Abstract
Introduction
Human platelet lysate (hPL) part of the growth factor cocktail derived from human platelets, which has been applied as a cell growth supplement. The production process is easier in comparison to platelet-rich plasma; thus, hPL is now considered for use in wound healing therapy. However, methods for preserving hPL for more than several months that maintain its bioactivity must be considered, especially for chronic wound treatment. The present study compared the effects of preservation for 9 months using a refrigerator or deep freezer.
Methods
We investigated three preservation conditions. In the C-hPL group, hPL was stored at −80 °C in a deep freezer for 9 months; in the CL-hPL group, hPL was cryopreserved for 9 months at −80 °C in a deep freezer then lyophilized; in the L-hPL group, lyophilized hPL was refrigerated at 4 °C for 9 months. The quantity and quality of growth factors in these three groups were measured by an ELISA and in fibroblast cell cultures. Then, gelatin hydrogel discs were impregnated with hPL and its effects with regard to the promotion of wound healing in mice were evaluated by histologic examinations.
Results
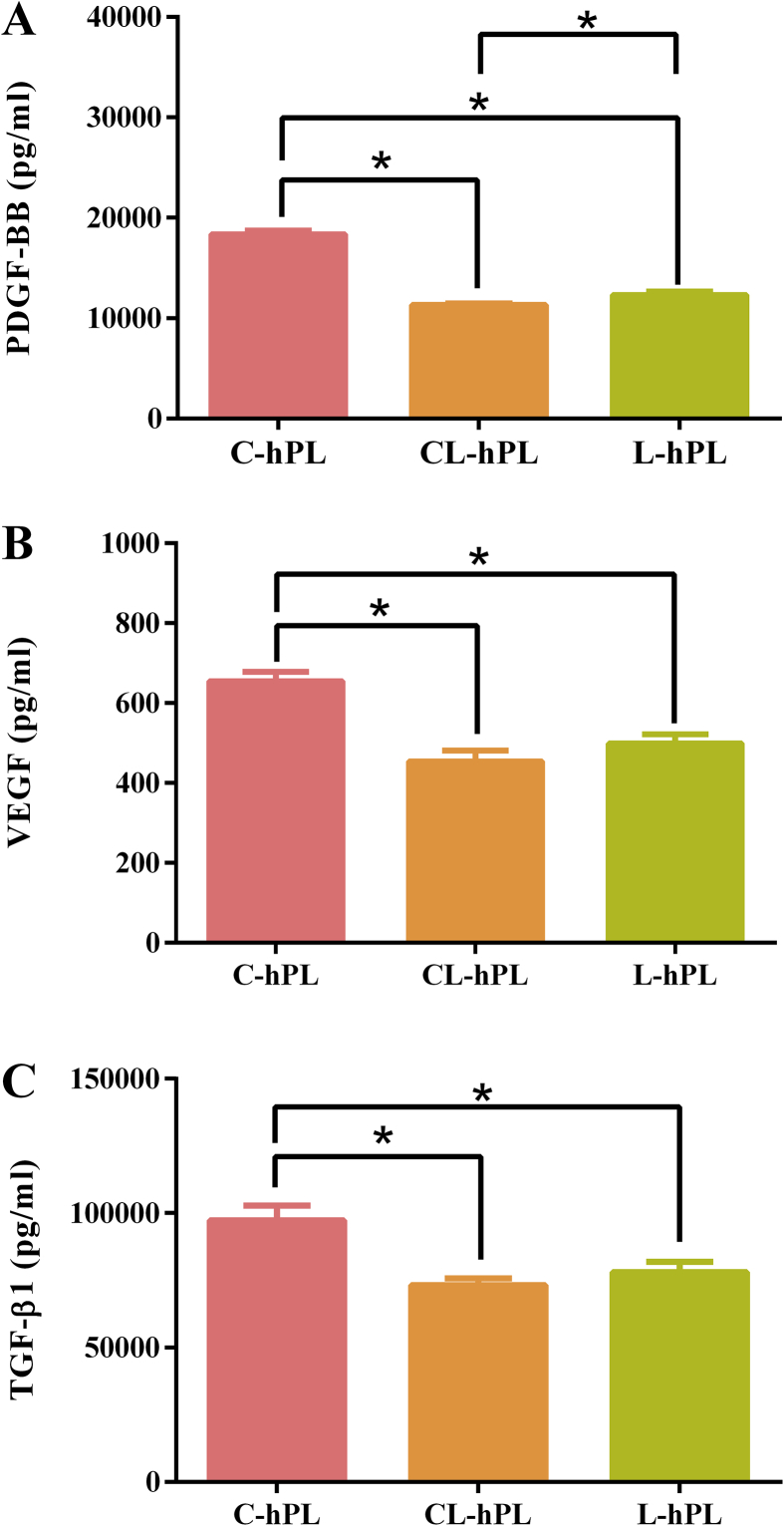
The PDGF-BB concentration in C-hPL, CL-hPL and L-hPL was 18,363 ± 370 pg/ml, 11,325 ± 171 pg/ml, and 12,307 ± 348 pg/ml, respectively; the VEGF concentration was 655 ± 23 pg/ml, 454 ± 27 pg/ml, and 499 ± 23 pg/ml, respectively; and the TGF-β1 concentration was 97,363 ± 5418 pg/ml, 73,198 ± 2442 pg/ml, and 78,034 ± 3885 pg/ml, respectively. In cell culture medium, fibroblast cell cultures were better supported in the hPL groups than in the fetal bovine serum group. In the histologic examination of the wound healing process, no differences were observed among the three preserved hPL groups with regard to epithelialization, or granulation tissue or capillary formation. The wounds in all groups had almost healed by day 14.
Conclusions
The stability of growth factors contained in lyophilized hPL is maintained at 4 °C for up to 9 months. This was a versatile preservation method that can be applied in clinical practice.
Keywords: Platelet lysate, Gelatin hydrogel, Cryopreservation, Lyophilization, Long-term preservation, Wound healing
Highlights
-
•
Efficacy of cryopreserved platelet lysate was maintained for 9 months at −80 °C.
-
•
Efficacy of lyophilized platelet lysate was maintained for 9 months at 4 °C.
-
•
Both cryopreserved PL and lyophilized PL supported cell growth and wound healing.
1. Introduction
Platelets are anucleated cells containing many types of growth factors, including platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β), vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF) in these alpha granules [1], [2]. Platelets play an important role in the beginning of the wound healing process. At the hemostasis phase, platelets are aggregated and activated to form a blood clot through binding to fibrinogen and to prevent blood loss at the injury site. At the same time, these growth factors are released from platelets and play an important role in the initiation of the wound repair process [3], [4], [5]. These growth factors from platelets have been used as cell growth supplements or clinical treatments for wound healing. Platelet-rich plasma (PRP) has commonly been used in clinical practice and is usually obtained from the patient's autologous blood [1], [6], [7]. Human platelet lysate (hPL) is a cocktail of growth factors prepared from donated human platelets and has mainly been used in in vitro experiments as a supplement for cell culture [8], [9], [10], [11], [12].
