Abstract
The co-presence of malignant pleural mesothelioma (MPM) and lung cancer is rare. We report a 70-year-old male with exposure to asbestos. Chest computed tomography revealed a right mediastinal mass combined with an enlarged ipsilateral lymph node and left pleural effusion. Transbronchial lung biopsy revealed lung adenocarcinoma. Thoracoscopic examination revealed multiple left pleural nodules, leading to the diagnosis of MPM. Despite aggressive anticancer drug therapy, he expired due to disease progression 2.5 years after diagnosis. Autopsy confirmed an epithelioid MPM in the left pleura. MPM comorbidity in patients diagnosed with lung cancer should be considered, especially in those exposed to asbestos.
Keywords: Malignant pleural mesothelioma, Lung cancer, Multiple primary cancer, Simultaneous, Autopsy
1. Introduction
Malignant pleural mesothelioma (MPM) is a relatively rare tumor closely related to asbestos exposure. In contrast, in the past decades, lung cancer has become the leading cause of death due to cancer in males worldwide. (Fitzmaurice C et al. ahead of print). Asbestos exposure is recognized as a risk factor for the development of both MPM and lung cancer [[1], [2], [3]]. However, the co-presence of MPM and lung cancer is rare [[2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17]]. Herein, we report a rare case of simultaneous presence of lung adenocarcinoma and MPM.
2. Case report
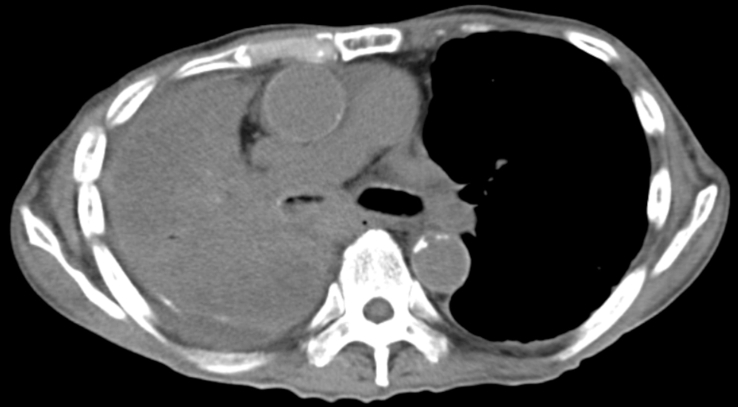
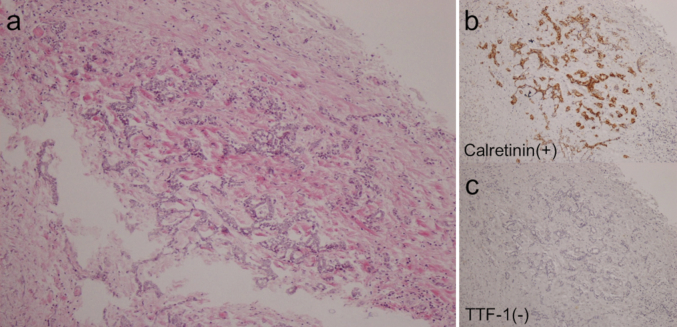
During a regular check-up, a chest X-ray revealed left pleural effusion in a 70-year-old male with chronic obstructive pulmonary disease. The patient had a history of smoking (65 packs per year) and exposure to asbestos. Chest computed tomography (CT) revealed the presence of a right mediastinal mass combined with an enlarged ipsilateral lymph node and left pleural effusion (Fig. 1). Positron emission tomography/CT using 18F-fluorodeoxyglucose (FDG) showed increased uptake in the right mediastinal mass (maximum standard uptake value: 7.31). However, it did not reveal thickening or increased uptake in the pleura. Fiberoptic bronchoscopy showed the presence of a submucosal tumor in the right main bronchus, and histopathological examination confirmed the type of the tumor as adenocarcinoma. The tumor cells were immunoreactive to adenocarcinoma markers, such as thyroid transcription factor-1 (TTF-1) and carcinoembryonic antigen (CEA). However, they were not immunoreactive to mesothelial markers, such as podoplanin (D2-40) and calretinin. Based on these findings, the patient was diagnosed with lung adenocarcinoma. Subsequently, a left thoracentesis was performed. The pleural fluid was bloody and showed high levels of hyaluronic acid (HA) (>80,000 ng/mL), cytokeratin 19 fragment (CYFRA) (466.9 ng/mL), and tissue polypeptide antigen (TPA) (7636 U/L), and low levels of CEA (1.2 ng/mL). The smear of the pleural fluid showed atypical cells forming papillary arrangements with hyperchromasia, indicative of adenocarcinoma or mesothelioma. Considering these findings and the history of asbestos exposure, it was suspected that the left pleural effusion was caused by the presence of an MPM. Thoracoscopic examination revealed the presence of multiple white small nodules on the left precordial parietal pleura and left diaphragm, and a coral-shaped nodule on the diaphragm (Fig. 2). Histopathologically, epithelioid atypical cells with anisokaryosis formed papillary