Abstract
Caroli syndrome, which is characterized by saccular and fusiform dilatation of the biliary ducts, is usually observed in association with autosomal recessive polycystic kidney disease (ARPKD). Although the diagnosis of ARPKD is generally easy to make in postnatal ultrasound, the diagnosis of Caroli syndrome may be challenging in prenatal ultrasound. Herein, we present a case of a 29-week fetus with ARPKD associated with Caroli syndrome in whom fetal magnetic resonance imaging was essential to identify the “central dot sign” within the dilated biliary ducts to confirm the prenatal diagnosis of Caroli syndrome and to increase our level of confidence in this diagnosis.
Keywords: Fetal MRI, Caroli syndrome, Autosomal recessive polycystic kidney disease
Introduction
Autosomal recessive polycystic kidney disease (ARPKD; OMIM entry 263200) is a rare ciliopathy with an incidence of 1:20000 live births. ARPKD is primarily caused by mutations in the Polycystic Kidney and Hepatic Disease 1 gene on chromosome 6 that encodes for the protein fibrocystin/polyductin (FPC) [1], [2], [3]. The protein FPC is expressed in the primary cilia of renal cells, mainly in the collecting ducts and thick ascending loops of Henle and in bile duct epithelia [3]. Defective FPC ultimately results in abnormal cystic dilation of renal collecting ducts and ductal plate malformations of the biliary tree, eventually leading to significant renal and hepatic morbidity and mortality. Up to 30% of patients die in the perinatal period, usually as a result of respiratory insufficiency from pulmonary hypoplasia [1], [3], [4], [5], [6]. Those children who survive go on to develop progressive renal failure and portal hypertension due to progressive liver fibrosis [3]. However, the phenotypic and clinical manifestations of ARPKD exhibit great variability. All patients with ARPKD invariably have a ductal plate malformation [3] and develop clinical manifestations of congenital hepatic fibrosis over time; in contrast, nonobstructive dilation of the biliary tree, Caroli syndrome, is seen in a subset of patients only [1]. Caroli syndrome is inherited in an autosomal recessive fashion and refers to the combination of dilated bile ducts and congenital hepatic fibrosis usually seen in association with ARPKD, although it may also be observed in association with other entities. Caroli syndrome should not be confused with Caroli disease. Caroli disease is rarer, is inherited in an autosomal dominant fashion or occurs sporadically, and refers to the isolated dilation of the biliary tree, which may be segmental [7], [8]. The “central dot sign” seen in association with Caroli disease and Caroli syndrome represents the central fibrovascular bundle, containing a portal vein radicle and an accompanying hepatic artery branch, within the lumen of a dilated bile duct seen in cross-section [8], [9], [10]. A review of the literature showed less than a handful of cases with the prenatal diagnosis of ARPKD in association with Caroli syndrome [11], [12], [13]. In addition, only a single case report by Castro et al [13] showed fetal magnetic resonance imaging (MRI) findings. However, the presence of the “central dot sign” [8], [14], a finding characteristic of Caroli syndrome seen on postnatal cross-sectional imaging, was not illustrated and not discussed or elaborated in the prenatal case reported by Castro et al [13]. Here, we present the fourth case of ARPKD associated with Caroli syndrome diagnosed prenatally. Our case was unique in that the infant survived and is doing well after undergoing renal and liver transplantation, in addition to the florid presentation of biliary ductal dilation demonstrated on fetal MRI images. To the best of our knowledge, there are no reports on the visualization of the “central dot sign” on fetal MRI to characterize Caroli syndrome.
Case report
A healthy 28-year-old female (gravida 6, para 3) at 27 weeks of gestation was referred for assessment after routine ultrasound showed enlarged kidneys and hepatomegaly in a male fetus. A detailed fetal ultrasound was carried out at 29 weeks of gestation and showed oligohydramnios. The kidneys were enlarged and echogenic (Fig. 1). The liver was also enlarged and revealed multiple cystic structures (Fig. 2). Ultrafast multiplanar fetal MRI better demonstrated the tubular nature of the cystic structures, and some of these structures showed the “central dot sign”; representing a portal vein branch protruding into the lumen of a dilated bile duct when imaged in cross-section [8], [14]. The “central dot sign” is highly associated with Caroli syndrome and aids in differentiating it from other causes of biliary ductal dilation (Fig. 3). In addition, fetal MRI showed low-lung volumes with relatively hypointense lungs and enlarged kidneys with increased signal on fluid-sensitive sequences. The prenatal imaging findings were consistent with ARPKD in association with Caroli syndrome [15]. The patient developed anhydramnios at 34 weeks of gestation, necessitating a cesarean delivery. Postnatally, renal and liver function remained initially stable. The kidneys were quite enlarged (Fig. 4), leading to labored breathing due to abdominal competition. In addition, due to recurrent metabolic abnormalities and worsening hypertension, the patient underwent left nephrectomy at 3 months of age. At 16 months of age, he underwent renal and liver transplantation. The patient is doing well 1 year after the transplant. Postnatal genetic testing revealed a missense alteration in the Polycystic Kidney and Hepatic Disease 1 gene c.8407T>C (p.Cys2803Arg).
