Abstract
Diabetes is considered as the most common metabolic disease affecting millions of people all around the world. Use of natural herbal medicines can be effective in treating diabetes. Zingerone (4-(4-hydroxy-3-methylphenyl) butan-2-one) a polyphenolic alkanone extracted from ginger has a broad spectrum of pharmacological properties and thus can be used as a promising candidate against various ailments. In the current study we aimed at demonstrating the protective effect of zingerone against diabetes mellitus and elucidating its possible mechanism. Five groups of animals (I-V) were made with ten animals each. Group I (control) was given normal saline orally. Group II (diabetic positive control) was given alloxan at the dose rate of 100 mg/kg bwt once. Group III and IV was given alloxan once at the dose rate of 100 mg/kg bwt. and received oral treatment of zingerone at a dose rate of 50 and 100 mg/kg bwt respectively daily for 21 days. Group V was given alloxan at the dose of 100 mg/kg bwt. and was treated with standard drug glibenclamide at the dose rate of 4.5 mg/kg bwt. daily for 21 days. According to our findings we confirmed that zingerone restrained the alloxan induced oxidative stress by increasing the activity of reduced glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX) and reducing the peroxidative damage. We also confirmed that zingerone suppressed the level of redox sensitive transcription factor NFκB and downregulated other downstream inflammatory cytokines like interleukins (IL1-β IL-2, IL-6) and tumor necrosis factor alpha (TNF-α). Moreover, the experimental findings suggested that zingerone improved the insulin levels. Taken together our results indicated that zingerone effectively ameliorated the diabetes induced complications which provide a strong theoretical basis for zingerone to be used clinically for treatment of diabetes.
Keywords: Alloxan, Diabetes, NFκB, Cytokines, Zingerone
1. Introduction
Diabetes mellitus is the fastest growing metabolic disease. It is spreading around the world as epidemic (Whiting et al.,2017) and victims of diabetes have major threat from the complications that arise after the onset of this metabolic syndrome. Diabetes prevalence is increasing across the globe with 8.8% prevalence in adults as estimated in 2015 (Ogurtsova et al., 2017). By 2040 the prevalence is predicted to rise to 10.4% which will definitely increase the health expenditure on diabetes (Ogurtsova et al., 2017). Diabetes mellitus is characterized by disturbed metabolism and a marked hyperglycaemia due to abnormal release and peripheral utilization of insulin hormone. This disease leads to multiple complications like increase in reactive oxygen species (Chen et al., 2018), impairment of scavenging enzymes (Choi and Ho, 2018), abnormal lipid profile (Alsharidah et al., 2017), hyperglycemia (Perry et al., 2018), impaired insulin secretion and action (Khan et al., 2009) and cellular damage induced by high oxidative stress (Capellini et al., 2010).
The compound alloxan which is a pyrimidine derivative causes “Alloxan Diabetes” which is insulin dependent similar to Type I diabetes mellitus, by selectively destroying insulin producing cells of the pancreas (Ibrahim and Abdelatif, 2011). Alloxan being a structural analogue of glucose enters the β-cells via GLUT-2 receptors and inhibits glucokinase, an enzyme essential for insulin secretion (Szkudelski, 2001). Alloxan has high affinity to SH group which causes decreased glutathione content and generation of an increased oxidative stress in the cell leading to increase in malonaldehyde (MDA) levels (Panahi, et al., 2017) and reactive oxygen species (ROS) (Chen et al., 2018). The proinflammatory cytokines like IL-1β, IL6, IL2 and TNF-α have a pivotal role in disruption of β-cell functions and aggrevation of diabetes mellitus symptoms (Banerjee and Saxen, 2012, Omodanis et al., 2017). There is induction of apoptotic cell death on exposure of pancreatic beta cells to IL-1β or along with IL-6 and TNF-α through the downstream activation of c-jun-N-terminal kinase (JNK) pathway or NF-κB (nuclear factor kappa B) signaling pathways (Welsh et al., 2005). NF-κB is a pleiotropic oxidant and a sensitive transcription factor, reported to play a key part in hyperglycemia induced oxidative stress. Previous studies have demonstrated the significant role of NF-κB in pathogenesis of diabetes (Arkan et al., 2005, Cai et al., 2005). NF-κB transcriptional factor might play a significant role in inducing diabetes due to its increased transcriptional activation in diabetic patients (Patel and Santani, 2009).
The common side effects of conventional pharmacological drugs used for treatment of diabetes are stomach disorders, diarrhea, kidney disorders, lactic acidosis and hepatotoxicity are well recognized (Nammour et al.,2003). So, the trend towards using traditional herbal medicinal products is continuously increasing Ethanolic extracts of leaves of Annona muricata showed antidiabetic actions which might be due to the presence of many potential antioxidative phytocompounds like chlorogenic and caffeic acids, procyanidins (epi)catechin, quercetin and kaempferol (Justino et al., 2018). In streptozotocin induced diabetic rats, chicoric acid derived from roots of Cichorium intybus causes a significant reduction in basal hyperglycemic conditions with improvisation of oral glucose tolerance due to its ability to scavenge free radicals like H2O2 (Ferrare et al., 2018). Entagenic acid, an aglycone present in seed kernels of E. phaseoloides possess a significant antidiabetic effect in type 2 diabetic mice by activation of downstresm AMP-activated protein kinase – Glucose Transporter (AMPK/GLUT4) pathway (Xiong et al., 2018).