arrangements. Furthermore, the tumor cells were immunoreactive to mesothelial markers (i.e., D2-40 and calretinin) and not immunoreactive to adenocarcinoma markers (i.e., TTF-1 and CEA). Based on these findings, the patient was also diagnosed with epithelioid mesothelioma. The definitive diagnosis for this patient was simultaneous lung adenocarcinoma (cT3N2M0, cStageШA) and MPM. However, examining the proliferation of cancer cells in the fibrous pleural tissue using specimens obtained through thoracoscopy was challenging. Hence, the possibility that these pleural lesions were reactive mesothelial hyperplasia rather than MPM could not be ruled out. Cisplatin (CDDP) and pemetrexed (PEM) were administered as first-line chemotherapy for six cycles, and PEM and bevacizumab (BEV) were administered as maintenance therapy. However, after 10 cycles of maintenance therapy, CT revealed right pleural effusion and enlargement of the right mediastinal lymph node. Docetaxel was administered as second-line chemotherapy. After two cycles of treatment, CT showed increased right pleural effusion. Pleurodesis was performed on the right side of the lung. Despite the administration of nivolumab as third-line chemotherapy for nine cycles, examination showed an increase in bilateral pleural effusion and further enlargement of the right mediastinal lymph node. Subsequently, carboplatin (CBDCA) and paclitaxel were administered as forth-line chemotherapy. After two cycles of treatment, an increase in left pleural effusion was detected. Pleurodesis was performed on the left side of the lung. The fifth line of chemotherapy consisted of CBDCA, PEM and BEV for one cycle, and PEM and BEV for five cycles (CBDCA was discontinued due to the occurrence of an adverse event [severe neutrophilia]). However, CT revealed an increase in right pleural effusion and findings indicative of lymphangitis carcinomatosa in the middle and lower lobes of the right lung. Subsequently, the administration of chemotherapy was discontinued. Eventually, the patient expired due to disease progression 2.5 years after diagnosis. An examination using CT prior to the patient's death showed right bronchial obstruction caused by the tumor, combined with enlargement of the mediastinal lymph node (Fig. 3). However, it did not show thickening of the left pleura (Fig. 3). A postmortem examination was performed. Macroscopically, the left parietal and visceral pleura and the left diaphragm showed slight thickening. Immunohistopathologically, mesothelial atypical cells positive for calretinin and negative for TTF-1 proliferated with ductal structures. Moreover, these cells infiltrated the fibrous pleural tissue (Fig. 4, Fig. 5). These results confirmed the diagnosis of epithelioid MPM. On the other hand, a tumor adjacent to the right main bronchus was macroscopically detected. Immunohistopathologically, the tumor was a well-differentiated adenocarcinoma, positive for TTF-1 and negative for calretinin (Fig. 6). The patient was diagnosed with right lung adenocarcinoma and lymph node metastasis. Moreover, the lung cancer had metastasized to the epicardium, right diaphragm, liver, and jejunum.
Fig. 1.
Chest CT revealed the presence of a right mediastinal mass combined with an enlarged right lymph node and left pleural effusion CT: computed tomography.
Fig. 2.
Thoracoscopic examination revealed the presence of multiple white small nodules on the left precordial parietal pleura (a) and a coral-shaped nodule on the diaphragm (b).