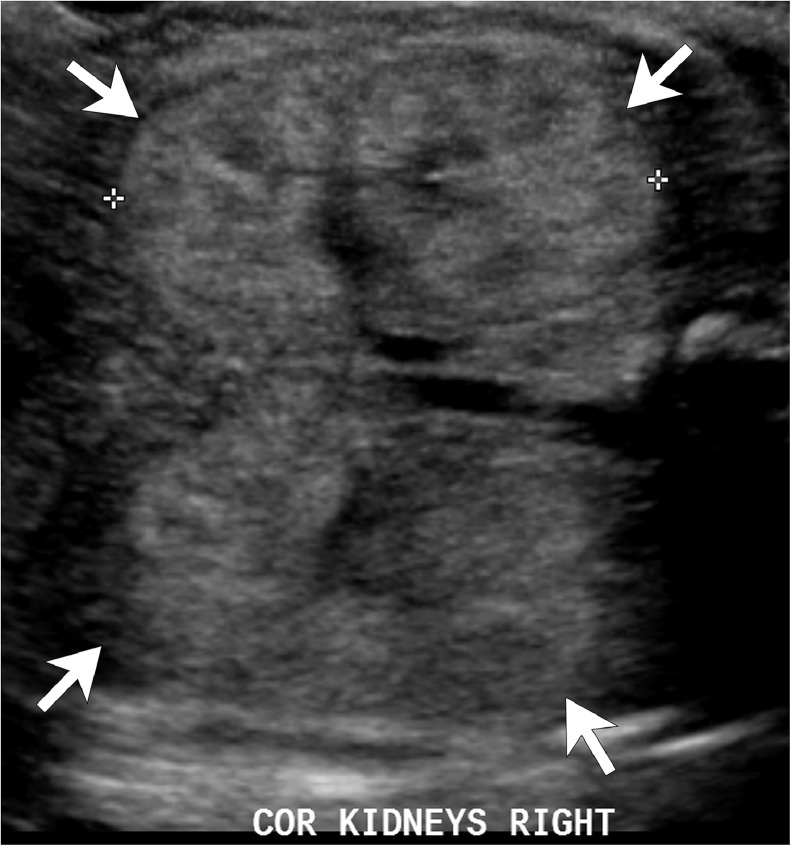
Fig. 1.
Fetal US at 29 weeks of gestation. Coronal US image shows bilateral renal enlargement. Both kidneys (arrows) show increased parenchymal echogenicity and loss of corticomedullary differentiation. US = ultrasound.
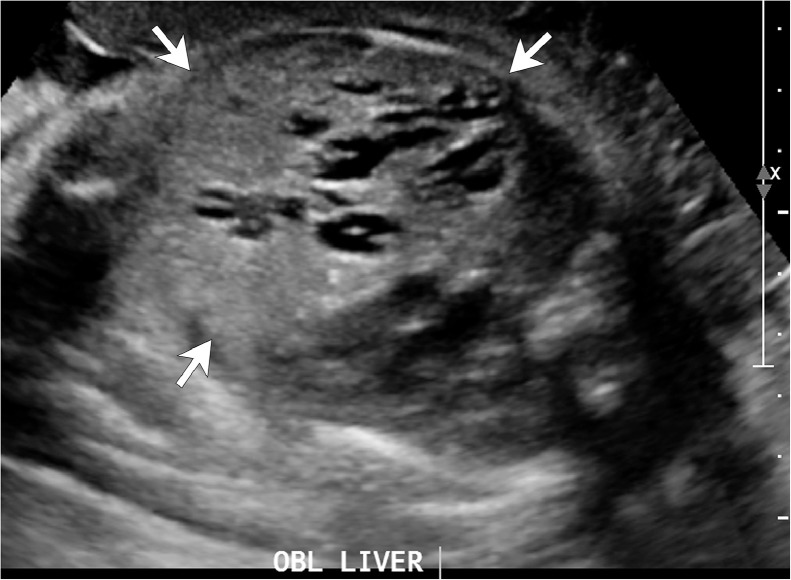
Fig. 2.
