Abstract
Desmoid fibromatosis is a rare, neoplastic tumor known for its aggressive local invasion and recurrence after surgery. Tumors can occur sporadically or associated with familial adenomatous polyposis. We present 3 cases of desmoid fibromatosis postpancreatectomy for pancreatic adenocarcinoma. All cases occurred within 3 years of diagnosis of pancreatic cancer, with subsequent extensive diagnostic work-up to rule out metastatic disease. No relationship between pancreatic cancer and desmoid fibromatosis is documented in the literature, with a postulated connection via mutations on the Wnt/APC/Beta-catenin pathway.
Abbreviations and Acronyms: CT, computed tomography; MRI, magnetic resonance imaging
We present 3 cases of desmoid fibromatosis mimicking metastatic recurrence postpancreatectomy for pancreatic adenocarcinoma.
Case 1
A 53-year-old woman with a history of abdominal pain underwent computed tomography (CT) and subsequent magnetic resonance imaging (MRI), which demonstrated a 2.2-cm mass in the body of the pancreas (Figure 1A). Endoscopic ultrasonography with fine-needle aspiration revealed pancreatic adenocarcinoma. The patient received neoadjuvant chemotherapy and chemoradiotherapy followed by definitive surgery. The surgery consisted of a subtotal distal pancreatectomy, splenectomy, lymphadenectomy, and cholecystectomy. Pathologic examination demonstrated a 1.5 × 1.0 × 1.0-cm fibrotic mass in the pancreatic body, with only a microscopic focus of residual adenocarcinoma measuring less than 1 mm with negative margins. There were no positive lymph nodes.
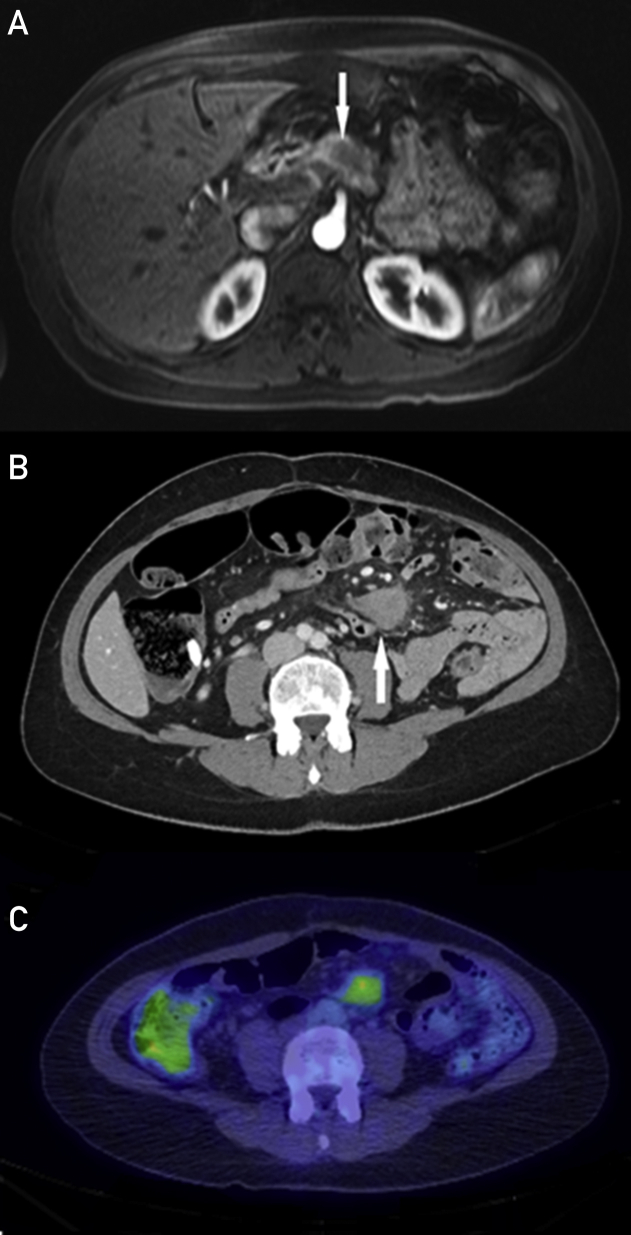
Figure 1.