Chronic skin wounds, such as diabetic ulcers, pressure ulcers, venous leg ulcers, currently represent a major healthcare burden for both professionals and patients. The long healing process is associated with high treatment costs, prolonged hospital stays, and affects the patient's quality of life. We are investigating the use of platelets and platelet-derived growth factors in the treatment chronic wounds, and have reported that PRP promotes the proliferation of human adipose-derived stem cells and human dermal fibroblasts [13], [14], and that PRP has an angiogenic effect on human umbilical vein endothelial cells (HUVECs) [15]. We also reported that we could preserve lyophilized autologous PRP by refrigeration at 4 °C and that refrigerated lyophilized PRP could be safely used in the clinical setting [16]. As for hPL, we applied a collagen/gelatin scaffold impregnated with various concentrations of lyophilized hPL from expired irradiated platelet concentrate to a murine wound healing model and showed its healing effects [9].
As mentioned above, in previous studies, we used a lyophilization technique to preserve PRP or regulated the concentrations of hPL, we have not evaluated the effective storage period, for which the bioactivity of hPL is maintained. The healing of chronic ulcers usually takes several months; thus, methods that can preserve PRP or hPL for more than several months, which also preserve its bioactivity, are required. For short-term preservation for periods of less than 1 month, bioactive substances can be preserved at 4 °C; however, for longer preservation, cryopreservation using liquid nitrogen, or at −20 °C or −80 °C using a deep freezer is recommended [17], [18]. On the other hand, it has been reported that lyophilized proteins can be stored at 4 °C or room temperature for longer periods of time [19], [20], [21]. In clinical practice, refrigeration at 4 °C would be desirable because a deep freezers are not installed in many hospitals and are associated with additional costs.
In the present study, we compared different methods for preserving hPL using a refrigerator or deep freezer. We set the preservation period of 9 months, the wound healing process for chronic wounds usually takes more than 6 months. We evaluated the bioactivity of preserved hPL in vitro and later in vivo using a murine wound healing model.
2. Materials and methods
2.1. Preparation of gelatin hydrogel (GH) sheets
We used gelatin hydrogel (GH) sheets in the animal experiments. GH sheets are bioabsorbable dressings that can sustain the release of bFGF (basic fibroblast growth factor) or PDGF-BB (platelet derived growth factor-BB) and we reported that GH sheets impregnated with PRPr (platelet-rich plasma releasate) accelerated murine wound healing [6]. GH sheets were prepared by the Department of Regeneration Science and Engineering Laboratory of Biomaterials, Institute for Frontier Medical Sciences, Kyoto University. In brief, acidic gelatin, with an isoelectric point of 5.0, isolated from bovine bone (Nitta Gelatin Co., Osaka, Japan) was used [6], [22]. Gelatin in 5% (w/v) aqueous solution was first cross-linked with glutaraldehyde at 4 °C for 12 h in polystyrene dishes. Then, the resulting hydrogel sheets were immersed in a glycine aqueous solution at 37 °C for 1 h to block the residual aldehyde. The hydrogel sheets were then rinsed with double-distilled water (DDW) then lyophilized. The dried hydrogels were sterilized with ethylene oxide gas.
2.2. Preparation and preservation of hPL
This study used 200 ml of expired irradiated platelet concentrate, leukocyte reduced (Ir-PC-LR) (Japanese Red Cross Society) that was supplied by Kansai Medical University Hospital at the expiration date. Our experiment protocol was approved by the ethics committee of Kansai Medical University (approval No. 1427).
At first, the Ir-PC-LR was activated with a freeze–thaw cycle at −80 °C and 37 °C, which was repeated three times. Then, activated platelet lysate was centrifuged at 3000 × g at 4 °C for 30 min to remove the platelet fragments. The supernatant was filtered through a 0.22-μm filter unit (Millex-GP; Merck Millipore Ltd., Bedford, MA, USA) and 2 U/ml of heparin sodium (5000 units/5 ml; Mochida Pharmaceutical Co., Tokyo, Japan) was added to prevent gelatinization. In the present study, this product was referred to as human platelet lysate (hPL) [8], [9], [10].
Next, we prepared three experimental groups. In the first group, hPL was divided into 3 ml aliquots and stored at −80 °C in a deep freezer for 9 months (C-hPL: the cryopreservation hPL group). In the second group, hPL cryopreserved for 9 months at −80 °C in a deep freezer then lyophilized using a lyophilize machine (FDU 2200; Tokyo Rikakikai Co, Ltd., Tokyo, Japan) according to the manufacturer's instructions and reconstituted to the original volume with saline (Fuso Pharmaceutical Industries, Ltd., Osaka, Japan) just before use (CL-hPL: the cryopreservation and lyophilization hPL group). In the third group, hPL was snap frozen in liquid nitrogen and lyophilized using FDU 2200 according to the manufacturer's instructions. Lyophilized hPL was refrigerated at 4 °C for 9 months and reconstituted to the original volume with saline just before the experiments (L-hPL: the lyophilization and refrigeration hPL group).
2.3. The quantitative analysis of the growth factors in preserved hPL
We evaluated the concentrations of three growth factors using an enzyme-linked immunosorbent assay (ELISA). We used a human PDGF-BB kit (DBB00), VEGF kit (DVE00) and TGF-β1 (DB100B) Quantikine ELISA kits (R&D Systems, Minneapolis, MN, USA). According to the manufacturer's instructions, for the measurement of PDGF-BB and VEGF samples require 20-fold dilution before use. For the measurement of TGF-β1, samples must be acid-activated by 1 N HCl and neutralized with 1.2 N NaOH/0.5 M HEPES before dilution. The activated sample is then diluted 400-fold before measurement.
Eight samples were obtained for the PDGF-BB, VEGF and TGF-β1 ELISA evaluations in each group. A total 40 wells was used for each growth factor, 16 wells (2 rows) were used for standard and 8 wells (1 row) for each group. Each sample was added to each well of the provided 96-well plates, which were coated with antibodies prior to use. They were then incubated, washed and the secondary antibody was added to the wells. After the addition of the substrate solution, the optical densities were evaluated at a test wavelength of 450 nm and a reference wavelength of 540 nm using an Enspire 2300 Multilabel Reader spectrophotometer (PerkinElmer Japan Co., Ltd., Yokohama, Japan) to evaluate the concentrations.
