + MTX | 1.727 | 0.204 | 11 (IC−) | 12 (46) | 1 | 2 | 16 | Alive (73) | |
| 6 | 10 | − | UR-CBT | 6/8 | FLU+MEL+TBI 4Gy | TAC + MTX | 1.706 | 0.134 | 10 (IC−) | 14 (55) | 2 | 2 | 21 | Alive (69) | |
| 7 | 11 | − | UR-CBT | 6/8 | FLU+MEL+TBI 4Gy | TAC + MTX | 1.149 | 0.020 | 15.5 (IC−) | 14 (39) | 1 | 11 | 22 | Alive (61) | |
| 8 | 4 | + | UR-CBT | 8/8 | FLU+MEL+TBI 4Gy | TAC + MTX | 2.030 | 0.119 | 13 (IC+) | 24 (36) | 6 | 21 | 21 | Alive (46) | |
| 9 | 9 | − | UR-CBT | 8/8 | FLU+MEL+TBI 4Gy | TAC + MTX | 1.690 | 0.089 | 14 (IC−) | 14 (26) | 2 | 3 | 20 | Alive (45) | |
| 10 | 10 | − | UR-CBT | 7/8 | FLU+MEL+TBI 4Gy | TAC + MTX | 2.000 | 0.099 | 12 (IC−) | 23 (23) | 4 | 14 | 29 | Dead (32) | |
| 11 | 10 | − | UR-CBT | 6/8 | FLU+MEL+TBI 4Gy | TAC + MTX | 1.429 | 0.026 | 15 (IC+) | 16.5 (1) | 2 | − | − | − | Alive (36) |
| 12 | 14 | + | UR-CBT | 6/8 | FLU+MEL+TBI 4Gy | TAC + MTX | 1.628 | 0.023 | 13 (IC−) | 14 (18) | 0 | 1 | − | 25 | Alive (26) |
| 13 | 14 | + | UR-CBT | 6/8 | FLU+MEL+TBI 4Gy | TAC + MTX | 1.438 | 0.022 | 0 (IC−) | 0 (8) | 0 | 0 | − | 20 | Alive (19) |
| 14 | 3 | + | UR-CBT | 7/8 | FLU+MEL+TBI 4Gy | TAC + MTX | 2.320 | 0.149 | 0 (IC−) | 0 (14) | 0 | 0 | 23 | Alive (29) | |
| 15 | 5 | − | UR-CBT | 6/8 | FLU+MEL+TBI 4Gy | TAC + MTX | 1.710 | 0.076 | 11 (IC+) | 15.5 (7) | 2 | 25 | − | 21 | Alive (20) |
| 16 | 5 | − | UR-CBT | 8/8 | FLU+MEL+TBI 4Gy | TAC + MTX | 1.930 | 0.116 | 20 (IC+) | 31 (7) | 2 | 22 | 19 | Alive (16) | |
SCT: stem cell transplantation, y.o.: years old, UR-CBT: unrelated cord blood transplantation, ID-SIB-BMT: bone marrow transplantation from an HLA identical sibling, HLA: human leukocyte antigen,
GVH: graft-versus-host, FLU: fludarabine 125 mg/m2, MEL: melphalan 140 mg/m2, TBI: total body irradiation, TAC: tacrolimus, MTX: methotrexate, CSA:cyclosporine,
IC−: No involvement of internal capsule, IC+: Involvement of internal capsule, VLCFA: very long chain fatty acid, NFS: Neurologic function score.
In ALD patients, the median Loes score before SCT was 12 (range 10.0–17.5) in fourteen symptomatic patients and 0 in two presymptomatic patients (cases 13, 14) who had a symptomatic older brother with the same ABCD1 gene mutation. White matter abnormality was observed in occipital and/or parietal lobes in 13 patients and the frontal lobe in one patient (case 8). Demyelination of the internal capsule with local Loes score of 1.0 was observed in five patients (cases 3, 8, 11, 15, 16). The median pre-SCT IQ of symptomatic patients was 83 (range 10–104), and those of the presymptomatic patients were 95 and 61. Adrenal dysfunction was observed in three patients and corticosteroid supplementation was necessary (cases 13, 15, 16). No patients received glyceryl trioleate or glyceryl trieucate (“Lorenzo's Oil”) before or after transplantation. Median neurologic function scale (NFS) before SCT was 1.5 (range 0–6).
