Abstract
Rheumatoid arthritis-related interstitial pneumonia with a usual interstitial pneumonia (RA-UIP) has a poor prognosis and a new treatment strategy is required. The antifibrotic agent nintedanib reduces the annual rate of decline in forced vital capacity (FVC) in idiopathic pulmonary fibrosis (IPF) patients. Recently, the potential efficacy of antifibrotic agents against chronic progressive fibrotic diseases including RA-UIP has been attracting attention.
A 74-year-old man diagnosed with IPF on high-resolution computed tomography (HRCT). His FVC was decreasing over time, and his exertional dyspnea and cough had progressed with progression of reticulation on imaging. He was treated with nintedanib, which resulted in decreased coughing together with a reduction in FVC decline, from −11.6%/year to −5.2%/year. A swollen joint appeared eight months after this intervention, and he was diagnosed with rheumatoid arthritis.
In this patient, nintedanib was effective against RA-UIP. This is the first case in which nintedanib was shown to be effective for RA-UIP.
1. Background
Rheumatoid arthritis-related interstitial pneumonia (RA-IP) has been investigated by applying the pathological classifications of idiopathic interstitial pneumonias (IIPs), and the histologic epidemiology and treatment outcomes have been intensively studied. The main treatment for RA-IP in the chronic phase is usually anti-inflammatory therapy, using corticosteroids or immunosuppressants. Rheumatoid arthritis-related interstitial pneumonia with a usual interstitial pneumonia pattern (RA-UIP) is considered to have a better prognosis than idiopathic UIP, idiopathic pulmonary fibrosis (IPF). On the other hand, RA-UIP shows poorer prognosis compared to RA-non UIP [1]; therefore, the development of a new treatment strategy for RA-UIP is required.
The antifibrotic agent nintedanib is a low molecular weight triple tyrosine kinase inhibitor [2]. It inhibits signal transmission at vascular endothelial growth factor (VEGF) receptors, platelet-derived growth factor (PDGF) receptors, and fibroblast growth factor (FGF) receptors associated with proliferation, migration, and transformation of fibroblasts involved in the pathology of IPF. In the INPULSIS trials, international phase III clinical trials of IPF patients, nintedanib was shown to significantly reduce the annual rate of decline in FVC [3].
Today, there is an expectation that antifibrotic agents will be effective for chronic progressive fibrotic diseases including RA-UIP.
We experienced a case of usual interstitial pneumonia (UIP) preceding rheumatoid arthritis (RA) with honeycombing on imaging that was successfully treated with nintedanib.
2. Case report
A 74-year-old man was referred to our hospital with abnormal chest X-ray findings in February 2015. He complained of dry cough, dyspnea on exertion (DOE) of modified Medical Research Council (mMRC) grade 1, and intermittent arthralgia. Initial significant findings were fine crackles in bilateral lower lung fields and tenderness of the metacarpophalangeal joint of the left second digit. Initial laboratory findings were as follows: rheumatoid factor 7.2 IU/ml, anti-cyclic citrullinated peptide antigen 818.9 IU/mL, and erythrocyte sedimentation rate (1 hour) 92 mm. Initial high-resolution computed tomography (HRCT) images showed typical UIP pattern with basal and subpleural lung dominant reticular opacities, interlobular septal thickness associated with honeycombing and traction bronchiectasis. Since the morphologic pattern showed UIP with no apparent cause, the diagnosis was IPF.
His symptoms gradually worsened over 2 years until January 2017; his dry cough increased, and DOE gradually worsened to mMRC grade 2. Chest HRCT images showed extended reticulation (Fig. 1B).