2.4. The evaluation of the bioactivity of preserved hPL
We evaluated the bioactivity of preserved hPL in three groups using a growth assay of fibroblasts. We prepared 1000 ml of DMEM medium (“Nissui”1; Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) supplemented with 3.5 g of D(+) Glucose, 1.8 mg of sodium bicarbonate (Wako Pure Chemical Industries Ltd., Osaka, Japan), 10,000 IU of penicillin and 10 mg of streptomycin (Penicillin-streptomycin; MP Biomedicals, LLC, Solon, OH, USA). We disseminated 1.0 × 104 human adult fibroblasts (HDFa; Gibco, Life Technologies Co., Ltd., Carlsbad, CA, USA) into each well of a 24-well plate (Iwaki, Asahi Glass Co., Ltd., Tokyo, Japan) and cultured them using 500 μl of DMEM with 10% FBS (Hyclone, Logan, UT, USA) overnight at 37 °C in a 5% CO2 atmosphere. The next day, we changed the medium to 500 μl of DMEM without FBS and incubated the plates overnight. We then changed the medium to DMEM, DMEM with 10% FBS, DMEM with 1% C-hPL, DMEM with 1% CL-hPL or DMEM with 1% L-hPL (n = 8 in each group) and incubated the plates for 48 h. Then, 50 μl of cell counting kit-8 (CCK-8) reagent (Dojindo Molecular Technologies, Kumamoto, Japan) was added to each well and the plate was incubated for 60 min. The absorbance was measured at 450 nm using an Enspire 2300 Multilabel Reader spectrophotometer (PerkinElmer Japan Co., Ltd., Yokohama, Japan).
2.5. Impregnation of GH disks with hPL
Supplied GH sheets were punched out using a 6-mm punch biopsy tool (Kai Industries Co., Ltd., Gifu, Japan) to prepare GH disks of 6 mm in diameter. The GH disks were then placed in 10-cm tissue culture dishes (Falcon, Corning Inc., Corning, NY, USA), and 100 μl of C-hPL or 100 μl of CL-hPL or 100 μl of L-hPL were dropped onto each disk (n = 21 in each group) using a micropipette and the disks were incubated overnight at 4 °C for impregnation [6], [9], [23], [24].
2.6. Animal wound models
Animal experiment on this study were performed according to protocols approved by the Animal Care and Use Committee of Kansai Medical University. Animals received humane care based on the “Guide for the Care and Use of Laboratory Animals, Eight Edition,” as published by National Research Council of the National Academies, 2011. The experiment protocol also approved by the ethics committee of Kansai Medical University with approval No. 1427. We used 8- to 9-week-old male C57bl6J/Jcl mice (n = 63) as a wound model to evaluate the effects of hPL in vivo. Prior to surgery, 5 animals were housed per cage in a temperature-controlled animal facility with a 12-h light/dark cycle. Mice were allowed to adapt in their environment for one week before the procedure. The animals had ad libitum access to food and water.
During the procedure, spontaneous breathing mice were anesthetized by isoflurane inhalation (Wako Pure Chemical Industries Ltd., Osaka, Japan) delivered by vaporizer (Forawick vaporizer; Muraco Medical Co., Ltd., Tokyo, Japan) at a standardized concentration to the outlet tube. Isoflurane was administered in a mixture with environmental air at a constant flow of 2 l/min. For the induction of anesthesia, animals were placed in a glass box (length, 22 cm; width, 9 cm; height, 11 cm), that was connected to an anesthetic gas mixture of 3% vaporized isoflurane for 45–60 s. As maintenance therapy, the concentration of isoflurane was reduced to 1.5%–2% to provide an appropriate depth of anesthesia during the surgical procedures.
Before the surgical procedure, the hair on the back of the mouse was shaved with an electric razor (Thrive; Daito Electric Machine Ind. Co., Ltd., Japan) and depilated with depilating cream (Kracie, Tokyo, Japan). A donut-shaped silicone skin splint (Fuji System Corp., Tokyo, Japan) of 16 mm in outer diameter, 10 mm in inner diameter, and 0.5 mm in thickness, was attached to the skin with binding adhesive (Aronalpha; Daiichi Sankyou, Osaka, Japan) and stitched with black nylon 5–0 (Bear Corporation, Osaka, Japan). This splinted wound model was used to prevent wound contraction and delay wound closure [6], [25]. Using a punch biopsy tool (Kai Industries Co., Ltd.) and Iris scissors, a full-thickness wound 6 mm in diameter including panniculus carnosus was made at the center of each splint. An GH disk from three groups was put on each wound (n = 21 in each group). The wound was covered by a semipermeable polyurethane film (Multifix Roll; Alcare Co., Ltd., Tokyo, Japan) and an elastic bandage (Elastopore, Nichiban Co., Ltd., Tokyo, Japan) to prevent contamination and damage to the wounds. After these procedures, the mice were placed in individual cages inside the institutional animal facility.
2.7. Wound healing evaluation
The mouse wound healing process was evaluated by gross photos and a histological assessment. Mice were sacrificed by carbon dioxide inhalation on days 4, 7, and 14 after surgery (n = 7 in each group). The dressings were removed and gross photos of the wound were taken with a digital camera (Olympus Tough TG-4; Olympus, Tokyo, Japan). Then, the wound specimens including the surrounding tissue were dissected and fixed in 10% formalin buffer solution (Wako Pure Chemical Industries, Ltd.) for histological staining. The paraffin sections were prepared at the center of each wound axially and hematoxylin and eosin (HE)-stained sections, Azan-stained sections, and anti-CD31-stained sections were prepared. As for the anti-CD31 staining, sections were deparaffinized and rehydrated, and then heat-induced antigen retrieval was performed in EDTA (Nichirei Biosciences Inc., Tokyo, Japan) at 98 °C for 20 min. After being cooled to room temperature, the sections were rinsed in distilled water and immersed in 130 mL methanol (CH3OH; Wako Pure Chemical Industries Ltd.) mixed with 4 mL hydrogen peroxide (H2O2; Wako Pure Chemical Industries Ltd.) for 10 min to block endogenous peroxidase activity. The sections were rinsed in TBST (Tris buffered saline with Tween-20; saline with Tween-20), and rabbit polyclonal antibody (Spring Bioscience, Fremont, CA, USA) at 1:200 was applied to sections and incubated at 4 °C overnight. The sections were then rinsed in TBST again, after which HRP-labeled secondary antibody (EnVision + System HRP-labeled polymer anti-rabbit; Dako Japan Co., Kyoto, Japan) was applied at room temperature for 30 min. The sections were rinsed in TBST again, exposed to DAB (3-30-diaminobenzidine tetrahydrochloride; Dako Japan Co., Ltd.), and counterstained with hematoxylin. After staining, histologic photographs were taken and analyzed using a Nanozoomer 2.0 HT whole-slide scanner with the NDP.view2 software program (Hamamatsu Photonics, Hamamatsu, Japan) at 40 × magnification.
