Summary
In transition metal-catalyzed asymmetric synthesis, enantioselectivity strongly depends on the structures of chiral ligands, so the development of new chiral ligands is crucial. Here, an efficient and highly enantioselective palladium-catalyzed intramolecular hydroarylation has been developed, and a new kind of N-heterocycles, 1H-pyrazolo[5,1-a]isoindol-2(8H)-ones containing a quaternary stereocenter, was prepared in high yields and excellent enantiomeric excess values. The reaction was effectively catalyzed by palladium-diphosphine complexes with numerous functional group tolerance, in which the newly developed axially chiral cyclic diphosphine ligands played key roles in the reactivity and enantioselectivity of the substrates. We believe that the cyclic diphosphine ligands with adjustable dihedral angles will find wide application in asymmetric synthesis.
Subject Areas: Chemistry, Catalysis, Organic Chemistry, Stereochemistry
Graphical Abstract

Highlights
-
•
Novel axially chiral cyclic diphosphine ligands
-
•
Excellent enantioselectivity
-
•
Wide substrate scope
-
•
Synthesis of a new kind of chiral N-heterocycles
Chemistry; Catalysis; Organic Chemistry; Stereochemistry
Introduction
Nitrogen-containing compounds widely occur in biologically active molecules including natural products (Ruiz-Sanchis et al., 2011), agrochemicals, and pharmaceuticals (Leeson and Springthorpe, 2007). In particular, over 90% of pharmaceuticals contain at least one nitrogen atom in their structures, so the development of efficient approaches to N-heterocycles is of paramount importance (Carey et al., 2006, Duggers et al., 2005). Compounds containing a l,8-diazabicyclo[3.3.0]octane skeleton exhibit diverse biological activities. For example, they are used as the androgen receptor modulator (Ullrich et al., 2014), angiotensin II receptor antagonist (Levin et al., 1994), and DNA topoisomerase inhibitor (Figure 1) (Katayama et al., 1999). However, 1H-pyrazolo[5,1-a]isoindol-2(8H)-ones as their derivatives have been ignored (Ivanovich et al., 2016). To the best of our knowledge, enantioselective synthesis of this kind of compounds containing a quaternary stereocenter has not been reported thus far.
Figure 1.
Selected Bioactive Compounds with a Diazabicyclo[3.3.0]octane Skeleton
Since the pioneering work by Cacchi and co-workers (Cacchi and Arcadi, 1983, Amorese et al., 1989; Cacchi, 1990, Arcadi et al., 1996), the palladium-catalyzed hydroarylation or reductive Heck reaction of aryl halides (pseudohalides) with alkenes has attracted much attention (Trost and Toste, 1999, Lee and Cha, 2001, Ichikawa et al., 2004, Dounay et al., 2008, Diethelm and Carreira, 2013, Schmidt and Hoffmann, 1991, Gottumukkala et al., 2011, Chen et al., 2012, Gao and Cook, 2012, Raoufmoghaddam et al., 2015). However, the development of highly enantioselective hydroarylation is still a great challenge, and only some examples of the enantioselective protocols have been reported till now (Minatti et al., 2007, Mannathan et al., 2017, Liu and Zhou, 2013, Yue et al., 2015, Shen et al., 2015, Kong et al., 2017). It is well known that the enantioselectivity highly depends on structures of chiral ligands in the transition-metal-catalyzed asymmetric synthesis, so the development of new chiral ligands is crucial (Tang and Zhang, 2003, Noyori and Ohkuma, 2001). In this regard, the axially chiral diphosphine ligands have been proved to be highly efficient in various enantioselective transformations (Qiu et al., 2006, Zhang et al., 2000, Sun et al., 2008, Wu et al., 2005, Pai et al., 2000, Jeulin et al., 2004a, Jeulin et al., 2004b, Genêt, 2003, Benincori et al., 2000, Tietze et al., 2000, Hatano et al., 2001, Graff et al., 2015). Recently, we have developed a kind of novel axially chiral cyclo-[1,1′-biphenyl]-2,2′-diols (CYCNOL) with adjustable dihedral angles (Zhang et al., 2016), and the chiral cyclic phosphoramidite ligands derived from CYCNOL have been successfully applied in iridium-catalyzed enantioselective arylation of unactivated racemic secondary allylic alcohols (Tian et al., 2017) and synthesis of dihydroimidazoquinazolinones (Peng et al., 2017). Inspired by the ligands we developed (Zhang et al., 2016, Tian et al., 2017, Peng et al., 2017), we herein report a palladium-catalyzed intramolecular enantioselective hydroarylation by elaborate tuning of newly developed axially chiral cyclic diphosphine ligands derived from CYCNOL.