Case 1. A, T1-weighted axial Vibe MR image after gadolinium administration. Hypoenhancing mass on arterial phase imaging in the pancreatic neck with subsequent resection demonstrating pancreatic adenocarcinoma (arrow). B, Axial contrast-enhanced CT demonstrating a well-defined, enhancing mass in the small bowel mesentery (arrow). C, F-18 FDG PET demonstrates a left mesenteric root mass with moderately increased FDG activity (SUV maximum, 3.8) suspicious for recurrent disease. Subsequent biopsy diagnosed desmoid fibromatosis. CT = computed tomography; FDG = fluorodeoxyglucose; MR = magnetic resonance; PET = positron emission tomography; SUV = standardized uptake value.
The patient had an uncomplicated course until surveillance CT 2 years postoperatively showed a new irregular, enhancing, soft tissue mass in the root of the mesentery measuring 3.4 × 2.6 cm (Figure 1B). Positron emission tomography revealed moderate increased fluorodeoxyglucose activity (standardized uptake value maximum, 3.8) and was considered highly suspicious for mesenteric root recurrent/metastatic disease (Figure 1C). A CT-guided percutaneous core needle biopsy specimen was consistent with desmoid-type fibromatosis, with molecular testing demonstrating a CTNNB1 (T41a) mutation. Subsequent imaging at 10 months postbiopsy revealed interval increased mass size, now measuring 6.3 × 5.3 cm (Figure 2A). Treatment with sulindac was initiated, but the mass continued to grow on surveillance imaging and became increasingly symptomatic with abdominal pain and dyspepsia. The patient underwent subsequent resection, which confirmed desmoid fibromatosis (Figure 2B).
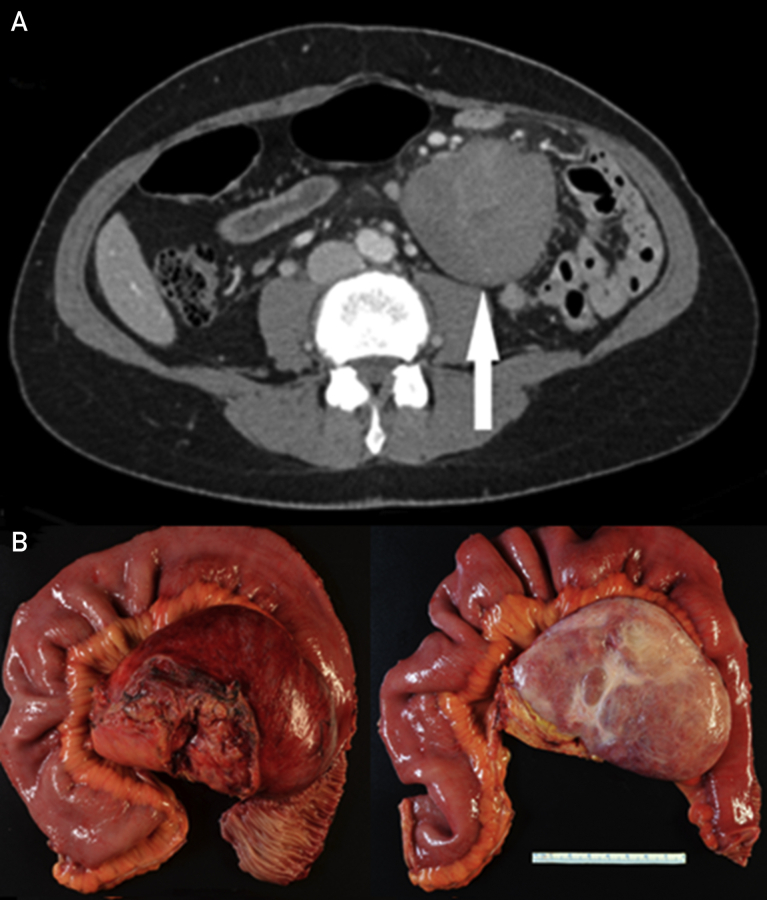
Figure 2.
Case 1. A, Repeated CT demonstrating a considerable increase in the size of the mesenteric desmoid (arrow). B, Gross resection specimen confirmed mesenteric desmoid fibromatosis. CT = computed tomography.