2.8. Assessment of the wound area
The wound area was evaluated by tracing the wound margin on a digital photograph with the Image J 1.50i software program (National Institutes of Health, Bethesda, MD, USA). The wound area was evaluated on days 0, 4, 7 and 14. The remaining wound area (%) on days 4, 7 and 14 was compared to the original wound area on day 0 in this study.
2.9. Assessment of neoepithelialization
HE-stained sections were used to evaluate the neoepithelialization of each wound on days 4, 7, and 14. The length was defined as the sum of the lengths of a line traced along the epithelium from the nearest hair follicle to the forefront of the neoepithelium at both sides. It is usually difficult to define the wound margin on HE sections, so we used the remaining hair follicle on the marginal wound as the starting point of the neoepithelium. When wounds were completely epithelized, neoepithelialization was estimated by tracing the epithelium between the hair follicles of both sides.
2.10. Assessment of granulation tissue formation
Azan-stained sections were used for the evaluation of granulation tissue formation. On days 4, 7, and 14, the area of granulation tissue was tracked between the upper new epithelium and the lower muscle layer. Besides the area, the thickness of the granulation tissue was also evaluated. The thickness between the upper and lower border of the granulation tissue was evaluated at three points: the center of each wound and 500 μm from the wound center on both sides. The mean of these three measurements was used for the analysis.
2.11. Assessment of newly formed capillaries and the capillary area
Newly formed capillaries on days 4, 7, and 14 were evaluated using anti-CD31-stained sections. The numbers and the total area of newly formed capillaries with a clearly visible lumen and erythrocytes inside were counted manually using the NDP.view2 software program. The stained capillaries in three squares of 200 μm × 200 μm at the center and both marginal wounds in each section were counted and the sum was used as the result.
2.12. Statistical analysis
All data are presented as the mean ± standard deviation. P values of <0.05 were considered to indicate statistical significance. We used the Tukey–Kramer multiple comparisons test, which was performed using Prism 7.03, to analyze the data (2017; GraphPad Software, Inc., San Diego, CA, USA).
3. Results
3.1. The comparison of growth factors in the preserved hPL groups
The concentrations of PDGF-BB, VEGF, and TGF-β1 in three groups are measured by ELISA (Fig. 1A–C). The C-hPL group had significantly higher concentrations of these growth factors in comparison to the CL-hPL and L-hPL groups (p < 0.05). The concentration of PDGF-BB in the L-hPL group was significantly higher than that of the CL-hPL group (p < 0.05).
Fig. 1.
The comparison of the concentrations of (A) PDGF-BB (B) VEGF and (C) TGF-β1 in the three groups. *p < 0.05.
3.2. The evaluation of bioactivity in the preserved hPL groups
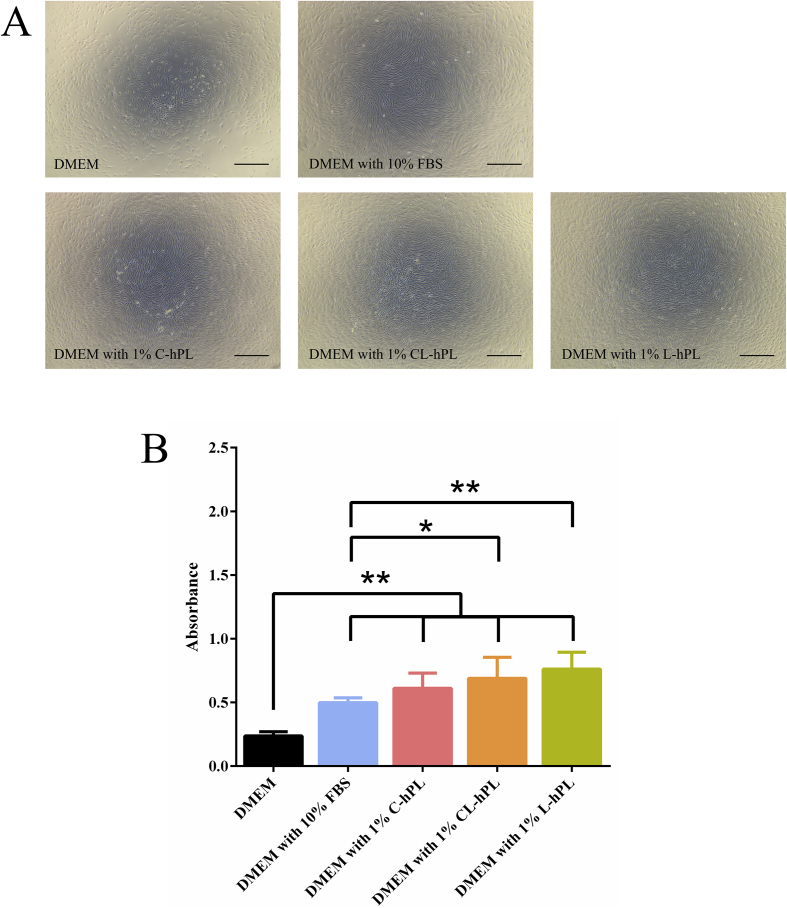
Microscopic view of the proliferation of fibroblasts after culturing for 48 h in all groups were taken (Fig. 2A). The DMEM with 10% FBS, DMEM with 1% C-hPL, DMEM with 1% CL-hPL, and DMEM with 1% L-hPL groups showed significantly higher proliferation in comparison to the DMEM group (p < 0.01). In addition, the DMEM with 1% CL-hPL group (p < 0.05) and DMEM with 1% L-hPL group (p < 0.01) showed higher proliferation in comparison to the DMEM with 10% FBS group (Fig. 2B).
Fig. 2.
(A) Fibroblast cell proliferation after 48 h of treatment with DMEM, DMEM with 10% FBS, DMEM with 1% C-hPL, or CL-hPL or L-hPL. The three hPL Groups showed higher proliferation in comparison to the DMEM and DMEM with FBS groups. Scale bar: 500 μm, Original magnification: 40 × . (B) The comparison of the proliferation in DMEM, DMEM with 10% FBS and DMEM with 1% preserved hPL groups. *p < 0.05; **p < 0.01.