Results and Discussion
Synthesis of Ligands
Racemic CYCNOL, Rac-CYC-8-NOL, Rac-CYC-9-NOL, and Rac-CYC-10-NOL, were prepared according to our previous procedures (Zhang et al., 2016). Subsequently, synthesis (following Zhou's protocol [Xie et al., 2003]) and resolution of our axially chiral cyclic diphosphine ligands were performed (Figure 2) (see Supplemental Information for details).
Figure 2.
Synthesis of Axially Chiral Cyclic Diphosphine Ligands
Crystal Structures of Ligands
Single crystals of the axially chiral cyclic diphosphine ligands (S)-CYC-8-BIPHP ((S)-E), (S)-CYC-9-BIPHP ((S)-F), and (S)-CYC-10-BIPHP ((S)-G) from mixed hexane and dichloromethane solvent were prepared, and their structures were unambiguously confirmed by X-ray diffraction analysis (see Supplemental Information, Data S1, S2, and S3 for details). According to the data from X-ray diffraction analysis, dihedral angles of the diphosphine ligands showed remarkable difference with a variety of ring sizes (Figure 3). It is known to all that the reactivity and enantioselectivity of substrates in the transition metal asymmetric synthesis are closely related to the structures of the ligands, such as the dihedral angles of axially chiral ligands.
Figure 3.
Crystal Structures and Dihedral Angles of Axially Chiral Cyclic Diphosphine Ligands (S)-E, (S)-F, and (S)-G
Optimization Study
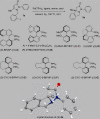
At first, palladium-catalyzed enantioselective hydroarylation of 1-(2-iodobenzyl)-5-methyl-2-phenyl-1H-pyrazol-3(2H)-one (1a) leading to (S)-3a-methyl-1-phenyl-3,3a-dihydro-1H-pyrazolo[5,1-a]isoindol-2(8H)-one (2a) was used as the model to optimize conditions including catalysts, ligands, tertiary amines, acids, solvents, and temperature. As shown in Table 1, seven ligands including four common diphosphine ligands, (S)- 2,2′- bis(diphenylphosphino)-1,1′-binaphthyl (BINAP), (R)- 5,5′-bis[di(3,5-di-t-butyl-4-methoxyphenyl)phosphino]-4,4′-bi-1,3-benzodioxole (DTBM-SEGPHOS), (S)-MeO-BIPNEP, and (S)- 7,7′-bis(diphenylphosphino)-2,2′,3,3′-tetrahydro-1,1′-spirobiindane (SDP), and our three cyclic diphosphine ligands, (S)-E, (S)-F, and (S)-G, were screened using Pd(trifluoroacetic acid [TFA])2 as the catalysts and N,N-dimethylbenzylamine/TFA as the hydride donors in N,N-dimethylacetamide (DMA) under a nitrogen atmosphere at 150°C for 24 hr (entries 1–7). We were pleased to find that the three cyclic diphosphine ligands, (S)-E, (S)-F, and (S)-G, all provided high yields with excellent enantiomeric excess (ee) values (entries 5–7), in which (S)-F was optimal (entry 6). Compared with the four common ligands, the advantage of our cyclo-[1,1′-biphenyl]diphosphine ligands, (S)-E, (S)-F, and (S)-G, is attributed to their combination of conformational rigidity and flexibility because they own the rigid biphenyl and the flexible full-carbon 6,6′-tethers. Meanwhile, the three cyclo-diphosphine ligands had little influence on the yields and ee values because of this factor. Single crystal of product 2a in entry 6 from mixed hexane and dichloromethane solvent was prepared, and its absolute configuration was determined to be S-form based on its single-crystal X-ray analysis (Table 1) (see Supplemental Information and Data S4 for details). Racemic 2a was obtained in 37% yield in the absence of ligand (entry 8). When other three tertiary amines, triethylamine, diisopropylethylamine, and proton sponge, were used instead of N,N-dimethylbenzylamine, lower ee values were observed (entries 9–11). Only a small amount of product 2a was found in the absence of amine (entry 12). Use of HOAc or HCOOH or absence of acid led to lower yields (entries 13–15). Two more palladium catalysts, Pd(dba)2 and Pd(OAc)2, were tested (entries 16 and 17), and they were inferior to Pd(TFA)2 (compare entries 6, 16, and 17). The effect of solvents was surveyed, and DMA proved to be a suitable solvent (compare entries 6, 18, and 19). When ligand (S)-F was increased from 7.5 mol % to 10 mol % (entry 20), the same yield and ee value were observed (compare entries 6 and 20). We attempted variation of temperature (entries 21 and 22), and the results showed that 150°C was a suitable temperature (compare entries 6, 21, and 22). According to the aforementioned results, we think that Pd(TFA)2 as the catalyst; (S)-E, (S)-F, and (S)-G as the ligands; N,N-dimethylbenzylamine/TFA as the hydride donor; and DMA as the solvent are suitable in the present palladium-catalyzed intramolecular enantioselective hydroarylation.