Case 2
A 51-year-old woman presented with abdominal pain. Subsequent CT demonstrated a large 11 × 7-cm complex mass centered in the pancreatic body (Figure 3A), with biopsy confirming locally advanced pancreatic ductal adenocarcinoma. The mass encased the celiac trunk and trifurcation of the splenic, common hepatic, and left gastric arteries and abutted the anterior surface of the superior mesenteric artery. The splenic vein was occluded, with the mass abutting the superior surface of the main portal vein. The patient received neoadjuvant chemoradiotherapy and subsequent definitive surgical management. Surgery entailed subtotal distal pancreatectomy, splenectomy, partial left adrenalectomy, partial gastrectomy, and cholecystectomy. Because of the vascular involvement, en-bloc celiac axis, and portal-superior mesenteric vein confluence, resection was performed with complex vascular reconstruction. Pathologic examination revealed a densely fibrotic mass measuring 3.5 × 3.5 × 2.2 cm, without any residual tumor identified. Resection margins were clear with no lymph node involvement.
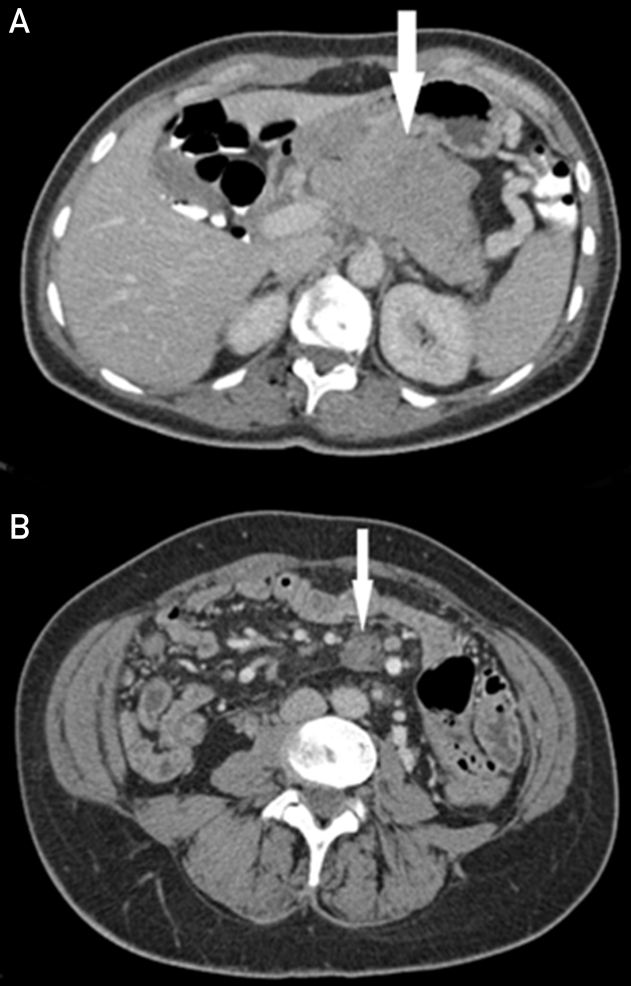
Figure 3.
Case 2. A, Axial, contrast-enhanced CT through the pancreas demonstrates a large mass in the pancreatic neck and body (arrow). This was subsequently resected with a diagnosis of pancreatic adenocarcinoma. B, Contrast-enhanced CT demonstrates a well-defined enhancing mass in the small bowel mesentery (arrow). Subsequent CT-guided biopsy diagnosed desmoid fibromatosis. CT = computed tomography.
Subsequent follow-up was uncomplicated until 3 years postoperatively when an ill-defined 2- cm enhancing mass was detected in the root of the small bowel mesentery (Figure 3B). Computed tomography–guided biopsy was performed, with pathologic examination revealing desmoid-type fibromatosis positive for CTNNB1 (T41a) mutation. Given the benign pathology and lack of symptoms, the patient is currently in surveillance and remains with no evidence of pancreatic cancer recurrence.