3.2. Engraftment and chimerism
Primary neutrophil engraftment was obtained in 11 patients with median 21 days (range 15–29), and five patients resulted in graft failure (cases 4, 11, 12, 13, 15). Cumulative incidence of engraftment by day 30 was 68.8% (95% confidence interval (CI) 35.3–84.9%). Four of the five patients who rejected the graft underwent second CBT at median 37 days (range 34–51) from first transplantation (cases 4, 12, 13, 15), but one patient with autologous recovery refused a second SCT (case 11). The conditioning regimen for second SCT was FLU (25 mg/m2/day × 2 days) + cyclophosphamide (CY, 1 g/m2/day × 2 days) and 3Gy TBI (n = 1) or FLU (25 mg/m2/day × 2 days) + MEL (50 mg/m2/day × 2 days) and 3Gy TBI (n = 3), and all four patients achieved engraftment within a median of 22 days (range 20–25).
A chimerism study of 11 patients with engraftment achieved >95% donor chimerism by day 28, but in the five patients with graft failure, donor chimerism declined after day 14 and ended with aplasia (n = 1) or autologous recovery (n = 4). One of the two patients who received bone marrow from an HLA identical sibling showed mixed chimerism after day 100 (case 5) and a donor lymphocyte infusion resulted in full chimerism.
3.3. GVHD and transplant related complications
Acute GVHD was observed in one case with grade I and the cumulative incidence of acute GVHD was 6.3%. Chronic GVHD was not observed in our study population. Transplant-related complications other than GVHD include engraftment syndrome (n = 2), and hemolytic anemia (n = 1), and these symptoms improved with supportive care. No bloodstream infection was observed, while viral reactivation was observed with HHV-6 (n = 1), CMV (n = 6), EBV (n = 1), and Parvo B19 (n = 1), but the symptoms disappeared with ganciclovir (n = 6) or supportive care.
3.4. OS and EFS
Five-year OS and EFS rates were 90.9% (95% CI, 50.8–98.7%) and 61.1% (95% CI, 32.7–80.5%), respectively, with a median follow-up of 45 months (range 16–91) as of October 2017.One patient died from an infection of an unknown origin 32 months after SCT and his Loes score was 23.0 at 24 months from SCT (case 10).
3.5. Neurological outcomes
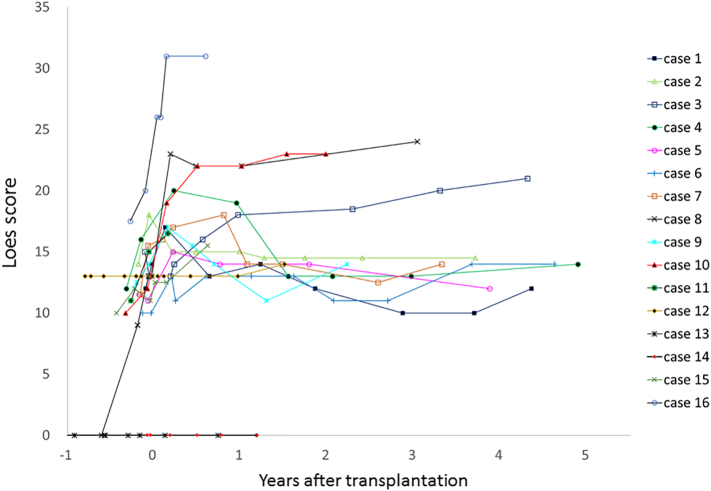
The MRI Loes score of patients before and after SCT is shown in Fig. 1. The Loes score of MRI after SCT was markedly different between patients with or without involvement of the internal capsule (IC) before SCT. In 10 of 11 patients without IC involvement (IC−), post-transplant Loes score stayed between 10 and 15 after 1.5 years from SCT and remained stable thereafter, but in all five patients with IC involvement (IC+) before SCT, it worsened by >15 from 1.9 to 7.4 months after SCT, i.e., the median post-transplant Loes score of symptomatic patients with IC+ was 21 (range 15.5–31), which was significantly higher than those with IC− (median 14, range 12–23) (P = 0.009). In our study population, six patients (cases 1, 2, 4, 5, 7, 9) showed a decreased Loes score after SCT compared to maximum Loes score at pre-transplant or the immediate post-transplant period. The score decreased by 3 to 6 (median 4) and the score stayed at 12 to 14 at their last examination.