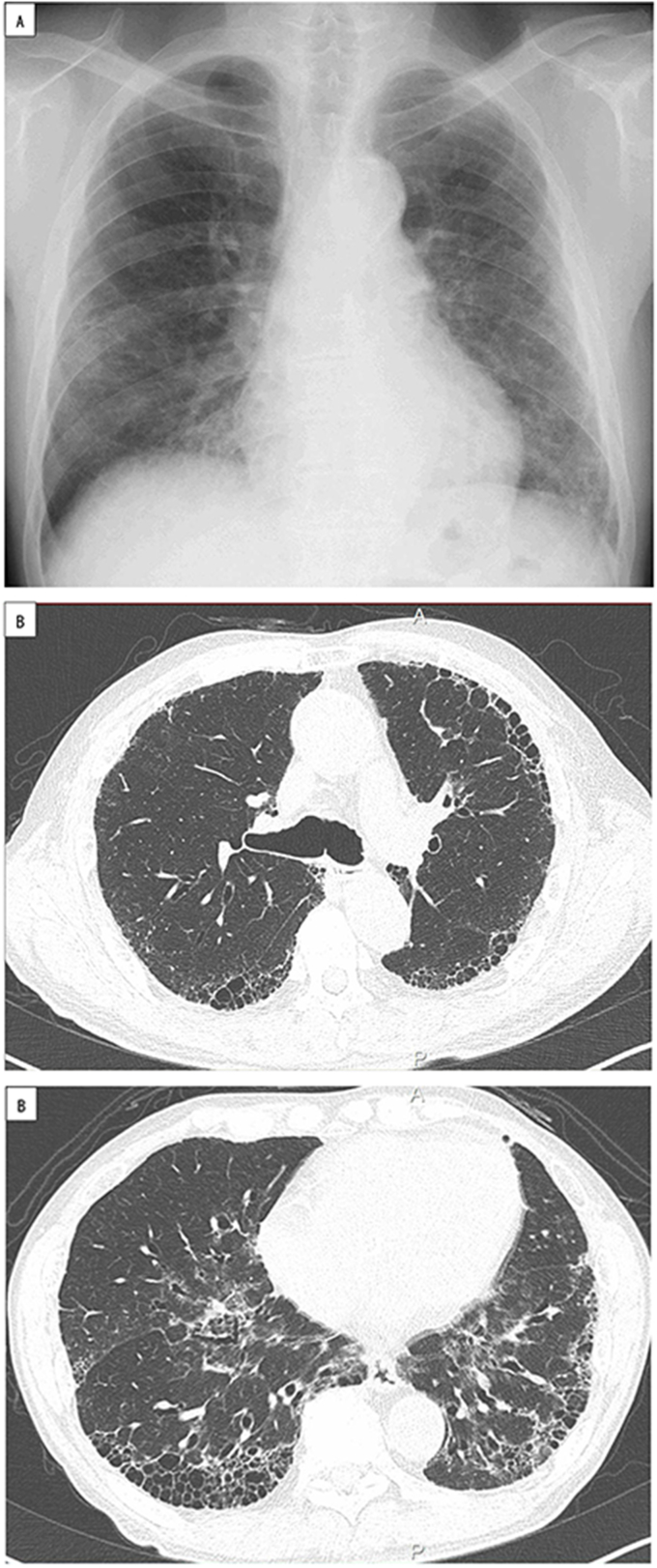
Fig. 1.
A. Chest X-ray. Reticular shadows are seen with bilateral lung base and subpleural predominance in February 2017. Decreased lung volume is also seen.
B. Chest HRCT. Reticular shadows with bilateral lung base and subpleural predominance, interlobular septal thickening, and traction bronchiectasis. Honeycombing is also seen in February 2017. Decreased lung volume in the lower lobes is seen from the resultant contractile changes.
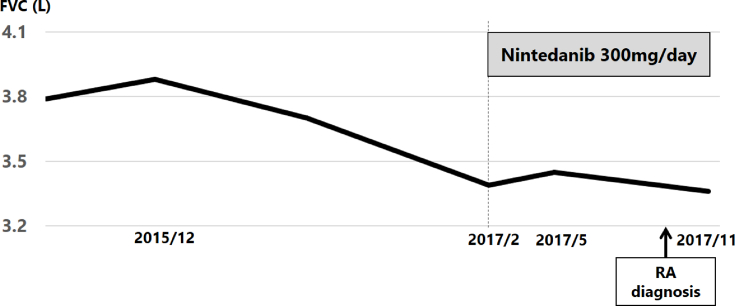
Pulmonary function test findings, especially FVC, also declined (Fig. 2). In February 2017, bronchoalveolar lavage (BAL) and transbronchial lung biopsy (TBLB) were performed. BALF cytology showed total cell counts of 2.71 × 105/mL, lymphocytes of 12%, and a CD4/CD8 ratio of 0.4. Histopathology showed old fibrosis and aggregated alveolar walls accompanying fibroblastic foci. At that time he had no arthralgia. Treatment was initiated with nintedanib 300 mg/day. It was generally well tolerated except for slight diarrhea. His dry cough improved gradually and diminished 8 months after he started treatment. Pulmonary function testing also improved; the gradual FVC decline in February recovered 60 mL 3 months after treatment initiation, followed by milder decline. FVC decreased by 490 ml 13 months after the initial visit (−12.6%/13 months) and by 90 ml after 6 months of treatment (−2.6%/6 months) (Fig. 2).
