Abstract
The main goal of the present work was to determine the nutraceutical potential of Asparagopsis taxiformis D. extracts from Madeira Archipelago south coast. Extraction methodologies consisted either/or in 72 hours stirring, at room temperature (M1), or 6 cycles of Soxhlet extraction (M2), both with re-extraction. Solvents used were distilled water, ethanol, methanol and ethyl acetate. M1 allowed to obtain the highest values for extraction yield (31.65 g.100g−1 dw) using water, whereas iodine content (3.37 g.100g−1 dw), TPC (1.71 g GAE.100g−1 dw) and chlorophyll a (45.96 mg.100g−1 dw) were obtained using ethanol, and TCC (36.23 mg.100g−1 dw) with methanol. Extracts that showed higher reduction activity in M1 were derived from ethanol extraction (1,908 mg AAE.100g−1 dw). Water and ethanol were the best solvents for higher DPPH scavenging activity in M2, both with same result (IC50 1.37 mg.mL−1). The lowest value of IC50 for chelating activity (1.57 mg.mL−1) was determined in M1, using ethyl acetate. The remaining residue was used to obtain other products, i.e. lipid extraction (M1, 2.05 g.100g−1 dw), carrageenans (M2, 21.18 g.100g−1 dw) and cellulose (M1, 23.81 g.100g−1 dw) with subsequent FTIR ATR analysis. Our results show that A. taxiformis is a valuable source of bioactive compounds. The M1 extraction methodology using ethanol is the most effective solvent to produce an iodine rich bioactive extract with potential of being used as a nutraceutical supplement. Also, we have demonstrated a possible downstream strategy that could be implemented for multiple compound extraction from A. taxiformis residue. This has a vital importance for future feasibility, when using this biomass as an industrial feedstock for multiple products production. Statistical analysis, using SPSS 24.0, was also performed and important correlations were found between assays and methods.
Keywords: Biochemistry, Food analysis, Nutrition, Food science
1. Introduction
Asparagopsis taxiformis, has been earlier researched by Kaliaperumal (2003) [1] and referred to as a “seaweed rich in iodine” which can be used to ameliorate goitre disease. McConnell and Fenical (1977) [2] identified the biological functions of the halogenated metabolites in A. taxiformis, which include bromine (Br) and iodine (I) in their molecular structures. These metabolites could be stored in specialized gland cells [3] and often function as defense compounds, correlated with environmental adaptations, due to A. taxiformis abundance in subtropical and tropical waters, where high seaweed herbivory occurs. In India, this species was found to accumulate 499.30 mg I/100g dw (dry weight) and its genus 556.70 mg I/100g dw [4]. In many areas of the world, soil surface is progressively becoming poorer in iodine content due to leaching processes [5]. The majority of the available iodine is supported by marine systems and marine organisms such as seaweeds, which can accumulate substantial quantities of iodine [6]. Chemical iodine species in seaweeds seems to be mainly , organic iodine and in minor quantity [7]. There is a widespread interest for functional foods that might promote health benefits, such as reducing the risk of chronic diseases and enhancing the ability to promote health, thus improving the quality of life. Seaweeds potential as a functional food can be used to produce supplements for the food industry [8]. To determine the nutraceutical quality of the extracts produced, nine parameters were assessed, namely extract yield, iodine, total phenolic compounds (TPC), flavonoids, chlorophyll a, total carotenoid content (TCC), reducing activity (RA), free radical scavenging assay (FRSA) and ferrous ion chelation (FIC). TPC are present in seaweeds as a major molecular group, with an important action in defending against bacteria, wounding or excessive radiation and contributing to the antioxidant activity of the tissue [9, 10]. These compounds are attractive due to their nutraceutical and antioxidative properties, showing bioactivity that is indicative of preventing pathologies caused by oxidative stress [11].
Numerous approaches are being developed for industrial crops or biomass applications, considering that the chemical composition of seaweeds and their fast growth enables a large set of strategies for biorefinary implementation. Nowadays, chemical production or extraction using seaweed as feedstock is mainly focused on single products, such as the extraction and purification of hydrocolloids, polysaccharides, xanthophylls, proteins and production of biofuels, discarding the remaining biomass [12]. Integrating sustainable strategies to cascade processing with efficient disintegration of biomass to obtain valuable biocompounds could be the answer for a profitable industry [13]. Taking this into consideration, a downstream process was also applied on the A. taxiformis residue after primary extraction. This remaining biomass is also known as PEAR (post-extracted algal residue). Three distinct compounds of commercial interest were extracted and quantified from PEAR, namely lipids, carrageenans and cellulose, as a first step towards a biorefinary strategy.
In this work, several solvents were tested, namely distilled water, ethanol, methanol and ethyl acetate and two distinct extraction processes for the development of seaweed nutraceutic production, using biomass from the south cost of Madeira. The directive 2009/32/CE for food applications allows the use of these solvents, under good working practices and quality control. Although methanol has some restrictions, it can be used if its maximum presence does not reach 10 mg/kg in the final product. FTIR-ATR was considered for spectra comparison between direct extraction and PEAR extraction of lipids, carrageenan and cellulose. This is a powerful technic, widely used for detection and characterization of various types of molecules, delivering a unique signature to each compound analyzed. The variability of transmittance along the correspondent wavenumber is caused due to the relative mass and geometry of the atoms. The conformation of the molecules causes the resonance between vibrations that further modulates the spectra [14].
2. Materials and methods
2.1. Seaweed biomass
Asparagopsis taxiformis (Delile) Trevisan de Saint-Léon 1845 samples were collected in a 10-meter maximum depth dive of the Madeiran south coastline, coordinates 32,646951 - 16,823967. Samples were collected in august 2016 and were transported in seawater and gently rinsed with filtered fresh water. Afterwards, a primary drying was applied in which seaweed was frozen at −35 °C and freeze-dried under reduced pressure (4 × 10−4 mbar), with a cooling trap set at −56 °C for 5 days. Samples were milled to 200 mesh particle size, vacuum packed and stored at −35 °C until use.
2.2. Preparation of extracts
Two methods of extract production were used. Ten grams of lyophilized A. taxiformis were added to 300 mL of one of the following solvents: distilled water, ethanol, methanol or ethyl acetate (1:30, w/v). The first extraction method (M1) included stirring the mixture in a borosilicate flask, under sonication, during 90 min. Afterwards, continuously stirring at 1,100 rpm was carried out at approximately 20 °C for 72 hours. Final suspension was centrifuged for 10 min at 7200×g. The supernatant was kept for analysis, as extract. In the second extraction method (M2) a soxhlet extractor was used to perform six complete extraction cycles. A re-extraction was performed in both methodologies and extracts were pooled together, according to the respective solvent and method. Extracts were partly evaporated in a rotary evaporator and dehydrated completely in an oven, at 40 °C. The post-extracted algal residue (PEAR) was recovered and dried in an oven, at 40 °C. Both extracts and PEAR were stored under vacuum at −35 °C until use.
2.3. Extract analysis
A. taxiformis extracts were subjected to nine analysis parameters. Total phenolic content (TPC) was determined using Folin Ciocalteu method described by Chew et al. (2008) [15] and results were expressed as grams of gallic acid equivalents (GAE) per 100 grams of dw of extract. Chlorophyll a and total carotenoids content (TCC) were determined according to Wellburn (1994) and Kumar et al. (2010) [16, 17]. Flavonoids were quantified according to Chan et al. (2015) [18], using aluminium chloride colorimetry and results were expressed as grams of quercetin equivalents (QE) per 100 grams dw of extract. The free radical-scavenging activity (FRSA) was measured using 2,2-diphenyl-1-picrylhydrazyl (DPPH) as described by Yen and Chen (1995) and Duan et al. (2006) [19, 20], also using butylated hydroxytoluene (BHT) as a positive control and results were expressed in milligrams IC50 per millilitre. Reducing activity was performed following Yuan et al. (2005) [21] work and results were expressed as grams of ascorbic acid equivalents (AAE) per 100 grams dw of extract. Ferrous ion chelating activity (FIC assay) was measured based on Chew et al. (2008) and Decker and Welch (1990) [15, 22], using EDTA as a positive control, results were expressed as milligrams IC50 per millilitre. All samples were analyzed in triplicates in all tests carried out.
2.3.1. Iodine quantification
Iodine quantification was initiated with seaweed incineration, adopting the method described by Mahesh et al. (1992) [23] with some modifications. About 0.5 g of sample is weighted into porcelain crucibles and 0.5 ml of potassium hydroxide solution (6M) mixed with a metal rod and placed in an oven at a temperature of 95 °C for 1h. After, it is added 0.5 ml of zinc sulphate solution (0.52M) and returned to the oven for a further 1 h at 95 °C. The crucibles were then placed in a muffle furnace to reach the 600 °C for a period of 2h. After, it is reconstituted in deionized water, filtered to remove particles and the volume adjusted accordingly. The oxidation process and spectrophotometric determination were performed according to Pino et al. (1996) [24] with some modifications. Aliquots of 0.2 ml of sample or standard was added to test tubes, followed by addition of 1.0 ml of ammonium persulfate (1M) to all tubes. The samples undergo a process of oxidation during 30 min in a water bath at a temperature of 95 °C. It was sequentially added 2 ml of arsenic acid (0.0253 M), 1 ml of sulfuric acid (1.25M) and 1 ml of water. The tubes are placed in a water bath at 32 °C for 10 min, then 0.5 ml of ceric ammonium sulfate (0.0158M) is added, vortexed and incubated exactly for 10 minutes in the water bath at the same temperature as before. A standard solution A is performed with to a concentration of 7.87 M (1000 μg/ml). Standard solution B is prepared by diluting the standard solution A to 100 times, to an iodine concentration of 78.74 mM (10.0 μg/ml). The working standards are prepared by diluting with distilled water in a volumetric flask of 100 ml amount of 1.0 ml, 2.0 ml, 3.0 ml, 4.0 ml and 5.0 ml of standard solution B resulting in the concentration of 0.78, 1.57, 2.36, 3.18, 3.94 mM (0.02, 0.04, 0.06, 0.08 and 0.10 μg/0.2 ml). After incubation the reactions are read at 410 nm in a spectrophotometer cuvettes of 10 mm. The calibration curve is prepared daily by placing % transmittance vs. iodine content in μg of iodine on the axis X.