Fig. 1.
Loes score of brain MRI in patients with adrenoleukodystrophy before and after stem cell transplantation.
Pre-and post-SCT MRI with Gadolinium (Gd) enhancement was done in 10 symptomatic ALD patients. Nine showed Gd enhancement before SCT, which was diminished (n = 6, cases 2, 3, 5, 6, 8, 12) or weaker (n = 2, cases 9, 10) between 2 and 4 months after SCT, except in one patient with graft failure (case 11).
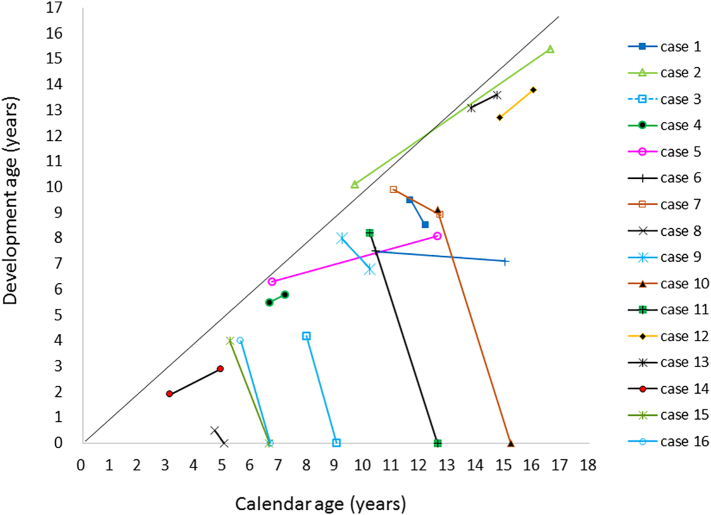
The development score of ALD patients before and after SCT is shown in Fig. 2. The median IQ of patients with IC− was 57(range 0–91) after SCT, but those with IC+ declined to a significantly lower level within 6 months after SCT(P < 0.001). Median NFS after SCT was 3 (range 0–25).
Fig. 2.
Development scores of patients with adrenoleukodystrophy.
3.6. Biochemical markers
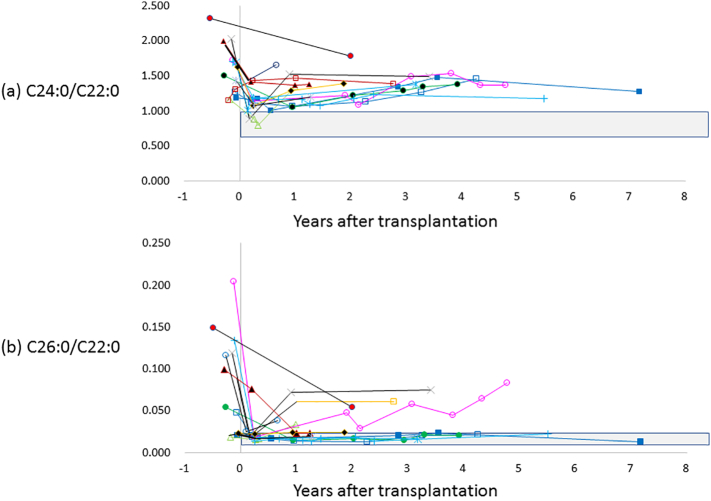
The level of VLCFA (C24:0/C22:0and C26:0/C22:0) was measured in 14 patients after transplantation (Fig. 3) and significantly decreased after SCT (P = 0.002, and P = 0.006, respectively) but was still higher than normal. The transition of VLCFA was not correlated with either involvement of internal capsule or neurological outcomes after transplantation.
Fig. 3.