Fig. 3.
Chest CT showed right bronchial obstruction due to the enlarged right mediastinal lymph node and bronchial tumor. However, it did not show thickening of the left pleura. CT: computed tomography.
Fig. 4.
Epithelioid malignant mesothelioma in the left parietal pleura detected at autopsy. (×10) (a) HE, hematoxylin and eosin; (b) Calretinin (c) TTF-1, thyroid transcription factor-1.
Fig. 5.
Epithelioid malignant mesothelioma in the left diaphragm detected at autopsy. (×10) (a) Calretinin (b) TTF-1, thyroid transcription factor-1.
Fig. 6.
Lung adenocarcinoma forming a right mediastinal mass detected at autopsy. (×10) (a) Calretinin (b) TTF-1, thyroid transcription factor-1.
3. Discussion
In this report, we present a rare case of simultaneous presence of lung adenocarcinoma and MPM. This case report highlights two clinical suggestions. Firstly, the possibility of MPM comorbidity should be considered in patients with lung cancer and pleural effusion, especially in those with history of asbestos exposure. Secondly, the treatment strategy for patients with simultaneous presence of MPM and lung adenocarcinoma, should be decided based on which disease is more likely to determine the prognosis of patients.
Asbestos exposure is an established risk factor for the development of both MPM and lung cancer [[1], [2], [3]]. However, the co-presence of MPM and lung cancer is rare. To the best of our knowledge, only 26 such cases have been previously reported in the literature (Table 1) [[2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17]]. A study investigating patients with malignant mesothelioma showed that the complication of lung cancer was detected in only 6 of 500 patients (1.2%) [3]. This low incidence of complications suggests that the pathogenesis of malignant mesothelioma caused by asbestos exposure differs from that of lung cancer [2,3].
Table 1.
Clinicopathological features of the co-presence of malignant pleural mesothelioma and lung cancer.
| Case No. | Reference | Age | Sex | Asbestos Exposure | Smoking History | MM Type | Pulmonary Type | Lung cancer | Mesothelioma | Survival time |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 62 | M | Y | Y | E | A | r | r | 6 weeks |
| 2 | 3 | 62 | M | Y | Y | S | A | unknown | unknown | unknown |
| 3 | 3 | 62 | M | Y | Y | B | A | unknown | unknown | unknown |
| 4 | 3 | 73 | M | Y | Y | E | A | unknown | unknown | unknown |
| 5 | 3 | 64 | M | Y | Y | S | Sq | unknown | unknown | unknown |
| 6 | 3 | 70 | M | Y | N | B | Sq | unknown | unknown | unknown |
| 7 | 3 | 72 | M | Y | Y | B | Sm | unknown | unknown | unknown |
| 8 | 4 | 55 | F | Y | unknown | E | A | L | unknown | 11 months |
| 9 | 5 | 63 | M | Y | Y | E | A | r | r | 6 weeks or less |
| 10 | 5 | 67 | M | N | Y | B | A | r | l | 6 weeks or less |
| 11 | 5 | 77 | M | unknown | unknown | B | A | r | unknown | 6 weeks or less |
| 12 | 6 | 62 | M | Y | Y | E | A | r | r | unknown |
| 13 | 7 | 68 | M | Y | N | unknown | A | r | r | unknown |
| 14 | 8 | 78 | F | Y | Y | unknown | Sq | r | r | 22 weeks |
| 15 | 9 | 64 | M | N | N | unknown | A | r | r | unknown |
| 16 | 10 | 60 | F | Y | Y | E | A | l | r | unknown |
| 17 | 11 | 83 | M | N | Y | E | A | l | l | unknown |
| 18 | 12 | 77 | M | Y | unknown | unknown | Sq | unknown | unknown | unknown |
| 19 | 12 | 69 | M | Y | unknown | unknown | unspecified | unknown | unknown | unknown |
| 20 | 13 | 81 | M | Y | Y | unknown | Sm | r | r | 9 months |
| 21 | 14 | 68 | M | Y | Y | B | undifferentiated | r | r | unknown |
| 22 | 15 | 62 | M | N | N | E | A | l | l | 26 weeks |
| 23 | 16 | 72 | M | Y | Y | unknown | A | r | r | unknown |
| 24 | 16 | 64 | M | Y | Y | unknown | A | l | r | unknown |
| 25 | 16 | 71 | M | Y | unknown | unknown | A | unknown | r | unknown |
| 26 | 17 | 76 | M | Y | unknown | unknown | Sq | l | l | 45 months |
A: adenocarcinoma, B: biphasic, E: epithelioid, l: left, MM: malignant mesothelioma, r: right, S: sarcomatous, Sm: small, Sq: squamous.