2.4. Downstream product analysis
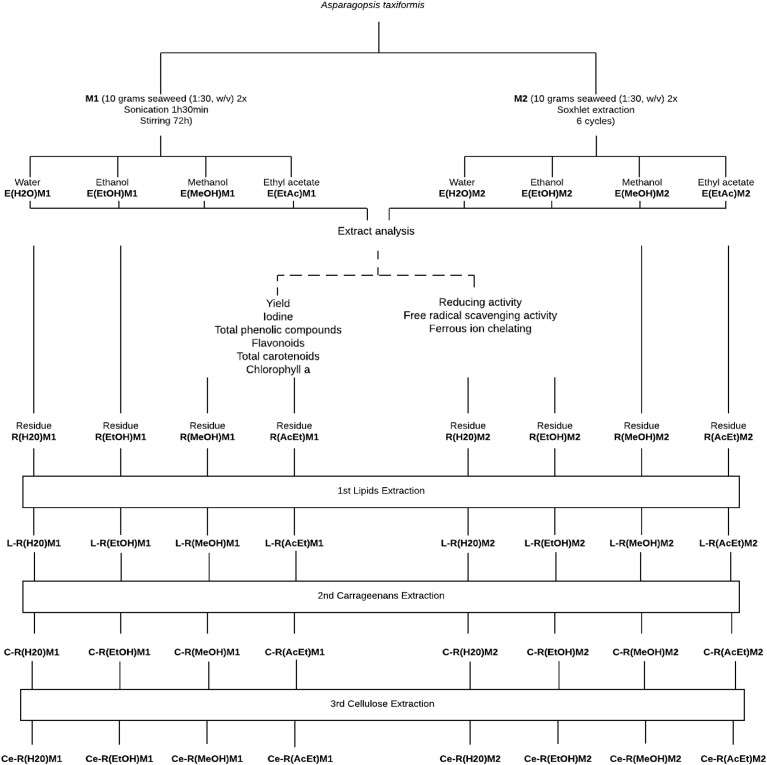
Lipids were quantified as described by Folch et al. (1957) [25]. Carrageenan was determined according to Tasende et al. (2012) [26] and cellulose was quantified following Baghel et al. (2015) [27]. In order to facilitate the comprehension of the multiple analysis and extractions performed in this work, a flowchart was created (Fig. 1). For extractions, residues and by-products a simple code was attributed, presenting results and discussion in a more perceptible way.
Fig. 1.
Schematic representation of extract production, using 4 solvents permitted by the food industry and two methodologies, with posterior extraction of lipids, carrageenan and cellulose from PEAR biomass. Codes were attributed to each product for simplicity purposes when discussing results.
FTIR-ATR spectra of the samples were obtained with a Perkin Elmer Spectrum Two coupled with a Diamond ATR accessory (DurasamplIR II, Smiths Detection, UK). Thirty-two scans were acquired in transmittance mode in the range of 4000–650 cm−1, with a wavenumber resolution of 1 cm−1. All samples were analyzed in triplicates in all tests carried out.
2.5. Statistical analysis
All values are expressed as mean of three replicates ± standard deviation. The statistical data analysis was performed, using SPSS 24.0 program for Windows. Data were analysed using one-way analysis of variance (ANOVA), and determined its homoscedasticity followed by Pearson's test (p ≤ 0.01) to assess correlations between means. Tukey's b test (p ≤ 0.01) was also performed to determine statistical variance between seaweeds in each parameter.
3. Results and discussion
In total, eight A. taxiformis extracts were assessed. These were produced using four distinct solvents (ethanol, methanol, water and ethyl acetate) considered safe for extracts production and two methods. Results for extract yield, iodine, TPC, flavonoids, chlorophyll a and TCC are presented in Table 1. For simplicity reasons, Fig. 1 was created, coding extractions, residues and by-products in an intuitive way, so it would became clear all of the steps involved in this research.
Table 1.
Yield of extract, iodine content and antioxidant composition from Asparagopsis taxiformis (Delile) Trevisan.
| Solvent | Extract Yield |
Iodine |
TPC |
Flavonoids |
Chlorophyll a |
TCC |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g (100 g)−1 in dw |
g (100 g)−1 in dw |
g GAE (100 g)−1 in dw |
g QE (100 g)−1 in dw |
mg (100 g)−1 in dw |
mg (100 g)−1 in dw |
|||||||
| M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | |
| Water | 31.65 ± 0.79a | 18.93 ± 0.69a | 1.71 ± 0.03a | 2.92 ± 0.05a | 0.62 ± 0.01a | 0.47 ± 0.04a | 0.04 ± 0a | 0.04 ± 0a | ND | ND | ND | 1.66 ± 0.26a |
| Ethanol | 10.92 ± 1.77b | 9.69 ± 0.21bc | 3.37 ± 0.01b | 3.13 ± 0.01b | 1.71 ± 0.13b | 1.43 ± 0.08b | 2.51 ± 0.03b | 3.51 ± 0.06b | 45.95 ± 0a | 14.34 ± 2.69a | 22.72 ± 0.69a | 23.23 ± 1.04b |
| Methanol | 11.98 ± 0.66b | 14.49 ± 4.09ac | 2.38 ± 0.07c | 1.82 ± 0.02c | 0.57 ± 0.02a | 0.52 ± 0.03a | 4.02 ± 0.11c | 2.85 ± 0.02c | 8.65 ± 1.98b | 34.80 ± 1.98b | 36.23 ± 1.05b | 26.62 ± 0.11c |
| Ethyl acetate | 5.32 ± 0.67c | 3.19 ± 0.06b | 0.69 ± 0.01d | 0.59 ± 0d | 0.07 ± 0.01c | ND | 24.25 ± 0.11d | 7.75 ± 0.04d | 8.10 ± 1.35b | 13.90 ± 1.96a | 36.13 ± 1.72b | 23.74 ± 0.67b |
Data are mean ± standard deviation in grams or milligrams per 100 grams of seaweed extract on a dry weight basis. All determinations were carried out in triplicate. Different letters within the same column indicate significant differences (p ≤ 0.01) determined in SPSS 24.0 using Tukey b test. Not detected (ND).
3.1. Extract yield
Extract yield was higher using distilled water (31.65 ± 0.79 g/100g dw) E(H2O)M1 and (18.93 ± 0.69 g/100g dw) for E(H2O)M2 (Table 1). Methanol and ethanol solvents also provided high extraction yields in E(MeOH)M2 (14.49 ± 4.09 g/100g dw) and E(EtOH)M1 (10.92 ± 1.77 g/100g dw), presented in Table 1. Mellouk et al. (2017) [28] also provided some insight of the antioxidant properties of A. taxiformis from the Algerian coast and water extraction resulted in the highest yield (24.0 g/100g dw), followed by methanol extract (21.3 g/100g dw) and ethanol extract (18.1g/100g dw). Although comparing A. taxiformis from different locations and time of harvest, the extract yield were in similar order with higher extraction yield using water, followed by methanol and ethanol solvents. In order to determine the in vitro anti-methanogenic activity of A. taxiformis extracts, Machado et al. (2016) [29] performed initial extractions with four different solvents, water, methanol, DCM and hexane. They have obtained higher yield using water and methanol, 24.9 g/100g dw and 10.2 g/100g dw, demonstrating some similarity with our results. Chan et al. (2015) [18] used similar solvents to produce extracts from Gracilaria changii, collected in Sarawak, Malaysia, varying from 13.06 ± 1.14 g/100g dw using ethanol (comparing with our data 10.92 ± 1.77 g/100g dw) and 3.15 ± 0.45 g/100g dw using ethyl acetate (comparing with our data 5.32 ± 0.67 g/100g dw).
3.2. Iodine quantification
For whole A. taxiformis, iodine content reached 1.16 ± 0.03 g/100g dw. The highest iodine content was detected using ethanol as solvent in M1, E(EtOH)M1 and M2 E(EtOH)M2 extractions, representing 3.37 ± 0.01 g/100g dw and 3.13 ± 0.01 g/100g dw, respectively (see Table 1). McConnell and Fenical (1977) [2] also used ethanol as a primary step to extract halogenated compounds from fresh A. taxiformis and Asparagopsis armata, obtaining high yield. Iodine is extensively integrated in these compounds, due to its biogenic functions. Statistical analysis showed that all values of iodine content in the extracts are independent, suggesting that the iodine has variable affinity, depending on the solvent used. The positive correlation (R2 = 0.77) (Table 2) between M1 and M2 extraction methods means that iodine extraction is strongly influenced by the solvents used in this work. Determining iodine content in seaweed extracts are of extreme importance, when considering these extracts to human supplementation. Iodine is essential for human physiology due to its integration in the composition of thyroid hormones T3 (3,5,3-triiodothyronine) and T4 (thyroxine or 3,5,3,5-tetraiodothyronine). These hormones are responsible for regulating key metabolic processes, such as catabolism of carbohydrates, lipids and protein, cellular respiration, thermoregulation, intermediary metabolism, and nitrogen retention [30]. Iodine plays an important role in human physiology, and it is known that its low intake is particularly harmful in childhood and pregnancy, contributing for developmental deficiencies and diseases. Iodine deficiency remains a major public health concern in many countries of the world [31]. Portugal is not an exception and the proximity to the sea does not avoid iodine deficiency. A study involving 3,631 pregnant women has shown that 83.2% in the mainland and 94.6% in Madeira and Azores Archipelagos had urinary iodine levels below 150 μg.L−1 [32], which is considered to be an indicator of insufficient iodine intake [33].
Table 2.