Transition of very long chain of fatty acid (VLCFA) of C24:0/C22:0 (a) and C26:0/C22:0 (b) before and after stem cell transplantation. Shaded area shows the normal range of VLCFA, which is 0.628–0.977 in C24:0/C22:0, and 0.003–0.006 in C26:0/C22:0.
4. Discussion
The prognosis of patients with ALD who develop neurological symptoms is dismal in terms of physical function and life expectancy [1]. Various therapeutic modalities including medication, SCT, or gene therapy were introduced in the clinical field [[2], [3], [4],16]. In ALD patients, Lorenzo's oil or various medications were tried, but no obvious positive effect was observed [2]. Gene therapy for ALD patients was introduced, but applied to only a small cohort of patients at an early disease stage, and not for large cohort or neurologically advanced patients [16]. Of these various treatment modalities, SCT is so far recognized as the only clinically effective treatment and it has been carried out since 1984 [4]. However, the clinical effect of SCT clearly depends on neurological status prior to transplantation as previously reported [5]. According to previous reports, when SCT was undergone for patients with advanced neurological symptoms, not only did the clinical symptoms deteriorate, but overall survival also declined, and it also decreased when SCT was performed from unrelated donors compared to related donors [5,17]. There are one or two possible reasons for these transplant outcomes. Regarding the conditioning regimen, busulfan was commonly used in SCT for patients with inborn errors of metabolism [5,18], but often causes hepatic damage and may also be neurotoxic to symptomatic ALD patients because it is easily permeable to the central nervous system, causing convulsions or other neurological damage [[19], [20], [21]]. To improve transplant outcomes of symptomatic ALD patients, other agents should be considered to eliminate busulfan as an agent in the conditioning regimen. Recently, SCT with a RIC regimen, consisting of fludarabine, melphalan and low dose TBI, was tried and promising results were reported [[6], [7], [8]]. This conditioning regimen may be a candidate for symptomatic ALD patients who are vulnerable to neurotoxic agents.
In SCT for symptomatic ALD patients, the immediate availability of a stem cell source is critically important because neurological symptoms progress rapidly and barely improve if transplant performed at an advanced stage. In the case of unrelated BMT, it takes at least four to five months from registration to transplant, but CB is readily available for patients who need urgent transplantation or for those who have no HLA matched related or unrelated donor. The other advantage of CB is the low incidence of acute and chronic GVHD, which are major causes of early or late mortality after transplantation [22]. In CBT, most patients receive CB units from an HLA mismatched donor and it is reported that the more the incompatibility increases, the higher the mortality [23]. However, in our study overall survival was not affected by HLA incompatibility. In contrast to these advantages, graft failure is more frequently observed in CBT than BMT. In our study, five patients lost the graft after a month from transplantation, but immediate second CBT was successful to achieve engraftment without any major transplant related complications, except for one patient who refused to receive the second graft. The short conditioning regimen used for second SCT in our patients is commonly applied for patients with graft failure after CBT [24] and was tolerated in our study population. To overcome graft failure, a more intensive conditioning regimen or improvement in GVHD prophylaxis is essential.
Out of 34 anatomical regions identified by the Loes scoring system [11], internal capsule is important because major neuron fibers transmit afferent sensory signals and efferent motor signals through this tract. In adult ALD patients, high mortality was reported when the internal capsule was involved before SCT [25]. In our study, all five patients with IC+ showed neurological deterioration soon after SCT, but 10 of 11 patients with IC− were neurologically stable, even though the follow-up period was relatively short. In our study, the pre-transplant Loes scores of all symptomatic patients were more than ten, which is considered an advanced neurological stage, but they tolerated transplant-related complications and IC involvement did not affect survival. As we have mentioned, six patients showed decreased Loes score compared to their maximum score at pre or immediate post-transplant period and their developmental score stayed at subnormal level without deterioration. The improvement in the Loes score of ALD patients after SCT was demonstrated in a case report [8] and this finding was also observed in our study.
We followed the transition of VLCFA after SCT and found that it came down to a lower level than pre-transplant as reported by Aubourg [26], but was still higher than normal level, although the VLCFA level after SCT in IC+ patients was similar to that of patients with IC−. This means that allogeneic SCT partially corrects VLCFA levels in ALD patients and possibly plays some role in stabilization of neurological symptoms, because a high level of VLCFA is known to be toxic to the oligodendrocyte, which supports the myelination of axons in the brain [27].