In cases of malignant cell detection in pleural effusions, the origin of these malignant cells (e.g., MPM or organ tumors such as lung cancer) should be considered. The pleural fluid frequently shows high concentrations of HA in patients with MPM. Pettersson et al. reported that using a cut-off level of 100 mg/L, the concentration of HA in the pleural fluid showed sensitivity of 73% and specificity of 90% for MPM [18]. Moreover, CYFRA and TPA are useful as positive markers for MPM, whereas CEA is useful as a negative marker [19,20]. In the present case, the patient had high levels of HA (>80,000 ng/mL), CYFRA (466.9 ng/mL) and TPA (7636 U/L), and normal CEA levels in the pleural fluid. Thus, we suspected that the pleural effusion was the result of the MPM rather than the lung cancer.
Attanoos et al. stated that extensive MPM may prevent the accurate clinicopathological recognition of concomitant thoracic neoplasms [3]. Therefore, performing cytological examination coupled with biomarker analysis of pleural effusion in patients with asbestos exposure is of crucial importance in reaching a definitive diagnosis of MPM.
The histopathological diagnosis of MPM is challenging because reactive mesothelial hyperplasia and pleural seeding of organ tumors (e.g., lung cancer) should be ruled out [21,22]. In such cases, the use of immunohistochemical methods is required to overcome this difficulty in diagnosing MPM histopathologically. For example, in the differential diagnosis of MPM and lung adenocarcinoma, calretinin and D2-40 are useful as positive markers for MPM. In contrast, TTF-1 and CEA are useful as negative markers [1,23]. However, the differential diagnosis of MPM and reactive mesothelial hyperplasia is challenging because these conditions cannot be differentiated through immunohistochemical methods and exhibit similar histological features. The proliferation of mesothelial cells in the fibrous pleural tissue most certainly favors malignancy [21]. Unfortunately, thoracoscopic examination under local anesthesia is characterized by limitations in terms of the size and depth of the obtained specimens. In the present case, it was difficult to examine the proliferation of mesothelial cells in the fibrous pleural tissue using specimens obtained through thoracoscopy. Hence, the antemortem diagnosis of MPM was not definitive. However, the autopsy confirmed the proliferation of mesothelial cells in the fibrous pleural tissue and a definitive diagnosis of MPM was reached.
Furthermore, in the present case, progression of MPM was not detected for 2.5 years after diagnosis. Among the aforementioned 26 cases with co-presence of MPM and lung cancer, survival data were available for nine patients [2,4,5,8,13,15,17]. Seven of those patients survived for <1 year [2,4,5,13,15], whereas only two patients (reported by Kishimoto et al. and Negi et al.) survived for >1 year (23 months and 45 months, respectively) [8,17]. However, in the case reported by Kishimoto, MPM was diagnosed 1 year after the diagnosis of lung cancer. Hence, the patient expired almost 1 year after the diagnosis of MPM. On the other hand, in a case reported by Negi et al., an initial lung cancer was diagnosed 12 months after the diagnosis of MPM and the patient was treated with chemoradiotherapy. Seven months later, a second lung cancer was diagnosed. Finally, the patient expired due to lung cancer 45 months after the diagnosis of MPM. Negi et al. stated that radiotherapy against lung cancer may have assisted in controlling the disease activity of the pre-existing mesothelioma. Moreover, in our case, the patient showed long-term survival