Statistical analysis using Pearson correlation to determine relationships between different parameters.
| Pearson's Correlation |
|||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extraction yield |
Iodine |
TPC |
Reduction activity |
FRSA |
FIC |
Chlorophyll a |
TCC |
Flavonoids |
PEAR Yield |
Lipids |
Carrageenans |
Cellulose |
|||||||||||||||
| M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | ||
| Extraction yield | M1 | 1 | ,849∗∗ | 0,028 | ,615∗ | 0,034 | 0,024 | −0,362 | −,738∗∗ | ,637∗ | −0,321 | −,831∗∗ | −,986∗∗ | −0,400 | −,587∗ | −,922∗∗ | −,936∗∗ | −,661∗ | −,877∗∗ | −,950∗∗ | −,850∗∗ | 0,227 | 0,153 | −,722∗ | −,876∗∗ | ,739∗∗ | ,624∗ |
| M2 | ,849∗∗ | 1 | 0,369 | ,661∗ | 0,226 | 0,267 | −0,129 | −0,289 | 0,257 | 0,102 | −0,489 | −,800∗∗ | −0,202 | −0,127 | −,705∗ | −,636∗ | −,843∗∗ | −,952∗∗ | −,924∗∗ | −,869∗∗ | −0,315 | −0,391 | −,915∗ | −,988∗∗ | ,755∗ | ,751∗ | |
| Iodine | M1 | 0,028 | 0,369 | 1 | ,770∗∗ | ,927∗∗ | ,951∗∗ | ,817∗∗ | ,584∗ | −,745∗∗ | 0,002 | 0,518 | 0,010 | ,765∗∗ | 0,254 | −0,110 | 0,194 | −,740∗∗ | −0,445 | −0,111 | −0,282 | −,813∗∗ | −,840∗∗ | −0,666 | −0,354 | 0,330 | −0,101 |
| M2 | ,615∗ | ,661∗ | ,770∗∗ | 1 | ,805∗∗ | ,801∗∗ | 0,497 | −0,062 | −0,193 | −0,399 | −0,117 | −,607∗ | 0,446 | −0,332 | −,717∗∗ | −0,471 | −,911∗∗ | −,812∗∗ | −,602∗ | −0,574 | −0,359 | −0,427 | −,894∗∗ | −,711∗ | ,738∗∗ | 0,166 | |
| TPC | M1 | 0,034 | 0,226 | ,927∗∗ | ,805∗∗ | 1 | ,993∗∗ | ,910∗∗ | 0,434 | −,700∗ | −0,331 | 0,450 | −0,045 | ,879∗∗ | −0,049 | −0,248 | 0,074 | −,626∗ | −0,346 | −0,034 | −0,137 | −0,558 | −,593∗ | −0,586 | −0,191 | 0,408 | −0,313 |
| M2 | 0,024 | 0,267 | ,951∗∗ | ,801∗∗ | ,993∗∗ | 1 | ,908∗∗ | 0,477 | −,724∗∗ | −0,272 | 0,477 | −0,026 | ,872∗∗ | 0,011 | −0,215 | 0,107 | −,648∗ | −0,359 | −0,036 | −0,165 | −,618∗ | −,648∗ | −0,579 | −0,208 | 0,369 | −0,287 | |
| Reduction activity | M1 | −0,362 | −0,129 | ,817∗∗ | 0,497 | ,910∗∗ | ,908∗∗ | 1 | ,658∗ | −,883∗∗ | −0,252 | ,732∗∗ | 0,342 | ,994∗∗ | 0,117 | 0,111 | 0,415 | −0,277 | 0,063 | 0,375 | 0,215 | −0,540 | −0,541 | −0,203 | 0,254 | 0,044 | −,610∗ |
| M2 | −,738∗∗ | −0,289 | ,584∗ | −0,062 | 0,434 | 0,477 | ,658∗ | 1 | −,921∗∗ | 0,511 | ,959∗∗ | ,791∗∗ | ,638∗ | ,798∗∗ | ,730∗∗ | ,897∗∗ | 0,005 | 0,342 | ,592∗ | 0,343 | −,805∗∗ | −,768∗∗ | 0,146 | 0,399 | −0,416 | −0,389 | |
| FRSA | M1 | ,637∗ | 0,257 | −,745∗∗ | −0,193 | −,700∗ | −,724∗∗ | −,883∗∗ | −,921∗∗ | 1 | −0,171 | −,942∗∗ | −,651∗ | −,872∗∗ | −0,547 | −0,505 | −,753∗∗ | 0,121 | −0,248 | −0,553 | −0,329 | ,750∗∗ | ,718∗∗ | 0,009 | −0,382 | 0,251 | 0,529 |
| M2 | −0,321 | 0,102 | 0,002 | −0,399 | −0,331 | −0,272 | −0,252 | 0,511 | −0,171 | 1 | 0,329 | 0,435 | −0,292 | ,872∗∗ | ,620∗ | 0,542 | 0,048 | 0,098 | 0,097 | −0,096 | −0,547 | −0,524 | 0,234 | −0,042 | −0,370 | 0,304 | |
| FIC | M1 | −,831∗∗ | −0,489 | 0,518 | −0,117 | 0,450 | 0,477 | ,732∗∗ | ,959∗∗ | −,942∗∗ | 0,329 | 1 | ,855∗∗ | ,737∗∗ | ,677∗ | ,744∗∗ | ,919∗∗ | 0,158 | 0,501 | ,748∗∗ | 0,557 | −,656∗ | −,606∗ | 0,237 | 0,595 | −0,496 | −,587∗ |
| M2 | −,986∗∗ | −,800∗∗ | 0,010 | −,607∗ | −0,045 | −0,026 | 0,342 | ,791∗∗ | −,651∗ | 0,435 | ,855∗∗ | 1 | 0,374 | ,678∗ | ,954∗∗ | ,969∗∗ | ,611∗ | ,834∗∗ | ,924∗∗ | ,805∗∗ | −0,312 | −0,243 | 0,678 | ,825∗∗ | −,753∗∗ | −,581∗ | |
| Chlorophyll a | M1 | −0,400 | −0,202 | ,765∗∗ | 0,446 | ,879∗∗ | ,872∗∗ | ,994∗∗ | ,638∗ | −,872∗∗ | −0,292 | ,737∗∗ | 0,374 | 1 | 0,091 | 0,135 | 0,430 | −0,203 | 0,131 | 0,435 | 0,271 | −0,479 | −0,475 | −0,140 | 0,341 | −0,002 | −,673∗ |
| M2 | −,587∗ | −0,127 | 0,254 | −0,332 | −0,049 | 0,011 | 0,117 | ,798∗∗ | −0,547 | ,872∗∗ | ,677∗ | ,678∗ | 0,091 | 1 | ,801∗∗ | ,811∗∗ | 0,057 | 0,246 | 0,372 | 0,108 | −,750∗∗ | −,697∗ | 0,094 | 0,185 | −0,501 | 0,069 | |
| TCC | M1 | −,922∗∗ | −,705∗ | −0,110 | −,717∗∗ | −0,248 | −0,215 | 0,111 | ,730∗∗ | −0,505 | ,620∗ | ,744∗∗ | ,954∗∗ | 0,135 | ,801∗∗ | 1 | ,945∗∗ | ,608∗ | ,774∗∗ | ,812∗∗ | ,665∗ | −0,331 | −0,254 | 0,630 | ,719∗ | −,800∗∗ | −0,347 |
| M2 | −,936∗∗ | −,636∗ | 0,194 | −0,471 | 0,074 | 0,107 | 0,415 | ,897∗∗ | −,753∗∗ | 0,542 | ,919∗∗ | ,969∗∗ | 0,430 | ,811∗∗ | ,945∗∗ | 1 | 0,417 | ,681∗ | ,827∗∗ | ,655∗ | −0,527 | −0,459 | 0,479 | ,696∗ | −,688∗ | −0,471 | |
| Flavonoids | M1 | −,661∗ | −,843∗∗ | −,740∗∗ | −,911∗∗ | −,626∗ | −,648∗ | −0,277 | 0,005 | 0,121 | 0,048 | 0,158 | ,611∗ | −0,203 | 0,057 | ,608∗ | 0,417 | 1 | ,931∗∗ | ,741∗∗ | ,770∗∗ | 0,540 | ,604∗ | ,979∗∗ | ,867∗∗ | −,710∗∗ | −0,477 |
| M2 | −,877∗∗ | −,952∗∗ | −0,445 | −,812∗∗ | −0,346 | −0,359 | 0,063 | 0,342 | −0,248 | 0,098 | 0,501 | ,834∗∗ | 0,131 | 0,246 | ,774∗∗ | ,681∗ | ,931∗∗ | 1 | ,929∗∗ | ,906∗∗ | 0,257 | 0,328 | ,942∗∗ | ,961∗∗ | −,778∗∗ | −,667∗ | |
| PEAR Yield | M1 | −,950∗∗ | −,924∗∗ | −0,111 | −,602∗ | −0,034 | −0,036 | 0,375 | ,592∗ | −0,553 | 0,097 | ,748∗∗ | ,924∗∗ | 0,435 | 0,372 | ,812∗∗ | ,827∗∗ | ,741∗∗ | ,929∗∗ | 1 | ,914∗∗ | −0,025 | 0,044 | ,750∗ | ,953∗∗ | −,720∗∗ | −,785∗∗ |
| M2 | −,850∗∗ | −,869∗∗ | −0,282 | −0,574 | −0,137 | −0,165 | 0,215 | 0,343 | −0,329 | −0,096 | 0,557 | ,805∗∗ | 0,271 | 0,108 | ,665∗ | ,655∗ | ,770∗∗ | ,906∗∗ | ,914∗∗ | 1 | 0,248 | 0,322 | 0,783 | ,964∗∗ | −,663∗ | −,721∗ | |
| Lipids | M1 | 0,227 | −0,315 | −,813∗∗ | −0,359 | −0,558 | −,618∗ | −0,540 | −,805∗∗ | ,750∗∗ | −0,547 | −,656∗ | −0,312 | −0,479 | −,750∗∗ | −0,331 | −0,527 | 0,540 | 0,257 | −0,025 | 0,248 | 1 | ,992∗∗ | 0,457 | 0,247 | 0,015 | −0,104 |
| M2 | 0,153 | −0,391 | −,840∗∗ | −0,427 | −,593∗ | −,648∗ | −0,541 | −,768∗∗ | ,718∗∗ | −0,524 | −,606∗ | −0,243 | −0,475 | −,697∗ | −0,254 | −0,459 | ,604∗ | 0,328 | 0,044 | 0,322 | ,992∗∗ | 1 | 0,505 | 0,315 | −0,085 | −0,128 | |
| Carrageenans | M1 | −,722∗ | −,915∗ | −0,666 | −,894∗∗ | −0,586 | −0,579 | −0,203 | 0,146 | 0,009 | 0,234 | 0,237 | 0,678 | −0,140 | 0,094 | 0,630 | 0,479 | ,979∗∗ | ,942∗∗ | ,750∗ | 0,783 | 0,457 | 0,505 | 1 | ,908∗∗ | −,757∗ | −0,602 |
| M2 | −,876∗∗ | −,988∗∗ | −0,354 | −,711∗ | −0,191 | −0,208 | 0,254 | 0,399 | −0,382 | −0,042 | 0,595 | ,825∗∗ | 0,341 | 0,185 | ,719∗ | ,696∗ | ,867∗∗ | ,961∗∗ | ,953∗∗ | ,964∗∗ | 0,247 | 0,315 | ,908∗∗ | 1 | −,641∗ | −,876∗∗ | |
| Cellulose | M1 | ,739∗∗ | ,755∗ | 0,330 | ,738∗∗ | 0,408 | 0,369 | 0,044 | −0,416 | 0,251 | −0,370 | −0,496 | −,753∗∗ | −0,002 | −0,501 | −,800∗∗ | −,688∗ | −,710∗∗ | −,778∗∗ | −,720∗∗ | −,663∗ | 0,015 | −0,085 | −,757∗ | −,641∗ | 1 | 0,402 |
| M2 | ,624∗ | ,751∗ | −0,101 | 0,166 | −0,313 | −0,287 | −,610∗ | −0,389 | 0,529 | 0,304 | −,587∗ | −,581∗ | −,673∗ | 0,069 | −0,347 | −0,471 | −0,477 | −,667∗ | −,785∗∗ | −,721∗ | −0,104 | −0,128 | −0,602 | −,876∗∗ | 0,402 | 1 | |
Statistical significance at 0.01 level (**) or at 0.05 level (*) bilateral, using Pearson correlation test in SPSS 24.0; Signalling (–) reveal the negative relation between parameters or in its absence, their positive correlation. Values presented are for R2.