Fetal US image shows an enlarged liver (arrows) with multiple tubular, cystic structures. US = ultrasound.
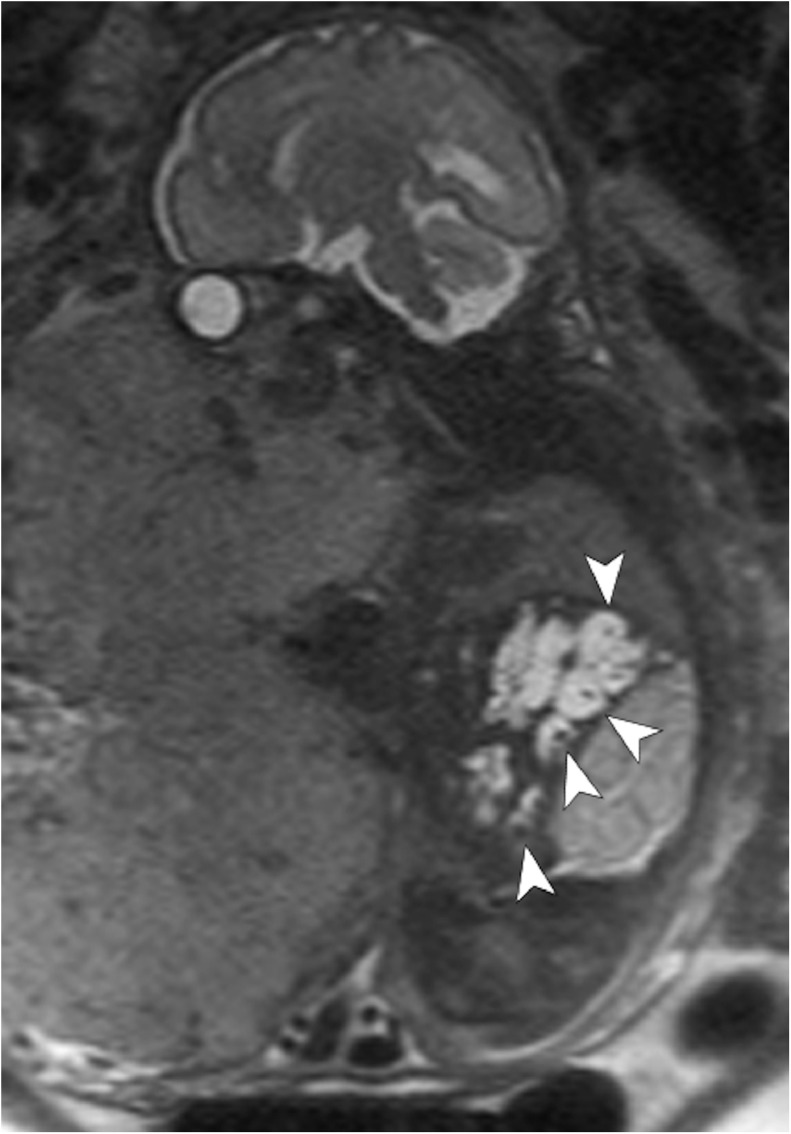
Fig. 3.
Sagittal T2-weighted fetal MR image demonstrates oligohydramnios; an enlarged, hyperintense right kidney; a small, hypointense right lung; and an enlarged liver with multiple dilated bile ducts (arrowheads) showing the “central dot sign”, which reflects the central fibrovascular bundle containing a portal vein radicle and an accompanying hepatic artery branch protruding into the lumen of dilated bile ducts. MR = magnetic resonance.