We transplanted two presymptomatic patients with genetic abnormality of the ABCD1 gene and elevated VLCFA. They had a symptomatic older brother and were carefully observed after diagnosis of the brother. They showed no neurological symptoms or MRI abnormality19 and 29 months after transplantation. In childhood onset ALD, neurological symptoms usually develop around 7 years old, but in some cases the start is 3 or 4 decades later as a different clinical entity of adrenomyeloneuropathy (AMN), or adult type ALD, even in the same family member who shares the same genetic abnormality [28].
As we have mentioned, early diagnosis is essential to achieve neurological improvement for ALD patients whatever treatment modality is applied. However, practically, early diagnosis of ALD is not easy because of its rarity and subtle clinical manifestation at an early stage. To overcome the difficulties of early diagnosis, newborn screening for ALD patients is essential and has already started in some countries [29,30]. This newborn screening is very encouraging in terms of early detection of ALD, although the incidence is quite low it does involve critical ethical issues of informed consent for late appearing symptoms, typical features of childhood onset ALD. Even so, the importance of newborn screening should be emphasized because regardless of how far treatment techniques progress, there is no or limited beneficial effect if done after neurological deterioration.
In conclusion, SCT with an RIC regimen for ALD is feasible in terms of safety and stabilization of neurological symptoms in patients without internal capsule involvement.
Conflicts of interest
The authors declare no conflicts of interest.
Author contributions
KK planned and designed this study. KK, RM, MW, AY, MH, SK, AN, SM, YS, NK, KS, KN, SD, HM, HS, KM, and NY treated patients and reviewed the patients' clinical data. YK and OO reviewed the MRI of ALD patients, MK performed neuropsychological assessment, and NS did the genetic analysis of ALD patients. KK, RM, MW, AY, MH, SK, AN, SM, YS, NK, KS, KN, SD, HM, HS, KM, YK, OO, MK, NS, and NY contributed to reporting this work.
Acknowledgements
The authors would like to thank Tetsushi Yoshikawa for virological analysis of HHV-6, EBV and CMV after SCT.
We thank all of the doctors who cared for patients before and after SCT: Dr. Yasuo Hachiya and Dr. Takashi Kaneko in Tokyo Metropolitan Children's Medical Center, Dr. Hiroshi Matsumoto in National Defense Medical College, Dr. Yukari Watanabe and Dr. Toru Kato in Okazaki City Hospital, Dr. Yasuhiro Takeshima and Dr. Akira Hayakawa in Kobe University, Dr. Yoshiteru Azuma in Nagoya University, Dr. Shiro Ozasa in Kumamoto University, Dr. Kyoko Suzuki in Juntendo University Urayasu Hospital, Dr. Yoshinori Okumura in Shizuoka Children's Hospital, Dr. Kenichi Inamura and Dr. Hiroto Akaike in Kawasaki Medical School, Dr. Pin Fee Chong in Fukuoka Children's Hospital, Dr. Akihiko Nakahara and Dr. Yasuhiro Kimoto in Miyazaki University, Dr. Norio Sakai and Dr. Noriko Miyamura in Osaka University, Dr. Hirokazu Kurahashi and Prof. Akihisa Okumura in Aichi Medical University, and Prof. Yasuyuki Suzuki in Gifu University.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
Koji Kato, Email: katok.pdc@gmail.com.
Shinsuke Kataoka, Email: s-kataoka@med.nagoya-u.ac.jp.
Atsushi Narita, Email: stanza-la-speranza@nifty.com.
Nozomu Kawashima, Email: kwnozomu@med.nagoya-u.ac.jp.
Sayoko Doisaki, Email: s-doisaki@med.nagoya-u.ac.jp.
Hideki Muramatsu, Email: hideki-muramatsu@med.nagoya-u.ac.jp.
Kimikazu Matsumoto, Email: matsumoto-kmk@ncchd.go.jp.
Yuka Koike, Email: yukkoike@bri.niigata-u.ac.jp.