3.3. Assessment of the wound area
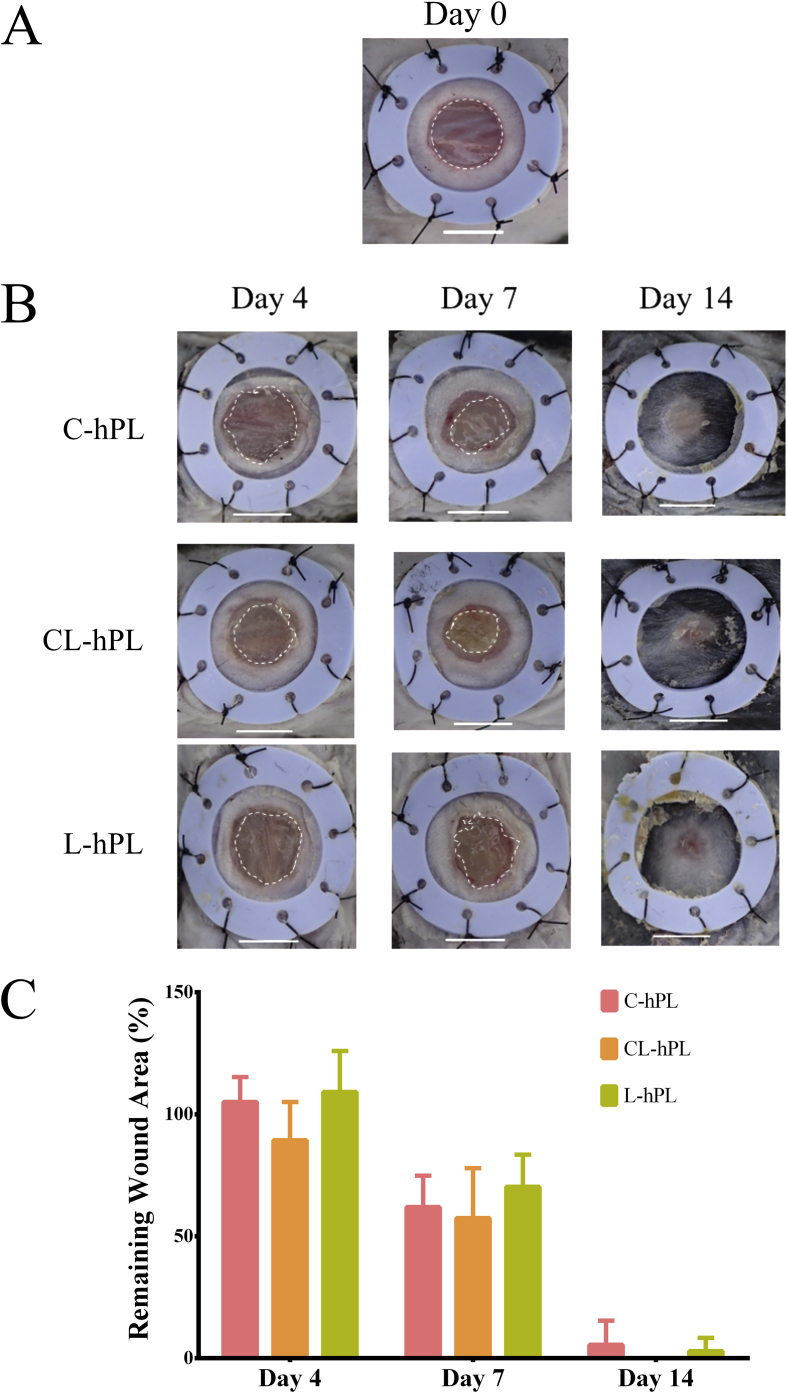
The original wound area was 6 mm in diameter on the day of surgery (Fig. 3A). Gross photos of all three groups on days 4, 7, and day 14 were taken. The remaining GH disks on the wounds were observed on days 4 and 7. The wounds were mostly healed by day 14 in all groups (Fig. 3B). No significant differences were observed in these three groups (Fig. 3C).
Fig. 3.
(A) A macroscopic view of a full-thickness wound of 6 mm in diameter on the day of surgery. The wound margin is indicated by the white broken line. Scale bar: 5 mm. (B) A macroscopic view of the wound healing process on days 4, 7 and 14 in the C-hPL, CL-hPL and L-hPL groups. Scale bar: 5 mm. (C) The comparison of the remaining wound area on days 4, 7 and 14.
3.4. The histological assessment of neoepithelialization
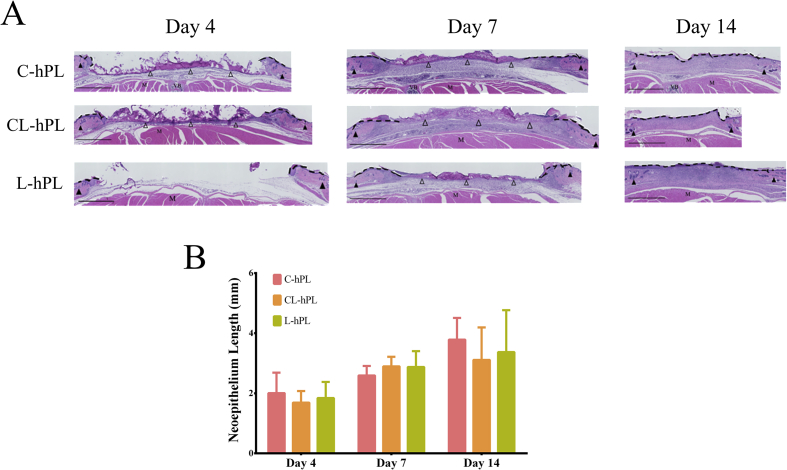
Micrographs of HE-stained sections were evaluated. The neoepithelium formed along the wound surface from the wound margin in all groups. The length of the neoepithelium on days 4, 7 and 14 was similar in all groups. The remaining GH disc was seen in all groups on days 4 and 7. On day 14, the GH disc had degraded in all groups (Fig. 4A). A statistical evaluation showed that the length of the epithelium did not differ among the groups to a statistically significant extent at any of the time points (Fig. 4B).
Fig. 4.
(A) Micrographs of HE-stained sections on days 4, 7 and 14. Neoepithelialization was measured above the muscle layer, between hair follicles. Black arrowheads indicate the hair follicles of the original wound margin. The black broken line indicates the evaluated neoepithelium. Open arrowheads indicate the remaining GH disks. M: muscle, VB: vertebral bone. Scale bar: 1 mm. (B) The comparison of the length of newly formed epithelium. Data presented in millimeters (mm). There were no significant differences among the groups.