Ginger (Zingiber officinale) is used as a condiment worldwide from times immemorial. Ginger is regarded to have many health benefits in traditional medicine. It is due to diverse phytochemical constituents present in ginger which lead to promising health benefits. Many active compounds like gingerols, shogaols, paradols and zingerone are present in ginger (Zhang et al., 2017). The unsaturated ketone moiety present in bioactive metabolites of ginger give strong antioxidant activity to it (Dugasani et al., 2010).
Zingerone or vanillyl acetone is present in significant concentration in ginger and is produced from gingerol or shogaols on thermal degradation or cooking by retro-aldol reaction (Ahmad et al., 2015, Takizawa et al., 2012) Zingerone belongs to phenolic alkanone group, having a wide range of essential pharmacological properties like anticancer (Vinothkumar et al., 2014), antioxidant (Rajan et al., 2013), anti-inflammatory (Kim et al., 2010), and antimicrobial activities (Ahmad et al., 2015). It has been reported that zingerone plays a significant role in treating diabetic nephropathy in STZ (Streptozocin) model (Rehman et al., 2018, Jothi et al., 2016). Zingerone shows vasorelaxatory response in aota of diabetic rat which might be due to stimulation of NO and guanylate cyclase which have prominent vasodilatory effects (Ghareib et al., 2016). Zingerone ameliorates diabetic nephropathy by downregulation of the NADPH oxidase 4 (NOX4) expression on administration of high glucose in kidneys and in HK-2 cells (Cui et al., 2018).
The conventional therapies available for controlling diabetes are usually expensive and often associated with adverse effects, therefore, various alternative therapies with anti-hyperglycemic effects are increasingly sought by patients. Keeping in mind the pharmacological effects of zingerone we hypothesized that zingerone may improve the pancreatic and liver functions through anti-hyperglycemia and through the inhibition of NF-κB mediated inflammatory processes in diabetic rats.
2. Material methods
2.1. Chemicals and reagents
Alloxan, Zingerone, reduced glutathione, nicotinamide adenine dinucleotide phosphate, glutathione reductase, oxidised glutathione were procured from Sigma Aldrich chemicals Pvt. Ltd. (USA). Rest of the chemicals used in the current study were of analytical grade.
2.2. Animals
Wistar male albino rats (160–200 g) were housed in the animal house of FVSc & AH, SKUAST-Kashmir, India. Animals were placed under standard controlled conditions of temperature (22 ± 2 °C) and humidity (55%) (Abdel-Kader et al., 2017). The maintenance of animals were in in accordance with specific guidelines prescribed by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and the current study was ratified by the Institutional animal ethics committee (IAEC) of FVSc, SKUAST-Kashmir, India (Au/FVS/PS-57/1224).
2.3. Induction of diabetes
After acclimatization period of seven days, induction of diabetes was done by through intra-peritoneal injection (I.P) of alloxan dissolved in 0.9% NaCl (Lee et al., 2008) at the dose of 100 mg/kg body weight. After I.P. administration of alloxan the rats were provided with 20% dextrose overnight to check the mortality of animals from hypoglycemic shock. The blood glucose levels and body weight and of animals were noted before and after induction. After 72 hrs of alloxan injection blood glucose levels were measured through UV spectrometric analysis. The animals having blood glucose level above 250 mg/dl were considered diabetic and were selected for the current study (Sathishsekar and Subramanian, 2005).
2.4. Treatment & experimental design
Adult wistar rats used in the present study were divided into 5 groups of 10 rats each. Animals in group II, III, IV and V were given alloxan once to induce diabetes and after seven days when the blood glucose levels were above the normal levels, the treatment of zingerone dissolved in aqueous solution (Kumar et al., 2014) was commenced and this was considered as first day of treatment. Zingerone dose was selected based OECD guidelines, where in 1/10th of LD50 is considered safe dose for chemopreventtive studies (Kumar et al., 2014, Rao et al., 2009). Group V was treated with standard drug, glibenclamide and dose was selected as 5 mg/kg b.wt (Akhter et al., 2011). The experimental design was formulated as shown in Table 1.
Table 1.
Effect of zingerone on blood glucose in different experimental groups.
| Group | No. of animals/group | Treatment regimen |
|---|---|---|
| Group 1 | N = 10 | Normal Control rats (no alloxan given) |
| Group II | N = 10 | Diabetic control group (Alloxan given @ 100 mg/kg b.wt. once intraperitoneally) Prince et al. (2004) |
| Group III | N = 10 | Diabetic rats (Alloxan given @ 100 mg/kg b.wt. once) and treated with zingerone (50 mg/kg b.w.) after seven days of alloxan treatment in an aqueous solution daily for 21 days using an oral gavage. |
| Group IV | N = 10 | Diabetic rats (Alloxan given @ 100 mg/kg b.wt. once) and treated with zingerone (100 mg/kg b.w.) after seven days of alloxan treatment in an aqueous solution daily for 21 days using an oral gavage |
| Group V | N = 10 | Diabetic rats (Alloxan given @ 100 mg/kg b.wt. once) and treated with glibenclamide orally at a dose of 4.5 mg/kg bwt once after induction of diabetes. |
2.4.1. Sample collection
At the end of experimental schedule, animals were sacrificed using diethyl ether anesthesia and blood was obtained by cardiac puncture. This blood was kept in clot activating vacutainers to obtain serum and centrifuged after clotting at 9000 rpm for 10 min to obtain clear serum. Serum obtained was collected in plastic vials for estimation of serum biochemical parameters and stored at −20 °C until assays were done. Liver was s was collected, cleaned and preserved in falcon tubes containing PBS, to study various estimations.