Osamu Onodera, Email: onodera@bri.niigata-u.ac.jp.
Makiko Kaga, Email: kaga@ncnp.go.jp.
Nobuyuki Shimozawa, Email: nshim@gifu-u.ac.jp.
Nao Yoshida, Email: nao-y@med.nagoya-u.ac.jp.
References
- 1.Moser H.W. Adrenoleukodystrophy: phenotype, genetics, pathogenesis and therapy. Brain. 1997;120:1485–1508. doi: 10.1093/brain/120.8.1485. [DOI] [PubMed] [Google Scholar]
- 2.Moser H.W., Raymond G.V., Koehler W. Evaluation of the preventive effect of glyceryl trioleate-trierucate ("Lorenzo's oil") therapy in X-linked adrenoleukodystrophy: results of two concurrent trials. Adv. Exp. Med. Biol. 2003;544:369–387. doi: 10.1007/978-1-4419-9072-3_47. [DOI] [PubMed] [Google Scholar]
- 3.Engelen M., Ofman R., Dijkgraaf M.G. Lovastatin in X-linked adrenoleukodystrophy. N. Engl. J. Med. 2010;362:276–277. doi: 10.1056/NEJMc0907735. [DOI] [PubMed] [Google Scholar]
- 4.Moser H.W., Tutschka P.J., Brown F.R., 3rd Bone marrow transplant in adrenoleukodystrophy. Neurology. 1984;34:1410–1417. doi: 10.1212/wnl.34.11.1410. [DOI] [PubMed] [Google Scholar]
- 5.Peters C., Charnas L.R., Tan Y. Cerebral X-linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood. 2004;104:881–888. doi: 10.1182/blood-2003-10-3402. [DOI] [PubMed] [Google Scholar]
- 6.Niizuma H., Uematsu M., Sakamoto O. Successful cord blood transplantation with reduced-intensity conditioning for childhood cerebral X-linked adrenoleukodystrophy at advanced and early stages. Pediatr. Transplant. 2012;16:E63–E70. doi: 10.1111/j.1399-3046.2011.01539.x. [DOI] [PubMed] [Google Scholar]
- 7.Awaya T., Kato T., Niwa A. Successful cord blood transplantation using a reduced-intensity conditioning regimen for advanced childhood-onset cerebral adrenoleukodystrophy. Pediatr. Transplant. 2011;15:E116–E120. doi: 10.1111/j.1399-3046.2009.01188.x. [DOI] [PubMed] [Google Scholar]
- 8.Okamura K., Watanabe T., Onishi T. Successful allogeneic unrelated bone marrow transplantation using reduced-intensity conditioning for the treatment of X-linked adrenoleukodystrophy in a one-yr-old boy. Pediatr. Transplant. 2009;13:130–133. doi: 10.1111/j.1399-3046.2008.00962.x. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi H., Watanabe N., Muramatsu H. Danaparoid as the prophylaxis for hepatic veno-occlusive disease after allogeneic hematopoietic stem cell transplantation in childhood hematological malignancy. Pediatr. Blood Cancer. 2010;55:1118–1125. doi: 10.1002/pbc.22645. [DOI] [PubMed] [Google Scholar]
- 10.Kato K., Sakaguchi H., Muramatsu H. Danaparoid reduces transplant-related mortality in stem cell transplantation for children. Pediatr. Transplant. 2018;22 doi: 10.1111/petr.13099. [DOI] [PubMed] [Google Scholar]
- 11.Loes D.J., Hite S., Moser H. Adrenoleukodystrophy: a scoring method for brain MR observations. Am. J. Neuroradiol. 1994;15:1761–1766. [PMC free article] [PubMed] [Google Scholar]
- 12.Moser H.W., Raymond G.V., Lu S.E. Follow-up of 89 asymptomatic patients with adrenoleukodystrophy treated with Lorenzo's oil. Arch. Neurol. 2005;62:1073–1080. doi: 10.1001/archneur.62.7.1073. [DOI] [PubMed] [Google Scholar]
- 13.Przepiorka D., Weisdorf D., Martin P. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 14.Shulman H.M., Sullivan K.M., Weiden P.L. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am. J. Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 15.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eichler F., Duncan C., Musolino P.L. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N. Engl. J. Med. 2017;377:1630–1638. doi: 10.1056/NEJMoa1700554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller W.P., Rothman S.M., Nascene D. Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: the largest single-institution cohort report. Blood. 2011;118:1971–1978. doi: 10.1182/blood-2011-01-329235. [DOI] [PubMed] [Google Scholar]