Fig. 2.
Treatment course: FVC is 3880 ml in December 2015 and decreases to 3390 ml in February 2017. Administration of nintedanib starts in February 2017. In May 2017, FVC has risen to 3450 ml, and in November 2017, it has decreased to 3360 ml.
In October 2017, his metacarpophalangeal joint of the left second digit developed swelling. At this time, he met the criteria of RA with a DAS 28 score of 3.61, showing moderate disease activity.
3. Discussion
RA-IP is generally treated using corticosteroids or other anti-inflammatory drugs, and treatment outcomes are good compared with IIPs, especially IPF. In RA-IP, however, cases that present a UIP pattern on histological classification based on imaging have a poor prognosis, and new treatment strategies are needed [1].
Nintedanib shows an antifibrotic effect by inhibiting the transmission of signals that promote proliferation and migration of fibroblasts in IPF patients. In the INPULSIS trials, it significantly reduced the annual rate of decline in FVC [3].
Among collagen disease patients, there is a group that presents with only interstitial pneumonia as an early sign [4]. Those in this group are described as having interstitial pneumonia preceding CVD. The interstitial pneumonia in this group is diagnosed as IIPs during the time until a definitive diagnosis of collagen disease is made with the appearance of other organ symptoms. Thus, it is difficult to differentiate between interstitial pneumonia preceding CVD and IIPs, and diagnostic methods, classification, and treatment methods for interstitial pneumonia preceding CVD have yet to be established.
In the criteria for inclusion in the INPULSIS trials, pathohistological diagnosis and a diagnosis of honeycomb lung on images were not always necessary. Thus, it is possible that a certain percentage of patients with interstitial pneumonia of known causes who present with a UIP pattern on evaluation of HRCT images are included. In particular, there is a possibility of including interstitial pneumonia preceding CVD under the clinical diagnosis of IPF, and nintedanib may also be effective in these interstitial pneumonia cases. In fact, nintedanib reduced the decline in FVC in the present patient, who had UIP preceding RA.
Nintedanib may be effective not only for IPF, but also for RA-UIP. In basic research, administration of nintedanib to a murine model of RA-IP was reported to inhibit the increase of collagen fibers in the lungs [5]. Future studies, including clinical trials, are awaited on the clinical efficacy of nintedanib on interstitial pneumonia other than IPF.
In this case, honeycomb lung was seen on imaging, and nintedanib was effective for UIP preceding RA. To the best of our knowledge, this is the first reported case in which nintedanib was shown to be effective for RA-UIP.
Declarations of interest
None.
References
- 1.Kim E.J., Elicker B.M., Maldonado F., Webb W.R. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur. Respir. J. 2010;35:1322–1328. doi: 10.1183/09031936.00092309. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhay N.I., Roth G.J., Hillberg F., Muller-Quernheim J. Inhibition of PDGF VEGF and FGF signaling attenuates fibrosis. Eur. Respir. J. 2007;29:976–985. doi: 10.1183/09031936.00152106. [DOI] [PubMed] [Google Scholar]
- 3.Richeldi L., du Bois R., Raghu G. Efficacy and safety of nintedanib in ibiopathic pulmonary fibrois. N. Engl. J. Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 4.Kono M., Nakamura Y., Enomoto N. Usual interstitial pneumonia preceding collagen vascular disease: a retrospective case control study of patients initially diagnosed with idiopathic pulmonary fibrosis. PloS One. 2014;9:1–10. doi: 10.1371/journal.pone.0094775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redente E.F., Aguilar M.A., Black B.P. Nintedanib reduces pulmonary fibrosis in a model of rheumatoid arthritis-associated interstitial lung disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2018;318:L998–L1009. doi: 10.1152/ajplung.00304.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]