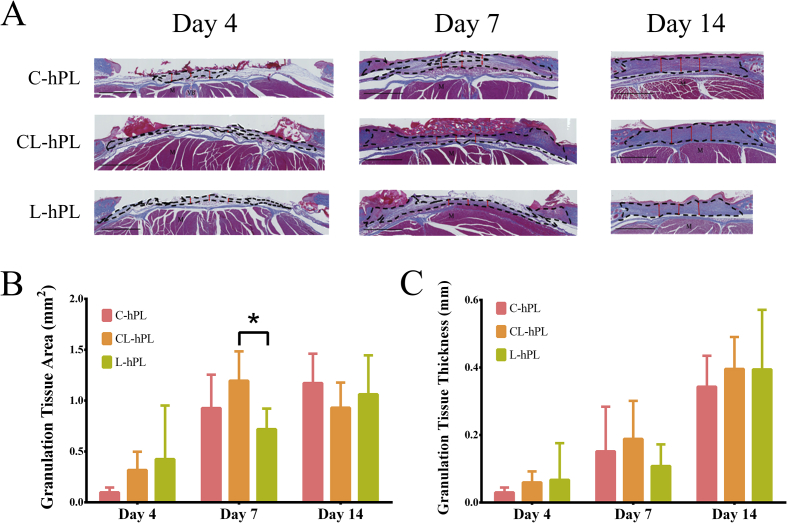
3.5. Histological assessment of granulation tissue
Micrographs of Azan-stained sections were used to evaluate the area and thickness of the granulation tissue that was stained in blue (Fig. 5A). In Azan-stained sections, the remaining GH disc was stained in red. The comparison of the area of the granulation tissue that had formed showed that the granulation area in the CL-hPL group was significantly larger than that in the L-hPL group on day 7 (p < 0.05); there were no significant differences among the groups on other days (Fig. 5B). The granulation tissue thickness did not differ among the groups at any of the time-points (Fig. 5C).
Fig. 5.
(A) Micrographs of Azan-stained sections of wounds on days 4, 7 and 14. Granulation tissue was stained in blue above the muscle layer. The black broken line indicates the formed granulation tissue. The red double-headed arrow indicates the evaluated thickness of the formed granulation tissue. M: muscle, VB: vertebral bone. Scale bar: 1 mm. (B) The comparison of the area of formed granulation tissue on days 4, 7 and 14. The granulation tissue of the CL-hPL group was significantly larger than that of the L-hPL group on day 7 (p < 0.05). *p < 0.05. (C) The comparison of the thickness of the formed granulation tissue on days 4, 7 and 14. The thickness of granulation tissue did not differ among the groups.
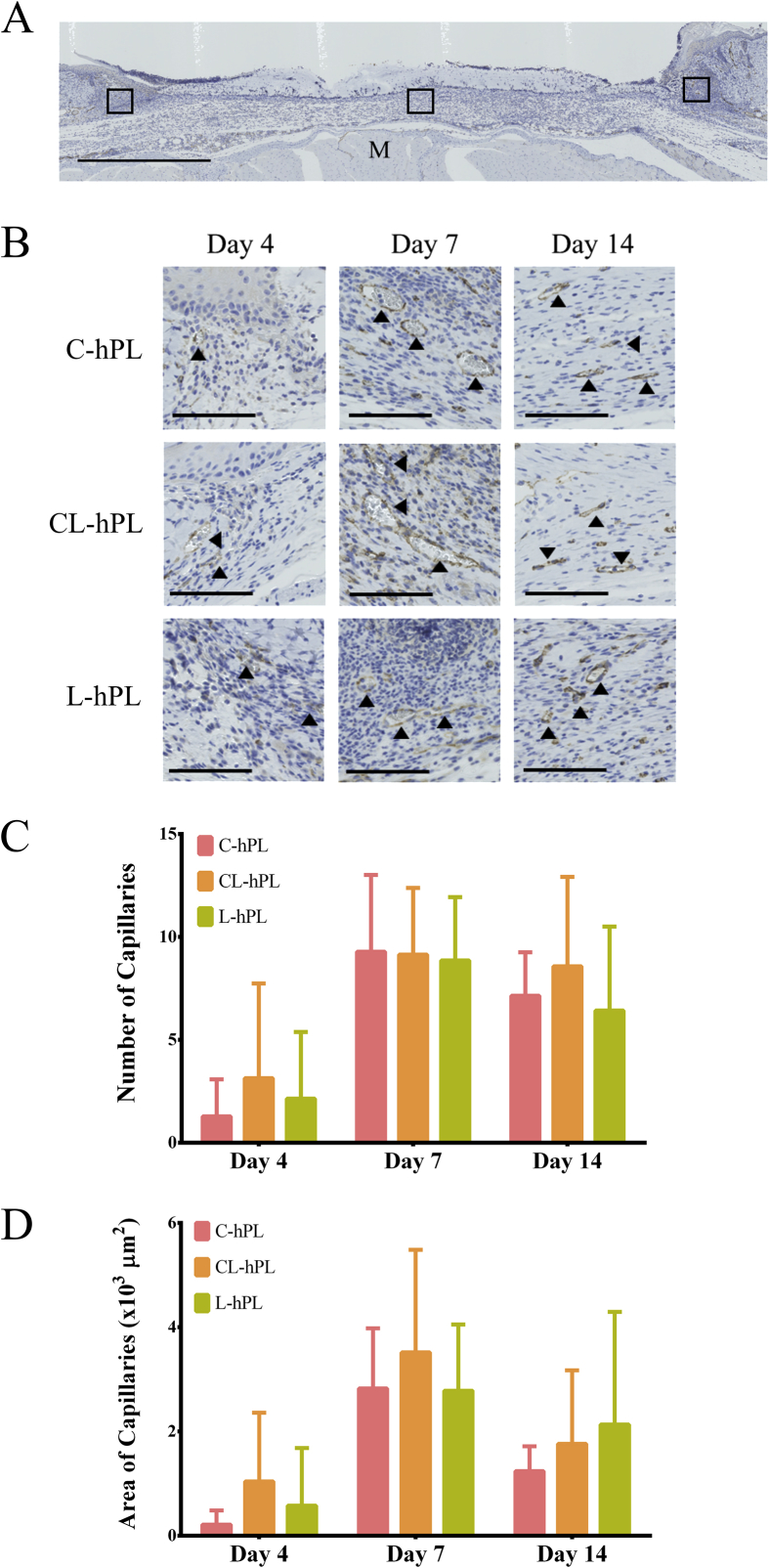
3.6. Histological assessment of newly formed capillaries
The number and area of the newly formed capillaries detected in three squares of 200 μm × 200 μm at the center and both marginal wounds in each section were counted and the sum was used for the analysis (Fig. 6A). Micrographs of anti-CD31-stained sections are shown in Fig. 6B. Newly formed capillaries were observed starting from day 4 in all groups. The peak of the neovascularization process was seen on day 7 in all groups (Fig. 6B). There were no differences among the groups with regard to the number or area of capillaries on days 4, 7 and 14 of wound healing (Fig. 6C–D).