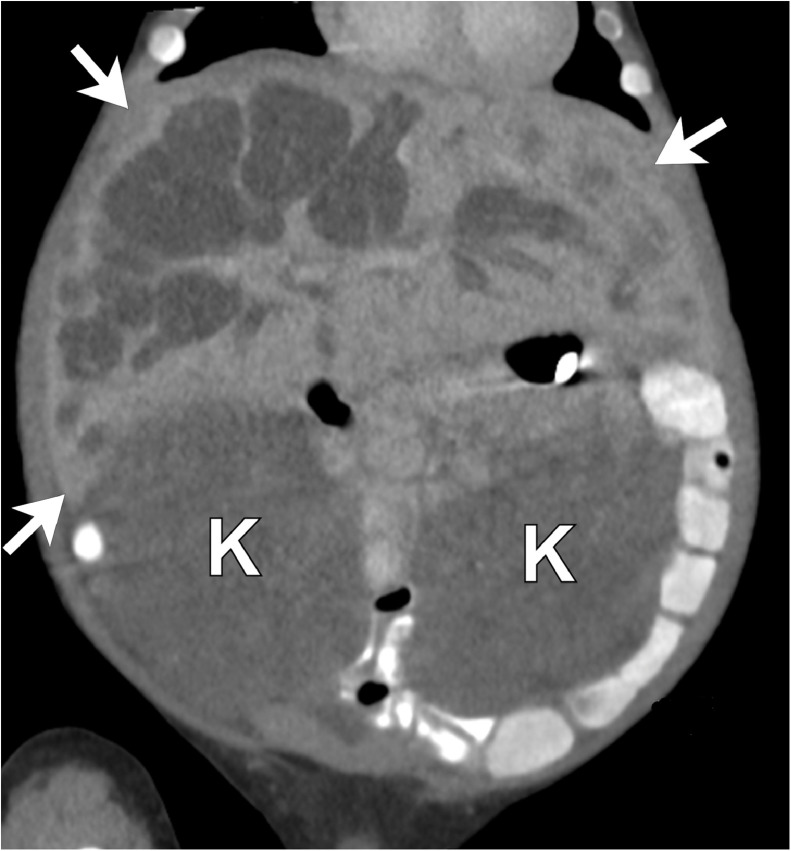
Fig. 4.
Postnatal coronal reformatted unenhanced CT image of the abdomen at 2.5 months of age shows an enlarged liver (arrows) with multiple dilated bile ducts. The cystic, saccular biliary dilatations communicate with the major biliary tree. The kidneys (K) are enlarged and diffusely low in attenuation due to increased water content within the dilated tubules. CT = computed tomography.
Discussion
ARPKD is an inherited disease characterized by nonobstructive dilation of the renal collecting ducts and ductal plate malformation of the biliary tree resulting in congenital hepatic fibrosis. A subset of patients develop Caroli syndrome, which is characterized by nonobstructive, saccular, and fusiform dilation of the biliary ducts. The presence of biliary ductal dilation predisposes these patients to recurrent ascending cholangitis and sepsis [1]. In addition, these patients have an increased risk for developing benign and malignant hepatic neoplasms, especially cholangiocarcinoma [2]. Calculi and bile stagnation within the dilated bile ducts explain recurrent cholangitis, stone formation, and liver abscesses in Caroli syndrome and Caroli disease patients [3]. In addition, complications related to portal hypertension, such as bleeding esophageal varices, are also seen [3], [8].
Visualization of the “central dot sign” in cross-sectional imaging studies is considered highly specific for the diagnosis of Caroli syndrome or Caroli disease, with the central dot reflecting the fibrovascular bundle within an abnormally dilated bile duct (Fig. 3) [8], [9], [14]. However, in the previously reported prenatal cases [11], [12], [13], there was no mention of the visualization of the “central dot sign”. Furthermore, in the case reported by Castro et al [13], the authors did not comment on their ability to identify the “central dot sign” on fetal MR. We were also unable to see the sign in the images provided in the case report. In addition, in 1 [12] of 3 previously reported cases, the authors were unable to diagnose ARPKD associated with Caroli syndrome in US prospectively.
Conclusion
In conclusion, we describe the fourth confirmed case in the literature of ARPKD in association with Caroli syndrome that was diagnosed prenatally. The MR images proved essential in the prenatal diagnosis of associated Caroli syndrome and significantly enhanced our level of confidence in the diagnosis by showing distinct “central dot signs” within dilated intrahepatic biliary ducts. The diagnosis was confirmed postnatally. The patient survived after renal and liver transplantation and is doing well. We suggest that larger scale studies are needed to assess the role of fetal MRI in patients with ARPKD at risk for concomitant Caroli syndrome.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2018.11.006.