Table 1.
Optimization of Conditions
 | |||||
|---|---|---|---|---|---|
| Entry | Ligand | Amine | Acid | Yield of 2a (%)a | ee of 2a (%)b |
| 1 | (S)-A | BnNMe2 | TFA | 68 | 23 |
| 2 | (R)-B | BnNMe2 | TFA | 31 | −59 |
| 3 | (S)-C | BnNMe2 | TFA | 63 | 28 |
| 4 | (S)-D | BnNMe2 | TFA | 73 | −2 |
| 5 | (S)-E | BnNMe2 | TFA | 70 | 96 |
| 6 | (S)-F | BnNMe2 | TFA | 76 | 97 |
| 7 | (S)-G | BnNMe2 | TFA | 73 | 96 |
| 8 | – | BnNMe2 | TFA | 37 | 0 |
| 9 | (S)-F | NEt3 | TFA | 76 | 93 |
| 10 | (S)-F | DIPEA | TFA | 75 | 92 |
| 11 | (S)-F | PS | TFA | 57 | 88 |
| 12 | (S)-F | – | TFA | 8 | 94 |
| 13 | (S)-F | BnNMe2 | HOAc | 48 | 95 |
| 14 | (S)-F | BnNMe2 | HCOOH | 37 | 96 |
| 15 | (S)-F | BnNMe2 | – | 35 | 96 |
| 16c | (S)-F | BnNMe2 | TFA | 51 | 95 |
| 17d | (S)-F | BnNMe2 | TFA | 63 | 96 |
| 18e | (S)-F | BnNMe2 | TFA | 62 | 96 |
| 19f | (S)-F | BnNMe2 | TFA | 56 | 95 |
| 20g | (S)-F | BnNMe2 | TFA | 76 | 97 |
| 21h | (S)-F | BnNMe2 | TFA | 38 | 97 |
| 22i | (S)-F | BnNMe2 | TFA | 76 | 96 |
Reaction conditions: under nitrogen atmosphere, 1-(2-iodobenzyl)-5-methyl-2-phenyl-1H-pyrazol-3(2H)-one (1a) (0.2 mmol, 1.0 equiv), Pd(TFA)2 (10 μmol, 5 mol%), ligand (15 μmol, 7.5 mol%), amine (1.0 mmol, 5 equiv), acid (0.4 mmol, 2 equiv), N,N-dimethylacetamide (DMA) (4.0 mL), temperature (150°C), time (24 hr) in a sealed Schlenk tube. Absolute configuration of (S)-2a was assigned by X-ray diffraction analysis.
PS, proton sponge; DMF, N,N-dimethylformamide; DMSO, dimethylsulfoxide.
Isolated yield.
The ee values were determined by high-performance liquid chromatography analysis.
Using Pd(dba)2 (10 μmol, 5 mol%) as the catalyst.
Using Pd(OAc)2 (10 μmol, 5 mol%) as the catalyst.
Using DMF (4.0 mL) as the solvent.
Using DMSO (4.0 mL) as the solvent.
Using (S)-F (20 μmol, 10 mol%) as the ligand.
The reaction was carried out at 130°C.
The reaction was carried out at 160°C.