Case 3
A 69-year-old man presented with distal duodenal obstruction secondary to a biopsy-proven pancreatic body adenocarcinoma with extrapancreatic extension inferiorly to involve the fourth portion of the duodenum. He underwent exploration at an outside facility and was found to have an isolated peritoneal metastasis and underwent palliative bypass of his duodenal obstruction. He then underwent extensive chemotherapy and subsequent chemoradiation. After more than 2 years of therapy with objective treatment response and no evidence of further metastatic disease, he underwent subtotal distal pancreatectomy, splenectomy, and lymphadenectomy. Pathologic examination revealed no residual active tumor, and all lymph nodes and margins were negative. On 9-month surveillance CT, a new mesenteric mass suggestive of metastatic disease was identified (Figure 4). A CT-guided biopsy specimen was most consistent with desmoid-type fibromatosis positive for CTNNB1 (T41a) mutation (Figure 5A, B). This patient is currently in observation with no evidence of pancreatic cancer recurrence.
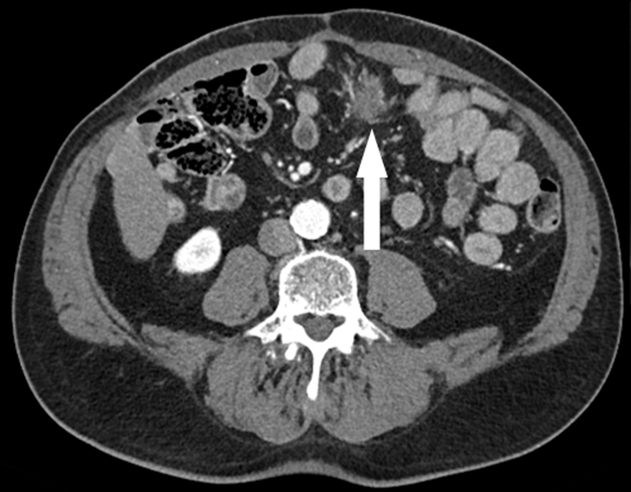
Figure 4.
Case 3. Computed tomography demonstrates a new irregular soft tissue mass in the root of the mesentery (arrow). Subsequent CT-guided biopsy diagnosed desmoid fibromatosis. CT = computed tomography.
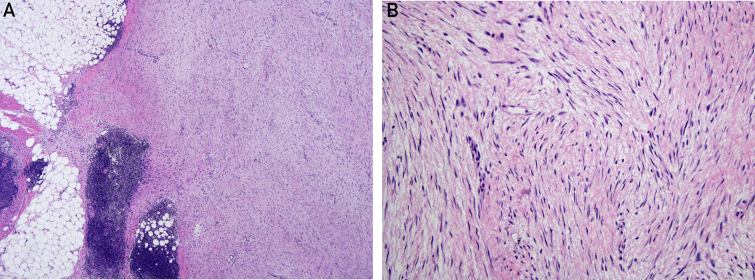
Figure 5.
Case 3. A, Low-power image (fibromatosis 4 × 3 lymphoid cuff edited) of a desmoid-type fibromatosis revealing a characteristic infiltrative border into adjacent adipose tissue (left) associated with peritumoral lymphoid aggregates. Note the uniform, long sweeping fascicles of tumor cells (right) (hematoxylin-eosin, original magnification ×4). B, High-power image (fibromatosis 20 × 2 edited). On high-power examination, the spindled tumor cells are bland, possess abundant fibrillary eosinophilic cytoplasm, and lack cytologic atypia (hematoxylin-eosin, original magnification ×20).
Discussion
Desmoid fibromatosis or aggressive fibromatosis is a rare neoplastic tumor arising from proliferation of mesenchymal stem cell progenitors.1 Although they do not metastasize, they have a tendency for aggressive local invasion and increased risk of recurrence after surgery.2
In a Finnish population, desmoid fibromatosis accounted for 3% of all soft tissue tumors, 0.03% of all neoplasms, and an incidence estimated at 2 to 4 new cases per year.3 In a separate study, age ranged from 15 to 60 years, with a peak at 30 years and a slightly increased female predilection. They occur along musculoaponeurotic structures, with most occurring intra-abdominally.4
Desmoid fibromatosis can occur sporadically or be associated with familial adenomatous polyposis. The sporadic subtype is more common, occurring 85% to 90% of the time.5, 6 Both desmoid fibromatosis subtypes are associated with specific mutations with relation to the Wnt/adenomatous polyposis coli/β-catenin pathway. β-Catenin is a proto-oncogene that regulates cell adhesion and transcription. There is an increase in nuclear accumulation of β-catenin in desmoid fibromatosis. In sporadic subtype, there is a somatic mutation on the CTNNB1 gene that affects the site of adenomatous polyposis coli/β-catenin interaction and ultimately the ability of adenomatous polyposis coli to drive β-catenin degradation. In familial adenomatous polyposis subtype, a truncated adenomatous polyposis coli leads to decreased adenomatous polyposis coli/β-catenin binding and resultant β-catenin accumulation.7 All 3 cases described are not associated with familial adenomatous polyposis and are therefore of the sporadic subtype. In each case, a CTNNB1 mutation in the T41a hotspot on exon 3 was described.