Appendix. Supplementary materials
References
- 1.Büscher R., Büscher A.K., Weber S., Mohr J., Hegen B., Vester U., Hoyer P.F. Clinical manifestations of autosomal recessive polycystic kidney disease (ARPKD): kidney-related and non-kidney-related phenotypes. Pediatr Nephrol. 2014;29(10):1915–1925. doi: 10.1007/s00467-013-2634-1. [DOI] [PubMed] [Google Scholar]
- 2.Sweeney W.E., Avner E.D. Diagnosis and management of childhood polycystic kidney disease. Pediatr Nephrol. 2011;26(5):675–692. doi: 10.1007/s00467-010-1656-1. [DOI] [PubMed] [Google Scholar]
- 3.Hartung E.A., Guay-Woodford L.M. Autosomal recessive polycystic kidney disease: a hepatorenal fibrocystic disorder with pleiotropic effects. Pediatrics. 2014;134(3):e833–e845. doi: 10.1542/peds.2013-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sweeney W.E., Avner E.D. Polycystic kidney disease, autosomal recessive. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., editors. GeneReviews(R) University of Washington, Seattle; Seattle, WA: 1993. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved. [Google Scholar]
- 5.Avni F.E., Garel C., Cassart M., D'Haene N., Hall M., Riccabona M. Imaging and classification of congenital cystic renal diseases. AJR Am J Roentgenol. 2012;198(5):1004–1013. doi: 10.2214/AJR.11.8083. [DOI] [PubMed] [Google Scholar]
- 6.Burgmaier K., Kunzmann K., Ariceta G., Bergmann C., Buescher A.K., Burgmaier M. Risk factors for early dialysis dependency in autosomal recessive polycystic kidney disease. JPediatr. 2018;199:22–28. doi: 10.1016/j.jpeds.2018.03.052. .e6. [DOI] [PubMed] [Google Scholar]
- 7.Wen J. Congenital hepatic fibrosis in autosomal recessive polycystic kidney disease. ClinTransl Sci. 2011;4(6):460–465. doi: 10.1111/j.1752-8062.2011.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brancatelli G., Federle M.P., Vilgrain V., Vullierme M.P., Marin D., Lagalla R. Fibropolycystic liver disease: CT and MR imaging findings. Radiographics. 2005;25(3):659–670. doi: 10.1148/rg.253045114. [DOI] [PubMed] [Google Scholar]
- 9.Perricone G. Image of the month: Caroli syndrome: central dot sign on CT. Am J Gastroenterol. 2015;110(4):497. doi: 10.1038/ajg.2014.253. [DOI] [PubMed] [Google Scholar]
- 10.Levy A.D., Rohrmann C.A., Jr., Murakata L.A., Lonergan G.J. Caroli's disease: radiologic spectrum with pathologic correlation. AJR AmJ Roentgenol. 2002;179(4):1053–1057. doi: 10.2214/ajr.179.4.1791053. [DOI] [PubMed] [Google Scholar]
- 11.Hussman K.L., Friedwald J.P., Gollub M.J., Melamed J. Caroli's disease associated with infantile polycystic kidney disease. Prenatal sonographic appearance. J Ultrasound Med. 1991;10(4):235–237. doi: 10.7863/jum.1991.10.4.235. [DOI] [PubMed] [Google Scholar]
- 12.Sgro M., Rossetti S., Barozzino T., Toi A., Langer J., Harris P.C. Caroli's disease: prenatal diagnosis, postnatal outcome and genetic analysis. Ultrasound Obstet Gynecol. 2004;23(1):73–76. doi: 10.1002/uog.943. [DOI] [PubMed] [Google Scholar]
- 13.Castro P.T., Matos A.P.P., Werner H., Daltro P., Fazecas T., Nogueira R. Prenatal diagnosis of Caroli disease associated with autosomal recessive polycystic kidney disease by 3-D ultrasound and magnetic resonance imaging. J Obstet Gynaecol Can. 2017;39(12):1176–1179. doi: 10.1016/j.jogc.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 14.Khalefa A.A., Alrasheed M., Saeedan M.B. Central dot sign. Abdom Radiol (NY) 2016;41(11):2289–2290. doi: 10.1007/s00261-016-0836-2. [DOI] [PubMed] [Google Scholar]
- 15.Nasu K., Yoshimatsu J., Anai T., Miyakawa I., Komatsu E., Maeda T. Magnetic resonance imaging of fetal autosomal recessive polycystic kidney disease. J Obstet Gynaecol Res. 1998;24(1):33–36. doi: 10.1111/j.1447-0756.1998.tb00049.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.