3.3. Antioxidant quantification
3.3.1. Total phenolic compounds (TPC)
The highest value for total phenolic content (TPC) (Table 1) was determined in ethanol extracts, E(EtOH)M1 (1.71 ± 0.13 g GAE/100g dw) and E(EtOH)M2 (1.43 ± 0.08 g GAE/100g dw). The TPC presence in ethyl acetate extracts varied from non-detectable quantity in E(EtAc)M2 to a slightly higher content, 0.07 ± 0.01 g GAE/100g for E(EtAc)M1, suggesting a weak affinity. Farvin and Jacobsen (2013) [34] evaluated eleven different seaweeds from the coast of Denmark and found that protocatechnic, gentisic and hydroxybenzoic phenolic acids were the major compounds in TPC for red and green seaweeds. A positive Pearson correlation (R2 = 0.80 to R2 = 0.95), presented in Table 2, between iodine and TPC was detected in the extracts. According to Hou et al. (2000) [35], iodine could be predominantly found in seaweed tissues bound with proteins, polyphenols and pigments.
3.3.2. Total flavonoid content (TFC)
Flavonoids, a subdivision of TPC, are secondary metabolites constituted by anthocyanidins, chalcones, flavones, flavanols, flavanones, isoflavones and flavonols, differentiated according to their biosynthetic origin and presenting a general structure of two aromatic rings linked together by a 3-carbon bridge [36]. Total flavonoid content (TFC) determined in the extracts was inversely related to the polarity of the solvents used in the extraction. Highest TFC content was determined in ethyl acetate extract (Table 1), E(EtAc)M1 24.25 ± 0.11 g QE/100g dw and lowest with water extraction, 0.04 ± 0.0 g QE/100g dw, both in E(H2O)M1 and E(H2O)M2. Solvent efficiency for TFC extraction in M1 apparently decreases from E(EtAc)M1 > E(MeOH)M1 > E(EtOH)M1 > E(H2O)M1, or in M2 from E(EtAc)M2 > E(EtOH)M2 > E(H2O)M2 > E(MeOH)M2. This suggests that flavonoids extraction, using M1 method is inversely correlated with the solvent polarity, ethyl acetate having strong affinity to flavonoids. According to Stankovic et al. (2011) [37], when using solvents like chloroform, dichloromethane, diethyl ether or ethyl acetate, polar flavonoids such as flavonols, methylated flavones, isoflavones and flavanones are extracted. Still, the use of alcohols or alcohol-water mixtures, allows the extraction of flavonoid glycosides and more polar aglycones. Tukey b test showed that all values of flavonoids were independent from each other in both extraction methods. The positive correlation in Table 2 (R2 = 0.931) between M1 and M2 methods suggests, for A. taxiformis, that solvents are more likely to influence flavonoid extraction than the extraction methods used.
3.3.3. Chlorophyll a
Chlorophylls are greenish pigments that have antioxidant bioactivity in consumed seaweed [38]. These pigments in processed food can be converted into compounds such as pheophytin, pyropheophytin and pheophorbide, known to have preventive action against cancer [8]. The only chlorophyll type found in red seaweeds is chlorophyll a [39] and maximum yield of this pigment was obtained using ethanol (Table 1), E(EtOH)M1 (45.95 ± 0 mg/100g dw) and methanol in E(MeOH)M2 (34.80 ± 1.98 mg/100g dw). When using water, no chlorophyll a was detected. Using Pearson's correlation analysis, no correlation could be found when comparing the two different methodologies, M1 and M2, in their chlorophyll content, indicating that chlorophyll a content is strongly dependent of the method applied to obtain the resulting extract. This effect can be easily observed when comparing chlorophyll a content in ethanol extract, E(EtOH)M1, 45.95 ± 0 mg/100g dw and in E(EtOH)M2, 14.34 ± 2.69 mg/100g dw, a decrease of 3.2 times. Methanol demonstrated the inverse behavior, extracting 8.65 ± 1.98 mg/100g dw in E(MeOH)M1 and increasing to 34.80 ± 1.98 mg/100g dw in E(MeOH)M2, a four time increase. This fact can be due to solvent efficiency influenced when heated in the Soxhlet extractor. Ethanol decreases its efficiency and methanol increases. Also, comparing the amount of chlorophyll a content in whole seaweed A. taxiformis, 28.81 ± 3.25 mg/100g dw, described in our previous work [40], only ethanol extract (45.95 ± 0 mg/100g dw) using E(EtOH)M1 and methanol extract (34.80 ± 1.98 mg/100g dw) using E(EtOH)M2, allowed a higher concentration of chlorophyll a, demonstrating the higher affinity of chlorophyll a for these two solvents, using different methods.
3.3.4. Total carotenoids content (TCC)
Carotenoids are terpenoid pigments and their oxygenated derivatives are called xanthophylls. These compounds have the ability to function as antioxidants, neutralizing reactive oxygen species (ROS) during metabolic processes [41]. Red seaweeds as A. taxiformis essentially produce lutein, α-carotene and zeaxanthin [8, 42]. Total carotenoids content (TCC) varied according to the solvents used to produce the extracts. The highest value was obtained with methanol extract (Table 1) in E(MeOH)M1, 36.23 ± 1.05 mg/100g dw and the lowest with water extract, with no amount detected. Comparing with the work of Chan et al. (2015) [18], the highest amount achieved in their work was using ethyl acetate extract, 73.44 ± 14.87 mg/100g dw and in our work, ethyl acetate was also very close to the highest value, 36.13 ± 1.72 mg/100g dw in E(EtAc)M1. This demonstrates that carotenoids in A. taxiformis have similar affinity to methanol and ethyl acetate when method 1 is applied and that ethyl acetate presents a high content of carotenoids, not only demonstrated in this work but also in the work of Chan et al. (2015) [18]. Therefore, it can be observed that the sequence of total carotenoids contents according to the solvents used is exactly the same when comparing the two different methods. M1 and M2 have the same order, E(MeOH) > E(EtAc) > E(EtOH) > E(H2O), but M1 has shown highest efficiency when extracting total carotenoids. In a previous work [40], we have demonstrated that A. taxiformis whole seaweed had 13.14 ± 2.63 mg/100g dw, and comparing this value with the ones obtained in the extracts, all of the extracts have higher concentration of TCC, except in water derived extracts, that presented from 1.66 ± 0.26 mg/100g in E(H2O)M2 to no amount detected in E(H2O)M1. Pearson's correlation evidenced a high degree of correlation between the two methods (R2 = 0.945) in Table 2, permitting to conjecture that TCC extraction is strongly defined by the solvent used and not from the methods applied in this work.
3.4. Antioxidant activity
The antioxidant activity, which includes reducing activity (RA), free radical scavenging activity (FRSA) and ferrous ion chelation (FIC), was also determined and results are presented in Table 3.
Table 3.
Antioxidant activity of extracts from Asparagopsis taxiformis (Delile) Trevisan.
| Solvent | Reducing Activity (RA) |
FRSA (DPPH) |
FIC |
|||
|---|---|---|---|---|---|---|
| mg AAE(100 g)−1 in dw |
mg IC 50 (mL)−1 |
mg IC 50 (mL)−1 |
||||
| M1 | M2 | M1 | M2 | M1 | M2 | |
| Water | 233.15 ± 5.15a | 174.38 ± 11.65a | 4.65 ± 0.29a | 1.37 ± 0.03a | 113.01 ± 10.62a | 74.00 ± 1.81a |
| Ethanol | 1908.44 ± 59.15b | 1156.86 ± 13.87b | 1.54 ± 0.07b | 1.37 ± 0.04a | 5.26 ± 0.27b | 10.49 ± 0.44b |
| Methanol | 584.46 ± 15.36c | 1161.47 ± 14.43b | 2.69 ± 0.03c | 1.64 ± 0.01b | 8.36 ± 0.29c | 10.07 ± 0.18b |
| Ethyl acetate | 409.60 ± 10.84d | 707.42 ± 98.78c | 3.62 ± 0.04d | 1.44 ± 0.08a | 1.57 ± 0.03d | 5.88 ± 0.26c |
Data are mean ± standard deviation in milligrams per 100 grams of seaweed on a dry weight basis, mg IC 50 (mL)−1. All determinations were carried out in triplicate. Different letters within the same column indicate significant differences (p ≤ 0.01) determined in SPSS 24.0 using Tukey b test.
3.4.1. Reducing activity (RA)
This antioxidant assay incorporates L-ascorbic acid as a standard and evaluates the potential of the compounds that comprise the extract to function as single electron transfer (SET) through primary antioxidation. The results are presented in L-ascorbic acid equivalents. The RA reaches the highest values of 1,908.44 ± 59.15 mg AAE/100g dw in E(EtOH)M1 and of 1,161.47 ± 14.43 mg AAE/100g dw in E(MeOH)M2 extracts. The lowest RA was obtained with distilled water E(H20)M1 and E(H2O)M2, 233.15 ± 5.15 and 174.38 ± 11.65 mg AAE/100g dw, respectively. RA in M1 shows a decrease in activity from E(EtOH)M1 > E(MeOH)M1 > E(EtAc)M1 > E(H2O)M1. In M2, there was a change in the most efficient solvent, presenting a different order, E(MeOH)M2 > E(EtOH)M2 > E(EtAc)M2 > E(H2O)M2. This discrepancy between M1 and M2 demonstrates the influence of the methods applied when comparing the same solvent extraction. Comparing ethanol extraction in the two methods employed, the antioxidant capacity assessed by RA obtained in E(EtOH)M1 was 1.7 times higher than E(EtOH)M2, distinguishing the simple stirring method for 72 hours with ultra-sounds as a better choice. In this assay we used BHT as a common antioxidant standard. The comparative RA of the extracts was between 573 and 52 times weaker than BHT, using E(H2O)M2 and E(EtOH)M1, respectively. Statistical analysis using Tukey b test showed that RA values in M1 were independent, each solvent having a different degree of reducing activity, but in M2 ethanol and methanol are closely related. Using Pearson's correlation (Table 2), it was determined that M1 has some degree of correlation with M2 (R2 = 0.658), suggesting that the solvents characterize the RA in the extract but methodology also imprints some variation.