Scope of the Investigation
After obtaining the optimized conditions, the substrate scope for the palladium-catalyzed intramolecular enantioselective hydroarylation of 1 was surveyed using (S)-F as the ligand. As shown in Figure 4, we first attempted variation of substituents R1 in 1; various alkyl groups including methyl, ethyl, propyl, isopropyl, cyclopropyl, cyclopentyl, phenethyl, and phenpropyl were feasible, and the reaction provided high reactivity (76%–83% yields) and excellent enantioselectivity (97%–99% ee) (see 2a-h). When substituents R1 in 1 were different substituted benzyls, their enantioselectivity was also excellent (98%–99% ee) (see 2i-m). Subsequently, variation of substituents R2 in 1 was investigated (see 2n-ad). For substituents R2 with different substituted phenyls, the influence of electronic effect including electron-donating (see 2n-t), slight electron-withdrawing (see 2u-w), and strong electron-withdrawing groups (see 2x-z) on the phenyl rings was slight, and high reactivity (74%–84% yields) and excellent enantioselectivity (97%–99% ee) of the substrates were observed. When substituents R2 were benzyl (see 2aa and 2ab) and cyclohexyl (see 2ac and 2ad), the reaction also afforded high yields and excellent ee values. Variation of substituents R3 on the phenyl rings was investigated, and excellent results were obtained (see 2ae-ah).
Figure 4.
Substrate Scope for Palladium-Catalyzed Asymmetric Cyclization of 1
Reaction conditions: under nitrogen atmosphere, 1-(2-iodobenzyl)-5-alkyl-2-alkyl-1H-pyrazol-3(2H)-one (1) (0.2 mmol, 1.0 equiv), Pd(TFA)2 (10 μmol, 5 mol%), (S)-F (15 μmol, 7.5 mol%), BnNMe2 (1.0 mmol, 5 equiv), TFA (0.4 mmol, 2 equiv), DMA (4.0 mL), temperature (150°C), time (24 hr) in a sealed tube. Isolated yield was obtained, and the ee values were determined by high-performance liquid chromatography analysis. Absolute configurations of products 2 were determined by comparing structure of (S)-2a (absolute configuration of (S)-2a was assigned by X-ray diffraction analysis). Bn, benzyl. See Transparent Methods for experimental details.
Next, influence of the cyclic diphosphine ligands, (S)-E, (S)-F, and (S)-G, with different dihedral angles was investigated (Figure 4), and we found that the different substrates exhibited slight difference in reactivity and enantioselectivity with variation of the ligands. For all the tested substrates, (S)-F containing nine-membered ring was a suitable ligand. For synthesis of 2b and 2y, (S)-G containing ten-membered ring showed slightly higher enantioselectivity than (S)-E, which contained an eight-membered ring and (S)-F. The present reaction showed tolerance of various functional groups including C-F, C-Cl, and C-Br bonds and ether, CF3, nitro, cyano, ester, and amide groups. It is worthwhile to note that substrates 1 have unactivated 2-iodobenzy unit. In fact, it was usually difficult for the reaction of the substrates with this unit in previous report, and an effective solution was the use of substituted 2-halobenzoyls with high reactivity as the alternatives of 2-iodobenzy unit (Shen et al., 2015). In addition, no erosion of ee values was observed at such high temperature (150°C). The results showed that our catalyst system was highly efficient in the present reaction.
Applications of the Method
A scale synthesis of (S)-2i was performed as example. As shown in Figure 5A, reaction of 1i (2.15 mmol, 1.0 g) under standard conditions provided (S)-2i in 82% yield with 98% ee without loss of yield and enantioselectivity. We attempted the reaction of aryl bromide 3 under the conditions (Figure 5B), and (S)-2a was obtained in 38% yield with 97% ee. Furthermore, reduction of (S)-2i with LiAlH4 provided (S)-4 in 95% yield with 98% ee without loss of ee (Figure 5C).
Figure 5.
Applications of the Method
(A) Scale synthesis of (S)-2i.
(B) Palladium-catalyzed asymmetric cyclization of 1-(2-bromobenzyl)-5-methyl-2-phenyl-1H-pyrazol-3(2H)-one (3).
(C) Reduction of (S)-2i.