and consequently, the lung adenocarcinoma determined his prognosis. When treating patients diagnosed with both MPM and lung adenocarcinoma, we should consider each prognosis of these two cancers. A possible reason for the absence of MPM progression is that MPM was diagnosed at an early stage. Furthermore, the possibility of mesothelioma in situ cannot be ruled out. Other possible reasons may be the relatively good prognosis of MPM in this patient and the good response to the administered chemotherapeutic regimens. Factors associated with a poor prognosis of MPM include the non-epithelial type, high white blood cell (WBC) count, low performance status (PS) and male gender [24,25]. Moreover, high levels of 18F-FDG uptake in mesothelioma cells have been associated with poor prognosis [26]. This patient had epithelioid MPM, normal WBC count, and normal 18F-FDG uptake in the left pleura, suggesting favorable prognosis. With regards to the chemotherapeutic regimens, in the present case, CDDP and PEM – effective against both types of cancers – were administered at first-line chemotherapy [27,28]. This was because the diagnosis of simultaneous MPM and lung adenocarcinoma had been reached prior to the initiation of treatment. Although drug regimens for the treatment of lung adenocarcinoma were mainly administered after first-line chemotherapy, MPM may have responded to the programmed cell death protein-1 (PD-1) inhibitor nivolumab. Studies have shown that programmed cell death ligand-1 (PD-L1) was significantly associated with reduced survival [29,30] in MPM. Moreover, PD-L1 has been associated with reduced outcome in various other types of cancer such as non-small cell lung cancer [31], esophageal cancer [32], and renal carcinoma [33]. Recently, the possibility of using nivolumab for the treatment of MPM has been suggested. In a multicenter phase 2 study conducted in France, nivolumab administered as second- or third-line chemotherapy showed a 12-week disease control rate of 42.6% (Scherpereel A et al. Second or third line Nivolumab (Nivo) versus Nivo plus Ipilimumab (Ipi) in Malignant Pleural Mesothelioma (MPM) patients: results of the IFCT-1501 MAPS2 randomized phase П trial. Presented at ASCO 2017. Unpublished data.). Moreover, a phase 2 study conducted in Japan showed that nivolumab had a 6-month response rate of 29.4% (Goto Y et al. A Phase П study of Nivolumab: A Multicenter, open-label, single arm study in malignant pleural mesothelioma (MERIT). WCLC 2017. Unpublished data). Considering that PEM or vinorelbine, often administered as second-line chemotherapy, show a response rate of approximately 15% [34,35], the use of nivolumab may be indicated for the treatment of MPM. However, the role of PD-L1 expression in predicting response to a PD-1 inhibitor in malignant mesothelioma remains controversial. In this case, MPM confirmed at postmortem examination showed a PD-L1 Tumor Proportion Score <1%.
In summary, we presented a rare case of simultaneous presence of lung adenocarcinoma and MPM. In patients with lung cancer and pleural effusion, especially in those with history of asbestos exposure, possible comorbidity with MPM should be considered. Moreover, we should consider each prognosis of MPM and lung adenocarcinoma when treating patients with both malignancies. Reaching a definitive diagnosis in such cases is crucial for optimal patient management.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
COI
We declare the authors have no conflict of interests.
Acknowledgments
The authors would like to thank the staff at Department of Pathology, Kobe City Medical Center West Hospital.