3.4.2. Free radical scavenging activity (FRSA)
FRSA uses DPPH as a stable radical to measure the ability of seaweed antioxidant compounds, e.g. carotenoids and chlorophyll, to scavenge and neutralize ROS and proton radicals, generated in tissues as result of oxidative stress [15]. It uses mechanisms based on the single electron transfer (SET) [43]. The IC50 values demonstrated that E(H2O)M2 and E(EtOH)M2 were the most efficient extracts (Table 3), with values of 1.37 ± 0.03 mg/ml and 1.37 ± 0.04 mg/ml, respectively. E(H20)M1 was the least efficient extract, having the highest value for IC50, 4.65 ± 0.29 mg/ml. It can be observed how two different methodologies greatly influence the performance of the final extract, although using the same solvent. In M1, a clear differentiation was detected, resulting in E(EtOH)M1 < E(MeOH)M1 < E(EtAc)M1 < E(H2O)M1, with E(EtOH)M1 needing 3 times less extract to develop the same activity as E(H2O)M1. For M2, although some variation occurred in the sequence, the values are very similar, demonstrating high efficiency. The crescent order for M2 is E(H2O)M2 < E(EtOH)M2 < E(EtAc)M2 < E(MeOH)M2, with the three first solvents presenting better results than E(EtOH)M1. This in an evidence that M2, independently of the solvent used, has a higher ability of producing extracts with better IC50 results. In M1, the results will vary greatly, depending of the solvent used to produce the extract. Mellouk et al. (2017) [28] also evaluated A. taxiformis of which water, methanol and ethanol extracts also produced good DPPH scavenging activity, using an extract concentration lower than 0.2 mg/ml. Statistical analysis was performed, and Tukey's b test determined that in M1 there are four distinct groups, highlighting the intrinsic capability of each solvent to produce a different extract with singular scavenging activity. In M2, only 2 distinct groups were formed, of which E(H2O)M2, E(EtOH)M2 and E(EtAc)M2 are one and E(MeOH)M2 the second group. No correlation between methods was determined using Pearson's test (Table 2), showing a clear independence and with no correlation between equal solvents.
3.4.3. Ferrous ion chelation (FIC)
The ferrous ion chelation (FIC) assay measures the ability of secondary antioxidants to inhibit oxidation through an indirect approach, in this case metal chelating [44]. It was demonstrated, for method 1 (Table 3), that E(EtAc)M1 developed 50% of chelating activity with the lowest concentration of extract, 1.57 mg/ml and E(H20)M1 the highest, 112.32 mg/ml. For method 2, E(EtAc)M2 presented the lowest concentration needed to achieve 50% of chelating activity, 5.88 mg/ml, and E(H20)M2 the highest concentration, 73.97 mg/ml. In M1, in increasing order of concentration to achieve 50 % of chelating activity, E(EtAc)M1 < E(EtOH)M1 < E(MeOH)M1 < E(H2O)M1. In M2, the order was slightly different, E(EtAc)M2 < E(MeOH)M2 < E(EtOH)M2 < E(H2O)M2. Except for water and ethyl acetate, the other extracts varied inversely, due to the change in the methodology. Statistical analysis using Tukey's b test resulted in four distinct groups in M1, showing independence in the results obtained in each extract and in M2 only three groups were formed, of which extracts produced with ethanol and methanol are in the same. Pearson's correlation (Table 2) between M1 and M2 is R2 = 0.855, showing strong relation between methodologies and attributing similar activity between different extracts, using the same solvent, in both methods. Also, when evaluating Pearson's correlation between parameters, FIC was determined to have a strong linear correlation with TCC, highlighting that carotenoid content is linked to the antioxidant capacity, when evaluating the ability of carotenoids to function as chelators, with a secondary antioxidant mechanism.
3.5. Downstream extractions
In order to test a downstream extraction, the remaining seaweed residue was kept and dried in an oven at 40 °C and weighed. Residue derived from seaweed extracts are denominated as PEAR (post-extracted algal residue) or simply as algal cake. This strategy was implemented to overcome some problems that often occur, which are the discharge of residue, low profitability and high input of biomass. This approach intends to understand how much can be extracted from PEAR, using multiple steps, to extract and purify high value seaweed derived products. Three extraction procedures in a sequential way were assessed. Each time PEAR was subjected to an extraction protocol, the residue was dried, grounded and weighed to determine the real yield that can be obtained from the subsequent residue. Lipids were primarily extracted from PEAR, followed by carrageenan and cellulose. Results are shown in Table 4.
Table 4.
Yield of PEAR and quantification of subsequent extraction of lipids, carrageenan's and cellulose from Asparagopsis taxiformis (Delile) Trevisan residue.
| Solvent | PEAR Yield |
Lipids |
Carrageenans |
Cellulose |
||||
|---|---|---|---|---|---|---|---|---|
| g (100 g)−1 in dw |
g (100 g)−1 in dw |
g (100 g)−1 in dw |
g (100 g)−1 in dw |
|||||
| M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | |
| Water | 58.73 ± 5.54a | 76.08 ± 1.87a | 1.80 ± 0.04a | 1.30 ± 0.03a | 3.75 ± 1.56a | 2.28 ± 0.47a | 23.81 ± 0.89a | 20.74 ± 0.68a |
| Ethanol | 75.43 ± 3.65b | 87.30 ± 1.18bc | 0.65 ± 0.06b | 0.58 ± 0.03b | 6.98 ± 1.56a | 14.30 ± 1.46b | 21.65 ± 2.69a | 18.13 ± 0.52b |
| Methanol | 72.47 ± 0.39c | 81.20 ± 5.59ab | 0.26 ± 0.03c | 0.35 ± 0.03c | 7.77 ± 1.83a | 6.85 ± 3.07a | 20.30 ± 0.89a | 20.59 ± 0.37a |
| Ethyl acetate | 82.71 ± 2.86d | 92.75 ± 1.00c | 2.05 ± 0.03d | 1.57 ± 0.08d | 20.78 ± 1.88b | 21.18 ± 0.81c | 18.59 ± 0.49a | 18.47 ± 0.54b |
Data are mean ± standard deviation in grams per 100 grams of seaweed residue on a dry weight basis. All determinations were carried out in triplicate.
Different letters within the same column indicate significant differences (p ≤ 0.01) determined in SPSS 24.0 using Tukey b test.
3.5.1. Residue quantification
R(EtAc)M2 presented the highest amount of PEAR as a starting material, 92.75 ± 1.00 g/100g dw and lowest from R(H2O)M1, 58.73 ± 5.54 g/100g dw. Using M1, in decreasing order of PEAR yield, R(EtAc)M1 > R(EtOH)M1 > R(MeOH)M1 > R(H2O)M1. In M2, the order was exactly the same only varying the quantity of PEAR yield. Statistical analysis, using Tukey's b analysis, showed that in M1, all PEAR yield formed statistically different groups and in M2 no individual groups were formed. We can assume that M1 makes a clear distinction between different PEAR yields, however this cannot be observed in M2, due to the variation of extraction using Soxhlet apparatus, defining its efficiency. This is intrinsically linked to the method rather than the solvent previously used. Pearson's correlation test demonstrated that M1 and M2 are close related (Table 2), which originated a correlation of R2 = 0.914. Also, it was determined that PEAR yield is positively correlated with cellulose, with flavonoids and TCC measured in the primary extract.
3.5.2. Lipids extraction
Lipids were the first product to be extracted from PEAR, using the solvent extraction procedure described by Folch et al. (1957) [25]. Highest lipid content was determined in L-R(EtAc)M1, 2.05 ± 0.03 g/100g dw and the lowest quantity in L-R(MeOH)M1, 0.26 ± 0.03 g/100g dw. For M1, resulted in the following sequence, in decreasing order of lipids quantity, L-R(EtAc)M1 > L-R(H2O)M1 > L-R(EtOH)M1 > L-R(MeOH)M1. The order was exactly the same in M2, with quantities varying slightly. We have also performed direct extraction of lipids, using same procedure, using whole seaweed A. taxiformis, and found that 5.42 ± 0.23 g/100g dw of lipids could be extracted. Comparing the results of downstream processing with direct extraction of whole seaweed A. taxiformis, we found that from whole seaweed, two to fourteen times higher lipids yield could be extracted. However, lipids extracted from PEAR could be a solution to increase profitability from the same biomass. According to Mellouk et al. (2017) [28], 2.85 g/100g dw of lipids could be extracted from Algerian A. taxiformis of which 91% are FAME, comprised of 23% saturated and 68% unsaturated fatty acids. The different yield obtained in our work could be due to different extraction technics or due to the natural variations in biocompounds within the A. taxiformis seaweed, that occur throughout the year or being located in different sites, in this case in the Atlantic Ocean and Mediterranean Sea, respectively. El-Baroty et al. (2007) [45], analysed the lipid composition concluding that ω3 linolenic acid is the major fatty acid of the lipid fraction in A. taxiformis and Mozaffarian (2005) [46] demonstrating that this fatty acid has the ability to prevent cardiovascular diseases. According to these previous studies, lipids extracted from A. taxiformis could became an important product when assessing its nutraceutical applicability.