Mechanism of the Reaction
According to the experiments mentioned above and previous references (Raoufmoghaddam et al., 2015, Minatti et al., 2007), a reaction pathway of this palladium-catalyzed intramolecular enantioselective hydroarylation is proposed in Figure 6. Oxidative addition of the aryl iodide 1 to the in situ-formed Pd(0) diphosphine complex leads to the Pd(II) intermediate I, and then anion exchange of I with the salt (BnNHMe2+-O2CCF3) provides II. Carbopalladation of the double bond in II yields the π-oxa-allyl palladium species III. A hydride transfer from the CH2 of benzyl in BnNMe2 to palladium gives the Pd(II) hydride complex IV leaving the iminium ion V. Reductive elimination of the Pd(II) hydride complex IV finally affords the target product (2) with regeneration of Pd(0)L*.
Figure 6.
Possible Mechanism for the Palladium-Catalyzed Intramolecular Asymmetric Hydroarylation
Extension of the Method
Furthermore, the palladium-catalyzed intramolecular asymmetric hydroarylation of o-iodobenzoyl derivatives (5) was attempted under conditions similar to those in (Figures 4 and 7), and we found that o-iodobenzoyl derivatives (5) exhibited higher reactivity and lower enantioselectivity than o-iodobenzy derivatives (1). Unfortunately, the factors that lead to lower enantioselectivity of 6 than 2 are unknown for us.
Figure 7.
Palladium-Catalyzed Intramolecular Asymmetric Hydroarylation of o-Iodobenzoyl Derivatives (5)
Limitations of Study
It should be pointed out that there are limitations to the present method including requirement of higher temperature and maladjustment of other common ligands.
Conclusions
In summary, we have developed an efficient and highly enantioselective palladium-catalyzed intramolecular hydroarylation, in which the reactivity and enantioselectivity of the substrates were tuned by our newly developed axially chiral cyclic diphosphine ligands and the new kind of N-heterocycles, 1H-pyrazolo[5,1-a]isoindol-2(8H)-ones containing a quaternary stereocenter, were prepared in high yields and excellent ee values with numerous functional group tolerance. We believe that our axially chiral cyclic diphosphine ligands with the adjustable dihedral angles will find wide application in asymmetric synthesis.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Dr. Haifang Li of the Department of Chemistry at Tsinghua University for her great help in high-resolution mass spectrometric analysis and the National Natural Science Foundation of China (Grant No. 21772108) for financial support.
Author Contributions
C.L. and H.F. conceived and design this subject; C.L. and X.Z. conducted the experimental work; C.L., X.Z., P.Z., H.Y., C.Z., and H.F. analyzed the results; C.L. and H.F. co-wrote the manuscript.
Declaration of Interests
There are no competing interests.
Published: December 21, 2018
Footnotes
Supplemental Information includes Transparent Methods, 312 figures, 1 Scheme, 4 tables, and 4 data files and can be found with this article online at https://doi.org/10.1016/j.isci.2018.11.018.
Data and Software Availability
Crystallographic data have been deposited in the Cambridge Crystallographic Data Center under accession numbers CCDC: 1842685, 1822026, 1842686, and 1822025.
Supplemental Information
References
- Amorese A., Arcadi A., Bernocchi E., Cacchi S., Cerrini S., Fedeli W., Ortar G. Conjugate addition vs vinylic substitution in palladium-catalyzed reaction of aryl halides with beta-substituted-alpha,beta-enones and beta-substituted-alpha,beta-enals. Tetrahedron. 1989;45:813–828. [Google Scholar]