The relationship of pancreatic adenocarcinoma with desmoid fibromatosis is not well established. The occurrence of desmoid fibroproliferation in 3 separate cases of pancreatic adenocarcinoma within the postoperative time frame is unlikely to be just coincidence. As discussed, desmoid fibromatosis, as well as many other malignancies, is associated with mutations on the Wnt/adenomatous polyposis coli/β-catenin pathway. Pancreatic adenocarcinoma has been demonstrated to exhibit increased nuclear accumulation of β-catenin, indicating an involvement of this pathway in its pathogenesis. A previous study demonstrated a mutation on exon 3 of the CTNNB1 gene in 2 of 31 patients with pancreatic adenocarcinoma.8 We present 3 cases of pancreatic adenocarcinoma with subsequent desmoid fibromatosis within a short time postoperatively that mimicked metastatic recurrence. This may indicate a relationship between the 2 entities, with the Wnt/adenomatous polyposis coli/β-catenin pathway providing a possible (although tenuous) connection.
There is a possible association between desmoid fibroproliferation and surgery or trauma.9 This association most commonly occurs in the abdominal wall and extremities and is known as cicatricial fibromatosis.10 A small number of cases of postoperative intra-abdominal desmoid fibroproliferation have been published.11 Given this low number vs the number of intraabdominal surgeries performed every day, the development of desmoid fibroproliferation postsurgery is therefore very rare. The Wnt/adenomatous polyposis coli/β-catenin pathway has again been postulated as an underlying etiological factor.12
Desmoid fibromatosis is typically suspected on imaging studies, with a pathological specimen required for definitive diagnosis. All 3 cases described were dense masses in the small bowel mesentery on contrast-enhanced CT. Typically, imaging features depend on vascularity, collagen content, degree of fibrosis, and fibroblastic proliferation.13 Although the abnormality may be noted on CT, MRI potentially offers better characterization and delineation. Computed tomography demonstrates a well-defined mass with variable enhancement pattern. The mass is typically isoattenuating to hyperattenuating to muscle on contrast-enhanced imaging.14 Desmoid fibromatosis on MRI typically returns low signal on T1-weighted sequences, with variable signal on T2-weighted sequences. Enhancement pattern is again variable. Classically, desmoid fibromatosis, like most fibrotic-type tumors, demonstrate enhancement with minimal washout. Homogeneous, inhomogeneous, or no considerable enhancement has been described.13, 15, 16 The appearances on fluorodeoxyglucose positron emission tomography/CT are not well defined. Previous studies describe larger masses as moderately hypermetabolic and smaller lesions hypometabolic. Mean and/or maximum standardized uptake value has been quoted as 3.1 to 4.1.17, 18
In the absence of known primary malignancy, the differential diagnosis on imaging is typically other soft tissue malignancies such as fibrosarcoma, rhabdomyosarcoma, synoviosarcoma, liposarcoma, and fibrous histiocytoma. Lymphoma is always a possibility in the mesentery, with metastatic disease also to be considered.19 In this case, a recurrence of the pancreatic malignancy was the major pathology to be excluded.
The treatment of desmoid fibromatosis has shifted in recent years from en-bloc resection to conservative management, particularly in asymptomatic masses with slow progression.20 En-bloc resection is associated with substantial recurrence in up to 60% of patients, with the recurrence at times larger than the initial pathology.21 Local therapies such as radiotherapy and cryoablation have been used with safe and effective outcomes.22, 23 Multiple systemic therapies such as nonsteroidal anti-inflammatory and antiestrogen therapies have been used, with varying results in predominantly small limited studies.24, 25, 26, 27 In 2 of the cases, a noninvasive approach was been used, with a nonsteroidal anti-inflammatory used in the first case because of rapid tumor expansion.