Fig. 6.
(A) The newly formed capillaries were counted in three squares of 200 μm × 200 μm at the center and both marginal wounds in each anti-CD31-stained section. M: muscle. Scale bar: 1 mm. (B) Micrographs of anti-CD31-stained sections of formed granulation tissue on days 4, 7 and 14 from each group. Black arrowheads indicate stained capillaries. Scale bar: 100 μm. (C) The comparison of the numbers of newly formed capillaries on days 4, 7 and 14. (D) The comparison of the area of newly formed capillaries on days 4, 7 and 14.
4. Discussion
In the present study, we compared the bioactivity of hPL in vitro and its wound healing effects in vivo after three different preservation methods using a refrigerator or deep freezer for 9 months.
With regard to the bioactivity in vitro, the concentrations of PDGF-BB, VEGF, and TGF-β1 in the C-hPL group were higher than those in the groups in which lyophilization was performed (CL-hPL and L-hPL). The decrease in growth factors in the CL-hPL and L-hPL groups probably occurred due to the loss of hPL in the lyophilization technique. In this process, hPL was powderized, so it was difficult to collect the powder completely. However, the bioactivity of the lyophilized hPL in the CL-hPL and L-hPL groups was sufficient for the proliferation of fibroblasts and the proliferation was superior to that observed with FBS. This suggests that hPL that had been refrigerated and lyophilized for 9 months (L-hPL group) maintained the same degree of bioactivity as cryopreserved hPL (C-hPL group).
With regard to the wound healing effect, we used GH sheets that could sustain positively charged growth factors in hPL because we could compare the healing effects in each group using a single dose method. The multiple administration of hPL might be affected by the method of preservation during an animal study. Our results showed no significant difference in the healing effects (epithelialization, granulation tissue formation and capillary formation) among the three groups. This suggests the in vivo bioactivity of hPL that had been refrigerated lyophilized for 9 months (L-hPL group) was maintained at a level that was similar to that observed in cryopreserved hPL (C-hPL group).
According to a study by Lai et al. [26], the proliferative effect of platelet-derivate growth factor cocktail is mainly from PDGF-BB. The concentrations of PDGF-BB in the C-hPL, CL-hPL and L-hPL groups were 18,363 ± 370 pg/ml, 11,325 ± 171 pg/ml and 12,307 ± 348 pg/ml respectively. Ito et al. [9] reported that the concentration of PDGF-BB was 10,000 pg/ml, and our other study [27] demonstrated that it was 17,432 ± 338 pg/ml. The concentration of PDGF-BB in this study is in the range of the other two studies, suggesting that the hPL used in this study was sufficient to promote wound healing. The temperature recommended for the long-term preservation of growth factors in liquid form is below −20 °C. The other alternative is to make it into a powder form and preserve it at 4 °C. The lyophilization process is a well-known method for turning a solid product into a powder form, after which it becomes more stable [18], [19], [20], [21]. With regard to the preservation period, Muraglia A et al. reported that they could preserve lyophilized platelet-rich plasma for up to 6 months [28]. In the present study, we could preserve the bioactivity of refrigerated lyophilized hPL for 9 months. The ability to preserve a lyophilized product at 4 °C would be desirable in clinical practice, because the cost of preserving lyophilized hPL is cheaper in comparison to cryopreservation using a deep freezer. Although it is difficult to prepare lyophilized hPL in many hospitals, lyophilized hPL can be transported more easily than cryopreserved hPL; thus, a processing facility could support this method of preservation.
Irradiated platelet concentrate, leukocyte reduced (Ir-PC-LR) provided by Japanese Red Cross Society was used in this study as the standardized platelet source for making hPL. Regarding the dispersion of each lot, the Japanese Red Cross Society measured several indicators, including the platelet count, pH level, lactate dehydrogenase percentage and hypotonic shock response of Ir-PC-LR on the day blood being drawn, and 3, 4, and 5 days later, and proved that these indicators were stable and similar in every Ir-PC-LR bag; thus, Ir-PC-LR could be the ideal source of platelets for regenerative therapy, including wound healing. In Japan, Ir-PC-LR is only allowed for use for transfusion, and not for other clinical treatments, such as regenerative therapy. However, we can purchase platelet concentrate for use in regenerative therapy from foreign companies in the future.
As we showed in the present study, the lyophilization of hPL was a preferable preservation method in terms of maintaining its bioactivity. In addition, we can manage the concentration of growth factors in hPL in the reconstitution process [9]. In summary, the lyophilization of hPL is a superior method for long-term preservation and would be a versatile method in clinical practice.
5. Conclusions
The quality of human platelet lysate that had been lyophilized and refrigerated at 4 °C was preserved for up to 9 months. This method may be useful for preserving human platelet lysate for use in wound treatment in the clinical setting.
Author contributions
N.M. and N.K. designed the research. N.M. and Y.T. prepared the materials. S.C.N. performed the in vitro experiments. S.C.N., T.M. and T.M.L. performed animal experiments. S.C.N., N.K. and K.K. analyzed the data. S.C.N., N.M. and K.K. wrote the paper. N.M. is the grant recipient.