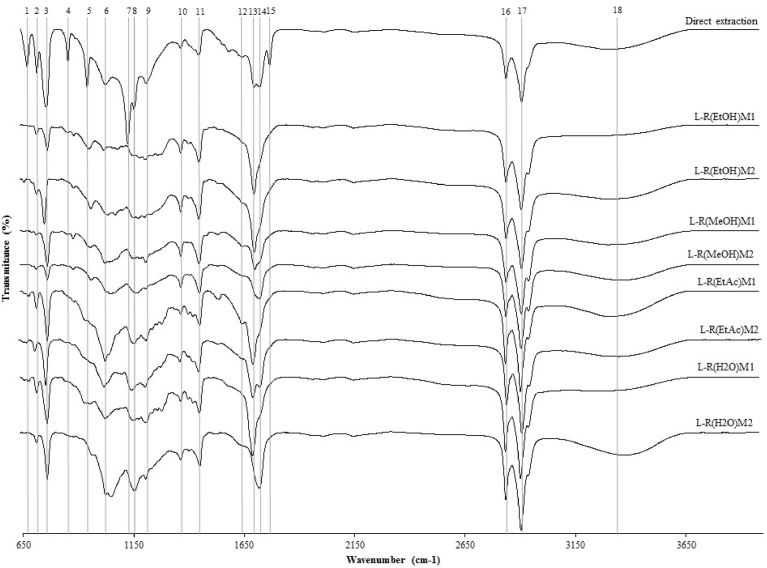
All lipid extractions were subjected to FTIR-ATR scan for comparative analysis (Fig. 2). A direct extraction of lipids from A. taxiformis was performed permitting a visual comparison between this extraction and lipids extracted from PEAR. Eighteen major peaks were identified from 650 to 4000 cm−1 wavenumber. This technique was used to evaluate the variability that occurs when extracting lipids from PEAR and perform a primary characterization of lipids extracted from A. taxiformis producing a fingerprint spectra. Peaks represent the percentage of transmittance in corresponding wavenumber measured in cm−1. The IR spectra is majorly divided in two groups. From wavenumber 650 to 1800 cm−1, fifteen major peaks can be observed and correlated with lipids polar head groups and from wavenumber 2800 to 4000 cm−1, a second group is observed, with 3 major peaks. Bands from 1 to 9 can also be correlated with high concentration of halogens in A. taxiformis [2]. According to Burreson et al. (1975) [47], which initially evaluated the haloforms in the essential oil of A. taxiformis, collected in Waikiki (Hawai), due to their interest in the odoriferous constituents, they have determined that the main component of this essential oil is the bromoform CHBr3, followed by smaller amounts of numerous chlorine and iodine haloforms. Recently, Machado et al. (2016) [29], evaluated whole and several extracts of A. taxiformis potential to inhibit methanogenesis. They have extracted a high yield of the bromoform CHBr3 using DCM in dried A. taxiformis (172.32 mg/100g dw) which successfully reduced the in vitro total gas production, using concentrations ≥ 5μM. Additionally, new highly brominated cyclopentenones were recently discovered in A. taxiformis extracts. These new molecules are called “Mahorones” and were discovered while Greff et al. (2014) [48] were initiating a chemical ecological study to determine the function of secondary metabolites in their interaction with native species.
Fig. 2.
FTIR ATR spectra, plotting wavenumber (cm−1) by transmittance (%), of lipids extracted using Folch et al. (1957) [40] methodology of whole seaweed (direct extraction) or from PEAR.
Band 2 at 720 cm−1 can be attributed to CH2 rocking and band 11 could also be attributed to CH2 bending. Band 16 and 17 are due to C-H stretching in CH3 and CH2. Peaks within 1070 and 1250 cm−1 (band 7 and 8) are corresponding to C—O—C stretching and band 14 to C O stretching in esters [14]. The wider peak (band 18) is due to some water content in lipid extract. Comparing the FTIR-ATR spectra, it becomes possible to visualize the fluctuation of major peaks intensity between direct extraction and lipid extracted from the downstream residue. This technic should be suitable for rapid quality control with further studies in lipid composition of A. taxiformis.
Statistical analysis, using Tukey's b test, demonstrated that all of the values in M1 and M2 are independent, presenting each PEAR as unique biomass in terms of lipid extractability. Pearson's correlation test showed that M1 and M2 have an R2 = 0.992 (Table 2), also showing that the lipid extraction is independent of this two methodologies and strongly dependent of the solvent primarily used.
3.5.3. Carrageenan extraction
Carrageenan was the second product to be extracted from PEAR and highest yield (Table 4) was obtained in C-R(EtAc)M2, 21.18 ± 0.81 g/100g dw and the lowest quantity obtained in C-R(H2O)M2, 2.28 ± 0.47 g/100g dw. The resulting sequence, in decreasing order in M1, was C-R(EtAc)M1 > C-R(MeOH)M1 > C-R(EtOH)M1 > C-R(H2O)M1. In this sequence, where the solvent has direct contact for 72 hours with the biomass, it can be observed that carrageenan yield increase is inversely correlated with the polarity index of the solvent used for extraction, having ethyl acetate 4.4, methanol 5.1, ethanol 5.2 and water 9.0. For M2, the polarity index was not as linear, and changed the order of the sequence resulting in C-R(EtAc)M2 > C-R(EtOH)M2 > C-R(MeOH)M2 > C-R(H2O)M2. We have performed the extraction of carrageenan from whole A. taxiformis and found that 20.11 ± 1.09 g/100g dw (data not shown) could be extracted. Comparing this result with PEAR carrageenan extraction, a quantity of one to nine times higher could be extracted from the whole seaweed that the amount which is extracted from PEAR. Although, PEAR could present a slightly higher result of carrageenan yield when comparing to whole seaweed due to the concentration process. When extracting several compounds from the initial biomass, this residue will inevitably become more concentrated in certain compounds that were not previously extracted. This will make carrageenan more concentrated in PEAR than in whole seaweed. C-R(EtAc)M1 and C-R(EtAc)M2 have carrageenan yield similar to whole seaweed and C-R(H2O)M2 about 8.8 times less than whole seaweed. Comparing with the work of Hung et al. (2009) [49], 49% of the dw of carrageenan-rich Kappaphycus alvarezii cultivated in Camranh Bay, Vietnam, are carrageenans. Although A. taxiformis and its extracts present far less carrageenan, bioactivity could be an important factor to assess special features that would increase its applicability.
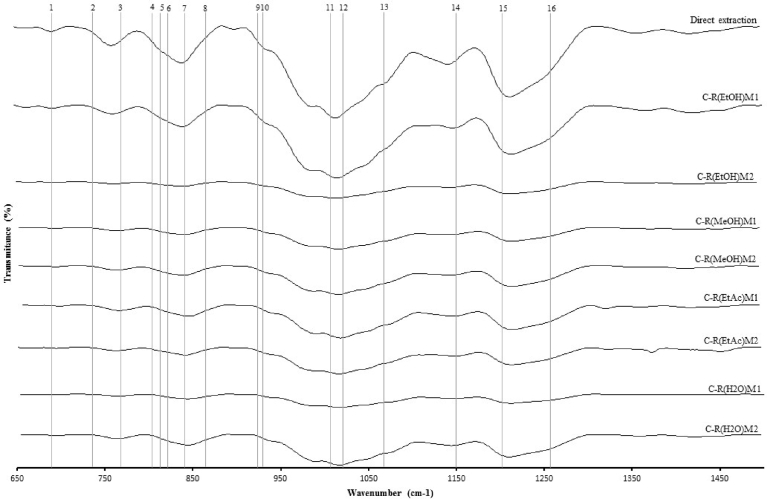
Carrageenan extracted from PEAR and obtained with a direct extraction were subjected to FTIR-ATR scan for comparative analysis between 650 to 1500 cm−1 (Fig. 3). Band 1 (694 cm−1), between band 2 (741 cm−1) and 3 (770 cm−1), peaks are usually assigned to skeleton bending of pyranose ring. This is well observed in direct extraction and C-R(EtOH)M1 spectra. According to Knutsen et al. (1994) [50], that implemented a nomenclature for red seaweed polysaccharides, absorption at 805 cm−1 (band 4) are manifestations of 3,6-anhydro-D-galactose-2-sulphate (DA2S) and can be assigned to iota hybridization of carrageenan. Band 4 appeared prominently as a shoulder in direct extraction and C-R(EtOH)M1 readings. In the same extractions, a strong absorption at 820 cm−1 (band 5) and 867 cm−1 (band 8), indicates the presence of D-galactose-6-sulphate (D6S), attributed to mu carrageenan, a precursor of kappa. At 825 cm−1 (band 6) is assigned to D-galactose-2,6-disulphate (D2S, 6S), the nu carrageenan, a precursor of iota. At 845 cm−1 (band 7), assigned to the presence of D-galactose-4-sulphate (G4S), is representative of kappa carrageenan. This appeared as a peak in direct extraction and C-R(EtOH)M1 and in a lesser extend in the other carrageenan extractions. The degree of hybridization between iota and kappa are usually performed, dividing the value obtained at 805 cm−1 by the value obtained at 845 cm−1, originating a ratio of carrageenan hybridization [51]. This is particularly important due to the variation that occurs with carrageenan that is constituted mostly by kappa hybridization, resulting in a strong but brittle gel and iota carrageenan in soft and elastic gel. Differences in these ratios, defines the application of carrageenan extractions, majorly in the food industry, for example, in the production of chocolate milk [52]. Ratios obtained were between 1.02 in C-R(EtOH)M2 and 1.19 in direct extraction, with C-R(EtOH)M1 obtaining the highest ratio, 1.11 within carrageenan extracted from PEAR. This indicates the majority of iota hybridization in carrageenan extracted from A. taxiformis. Absorption of IR between 928 cm−1 (band 9) and 933 cm−1 (band 10) with a shoulder at 1070 cm−1 (band 13) represents the presence of 3,6 anhydro-D-galactose (DA). Absorption between 1010 cm−1 (band 11) and 1030 cm−1 (band 12) with an additional band at 1150 cm−1 (band 14) may be attributed to C—C and C—O stretching vibrations of pyranoid ring, common to all polysaccharides [53]. Considering absorptions of IR between 1210 cm−1 (band 15) and 1260 cm−1 (band 16), it is attributed to sulphate ester (O—SO−3) (S). This is particularly important due to the rheological properties that are influenced by the degree of sulfation within the polymer. This characterization can be well observed in direct extraction and C-R(EtOH)M1. Carrageenan extracted from C-R(EtOH)M1 presents a pattern very similar to carrageenan extracted from A. taxiformis direct extraction.
Fig. 3.
FTIR ATR spectra, plotting wavenumber (cm−1) by transmittance (%), of carrageenan extracted using Tasende et al. (2012) [41] methodology of whole seaweed (direct extraction) or from PEAR.
Statistical analysis using Pearson correlation (Table 2), demonstrated an inverse proportionally between extraction yield and carrageenan extraction yield but positively related with flavonoids content in the primary extract. Tukey's b test showed that in M1, 2 groups are formed, C-R(H20)M1, C-R(EtOH)M1 and C-R(MeOH)M1 and second group only C-R(EtAc)M1. For M2, 3 groups were formed, of which one was formed by C-R(H20)M2 and C-R(MeOH)M2, C-R(EtOH)M2 and C-R(EtAc)M2.