In conclusion, we present 3 cases of pancreatic adenocarcinoma treated with pancreatectomy after neoadjuvant therapy that postoperatively developed desmoid fibromatosis mimicking tumor recurrence. Image-guided biopsy is able to yield tissue sufficient to make a pathological diagnosis in these perplexing cases. The potential association of the 2 entities with the Wnt/adenomatous polyposis coli/β-catenin pathway highlights an intriguing consideration for further investigation.
Footnotes
Potential Competing Interests: The authors report no competing interests.
References
- 1.Wu C., Amini-Nik S., Nadesan P., Stanford W.L., Alman B.A. Aggressive fibromatosis (desmoid tumor) is derived from mesenchymal progenitor cells [published correction appears in Cancer Res. 2011;71(18):6084] Cancer Res. 2010;70(19):7690–7698. doi: 10.1158/0008-5472.CAN-10-1656. [DOI] [PubMed] [Google Scholar]
- 2.Fallen T., Wilson M., Morlan B., Lindor N.M. Desmoid tumors -- a characterization of patients seen at Mayo Clinic 1976-1999. Fam Cancer. 2006;5(2):191–194. doi: 10.1007/s10689-005-5959-5. [DOI] [PubMed] [Google Scholar]
- 3.Reitamo J.J., Häyry P., Nykyri E., Saxén E. The desmoid tumor, I: incidence, sex-, age- and anatomical distribution in the Finnish population. Am J Clin Pathol. 1982;77(6):665–673. doi: 10.1093/ajcp/77.6.665. [DOI] [PubMed] [Google Scholar]
- 4.Mankin H.J., Hornicek F.J., Springfield D.S. Extra-abdominal desmoid tumors: a report of 234 cases. J Surg Oncol. 2010;102(5):380–384. doi: 10.1002/jso.21433. [DOI] [PubMed] [Google Scholar]
- 5.Huss S., Nehles J., Binot E., et al. β-catenin (CTNNB1) mutations and clinicopathological features of mesenteric desmoid-type fibromatosis. Histopathology. 2013;62(2):294–304. doi: 10.1111/j.1365-2559.2012.04355.x. [DOI] [PubMed] [Google Scholar]
- 6.Le Guellec S., Soubeyran I., Rochaix P., et al. CTNNB1 mutation analysis is a useful tool for the diagnosis of desmoid tumors: a study of 260 desmoid tumors and 191 potential morphologic mimics. Mod Pathol. 2012;25(12):1551–1558. doi: 10.1038/modpathol.2012.115. [DOI] [PubMed] [Google Scholar]
- 7.Li J., Wang C.Y. TBL1-TBLR1 and beta-catenin recruit each other to Wnt target-gene promoter for transcription activation and oncogenesis. Nat Cell Biol. 2008;10(2):160–169. doi: 10.1038/ncb1684. [DOI] [PubMed] [Google Scholar]
- 8.Zeng G., Germinaro M., Micsenyi A., et al. Aberrant Wnt/beta-catenin signaling in pancreatic adenocarcinoma. Neoplasia. 2006;8(4):279–289. doi: 10.1593/neo.05607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren S. Minimal criteria required to prove causation of traumatic or occupational neoplasms. Ann Surg. 1943;117(4):585–595. doi: 10.1097/00000658-194304000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen P.W. The fibromatoses: a clinicopathologic classification based on 140 cases. Am J Surg Pathol. 1977;1(3):255–270. [PubMed] [Google Scholar]
- 11.Shih L.Y., Wei C.K., Lin C.W., Tseng C.E. Postoperative retroperitoneal desmoid tumor mimics recurrent gastrointestinal stromal tumor: a case report. World J Gastroenterol. 2012;18(42):6172–6176. doi: 10.3748/wjg.v18.i42.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lips D.J., Barker N., Clevers H., Hennipman A. The role of APC and beta-catenin in the aetiology of aggressive fibromatosis (desmoid tumors) Eur J Surg Oncol. 2009;35(1):3–10. doi: 10.1016/j.ejso.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Sundaram M., McGuire M.H., Schajowicz F. Soft-tissue masses: histologic basis for decreased signal (short T2) on T2-weighted MR images. AJR Am J Roentgenol. 1987;148(6):1247–1250. doi: 10.2214/ajr.148.6.1247. [DOI] [PubMed] [Google Scholar]