- 18.Beam D., Poe M.D., Provenzale J.M. Outcomes of unrelated umbilical cord blood transplantation for X-linked adrenoleukodystrophy. Biol. Blood Marrow Transplant. 2007;13:665–674. doi: 10.1016/j.bbmt.2007.01.082. [DOI] [PubMed] [Google Scholar]
- 19.Barker C.C., Butzner J.D., Anderson R.A. Incidence, survival and risk factors for the development of veno-occlusive disease in pediatric hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2003;32:79–87. doi: 10.1038/sj.bmt.1704069. [DOI] [PubMed] [Google Scholar]
- 20.Hassan M. The role of busulfan in bone marrow transplantation. Med. Oncol. 1999;16:166–176. doi: 10.1007/BF02906128. [DOI] [PubMed] [Google Scholar]
- 21.Vassal G., Deroussent A., Hartmann O. Dose-dependent neurotoxicity of high-dose busulfan in children: a clinical and pharmacological study. Cancer Res. 1990;50:6203–6207. [PubMed] [Google Scholar]
- 22.Ballen K.K., Gluckman E., Broxmeyer H.E. Umbilical cord blood transplantation: the first 25 ye2rs and beyond. Blood. 2013;122:491–498. doi: 10.1182/blood-2013-02-453175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallhi K.K., Smith A.R., DeFor T.E. Allele-level HLA matching impacts key outcomes following umbilical cord blood transplantation for inherited metabolic disorders. Biol. Blood Marrow Transplant. 2017;23:119–125. doi: 10.1016/j.bbmt.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu I., Kobayashi H., Nasu K. Successful engraftment of cord blood following a one-day reduced-intensity conditioning regimen in two patients suffering primary graft failure and sepsis. Bone Marrow Transplant. 2009;44:617–618. doi: 10.1038/bmt.2009.69. [DOI] [PubMed] [Google Scholar]
- 25.Kühl J.S., Suarez F., Gillett G.T. Long-term outcomes of allogeneic haematopoietic stem cell transplantation for adult cerebral X-linked adrenoleukodystrophy. Brain. 2017;140:953–966. doi: 10.1093/brain/awx016. [DOI] [PubMed] [Google Scholar]
- 26.Aubourg P., Blanche S., Jambaqué I. Reversal of early neurologic and neuroradiologic manifestations of X-linked adrenoleukodystrophy by bone marrow transplantation. N. Engl. J. Med. 1990;322:1860–1866. doi: 10.1056/NEJM199006283222607. [DOI] [PubMed] [Google Scholar]
- 27.Schönfeld P., Reiser G. Brain lipotoxicity of phytanic acid and very long-chain fatty acids. Harmful cellular/mitochondrial activities in refsum disease and X-linked adrenoleukodystrophy. Aging Dis. 2016;7:136–149. doi: 10.14336/AD.2015.0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiesinger C., Eichler F.S., Berger J. The genetic landscape of X-linked adrenoleukodystrophy: inheritance, mutations, modifier genes, and diagnosis. Appl. Clin. Genet. 2015;8:109–121. doi: 10.2147/TACG.S49590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu C., Iwamoto T., Igarashi J. Application of a diagnostic methodology by quantification of 26:0 lysophosphatidylcholine in dried blood spots for Japanese newborn screening of X-linked adrenoleukodystrophy. Mol. Genet. Metab. Rep. 2017;12:115–118. doi: 10.1016/j.ymgmr.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogel B.H., Bradley S.E., Adams D.J. Newborn screening for X-linked adrenoleukodystrophy in New York State: diagnostic protocol, surveillance protocol and treatment guidelines. Mol. Genet. Metab. 2015;114:599–603. doi: 10.1016/j.ymgme.2015.02.002. [DOI] [PubMed] [Google Scholar]