2.5. Preparation of Post-mitochondrial supernatant preparation (PMS) for estimation of various parameters
PMS prepration was done as per Khan et al., 2012. After sacrifising the animals, livers were removed readily, cleaned by perfusing with ice cold 0.85% NaCl solution and homogenized in phosphate buffer (0.1 M, pH 7.4) using a homogeniser. Following complete homogenization, the homogenate obtained was filtered through muslin cloth and the filtrate was centrifuged at 3000 rpm for 10 min at 48 °C. This was followed by recentrifugating the supernatant at 12,000 rpm for 20 min at 48 °C. The supernatant now obtained called as post mitochondrial supernatant (PMS) was used for further estimation of different parameters (Rehman et al., 2013).
2.6. Estimation of different parameters in serum
2.6.1. Estimation of glucose concentration
Fasting Blood glucose level was monitored after every week of study using commercially available blood glucose kits (Accurax).
2.6.2. Estimation of insulin levels
Fasting Insulin level in serum was estimated by using ELISA (Enzyme Linked Immunosorbent Assay) method using ELISA kit (Calbiotec).
2.6.3. Lipid profile
Serum was collected and the levels of Triglycerides (TG), Total cholesterol (TC) and HDL-cholesterol were measured using kit (Genzyme Diagnostics). However LDL-cholesterol was calculated as the remaining difference of total cholesterol and HDL-cholesterol.
2.6.4. Glycosylatedhemoglobin (GHb)
GlycosylatedHb was determined using commercial kit (Elabscience) according to the enclosed guidelines.
2.6.5. Estimation of serum α-Amylase
Serum α-amylase concentration was analysed by using commercially available diagnostic kit (Span diagnostic).
2.6.6. Assay of total protein
Serum level of total protein was estimated by using commercially available diagnostic kit (Accurex).
2.6.7. Estimation of SGPT SGOT and ALP
Serum levels of SGPT, SGOT, and ALP were estimated using commercially available kits (Accurex).
2.7. Lipid peroxidation level
Lipid peroxidation was estimated in PMS by the method of Wright et al. (1981). The results were expressed as the nmol MDA formed/g tissue by using a molar extinction coefficient of 1·56 × 105/M per cm.
2.8. Antioxidant enzyme assay
2.8.1. Measurement of SOD activity
PMS was used and the SOD activity was analysed by using the method of Marklund and Marklund, 1974. Units of SOD activity were expressed as IU/L.
2.8.2. Assay for catalase activity
The catalase activity in PMS was assessed by the method of Claiborne (1985). The CAT activity was calculated in terms of nmol H2O2 consumed/min per mg protein.
2.8.3. Assay for reduced glutathione (GSH) activity
The GSH activity was assessed in PMS by the method of Carlberg and Mannervik, 1975. The GSH content was calculated as mmol 5,50-dithio-bis-(2-nitrobenzoic acid) conjugate formed/g tissue using a molar extinction coefficient of 13·6 × 103/M per cm.
2.8.4. Assay for glutathione peroxidase activity
glutathione peroxidase (GPx) activity was assessed by following mehod of Mohandas et al., 1984 and the levels were calculated as nmol NADPH oxidised/min per mg protein with the molar extinction coefficient of 6·22 × 103/M per cm.
2.9. Immunoassay for NF-κB estimation
Serum was used to estimate the NF-κB level using an ELISA kit (NF-κB p65 ELISA, Invitrogen Corporation, CA, USA) according to protocol of the manufacturer. Standard available with the commercial kit was used to obtain the standard curve for the NF-κB and plates were read at 450 nm in a Multiscan EX microplate reader (Thermo).
2.10. Immunoassay for estimation of TNF-α, ILI-β, IL-2 and IL-6
Concentrations of TNF-α, ILI-β, IL-2 and IL-6 were estimated in serum using ELISA kits for all these markers provided by Invitrogen Corporation, CA, USA., following the manufacturer’s protocol. Standard curves were obtained using available standards and plates were read at 450 nm in a Multiscan EX microplate reader (Thermo).
2.11. Statistical analysis
Statistical analysis of all the obtained data was done using SPSS 20.0. The data was subjected to one way ANOVA followed by Tukey Kramer test. All the results are presented as mean ± SE.
3. Results
3.1. Effect of zingerone on serum glucose levels
There was marked (p < 0.001) rise in serum glucose levels in rats in the diabetic group (Group II) as compared to levels in normal control group (Group I). Administration of zingerone in diabetic rats showed a prominent hypoglycemic effect. The level of fasting blood glucose in the rats was monitored every week (0, 1, 2 and 3 weeks). After 1st week of treatment with zingerone blood glucose levels started reducing in diabetic animals of group III and group IV with different doses of zingerone. Animals treated with 100 mg/kg bwt zingerone showed prominent reduction in glucose levels as compared to animals treated with 50 mg/kg bwt. After 2nd week of continuous treatment with zingerone blood glucose levels again started decreasing in significant manner as compared to 1st week of treatment. Finally at the end of treatment (21 Days) blood glucose levels showed marked reduction in zingerone treated diabetic groups as compared to diabetic control rats. 100 mg/kg bwt of zingerone was more effective as compared to 50 mg /kg bwt because 50% reduction of glucose level occurred in group IV at the end of study (21 days) (Table 2).