3.5.4. Cellulose extraction
Thirdly, we have successfully extracted cellulose from the remaining residue and the highest cellulose value is presented in Ce-R(H20)M1, 23.81 ± 0.89 g/100g dw and the lowest in Ce-R(EtOH)M2, 18.13 ± 0.52 g/100g dw (Table 4). The sequence, from the highest content to the lowest was Ce-R(H2O)M1 > Ce-R(EtOH)M1 > Ce-R(MeOH)M1 > Ce-R(EtAc)M1. Here, we can observe that the extraction yield of cellulose is proportional to the polarity index of the solvents used. For M2, this was not observed, and the sequence resulted in Ce-R(H2O)M2 > Ce-R(MeOH)M2 > Ce-R(EtAc)M2 > Ce-R(EtOH)M2. We also determined the cellulose content from whole seaweed A. taxiformis, and determined that 9.76 ± 0.15 g/100g dw could be obtained from direct extraction. Comparing with PEAR residue, cellulose could be extracted from PEAR 1.9 to 2.4 times higher than from direct extraction. The motive of the increase ratio, comparing PEAR with whole seaweed, is due to an increase of cellulose concentration in final PEAR, due to selectively extracting other compounds. In each time that we extracted a different compound from initial PEAR, we would dry, grind and weight, and each time we did this, cellulose would get concentrated in final PEAR. According to Siddhanta et al. (2009) [54], 2.5–12.5% of cellulose could be determined in 12 different seaweeds from India, evidencing the potential that PEAR has in extracting and purifying cellulose. Long polymeric chains of cellulose can be extracted from seaweed and hydrolyzed forming nanocellulose [55]. Nanocristaline cellulose has an enormous potential, with multiple applications such as the production of films with barrier properties, biocomposites, and systems for controlled drug release [56], which justifies the interest of extracting cellulose from PEAR.
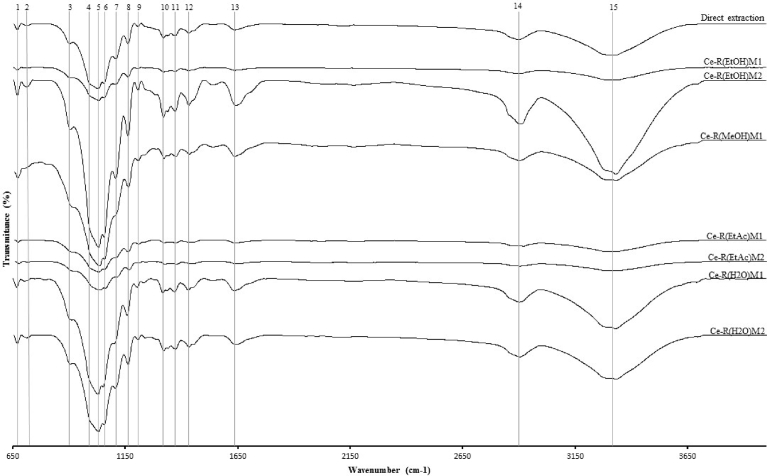
Using FTIR ATR analysis we developed a fingerprint spectra for cellulose extracted from red seaweed A. taxiformis (Fig. 4). Cellulose extracted and purified from PEAR was compared with cellulose obtained from direct extraction from whole seaweed A. taxiformis. Fifteen major bands were identified from 650 to 4000 cm−1 in FTIR ATR spectra which can be used for comparability. Decrease of transmittance % at 900 cm−1 (band 3) are due to the absorption of IR from glycosidic linkages between the anhydroglucose rings in the cellulose. This peak can be observed in every cellulose extracted from PEAR but better distinguish in Ce-R(EtOH)M2. Between 1000 cm−1 (band 4) and 1030 (band 5) decreases in transmittance could be due to C—O—C bending in which Ce-R(EtOH)M2 demonstrates prominently. At 1054 cm−1 (band 6) a strong decrease of transmittance could be observed and can be assigned to the skeletal vibration of C—O—C pyranose ring skeleton in cellulose fibers. This peak was visible in all of the cellulose extracted from PEAR and directly extracted from whole seaweed but with different intensities. At 1160 cm−1 (band 8), a decrease in transmittance can be assigned to C—O—C asymmetric stretching of cellulose. This can be well observed in cellulose obtained directly from whole seaweed but more distinguish in Ce-R(EtOH)M2. A decrease in transmittance at 1432 cm−1 (band 12) could be due to C-H bending and at 1642 cm−1 (band 13) IR absorbed could be assigned to O-H bending in water molecules. An sp3 C-H stretching vibration was detected at 2903 cm−1 (band 14) and a broad peak appeared at 3316 cm−1 (band 15) which could be assigned as a specific absorbance of O—H stretching, demonstrating an hydrophilic tendency of the cellulose fibers [57]. This peak is visible in all cellulose extracted from PEAR and whole seaweed A. taxiformis but prominently visible in Ce-R(EtOH)M2.
Fig. 4.
FTIR ATR spectra, plotting wavenumber (cm−1) by transmittance (%), of cellulose extracted using Baghel et al. (2015) [42] methodology of whole seaweed (direct extraction) or from PEAR.
Statistical analysis showed that applying Tukey's b test, M1 only formed one group, suggesting that these values are not statistically different and in M2 two groups were formed. One group includes Ce-R(H2O)M2 and Ce-R(MeOH)M2, the second group the remaining Ce-R(EtOH)M2 and Ce-R(EtAc)M2. The Pearson's correlation test (Table 2) demonstrated that there isn't correlation between methodologies, and cellulose is negatively correlated with residue yield proving the statement previously described that cellulose will be increasingly concentrated after supplement, lipids and carrageenan extraction.
4. Conclusion
The perspective of this research work was to increase the knowledge of the nutraceutical potential of A. taxiformis from Madeira archipelago, as a whole or, its extracts. Also, the development of a downstream strategy, to develop procedures to purify valuable compounds from A. taxiformis PEAR, was performed, attributing applicability and yield of important products to remaining residue. This seaweed demonstrated to be an excellent source of natural iodine, utilizing four solvents permitted in the food industry with two distinct methodologies. Method 1 demonstrated to be the most predictable, since certain parameters could be related with the polarity index of the solvents used. Since our main effort was to produce an iodine rich extract, ethanol demonstrated to be the most efficient solvent in having the most concentrated iodine content. Evaluation of the antioxidant compounds in E(EtOH)M1 resulted in the highest values for TPC and chlorophyll a content. Also, in three parameters analysed for antioxidant capacity, E(EtOH)M1 developed the best results for RA and FRSA antioxidant capacity tests. For the downstream strategy adopted in this work, lipids could be further studied to determine their fatty acids composition, carrageenan studied in their gelling properties, purity and bioactivity. Cellulose could be the precursor from low to high end products, since this could be the starting material to produce paper, bioethanol or micro and nano-crystalline cellulose, a multifunctional ingredient in the food and pharmaceutical industry. When targeting iodine rich extracts, we have determined, using statistical analysis, that iodine content is positively correlated with TPC and negatively correlated with flavonoids, allowing us to extrapolate in the future, which extracts would have higher concentration of iodine based on TPC or flavonoids analysis. FTIR ATR analysis demonstrated high potential for spectra comparison and quality control due to its simplicity and time saving. It produced a fingerprint signature for lipids, carrageenan and cellulose extracted directly from whole seaweed A. taxiformis, used for comparison between same products extracted from PEAR.
Declarations
Author contribution statement
Nuno Nunes: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Sofia Valente: Performed the experiments.
Sónia Ferraz: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Maria do Carmo Barreto: Conceived and designed the experiments.
Miguel Carvalho: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by ISOPlexis, University of Madeira; Blue Iodine II “Boost Blue economy through market uptake an innovative seaweed bioextract for iodine fortification II” (733552, H2020-SMEInst-2016-2017); DemoBlueAlgae “Desenvolvimento de metodologias e optimização dos processos de cultivo e processamento de macroalgas para a indústria e economia azul” PROCiência 2020 (M1420-01-0247-FEDER000002); MACBIOBLUE “Proyecto demostrativo y de transferencia tecnológica para ayudar a las empresas a desarrollar nuevos productos y procesos en el ámbito de la Biotecnología Azul de la Macaronesia” (MAC/1.1b/086); and ARDITI - Regional Agency for the Development of Research Technology and Innovation (M14-20 - 09-5369-FSE-000001- Doctorate in Business and UBQ II, Unidade de Bioquímica company).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Kaliaperumal N. Vol. 3. SDMRI Research Publication; 2003. pp. 33–42. (Products from Seaweeds). [Google Scholar]
- 2.McConnell O., Fenical W. Halogen chemistry of the red alga Asparagopsis. Phytochemistry. 1977;16:367–374. [Google Scholar]
- 3.Paul N.A., Cole L., De Nys R., Steinberg P.D. Ultrastructure of the gland cells of the red alga Asparagopsis armata (Bonnemaisoniaceae) J. Phycol. 2006;42:637–645. [Google Scholar]
- 4.James P.S.B.R. Seaweed research and utilization in India. CMFRI Bull. 1987;41:1–116. [Google Scholar]
- 5.EFSA . Intake Levels Vitam. Miner.; 2006. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Folate (Expressed on 19 October 2000), Tolerable up; pp. 51–58. [Google Scholar]
- 6.Saenko G.N., Kravtsova Y.Y., V Ivanenko V., Sheludko S.I. Marine biology concentration of iodine and bromine by plants in the seas of Japan and Okhotsk. Mar. Biol. 1978;47:243–250. [Google Scholar]
- 7.Hou X., Chai C., Qian Q., Yan X., Fan X. Determination of chemical species of iodine in some seaweeds (I) Sci. Total Environ. 1997;204:215–221. [Google Scholar]
- 8.Holdt S.L., Kraan S. Bioactive compounds in seaweed: functional food applications and legislation. J. Appl. Phycol. 2011;23:543–597. [Google Scholar]
- 9.Chan E.W.C., Lim Y.Y., Chew Y.L. Antioxidant activity of Camellia sinensis leaves and tea from a lowland plantation in Malaysia. Food Chem. 2007;102:1214–1222. [Google Scholar]
- 10.Cox S., Abu-Ghannam N., Gupta S. An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. Int. Food Res. J. 2010;17:205–220. http://arrow.dit.ie/schfsehart/33 [Google Scholar]
- 11.Okuzumi J., Takahashi T., Yamane T., Kitao Y., Inagake M., Ohya K., Nishino H., Tanaka Y. Inhibitory effects of fucoxanthin, a natural carotenoid, on N-ethyl-N'-nitro-N-nitrosoguanidine-induced mouse duodenal carcinogenesis. Cancer Lett. 1993;68:159–168. doi: 10.1016/0304-3835(93)90142-v. [DOI] [PubMed] [Google Scholar]