Acknowledgment
This work was supported by JSPS (Japan Society for the Promotion of Science) KAKENHI (Grant-in-Aid for Scientific Research B) with Grant Number 24390399.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Hokugo A., Ozeki M., Kawakami O., Sugimoto K., Mushimoto K., Morita S. Augmented bone regeneration activity of platelet-rich plasma by biodegradable gelatin hydrogel. Tissue Eng. 2005;11:1224–1233. doi: 10.1089/ten.2005.11.1224. [DOI] [PubMed] [Google Scholar]
- 2.Pietramaggiori G., Kaipainen A., Czeczuga J.M., Wagner C.T., Orgill D.P. Freeze-dried platelet-rich plasma shows beneficial healing properties in chronic wounds. Wound Repair Regen. 2006;14(5):573–580. doi: 10.1111/j.1743-6109.2006.00164.x. [DOI] [PubMed] [Google Scholar]
- 3.Gurtner G.C. Wound healing: normal and abnormal. In: Thorne C.H., Beasley R.W., Aston S.J., Bartlett S.P., Gurtner G.C., Spear S.L., editors. Vol. 6. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 15–22. (Grabb and Smith's plastic surgery). [Google Scholar]
- 4.Chiara Barsotti M., Losi P., Briganti E., Sanguinetti E., Magera A., Al Kayal T. Effect of platelet lysate on human cells involved in different phases of wound healing. PLoS ONE. 2013;8(12) doi: 10.1371/journal.pone.0084753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranzato E., Mazzucco L., Patrone M., Burlando B. Platelet lysate promotes in vitro wound scratch closure of human dermal fibroblasts: different roles of cell calcium, P38, ERK and PI3K/AKT. J Cell Mol Med. 2009;13(8B):2030–2038. doi: 10.1111/j.1582-4934.2008.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Notodihardjo P.V., Morimoto N., Kakudo N., Matsui M., Sakamoto M., Liem P.H. Gelatin hydrogel impregnated with platelet-rich plasma releasate promotes angiogenesis and wound healing in murine model. J Artif Organs. 2015;18(1):64–71. doi: 10.1007/s10047-014-0795-8. [DOI] [PubMed] [Google Scholar]
- 7.Matsui M., Tabata Y. Enhanced angiogenesis by multiple release of platelet-rich plasma contents and basic fibroblast growth factor from gelatin hydrogels. Acta Biomater. 2012;8:1792–1801. doi: 10.1016/j.actbio.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Jonsdottir-Buch S.M., Lieder R., Sigurjonsson O.E. Platelet lysates produced from expired platelet concentrates support growth and osteogenic differentiation of mesenchymal stem cells. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0068984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito R., Morimoto N., Pham L.H., Taira T., Kawai K., Suzuki S. Efficacy of the controlled release of concentrated platelet lysate from a collagen/gelatin scaffold for dermis-like tissue regeneration. Tissue Eng. 2013;19:1398–1405. doi: 10.1089/ten.TEA.2012.0375. [DOI] [PubMed] [Google Scholar]
- 10.Strandberg G., Sellberg F., Sommar P., Ronaghi M., Lubenow N., Knutson F. Standardizing the freeze-thaw preparation of growth factors from platelet lysate. Transfusion. 2017;57:1058–1065. doi: 10.1111/trf.13998. [DOI] [PubMed] [Google Scholar]
- 11.Fortunato T.M., Beltrami C., Emanueli C., De Bank P.A., Pula G. Platelet lysate gel and endothelial progenitors stimulate microvascular network formation in vitro: tissue engineering implications. Sci Rep. 2016;6:25326. doi: 10.1038/srep25326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gstraunthaler G., Rauch C., Feifel E., Lindl T. Preparation of platelet lysates for mesenchymal stem cell culture media. J Stem Cell Res Rev Rep. 2015;2(1):1021. [Google Scholar]
- 13.Kakudo N., Minakata T., Mitsui T., Kushida S., Notodihardjo F.Z., Kusumoto K. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg. 2008;122(5):1352–1360. doi: 10.1097/PRS.0b013e3181882046. [DOI] [PubMed] [Google Scholar]
- 14.Hara T., Kakudo N., Morimoto N., Ogawa T., Lai F., Kusumoto K. Platelet-rich plasma stimulates human dermal fibroblast proliferation via a Ras-dependent extracellular signal-regulated kinase 1/2 pathway. J Artif Organs. 2016;19(4):372–377. doi: 10.1007/s10047-016-0913-x. [DOI] [PubMed] [Google Scholar]
- 15.Kakudo N., Morimoto N., Kushida S., Ogawa T., Kusumoto K. Platelet-rich plasma releasate promotes angiogenesis in vitro and in vivo. Med Mol Morphol. 2014;47(2):83–89. doi: 10.1007/s00795-013-0045-9. [DOI] [PubMed] [Google Scholar]
- 16.Morimoto N., Kakudo N., Ogura T., Hara T., Matsui M., Yamamoto M. Easy-to-use preservation and application of platelet-rich plasma in combination wound therapy with a gelatin sheet and freeze-dried platelet-rich plasma: a case report. Eplasty. 2016;16:202–207. [PMC free article] [PubMed] [Google Scholar]
- 17.Altaie A., Owston H., Jones E. Use of platelet lysate for bone regeneration-are we ready for clinical translation? World J Stem Cell. 2016;8(2):47–55. doi: 10.4252/wjsc.v8.i2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramirez-Santana C., Perez-Cano F.J., Audi C., Castell M., Moretones M.G., Lopez-Sabater M.C. Effects of cooling and freezing storage on the stability of bioactive factors in human colostrum. J Dairy Sci. 2012;95:2319–2325. doi: 10.3168/jds.2011-5066. [DOI] [PubMed] [Google Scholar]
- 19.Arakawa T., Prestrelski S.J., Kenney W.C., Carpenter J.F. Factors affecting short-term and long-term stabilities of proteins. Adv Drug Deliv Rev. 1993;10:1–28. doi: 10.1016/s0169-409x(00)00144-7. [DOI] [PubMed] [Google Scholar]
- 20.Pikal M.J. Mechanism of protein stabilization during freeze-drying storage: the relative importance of thermodynamic stabilization and glassy state relaxation dynamics. In: Rey L., May J.C., editors. Freeze-drying/lyophilization of pharmaceutical and biological products. Informa Healthcare; New York: 2010. pp. 198–232. [Google Scholar]
- 21.Nireesha G.R., Divya L., Sowmya C., Venkateshan N., Niranja Babu M., Lavakumar V. Lyophilization/freeze drying - a review. IJNTPS. 2013;3(4):87–98. [Google Scholar]
- 22.Tabata Y., Nagano A., Ikada Y. Biodegradation of hydrogel Carrier incorporating fibroblast growth factor. Tissue Eng. 1999;5:127–138. doi: 10.1089/ten.1999.5.127. [DOI] [PubMed] [Google Scholar]
- 23.Tabata Y., Ikada Y. Protein release from gelatin matrices. Adv Drug Deliv Rev. 1998;31:287–301. doi: 10.1016/s0169-409x(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto M., Ikada Y., Tabata Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J Biomater Sci Polym Ed. 2001;12(1):77–88. doi: 10.1163/156856201744461. [DOI] [PubMed] [Google Scholar]
- 25.Galiano R.D., Michaels J., Dobryansky M., Levine J.P., Gurtner G.C. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12:485–492. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 26.Lai F., Kakudo N., Morimoto N., Taketani S., Hara T., Ogawa T. Platelet-rich plasma enhances the proliferation of human adipose stem cells through multiple signaling pathways. Stem Cell Res Ther. 2018;9:107. doi: 10.1186/s13287-018-0851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Notodihardjo S.C., Morimoto N., Kakudo N., Mitsui T., Le T.M., Tabata Y. Efficacy of gelatin hydrogel impregnated with concentrated platelet lysate in murine wound healing. J Surg Res. 2019;234:190–201. doi: 10.1016/j.jss.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 28.Muraglia A., Ottonello C., Spano R., Dozin B., Strada P., Grandizio M. Biological activity of a standardized freeze-dried platelet derivative to be used as cell culture medium supplement. Platelets. 2013;25(3):1–10. doi: 10.3109/09537104.2013.803529. [DOI] [PubMed] [Google Scholar]