Table 2.
Effect of zingerone on blood glucose in different experimental groups.
| Treatment regimen | Glucose on Day 7 | Glucose on Day 14 | Glucose on Day 21 |
|---|---|---|---|
| Group I | 86.83 ± 2.63 | 88.06 ± 3.30 | 88.34 ± 2.8 |
| Group II | 273.4 ± 8.80*** | 260.2 ± 10.15*** | 245.00 ± 11.12*** |
| Group III | 189.5 ± 3.59## | 168 ± 4.18## | 144.7 ± 4.26## |
| Group IV | 162.8 ± 6.84### | 142.8 ± 4.43### | 122.6 ± 1.80### |
| Group V | 86.83 ± 2.63### | 118.1 ± 2.56### | 117.5 ± 2.40### |
Group-I: normal control; Group-II: diabetic control; Group-III: diabetic rats treated with zingerone (50 mg/kg bw/day); Group-IV: diabetic rats treated with zingerone (100 mg/kg bw/day); Group-V: Diabetic rats treated with glibenclamide 4.5 mg/kg bw/day). Data are represented as mean of 10 rats± S.E.M.
ns: Non significant.
P < 0.001: Significant difference between control and treated groups.
P < 0.01: Significant difference between Diabetic control and treated groups.
P < 0.001: Significant difference between Diabetic control and treated group.
3.2. Effect of zingerone treatment on serum insulin
In the current study there was a decrease in fasting serum insulin levels in group II. However, group III treated with zingerone at the dose of 50 mg/kg bwt showed considerable improvement in insulin levels while treatment with 100 mg/kg bwt of zingerone in group IV showed significant increase in serum insulin levels as compared to group II (Fig. 1).
Fig. 1.
Effect of treatment of Zingerone on insulin levels in different experimental groups: Group-I: normal control; Group-II: diabetic control; Group-III: diabetic rats treated with zingerone (50 mg/kg bw/day); Group-IV: diabetic rats treated with zingerone (100 mg/kg bw/day); Group-V: Diabetic rats treated with glibenclamide 4.5 mg/kg bw/day). Data are represented as mean of 10 rats ± S.E.M. ***P < 0.001: Significant difference between control and treated groups. #P < 0.05: Significant difference between Diabetic control and treated groups ##P < 0.01: Significant difference between Diabetic control and treated groups. ###P < 0.001: Significant difference between Diabetic control and treated group. ns: Non significant.
3.3. Effect of zingerone treatment on serum amylase
In the current study there was significant decrease in the concentrations of serum amylase in group II as compared to group I. However, a significant increase in serum levels of amylase was observed in group IV treated with 100 mg/kg bwt of zingerone (Fig. 2).
Fig. 2.
Effect of Zingerone on serum amylase levels in different experimental groups: Group-I: normal control; Group-II: diabetic control; Group-III: diabetic rats treated with zingerone (50 mg/kg bw/day); Group-IV: diabetic rats treated with zingerone (100 mg/kg bw/day); Group-V: Diabetic rats treated with glibenclamide 4.5 mg/kg bw/day). Data are represented as mean of 10 rats ± S.E.M. ***P < 0.001: Significant difference between control and treated groups. #P < 0.05: Significant difference between Diabetic control and treated groups ##P < 0.01: Significant difference between Diabetic control and treated groups. ###P < 0.001: Significant difference between Diabetic control and treated group. ns: Non significant.
3.4. Effect of zingerone on lipid profile in different experimental groups
There was a significant increase in levels of TC, TG, LDL-C and a concomitant decrease in levels HDL-C level in serum of animals of group II as compared to group I. In treatment group III there was a significant decrease (p < 0.01) in levels of TC, TG and LDL-C, however group IV showed a highly significant decrease (p < 0.001) in the concentrations of TC, TG and LDL-C levels with a significant increase in HDL-C levels. There was a significant increase in HDL-C levels in group IV (Table 3).
Table 3.
Effect of Zingerone on Total cholesterol (TC), High density, lipoprotein (HDL-C), Low density lipoprotein (LDL-C) and Triglycerides (TG) in different experimental groups.
| Treatment regimen | TC(mg/dl) | HDL-C(mg/dl) | LDL-C(mg/dl) | TG(mg/dl) |
|---|---|---|---|---|
| Group I | 87.65 ± 5.32 | 31.00 ± 3.4 | 51.14 ± 1.85 | 86.26 ± 6.73 |
| Group II | 224.6 ± 14.6*** | 16.78 ± 0.79*** | 94.21 ± 5.87*** | 170.3 ± 12.5*** |
| Group III | 181.1 ± 6.69# | 23.82 ± 1.21 ns | 91.48 ± 7.75 ns | 139.5 ± 7.61ns |
| Group IV | 177.4 ± 11.5## | 25.02 ± 2.10# | 68.26 ± 3.12## | 122.41 ± 14.0# |
| Group V | 97.87 ± 6.5### | 28.75 ± 1.65## | 55.40 ± 5.19### | 95.97 ± 8.48## |
Group-I: normal control; Group-II: diabetic control; Group-III: diabetic rats treated with zingerone (50 mg/kg bw/day); Group-IV: diabetic rats treated with zingerone (100 mg/kg bw/day); Group-V: Diabetic rats treated with glibenclamide 4.5 mg/kg bw/day). Data are represented as mean of 10 rats± S.E.M.