- Arcadi A., Cacchi S., Fabrizi G., Marinelli F., Pace P. The palladium-catalysed vinylic substitution of vinyl triflates with beta-substituted-alpha,beta-unsaturated carbonyl compounds. An application to the synthesis of cardenolides. Tetrahedron. 1996;52:6983–6996. [Google Scholar]
- Benincori T., Cesarotti E., Piccolo O., Sannicolo F. 2,2',5,5'-Tetramethyl-4,4'-bis(diphenylphoshino)-3,3'-bithiophene: a new, very efficient, easily accessible, chiral biheteroaromatic ligand for homogeneous stereoselective catalysis. J. Org. Chem. 2000;65:2043–2047. doi: 10.1021/jo991533x. [DOI] [PubMed] [Google Scholar]
- Cacchi S. The palladium-catalyzed hydroarylation and hydrovinylation of carbon carbon multiple bonds - new perspectives in organic-synthesis. Pure Appl. Chem. 1990;62:713–722. [Google Scholar]
- Cacchi S., Arcadi A. Palladium-catalyzed conjugate addition type reaction of aryl iodides with alpha,beta-unsaturated ketones. J. Org. Chem. 1983;48:4236–4240. [Google Scholar]
- Carey J.S., Laffan D., Thomson C., Williams M.T. Analysis of the reactions used for the preparation of drug candidate molecules. Org. Biomol. Chem. 2006;4:2337–2347. doi: 10.1039/b602413k. [DOI] [PubMed] [Google Scholar]
- Chen J.-Q., Xie J.-H., Bao D.-H., Liu S., Zhou Q.-L. Total synthesis of (-)-galanthamine and (-)-lycoramine via catalytic asymmetric hydrogenation and intramolecular reductive Heck cyclization. Org. Lett. 2012;14:2714–2717. doi: 10.1021/ol300913g. [DOI] [PubMed] [Google Scholar]
- Diethelm S., Carreira E.M. Total synthesis of (+/-)-gelsemoxonine. J. Am. Chem. Soc. 2013;135:8500–8503. doi: 10.1021/ja403823n. [DOI] [PubMed] [Google Scholar]
- Dounay A.B., Humphreys P.G., Overman L.E., Wrobleski A.D. Total synthesis of the strychnos alkaloid (+)-minfiensine: tandem enantioselective intramolecular heck-iminium ion cyclization. J. Am. Chem. Soc. 2008;130:5368–5377. doi: 10.1021/ja800163v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggers R.W., Ragan J.A., Brown Ripin D.H. Survey of GMP bulk reactions run in a research facility between 1985 and 2002. Org. Process. Res. Dev. 2005;9:253–258. [Google Scholar]
- Gao P., Cook S.P. A Reductive-Heck approach to the hydroazulene ring system: a formal synthesis of the englerins. Org. Lett. 2012;14:3340–3343. doi: 10.1021/ol3013167. [DOI] [PubMed] [Google Scholar]
- Genêt J.-P. Asymmetric catalytic hydrogenation. design of new Ru catalysts and chiral ligands: from laboratory to industrial applications. Acc. Chem. Res. 2003;36:908–918. doi: 10.1021/ar020152u. [DOI] [PubMed] [Google Scholar]
- Gottumukkala A.L., de Vries J.G., Minnaard A.J. Pd-NHC catalyzed conjugate addition versus the Mizoroki-Heck reaction. Chem. Eur. J. 2011;17:3091–3095. doi: 10.1002/chem.201003643. [DOI] [PubMed] [Google Scholar]
- Graff J., Łastawiecka E., Guénée L., Leroux F., Alexakis A. Asymmetric bromine–lithium exchange: application toward the synthesis of new biaryl-diphosphine ligands. Adv. Synth. Catal. 2015;357:2833–2839. [Google Scholar]
- Hatano M., Terada M., Mikami K. Highly enantioselective palladium-catalyzed ene-type cyclization of a 1,6-enyne. Angew. Chem. Int. Ed. 2001;40:249–253. doi: 10.1002/1521-3773(20010105)40:1<249::AID-ANIE249>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Ichikawa M., Takahashi M., Aoyagi S., Kibayashi C. Total synthesis of (-)-incarvilline, (+)-incarvine C, and (-)-incarvillateine. J. Am. Chem. Soc. 2004;126:16553–16558. doi: 10.1021/ja0401702. [DOI] [PubMed] [Google Scholar]
- Ivanovich R.A., Clavette C., Vincent-Rocan J.-F., Roveda J.-G., Gorelsky S.I., Beauchemin A.M. Intramolecular alkene aminocarbonylation using concerted cycloadditions of amino-isocyanates. Chem. Eur. J. 2016;22:7906–7916. doi: 10.1002/chem.201600574. [DOI] [PubMed] [Google Scholar]