References
- 1.Scherpereel A., Astoul P., Baas P. Guidelines of the European respiratory society and the European society of thoracic surgeons for the management of malignant pleural mesothelioma. Eur. Respir. J. 2010;35:479–495. doi: 10.1183/09031936.00063109. [DOI] [PubMed] [Google Scholar]
- 2.Cagle P.T., Wessels R., Greenberg S.D. Concurrent mesothelioma and adenocarcinoma of the lung in a patient with asbestosis. Mod. Pathol. 1993;6:438–441. [PubMed] [Google Scholar]
- 3.Attanoos R.L., Thomas D.H., Gibbs A.R. Synchronous diffuse malignant mesothelioma and carcinomas in asbestos-exposed individuals. Histopathology. 2003;43:387–392. doi: 10.1046/j.1365-2559.2003.01685.x. [DOI] [PubMed] [Google Scholar]
- 4.Okumura T., Okada M., Tsuji M. Mesothelioma with lung cancer complicating asbestosis. Acta Pathol. Jpn. 1980;30:579–590. doi: 10.1111/j.1440-1827.1980.tb01353.x. [DOI] [PubMed] [Google Scholar]
- 5.Allen T.C., Moran C. Synchronous pulmonary carcinoma and pleural diffuse malignant mesothelioma. Arch. Pathol. Lab Med. 2006;130:721–724. doi: 10.5858/2006-130-721-SPCAPD. [DOI] [PubMed] [Google Scholar]
- 6.Thomas D.H., Attanoos R.L., Gibbs A.R. Coexistent atypical adenomatous hyperplasia, primary lung adenocarcinoma and pleural mesothelioma in an asbestos-exposed subject. Histopathology. 2004;45:540–542. doi: 10.1111/j.1365-2559.2004.01939.x. [DOI] [PubMed] [Google Scholar]
- 7.Imenpour H., Ivaldi G.P., Brianti A., Pastorino G., Biggi S., Auriati L., Simonassi C. Synchronous occurrence of pulmonary adenocarcinoma and pleural diffuse malignant mesothelioma. Pathologica. 2013;105:353–356. [PubMed] [Google Scholar]
- 8.Kishimoto T. A case of triple malignancies (gastric cancer, lung cancer and malignant pleural mesothelioma) after asbestos exposure. Nihon Kokyuki Gakkai Zasshi. 2003;41:304–309. (in Japanese) [PubMed] [Google Scholar]
- 9.Maeda R., Isowa N., Onuma H., Miura H., Tokuyasu H., Kawasaki Y. Minute localized malignant pleural mesothelioma coexisting with multiple adenocarcinomas. Gen. Thorac. Cardiovasc. Surg. 2010;58:91–94. doi: 10.1007/s11748-009-0466-5. [DOI] [PubMed] [Google Scholar]
- 10.Özbudak İ.H., Ö Özbudak, Arslan G., Erdoğan A., Özbılım G. Metachronous malignant mesothelioma and pulmonary adenocarcinoma. Turk. Patoloji Derg. 2013;29:83–86. doi: 10.5146/tjpath.2013.01156. [DOI] [PubMed] [Google Scholar]
- 11.Haber Steven E. Synchronous malignant pleural mesothelioma and pulmonary carcinoma in a woman without evidence of asbestos exposure. Resp. Med. CME. 2010;3:160–161. [Google Scholar]
- 12.Bianchi C., Bianchi T., Ramani L. Malignant mesothelioma of the pleura and other malignancies in the same patient. Tumori. 2007;93:19–22. doi: 10.1177/030089160709300104. [DOI] [PubMed] [Google Scholar]
- 13.Lee A.H., Soomro I.N. Collision tumor of the pleura composed of small cell carcinoma and malignant mesothelioma. Histopathology. 2004;45:305–306. doi: 10.1111/j.1365-2559.2004.01900.x. [DOI] [PubMed] [Google Scholar]
- 14.Flood T.A., Sekhon H.S., Seely J.M., Shamji F.M., Gomes M.M. Spontaneous pneumothorax and lung carcinoma: should one consider synchronous malignant pleural mesothelioma? J. Thorac. Oncol. 2009;4:770–772. doi: 10.1097/JTO.0b013e3181a52c3f. [DOI] [PubMed] [Google Scholar]
- 15.Tsuzuki T., Ninomiya H., Natori Y., Ishikawa Y. Coalescent pleural malignant mesothelioma and adenocarcinoma of the lung, involving only minor asbestos exposure. Pathol. Int. 2008;58:451–455. doi: 10.1111/j.1440-1827.2008.02253.x. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki Y., Cagle P.T., Wessels R., Greenberg S.D. Concurrent mesothelioma and adenocarcinoma of the lung in a patient with asbestosis. Mod. Pathol. 1994;7:888–889. [PubMed] [Google Scholar]