P < 0.001: Significant difference between control and treated groups.
P < 0.05: Significant difference between Diabetic control and treated groups
P < 0.01: Significant difference between Diabetic control and treated groups.
P < 0.001: Significant difference between Diabetic control and treated group
: Non significant.
3.5. Effect of zingerone treatment on ALT, AST, ALP and total protein
There was a significant increase in the levels of ALT, AST and ALP in the serum of group II animals as compared to group I. There was significant decrease in the levels of these enzymes in serum in group IV of 100 mg/kgbwt of zingerone. There was no significant decrease in serum total protein in group II as compared to normal group. Zingerone administered at dose of 100 mg/kg bwt rats of group IV showed a considerable increase (p < 0.05) in the serum total proteins levels (Table 4).
Table 4.
Effect of treatment of Zingerone on ALT, AST, ALP and Protein in different experimental groups.
| Treatment regimen | ALT | AST | ALP | Total protein |
|---|---|---|---|---|
| Group I | 75.21 ± 2.86 | 115.21 ± 4.57 | 142.26 ± 1.32 | 4.41 ± 0.30 |
| Group II | 117 ± 9.05*** | 275.11 ± 15.1*** | 194.92 ± 2.37*** | 3.36 ± 0.21*** |
| Group III | 96.01 ± 6.86# | 158.61 ± 8.62# | 174.65 ± 1.84# | 4.68 ± 0.37# |
| Group IV | 79.78 ± 4.89# | 137 ± 3.74## | 162.58 ± 1.28## | 5.07 ± 0.42## |
| Group V | 79.83 ± 3.29## | 111.41 ± 11.9## | 167.87 ± 0.97## | 3.83 ± 0.33ns |
Group-I: normal control; Group-II: diabetic control; Group-III: diabetic rats treated with zingerone (50 mg/kg bw/day); Group-IV: diabetic rats treated with zingerone (100 mg/kg bw/day); Group-V: Diabetic rats treated with glibenclamide 4.5 mg/kg bw/day). Data are represented as mean of 10 rats± S.E.M.
P < 0.001: Significant difference between control and treated groups.
P < 0.05: Significant difference between Diabetic control and treated groups.
P < 0.01: Significant difference between Diabetic control and treated groups.
: Non significant.
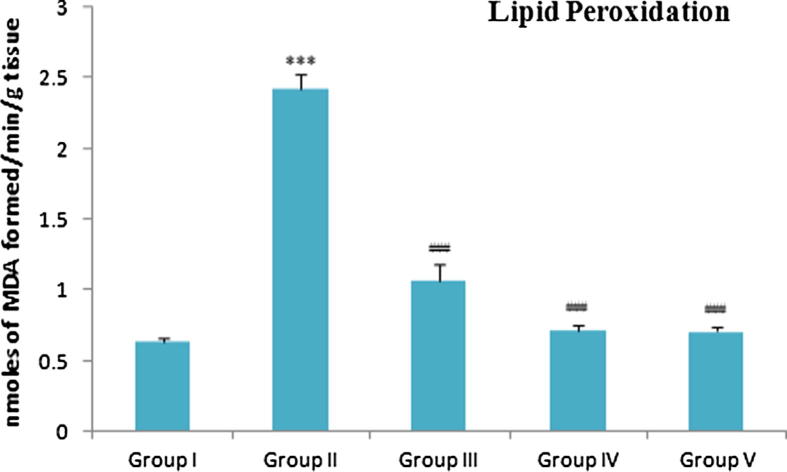
3.6. Effect of zingerone treatment on LPO levels
Malonaldehyde (MDA) is the stable product of lipid peroxidation. Alloxan administration increased the vulnerability of cellular membranes for lipid peroxidation. In the current study, group II, showed a remarkable increase in MDA levels as compared to group I. However, a significant reduction in levels of MDA were observed in group III and group IV. Group V also showed a considerable decrease in MDA level in comparison to group II (Fig. 3).
Fig. 3.
Effect of Zingerone on MDA levels in different experimental group: Group-I: normal control; Group-II: diabetic control; Group-III: diabetic rats treated with zingerone (50 mg/kg bw/day); Group-IV: diabetic rats treated with zingerone (100 mg/kg bw/day); Group-V: Diabetic rats treated with glibenclamide 4.5 mg/kg bw/day). Data are represented as mean of 10 rats± S.E.M. ***P < 0.001: Significant difference between control and treated groups. #P < 0.05: Significant difference between Diabetic control and treated groups ##P < 0.01: Significant difference between Diabetic control and treated groups. ###P < 0.001: Significant difference between Diabetic control and treated group. ns: Non significant.
3.7. Effect of zingerone treatment on antioxidant enzymes
Alloxan administration caused marked reduction in the in the activity of scavenging enzymes viz SOD, CAT and GPx and also decreased the reduced glutathione (GSH) levels in group II. These adverse changes were reversed to near normal values by the treatment of zingerone in group III and group IV as well as glibenclamide treated diabetic group V. However, zingerone at the dose of 100 mg/kg bwt in group IV caused remarkable recovery in the activity of all these antioxidant parameters (Table. 5).
Table 5.