- Jeulin S., Duprat de Paule S., Ratovelomanana-Vidal V., Genêt J.-P., Champion N., Dellis P. Difluorphos, an electron-poor diphosphane: a good match between electronic and steric features. Angew. Chem. Int. Ed. 2004;43:320–325. doi: 10.1002/anie.200352453. [DOI] [PubMed] [Google Scholar]
- Jeulin S., Duprat de Paule S., Ratovelomanana-Vidal V., Genêt J.-P., Champion N., Dellis P. Chiral biphenyl diphosphines for asymmetric catalysis: stereoelectronic design and industrial perspectives. Proc. Natl. Acad. Sci. U S A. 2004;101:5799–5804. doi: 10.1073/pnas.0307620101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama H., Kawada Y., Kaneko K., Oshiyama T., Takatsu N. Studies on the drug against refractory diseases. Part I - synthetic inhibitors of DNA topoisomerase I and II. Chem. Pharm. Bull. 1999;47:48–53. doi: 10.1248/cpb.47.48. [DOI] [PubMed] [Google Scholar]
- Kong W., Wang Q., Zhu J. Water as a hydride source in palladium-catalyzed enantioselective reductive Heck reactions. Angew. Chem. Int. Ed. 2017;56:3987–3991. doi: 10.1002/anie.201700195. [DOI] [PubMed] [Google Scholar]
- Lee K., Cha J.K. Formal synthesis of (+)-phorbol. J. Am. Chem. Soc. 2001;123:5590–5591. doi: 10.1021/ja010643u. [DOI] [PubMed] [Google Scholar]
- Leeson P.D., Springthorpe B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat. Rev. Drug Discov. 2007;6:881–890. doi: 10.1038/nrd2445. [DOI] [PubMed] [Google Scholar]
- Levin J.I., Venkatesan A.M., Chan P.S., Bailey T.K., Vice G., Coupet J. 6-Substituted quinazolinone angiotensin-II receptor antagonists. Bioorg. Med. Chem. Lett. 1994;4:1819–1824. [Google Scholar]
- Liu S., Zhou J. Desymmetrization of cyclic olefins via asymmetric Heck reaction and hydroarylation. Chem. Commun. (Camb.) 2013;49:11758–11760. doi: 10.1039/c3cc47551d. [DOI] [PubMed] [Google Scholar]
- Mannathan S., Raoufmoghaddam S., Reek J.N.H., de Vries J.G., Minnaard A.J. Enantioselective intramolecular reductive Heck reaction with a palladium/monodentate phosphoramidite catalyst. ChemCatChem. 2017;9:551–554. [Google Scholar]
- Minatti A., Zheng X., Buchwald S.L. Synthesis of chiral 3-substituted indanones via an enantioselective reductive-Heck reaction. J. Org. Chem. 2007;72:9253–9558. doi: 10.1021/jo701741y. [DOI] [PubMed] [Google Scholar]
- Noyori R., Ohkuma T. Asymmetric catalysis by architectural and functional molecular engineering: practical chemo- and stereoselective hydrogenation of ketones. Angew. Chem. Int. Ed. 2001;40:40–73. [PubMed] [Google Scholar]
- Pai C.-C., Lin C.-W., Lin C.-C., Chen C.-C., Chan A.S.C. Highly effective chiral dipyridylphosphine ligands: synthesis, structural determination, and applications in the Ru-catalyzed asymmetric hydrogenation reactions. J. Am. Chem. Soc. 2000;122:11513–11514. [Google Scholar]
- Peng F., Tian H., Zhang P., Liu C., Wu X., Yuan X., Yang H., Fu H. Iridium-catalyzed enantioselective synthesis of dihydroimidazoquinazolinones by elaborate tuning of chiral cyclic ligands. Org. Lett. 2017;19:6376–6379. doi: 10.1021/acs.orglett.7b03230. [DOI] [PubMed] [Google Scholar]
- Qiu L., Kwong F.Y., Wu J., Lam W.H., Chan S., Yu W.-Y., Li Y.-M., Guo R., Zhou Z., Chan A.S.C. A new class of versatile chiral-bridged atropisomeric diphosphine ligands: remarkably efficient ligand syntheses and their applications in highly enantioselective hydrogenation reactions. J. Am. Chem. Soc. 2006;128:5955–5965. doi: 10.1021/ja0602694. [DOI] [PubMed] [Google Scholar]
- Raoufmoghaddam S., Mannathan S., Minnaard A.J., de Vries J.G., Reek J.N.H. Palladium(0)/NHC-catalyzed reductive Heck reaction of enones: a detailed mechanistic study. Chem. Eur. J. 2015;21:18811–18820. doi: 10.1002/chem.201503217. [DOI] [PubMed] [Google Scholar]