- 17.Negi Y., Kuribayashi K., Doi H. Double cancer comprising malignant pleural mesothelioma and squamous cell carcinoma of the lung treated with radiotherapy: a case report. Mol.Clin.Oncol. 2018;9:181–186. doi: 10.3892/mco.2018.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pettersson T., Fröseth B., Riska H., Klockars M. Concentration of hyaluronic acid in pleural fluid as a diagnostic aid for malignant mesothelioma. Chest. 1988;94:1037–1039. doi: 10.1378/chest.94.5.1037. [DOI] [PubMed] [Google Scholar]
- 19.Paganuzzi M., Onetto M., Paola Marroni. Diagnostic value of CYFRA 21-1 tumor marker and CEA in pleural effusion due to mesothelioma. Chest. 2001;119:1138–1142. doi: 10.1378/chest.119.4.1138. [DOI] [PubMed] [Google Scholar]
- 20.Parazzi F., Faravelli B., Gall L. Tissue polypeptide antigen (TPA) in pleural effusions. Tumori. 1987;73:33–36. doi: 10.1177/030089168707300106. [DOI] [PubMed] [Google Scholar]
- 21.Cagle P.T., Churg A. Differential diagnosis of benign and malignant mesothelial proliferations on pleural biopsies. Arch. Pathol. Lab Med. 2005;129:1421–1427. doi: 10.5858/2005-129-1421-DDOBAM. [DOI] [PubMed] [Google Scholar]
- 22.Husain A.N., Colby T., Ordonez N. International mesothelioma interest group. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the international mesothelioma interest group. Arch. Pathol. Lab Med. 2013;137:647–667. doi: 10.5858/arpa.2012-0214-OA. [DOI] [PubMed] [Google Scholar]
- 23.Ordóñez N.G. Immunohistochemical diagnosis of epithelioid mesothelioma: an update. Arch. Pathol. Lab Med. 2005;129:1407–1414. doi: 10.5858/2005-129-1407-IDOEMA. [DOI] [PubMed] [Google Scholar]
- 24.Curran D., Sahmoud T., Therasse P., van Meerbeeck J., Postmus P.E., Giaccone G. Prognostic factors in patients with pleural mesothelioma: the European organization for research and treatment of cancer experience. J. Clin. Oncol. 1998;16:145–152. doi: 10.1200/JCO.1998.16.1.145. [DOI] [PubMed] [Google Scholar]
- 25.Edwards J.G., Abrams K.R., Leverment J.N., Spyt T.J., Waller D.A., O'Byrne K.J. Prognostic factors for malignant mesothelioma in 142 patients: validation of CALGB and EORTC prognostic scoring systems. Thorax. 2000;55:731–735. doi: 10.1136/thorax.55.9.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flores R.M., Akhurst T., Gonen M. Positron emission tomography predicts survival in malignant pleural mesothelioma. J. Thorac. Cardiovasc. Surg. 2006;132:763–768. doi: 10.1016/j.jtcvs.2006.03.068. [DOI] [PubMed] [Google Scholar]
- 27.Vogelzang N.J., Rusthoven J.J., Symanowski J. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 28.Scagliotti G.V., Parikh P., von Pawel J. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen B.H., Montgomery R., Fadia M., Wang J., Ali S. PD-L1 expression associated with worse survival outcome in malignant pleural mesothelioma. Asia Pac. J. Clin. Oncol. 2018;14:69–73. doi: 10.1111/ajco.12788. [DOI] [PubMed] [Google Scholar]
- 30.Cedrés S., Ponce-Aix S., Zugazagoitia J. Analysis of expression of programmed cell death 1 ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM) PloS One. 2015;10 doi: 10.1371/journal.pone.0121071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang A., Wang H.Y., Liu Y. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur. J. Surg. Oncol. 2015;41:450–456. doi: 10.1016/j.ejso.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 32.Ohigashi Y., Sho M., Yamada Y. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin. Canc. Res. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 33.Thompson R.H., Gillett M.D., Cheville J.C. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jassem J., Ramlau R., Santoro A. Phase III trial of pemetrexed plus best supportive care compared with best supportive care in previously treated patients with advanced malignant pleural mesothelioma. J. Clin. Oncol. 2008;26:1698–1704. doi: 10.1200/JCO.2006.09.9887. [DOI] [PubMed] [Google Scholar]
- 35.Stebbing J., Powles T., McPherson K. The efficacy and safety of weekly vinorelbine in relapsed malignant pleural mesothelioma. Lung Canc. 2009;63:94–97. doi: 10.1016/j.lungcan.2008.04.001. [DOI] [PubMed] [Google Scholar]