Effects of treatment of Zingerone on various antioxidant enzymes in different experimental groups.
| Treatment regimen | GSH (n mol GSH/gm tissue) | Catalase (nanomol H2O2 Consumed/min/mg protein) | SOD (IU/L) | GPX (nanomol NADPH oxidized/min/mg protein) |
|---|---|---|---|---|
| Group I | 82.73 ± 8.79 | 674.4 ± 40.16 | 5.38 ± 0.81 | 6.42 ± 0.17 |
| Group II | 38.76 ± 9.76*** | 315 ± 28.11*** | 2.17 ± 0.13*** | 3.03 ± .0.14*** |
| Group III | 52.24 ± 8.73## | 480.0 ± 20.70## | 3.56 ± 0.19# | 4.12 ± 0.29## |
| Group IV | 72.84 ± 9.14### | 640.8 ± 15.71### | 4.33 ± 0.13### | 5.59 ± 0.16### |
| Group V | 75.72 ± 10.14### | 659.3 ± 38.71### | 4.96 ± 0.10### | 5.84 ± 0.14### |
Group-I: normal control; Group-II: diabetic control; Group-III: diabetic rats treated with zingerone (50 mg/kg bw/day); Group-IV: diabetic rats treated with zingerone (100 mg/kg bw/day); Group-V: Diabetic rats treated with glibenclamide 4.5 mg/kg bw/day). Data are represented as mean of 10 rats± S.E.M.
P < 0.001: Significant difference between control and treated groups. (#P < 0.05): Significant difference between Diabetic control and treated groups,
P < 0.01: Significant difference between Diabetic control and treated groups.
P < 0.001: Significant difference between Diabetic control and treated group.
3.8. Effect of zingerone on glycosylation of hemoglobin
Alloxan administration caused significant increase in hemoglobin glycosylation. Zingerone treatment in group III did not exhibit any marked improvement. However, zingerone administration in group IV showed a marked reduction in Hb glycosylation (Fig. 4).
Fig. 4.
Effect of Zingerone on Glycosylation of Hb levels in different experimental groups: Group-I: normal control; Group-II: diabetic control; Group-III: diabetic rats treated with zingerone (50 mg/kg bw/day); Group-IV: diabetic rats treated with zingerone (100 mg/kg bw/day); Group-V: Diabetic rats treated with glibenclamide 4.5 mg/kg bw/day). Data are represented as mean of 10 rats ± S.E.M. ***P < 0.001: Significant difference between control and treated groups. #P < 0.05: Significant difference between Diabetic control and treated groups ##P < 0.01: Significant difference between Diabetic control and treated groups. ###P < 0.001: Significant difference between Diabetic control and treated group. ns: Non significant.
3.9. Effect of zingerone treatment on serum NF-κBp65
NFκB has been shown to have significant role in induction of diabetes mellitus. In the present study levels of NFκB were marked increased in group II as compared to group I (Fig. 5). Group III which was administered with zingerone at the dose of 50 mg/kg bwt revealed a significant attenuation of NF-κBp65 levels in the liver PMS. However 100 mg/kg bwt of zingerone in group IV showed more profound effects.
Fig. 5.
Effect of Zingerone on NF-κB-p65 levels in different experimental groups: Group-I: normal control; Group-II: diabetic control; Group-III: diabetic rats treated with zingerone (50 mg/kg bw/day); Group-IV: diabetic rats treated with zingerone (100 mg/kg bw/day); Group-V: Diabetic rats treated with glibenclamide 4.5 mg/kg bw/day). Data are represented as mean of 10 rats ± S.E.M. ***P < 0.001: Significant difference between control and treated groups. #P < 0.05: Significant difference between Diabetic control and treated groups ##P < 0.01: Significant difference between Diabetic control and treated groups. ###P < 0.001: Significant difference between Diabetic control and treated group. ns: Non significant.
3.10. Effect of zingerone on IL1-β, IL-6, IL-2 and TNF-α
In the current study there were marked increase in IL1-β, IL-6, IL-2 (Table 6) and TNF-α (Fig. 6) levels in group II as compared to group I. A significant decrease in the levels of IL1-β, IL-6, IL-2 and TNF-α was observed in group III & IV treated with zingerone at the dose range of 50 and 100 mg/kg bwt respectively (Table 6; Fig. 6).
Table 6.
Effect of Zingerone on serum levels of inflammatory cytokines (IL-1β, IL-6 and IL-2).
| Group-I | Group-II | Group-III | Group-IV | Group-V | |
|---|---|---|---|---|---|
| IL-1β (pg/ml) | 840.24 ± 27.6 | 2533.28 ± 55.1*** | 1872.58 ± 41.9# | 1001.92 ± 41.4### | 921.32 ± 39.1### |
| IL-6 (pg/ml) | 973.11 ± 32.6 | 2681.52 ± 59.9*** | 1471.42 ± 49.2## | 1135.71 ± 61.3### | 992.57 ± 46.2### |
| IL-2(pg/ml) | 232.13 ± 44.6 | 942.18 ± 65.7*** | 743.03 ± 31.5## | 357.81 ± 59.3### | 239.71 ± 27.1### |
Group-I: normal control; Group-II: diabetic control; Group-III: diabetic rats treated with zingerone (50 mg/kg bw/day); Group-IV: diabetic rats treated with zingerone (100 mg/kg bw/day); Group-V: Diabetic rats treated with glibenclamide 4.5 mg/kg bw/day). Data are represented as mean of 10 rats± S.E.M.
P < 0.001: Significant difference between control and treated groups.