- Ruiz-Sanchis P., Savina S.A., Albericio F., Álvarez M. Structure, bioactivity and synthesis of natural products with hexahydropyrrolo[2,3-b]indole. Chem. Eur. J. 2011;17:1388–1408. doi: 10.1002/chem.201001451. [DOI] [PubMed] [Google Scholar]
- Schmidt B., Hoffmann H.M.R. On the way to aflatoxins and related structure types - regio-controlled annulations by application of homogenous palladium catalysis, urethane tether and ortho,ortho'-diiodine effect. Tetrahedron. 1991;47:9357–9368. [Google Scholar]
- Shen C., Liu R.-R., Fan R.-J., Li Y.-L., Xu T.-F., Gao J.-R., Jia Y.-X. Enantioselective arylative dearomatization of indoles via Pd-catalyzed intramolecular reductive Heck reactions. J. Am. Chem. Soc. 2015;137:4936–4939. doi: 10.1021/jacs.5b01705. [DOI] [PubMed] [Google Scholar]
- Sun X., Zhou L., Li W., Zhang X. Convenient divergent strategy for the synthesis of tunephos-type chiral diphosphine ligands and their applications in highly enantioselective Ru-catalyzed hydrogenations. J. Org. Chem. 2008;73:1143–1146. doi: 10.1021/jo702068w. [DOI] [PubMed] [Google Scholar]
- Tang W., Zhang X. New chiral phosphorus ligands for enantioselective hydrogenation. Chem. Rev. 2003;103:3029–3070. doi: 10.1021/cr020049i. [DOI] [PubMed] [Google Scholar]
- Tian H., Zhang P., Peng F., Yang H., Fu H. Chiral cyclic ligand-enabled iridium-catalyzed asymmetric arylation of unactivated racemic allylic alcohols with anilines. Org. Lett. 2017;19:3775–3778. doi: 10.1021/acs.orglett.7b01631. [DOI] [PubMed] [Google Scholar]
- Tietze L.F., Thede K., Schimpf R., Sannicolò F. Enantioselective Synthesis of tetrahydroisoquinolines and benzazepines by silane terminated heck reactions with the chiral ligands (+)-TMBTP and (R)-BITIANP. Chem. Commun. 2000:583–584. [Google Scholar]
- Trost B.M., Toste F.D. Palladium-catalyzed kinetic and dynamic kinetic asymmetric transformation of 5-acyloxy-2-(5H)-furanone. Enantioselective synthesis of (-)-aflatoxin B lactone. J. Am. Chem. Soc. 1999;121:3543–3544. doi: 10.1021/ja020988s. [DOI] [PubMed] [Google Scholar]
- Ullrich T., Sasmal S., Boorgu V., Pasagadi S., Cheera S., Rajagopalan S., Bhumireddy A., Shashikumar D., Chelur S., Belliappa C. 3-Alkoxy-pyrrolo[1,2-b]pyrazolines as selective androgen receptor modulators with ideal physicochemical properties for transdermal administration. J. Med. Chem. 2014;57:7396–7411. doi: 10.1021/jm5009049. [DOI] [PubMed] [Google Scholar]
- Wu J., Ji J.-X., Chan A.S.C. A Remarkably effective copper(II)-dipyridylphosphine catalyst system for the asymmetric hydrosilylation of ketones in air. Proc. Natl. Acad. Sci. U S A. 2005;102:3570–3575. doi: 10.1073/pnas.0409043102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J.-H., Wang L.-X., Fu Y., Zhu S.-F., Fan B.-M., Duan H.-F., Zhou Q.-L. Synthesis of spiro diphosphines and their application in asymmetric hydrogenation of ketones. J. Am. Chem. Soc. 2003;125:4404–4405. doi: 10.1021/ja029907i. [DOI] [PubMed] [Google Scholar]
- Yue G., Lei K., Hirao H., Zhou J. Palladium-catalyzed asymmetric reductive Heck reaction of aryl halides. Angew. Chem. Int. Ed. 2015;54:6531–6535. doi: 10.1002/anie.201501712. [DOI] [PubMed] [Google Scholar]
- Zhang P., Yu J., Peng F., Wu X., Jie J., Liu C., Tian H., Yang H., Fu H. Development of axially chiral cyclo-biaryldiol ligands with adjustable dihedral angles. Chem. Eur. J. 2016;22:17477–17484. doi: 10.1002/chem.201603706. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Qian H., Longmire J., Zhang X. Synthesis of chiral bisphosphines with tunable bite angles and their applications in asymmetric hydrogenation of beta-ketoesters. J. Org. Chem. 2000;65:6223–6226. doi: 10.1021/jo000462v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.