- 14.Einstein D.M., Tagliabue J.R., Desai R.K. Abdominal desmoids: CT findings in 25 patients. AJR Am J Roentgenol. 1991;157(2):275–279. doi: 10.2214/ajr.157.2.1853806. [DOI] [PubMed] [Google Scholar]
- 15.Hamlin D.J., Paige R., Pettersson H., Bland K.I. Magnetic resonance characteristics of an abdominal desmoid tumor. Comput Radiol. 1986;10(1):11–13. doi: 10.1016/0730-4862(86)90014-4. [DOI] [PubMed] [Google Scholar]
- 16.Azizi L., Balu M., Belkacem A., Lewin M., Tubiana J.-M., Arrivé L. MRI features of mesenteric desmoid tumors in familial adenomatous polyposis. AJR Am J Roentgenol. 2005;184(4):1128–1135. doi: 10.2214/ajr.184.4.01841128. [DOI] [PubMed] [Google Scholar]
- 17.Kasper B., Dimitrakopoulou-Strauss A., Strauss L.G., Hohenberger P. Positron emission tomography in patients with aggressive fibromatosis/desmoid tumours undergoing therapy with imatinib. Eur J Nucl Med Mol Imaging. 2010;37(10):1876–1882. doi: 10.1007/s00259-010-1498-x. [DOI] [PubMed] [Google Scholar]
- 18.Xu H., Koo H.J., Lim S., et al. Desmoid-type fibromatosis of the thorax: CT, MRI, and FDG PET characteristics in a large series from a tertiary referral center. Medicine (Baltimore) 2015;94(38):e1547. doi: 10.1097/MD.0000000000001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casillas J., Sais G.J., Greve J.L., Iparraguirre M.C., Morillo G. Imaging of intra- and extraabdominal desmoid tumors. Radiographics. 1991;11(6):959–968. doi: 10.1148/radiographics.11.6.1749859. [DOI] [PubMed] [Google Scholar]
- 20.Bonvalot S., Eldweny H., Haddad V., et al. Extra-abdominal primary fibromatosis: aggressive management could be avoided in a subgroup of patients. Eur J Surg Oncol. 2008;34(4):462–468. doi: 10.1016/j.ejso.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Crago A.M., Denton B., Salas S., et al. A prognostic nomogram for prediction of recurrence in desmoid fibromatosis. Ann Surg. 2013;258(2):347–353. doi: 10.1097/SLA.0b013e31828c8a30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keus R.B., Nout R.A., Blay J.Y., et al. Results of a phase II pilot study of moderate dose radiotherapy for inoperable desmoid-type fibromatosis--an EORTC STBSG and ROG study (EORTC 62991-22998) Ann Oncol. 2013;24(10):2672–2676. doi: 10.1093/annonc/mdt254. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz J.J., Schmit G.D., Atwell T.D., et al. Percutaneous cryoablation of extraabdominal desmoid tumors: a 10-year experience. AJR Am J Roentgenol. 2016;207(1):190–195. doi: 10.2214/AJR.15.14391. [DOI] [PubMed] [Google Scholar]
- 24.Fiore M., Colombo C., Radaelli S., et al. Hormonal manipulation with toremifene in sporadic desmoid-type fibromatosis. Eur J Cancer. 2015;51(18):2800–2807. doi: 10.1016/j.ejca.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 25.Tsukada K., Church J.M., Jagelman D.G., et al. Noncytotoxic drug therapy for intra-abdominal desmoid tumor in patients with familial adenomatous polyposis. Dis Colon Rectum. 1992;35(1):29–33. doi: 10.1007/BF02053335. [DOI] [PubMed] [Google Scholar]
- 26.Patel S.R., Evans H.L., Benjamin R.S. Combination chemotherapy in adult desmoid tumors. Cancer. 1993;72(11):3244–3247. doi: 10.1002/1097-0142(19931201)72:11<3244::aid-cncr2820721118>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 27.Azzarelli A., Gronchi A., Bertulli R., et al. Low-dose chemotherapy with methotrexate and vinblastine for patients with advanced aggressive fibromatosis. Cancer. 2001;92(5):1259–1264. doi: 10.1002/1097-0142(20010901)92:5<1259::aid-cncr1446>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]