P < 0.05: Significant difference between Diabetic control and treated groups.
P < 0.01: Significant difference between Diabetic control and treated groups.
P < 0.001: Significant difference between Diabetic control and treated group.
Fig 6.
Effect of Zingerone on TNF-α levels in different experimental groups: Group-I: normal control; Group-II: diabetic control; Group-III: diabetic rats treated with zingerone (50 mg/kg bw/day); Group-IV: diabetic rats treated with zingerone (100 mg/kg bw/day); Group-V: Diabetic rats treated with glibenclamide 4.5 mg/kg bw/day). Data are represented as mean of 10 rats± S.E.M. ***P < 0.001: Significant difference between control and treated groups. #P < 0.05: Significant difference between Diabetic control and treated groups ##P < 0.01: Significant difference between Diabetic control and treated groups. ###P < 0.001: Significant difference between Diabetic control and treated group. ns: Non significant.
4. Discussion
The current study is the first report to demonstrate the potential of zingerone to suppress alloxan induced diabetes in Wistar rats. In this study, we have observed that treatment with zingerone protects the diabetes in Wistar rats. This protection occurs by regulating oxidative stress, decreasing MDA levels, restoring liver function enzymes and inhibiting pro-inflammatory cytokines.
Different model systems like alloxan, streptozotocin, viruses, hormones like dexamethasone, adrenaline and dithizone are available to induce diabetes artificially (Lenzen, 2008, Qaisar et al., 2017, Thounaojam et al., 2017). In the present study alloxan was used to produce marked diabetic effects in animals. Alloxan, a β-cell cytotoxin is used to induce chemical diabetes or Alloxan diabetes by development of oxidative stress inside the cell niche, thereby damaging β-cell which leads to decreased insulin secretion (Rohilla and Ali, 2012). It has been proposed that due to inhibition of glucokinase enzyme activity, there is a decrease in endogenous release of insulin and utilization of insulin which inturn creates hyperglycaemic conditions (Szkudelski, 2001).
Administration of zingerone in diabetic rats for a period of 21 days showed a prominent hypoglycemic effect (Table 1). The hypoglycemic effect of zingerone may be attributed to the regeneration of endocrine pancreatic cellular architecture leading to normal β-cells activity and subsequent release of insulin which inturn might be due to strong antioxidant properties of zingerone (Rao and Rao, 2010). Also increased ROS production leads to derailment of the antioxidant potential inside the cell leading to lipid peroxidation (Petersen et al., 2017). Generation of free radicles due to hyperglycemia weakens the antioxidant defense mechanisms which in-turn cause damage to cellular enzymes and organelles. It also results in elevated MDA levels and development of insulin resistance (Verma et al., 2018). MDA, an aldehyde is regarded as a biomarker for assessing lipid peroxidations as it is formed due to oxidation of membrane lipids and their degeneration (Yigit et al., 2018). In the current study, Alloxan treatment caused a significant elevation in MDA levels suggesting increased production of free radicals which are in agreement with the findings of Nia et al., 2018 which reported increased levels of MDA in liver in diabetic Wistar rats (Fig. 3).
Antioxidative enzymes like CAT, SOD and GPx have a key role in protection of cell against free radical damage. In the current study, alloxan caused a significant decrease in the levels of all these enzymes in the PMS in the diabetic group (group II) as compared to the normal control group (group I) (Table 2). It has been reported earlier that diabetes has a negative effect on the antioxidant enzyme activities, which is attributed to increase in reactive oxygen species (ROS) leading to development and progression of diabetes mellitus (Suryawanshi et al., 2006). These effects may be due to hyperglycemia which increases oxidative stress via over production of ROS, which is generally found in diabetes and contribute to organ injury in liver as well. In the present study, we observed diabetic rats treated with zingerone showed an increase in the activity of CAT, GSH, GPx and SOD as compared to untreated diabetic rats which is in agreement with previous reports (Hemalatha and Prince, 2015, Rajan et al., 2013). The hyperglycemia induced by alloxan also leads to increase in glycosylation of a wide range of proteins including Haemoglobin (Hb) (Reddy, 2018). Administration of zingerone slightly decreased glycosylated Hb content in diabetic rats (Fig. 4) yet the decrease in glycosylation of Hb by zingerone administration was not markedly observed in our study. Regulation of hepatic glucose (HGO) is mediated mainly by gluconeogenesis or glycogenolysis.
Pancreas is a vital organ involved in metabolism of glucose through its endocrine (insulin) and exocrine (amylase) secretions. Insulin the main hormone responsible for glucose metabolism is secreted by β-cells of pancreas. The increase in the serum levels of insulin in zingerone treated diabetic rats (Fig. 1) may be attributed to the regenerative effect of zingerone on pancreas and stimulation of insulin release. In consistency with our findings Chakraborty et al., 2012 revealed that ginger and its active ingredients are responsible for modulating insulin release from β-cells resulting in lowering blood glucose levels. Exocrine pancreatic activity is checked by the concentration of α-amylase in the serum. Any impairment in the pancreatic exocrine cells may be indicated by the decrease in the serum α-amylase activity (Hardt et al., 2000, Shimizu et al., 2000). Zingerone treated diabetic rats showed profound elevation in serum amylase levels (Fig. 2). The improvement in serum amylase observed in zingerone treated rats might be due to regenerative effect of zingerone on islets of pancreas and stimulation of insulin secretions.