- 12.Van Hal J.W., Huijgen W.J.J., López-Contreras A.M. Opportunities and challenges for seaweed in the biobased economy. Trends Biotechnol. 2014;32:231–233. doi: 10.1016/j.tibtech.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert-López B., Mendiola J.A., Fontecha J., van den Broek L.A.M., Sijtsma L., Cifuentes A., Herrero M., Ibáñez E. Downstream processing of Isochrysis galbana: a step towards microalgal biorefinery. Green Chem. 2015;17:4599–4609. [Google Scholar]
- 14.Derenne A., Vandersleyen O., Goormaghtigh E. Lipid quantification method using FTIR spectroscopy applied on cancer cell extracts. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2014;1841:1200–1209. doi: 10.1016/j.bbalip.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Chew Y.L., Lim Y.Y., Omar M., Khoo K.S. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT Food Sci. Technol. 2008;41:1067–1072. [Google Scholar]
- 16.Wellburn A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994;144:307–313. [Google Scholar]
- 17.Kumar J.I.N., Kumar R.N., Bora A., Amb M.K., Chakraborthy S. An evaluation of the pigment composition of eighteen marine Macroalgae collected from Okha Coast, Gulf of Kutch, India. Our Nat. 2010;7:48–55. [Google Scholar]
- 18.Chan P.T., Matanjun P., Yasir S.M., Tan T.S. Antioxidant activities and polyphenolics of various solvent extracts of red seaweed, Gracilaria changii. J. Appl. Phycol. 2015;27:2377–2386. [Google Scholar]
- 19.Yen G.-C., Chen H.-Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995;43:27–32. [Google Scholar]
- 20.Duan X.J., Zhang W.W., Li X.M., Wang B.G. Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. Food Chem. 2006;95:37–43. [Google Scholar]
- 21.Yuan Y.V., Bone D.E., Carrington M.F. Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem. 2005;91:485–494. doi: 10.1016/j.fct.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Decker E.A., Welch B. Role of Ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990;38:674–677. [Google Scholar]
- 23.Mahesh D.L., Deosthale Y.G., Rao B.S.N. A sensitive kinetic assay for the determination of iodine in foodstuffs. Food Chem. 1992;43:51–56. [Google Scholar]
- 24.Pino S., Fang S.L., Braverman L.E. Ammonium persulfate: a safe alternative oxidizing reagent for measuring urinary iodine. Clin. Chem. 1996;42:239–243. [PubMed] [Google Scholar]
- 25.Folch J., Lees M., Stanley G.H.S. A simple method for the isolation and purification of total lipids from animal animal tissues. J. Biol. Chem. 1957;226:497–509. http://www.jbc.org/content/226/1/497.citation [PubMed] [Google Scholar]
- 26.Tasende M.G., Cid M., Fraga M.I. Spatial and temporal variations of Chondrus crispus (Gigartinaceae, Rhodophyta) carrageenan content in natural populations from Galicia (NW Spain) J. Appl. Phycol. 2012;24:941–951. [Google Scholar]
- 27.Baghel R.S., Trivedi N., Gupta V., Neori A., Reddy C.R.K., Lali A., Jha B. Biorefining of marine macroalgal biomass for production of biofuel and commodity chemicals. Green Chem. 2015;17:2436–2443. [Google Scholar]
- 28.Mellouk Z., Benammar I., Krouf D., Goudjil M., Okbi M., Malaisse W. Antioxidant properties of the red alga Asparagopsis taxiformis collected on the North West Algerian coast. Exp. Ther. Med. 2017;13:3281–3290. doi: 10.3892/etm.2017.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machado L., Magnusson M., Paul N.A., Kinley R., de Nys R., Tomkins N. Identification of bioactives from the red seaweed Asparagopsis taxiformis that promote antimethanogenic activity in vitro. J. Appl. Phycol. 2016;28:3117–3126. [Google Scholar]
- 30.Pucci E., Chiovato L., Pinchera A. Thyroid and lipid metabolism. Int. J. Obes. Relat. Metab. Disord. 2000;24(Suppl. 2):S109–S112. doi: 10.1038/sj.ijo.0801292. [DOI] [PubMed] [Google Scholar]
- 31.Andersson M., Karumbunathan V., Zimmermann M.B. Global iodine status in 2011 and trends over the past decade. J. Nutr. 2012;142:744–750. doi: 10.3945/jn.111.149393. [DOI] [PubMed] [Google Scholar]
- 32.Limbert E., Prazeres S., São Pedro M., Madureira D., Miranda A., Ribeiro M., De Castro J.J., Carrilho F., Oliveira M.J., Reguengo H., Borges F. Iodine intake in Portuguese pregnant women: results of a countrywide study. Eur. J. Endocrinol. 2010;163:631–635. doi: 10.1530/EJE-10-0449. [DOI] [PubMed] [Google Scholar]
- 33.Andersson M., De Benoist B., Darnton-Hill I., Delange F. WHO; Geneva: 2007. Iodine Deficiency in Europe: a Continuing Public Health Problem; pp. 1–86. [Google Scholar]
- 34.Sabeena Farvin K.H., Jacobsen C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013;138:1670–1681. doi: 10.1016/j.foodchem.2012.10.078. [DOI] [PubMed] [Google Scholar]
- 35.Hou X., Yan X., Chai C. Chemical species of iodine in some seaweeds II. Iodine-bound biological macromolecules. J. Radioanal. Nucl. Chem. 2000;245:461–467. [Google Scholar]
- 36.Seleem D., Pardi V., Murata R.M. Archives of oral biology review of flavonoids: a diverse group of natural compounds with anti- Candida albicans activity in vitro. Arch. Oral Biol. 2017;76:76. doi: 10.1016/j.archoralbio.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 37.Stankovic M.S., Niciforovic N., Topuzovic M., Solujic S. Total phenolic content, flavonoid concentrations and antioxidant activity, of the whole plant and plant parts extracts from Teucrium montanum L. var. montanum, f. supinum (L.) reichenb, Biotechnol. Biotechnol. Equip. 2011;25:2222–2227. [Google Scholar]
- 38.Lanfer-Marquez U.M., Barros R.M.C., Sinnecker P. Food Res. Int. 2005. Antioxidant activity of chlorophylls and their derivatives; pp. 885–891. [Google Scholar]
- 39.Takaichi S. Distributions, biosyntheses and functions of carotenoids in algae. Agro Food Ind. Hi-Tech. 2013;24:55–58. [Google Scholar]
- 40.Nunes N., Ferraz S., Valente S., Barreto M. do C., Pinheiro de Carvalho M.A.A. Biochemical composition, nutritional value, and antioxidant properties of seven seaweed species from Madeira archipelago. J. Appl. Phycol. 2017 [Google Scholar]
- 41.von Elbe J., Schwartz S. In: Food Chem. Dekker M., editor. CRC Press; New York: 1996. Colorants; pp. 651–722. [Google Scholar]
- 42.Pereira L. In: Springer Handbook of Marine Biotechnology, Part II. Kim Se-Kwon., editor. Springer; Berlin, Heidelberg: 2015. Chapter 6 – seaweed flora of the European North Atlantic and Mediterranean; pp. 65–178. [Google Scholar]
- 43.Prior R.L., Wu X., Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 44.Kristinsson H.G., editor. Antioxidants and Functional Components in Aquatic Foods. Wiley-Blackwell; Reykjavik, Iceland: 2014. [Google Scholar]
- 45.El-Baroty G.S., Moussa M.Y., Shallan M., Ali M., Sabh Z., Shalaby E. Contribution to the aroma , biological activities, minerals, protein, pigments and lipid contents of the red Alga : Asparagopsis taxiformis (Delile) Trevisan. J. Appl. Sci. Res. 2007;3:1825–1834. [Google Scholar]
- 46.Mozaffarian D. Does alpha-linolenic acid intake reduce the risk of coronary heart disease? A review of the evidence. Altern. Ther. Health Med. 2005;11:24–31. [PubMed] [Google Scholar]
- 47.Burreson B.J., Moore R.E., Roller P. Haloforms in the essential oil of the alga Asparagopsis taxiformis (Rhodophyta) Tetrahedron Lett. 1975:473–476. [Google Scholar]
- 48.Greff S., Zubia M., Genta-Jouve G., Massi L., Perez T., Thomas O.P. Mahorones, highly brominated cyclopentenones from the red alga Asparagopsis taxiformis. J. Nat. Prod. 2014;77:1150–1155. doi: 10.1021/np401094h. [DOI] [PubMed] [Google Scholar]
- 49.Hung L.D., Hori K., Nang H.Q., Kha T., Hoa L.T. Seasonal changes in growth rate, carrageenan yield and lectin content in the red alga Kappaphycus alvarezii cultivated in Camranh Bay, Vietnam. J. Appl. Phycol. 2009;21:265–272. [Google Scholar]
- 50.Knutsen S.H., Myslabodski D.E., Larsen B., Usov A.I. A modified system of nomenclature for red algal Galactans. Bot. Mar. 1994;37:163–170. [Google Scholar]
- 51.Pereira L., Mesquita J.F. Carrageenophytes of occidental Portuguese coast: 1-spectroscopic analysis in eight carrageenophytes from Buarcos bay. Biomol. Eng. 2003;20:217–222. doi: 10.1016/s1389-0344(03)00056-x. [DOI] [PubMed] [Google Scholar]
- 52.Villanueva R.D., Mendoza W.G., Rodrigueza M.R.C., Romero J.B., Montaño M.N.E. Structure and functional performance of gigartinacean kappa-iota hybrid carrageenan and solieriacean kappa-iota carrageenan blends. Food Hydrocolloids. 2004;18:283–292. [Google Scholar]
- 53.Gómez-Ordóñez E., Rupérez P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocolloids. 2011;25:1514–1520. [Google Scholar]
- 54.Siddhanta A.K., Prasad K., Meena R., Prasad G., Mehta G.K., Chhatbar M.U., Oza M.D., Kumar S., Sanandiya N.D. Profiling of cellulose content in Indian seaweed species. Bioresour. Technol. 2009;100:6669–6673. doi: 10.1016/j.biortech.2009.07.047. [DOI] [PubMed] [Google Scholar]
- 55.Chen Y.W., Lee H.V., Juan J.C., Phang S.M. Production of new cellulose nanomaterial from red algae marine biomass Gelidium elegans. Carbohydr. Polym. 2016;151:1210–1219. doi: 10.1016/j.carbpol.2016.06.083. [DOI] [PubMed] [Google Scholar]
- 56.Ditzel F.I., Prestes E., Carvalho B.M., Demiate I.M., Pinheiro L.A. Nanocrystalline cellulose extracted from pine wood and corncob. Carbohydr. Polym. 2017;157:1577–1585. doi: 10.1016/j.carbpol.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 57.Singh S., Gaikwad K.K., Il Park S., Lee Y.S. Microwave-assisted step reduced extraction of seaweed (Gelidiella aceroso) cellulose nanocrystals. Int. J. Biol. Macromol. 2017;99:506–510. doi: 10.1016/j.ijbiomac.2017.03.004. [DOI] [PubMed] [Google Scholar]