Diabetic hyperlipidemia can be regarded for promoting cardiovascular diseases and the management of diabetic dyslepidemia is a key factor in treating diabetes (Kaga et al., 2018). The significant elevation of Triglycerides (TGs), LDL-C and total cholesterol in diabetic rats (group II) in the present study was similar to what was reported previously (Ahmadvand et al., 2014). Insulin deficiency in diabetic rats leads to dearrangement of lipid metabolism which is reflected in terms of elevation of TG, LDL-C and total cholesterol. Drastic reduction in HDL-C was observed in group II. This may be due to protein apoB asoociated with HDL-C which triggers hydrolysis of triglycerides stored in the adipocytes and efflux them into systemic circulation leading to decrease in serum HDL-C levels (Rifai and Warnick, 2006). Zingerone administration in diabetic rats markedly decreased the levels of TG, LDL-C and cholesterol and improved the levels of HDL-C (Table. 3). This is in agreement with the findings of Narayanan and Jesudoss (2016) in which administration of zingerone was found to improve the lipid profile in rats fed with fructose enriched diet.
Both type 1 diabetes and type 2 diabetes are associated with ketosis as the cells utilize fats and lipids instead of glucose due to non-availability of glucose inside the cells leading to harsh consequences in diabetic patients (Kitabchi et al., 2009). In our study there was an increase in the serum levels of AST, ALT and ALP in diabetic group (group II) which might be due to degeneration of the liver architecture and subsequent release of these enzymes in the serum (Table. 4). However, treatment with zingerone effectively decreased the alloxan induced levels of these liver enzymes in serum indicating the hepatoprotective effects of zingerone against alloxan induced diabetes and these results are in agreement with recent findings of Cheong et al. (2016).
NF-κB is an important transcriptional factor sensitive to abnormal oxidative potential due to free radical production, cytokines, obesity and glucose concentration inside the cell milieu (Gilmore, 2006). NF-κB belongs to Rel family of transcription factors and are conserved throughout the evolution (Birbach et al., 2002). It controls the metabolism of many vital cellular processes related to inflammatory modulations such as cytokine production, immunomodulations and cell survival. Stimulation of NF-κB leads to overexpression of many genes coding for cytokines, chemokines and pro-fibrogenic which evoke an inflammatory reaction molecules during diabetes (Patel and Santani, 2009). It has been reported that NF-κB plays a significant role in tissue injuries and different inflammations of vital organs of the body. TNF-α cytokine leads to the activation of NF-Κb and facilitates its entry inside the nucleus for its appropriate targeted action (Jayachandran et al., 2018). In tissue injuries and inflammation thereis Many natural agents like ginger, curcumin, etc., prove to be useful in treating diabetes by inhibiting NF-κB pathway (Kim et al., 2010). Zingerone has also been found to suppress inflammatory response (Xie et al., 2014, Hsiang et al., 2015). In our study, there was inhibition of NF-κB activity in diabetic rats treated with zingerone suggests that zingerone ezhibits its antidiabetic potential by downregulating the NF-κB mediated signaling pathways (Fig. 6).
TNF-α is regarded as the marker inflammatory cytokine being associated with various metabolic diseases including diabetes (Conti et al., 2018). NF-κB has the key role in regulating the TNF-α metabolism and is known to cause insulin resistance by phosphorylation of serine residue of insulin receptor substrate-1 (IRS1). In obese patients, there is a marked induction of TNF-α in the adipose tissues leading to an increase in insulin sensitivity (Shimobayashi et al., 2018). Jimenez et al. (2017) reported that there is a prominent upregulation of proinflammatory cytokines which can lead to pancreatic beta cell destruction and hyperglycemia effects in diabetes mellitus. In accordance with this reports, our study shows that exposure of alloxan to rats leads to generation of acute inflammatory response which might be mediated via induction of NFκB, TNF-α IL-1β, IL-6 and IL-2. Recently it was reported that zingerone treatment ameliorated the inflammatory mediators in diabetic rats (Hany et al., 2017). In our study, zingerone treatment was able to alleviate the abnormal levels of these inflammation related markers in diabetic rats (Fig 6, Fig. 7: Table 5).
Fig. 7.
Summary of Mechanism (Possible Targets) involved in anti-diabetic effect of zingerone.
5. Conclusion
We found that the free radical mediated damage induced by alloxan was reversed by treatment with zingerone to a significant extent. Zingerone in the current study exhibited its hypoglycemic effect in diabetic rats by increasing the antioxidative defensive enzymes, decreasing lipid peroxidation, suppression of proinflammatory cytokines and regulating NFκB levels (Fig. 7). Zingerone at the dose of 100 mg/kg bwt has shown potent protective effect. More studies needs to be conducted in future in order to elucidate the molecular mechanism involved in antidiabetic effect of zingerone.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Kader M.S., Alanazi M.T., Bin Saeedan A.S., Al-Saikhan F.I., Abubaker M., Hamad A.M. Hepatoprotective and nephroprotective activities of Juniperus sabina L. aerial parts. J. Pharm. Pharmacogn. Res. 2017;5:29–39. [Google Scholar]
- Ahmad B., Rehman M.U., Amin I., Arif A., Rasool S., Bhat S.A., Afzal I., Hussain I., Bilal S., Mir M.U. A review on pharmacological properties of zingerone (4-(4-hydroxy-3-methoxyphenyl)-2-butanone) Scientific World J. 2015;10:114–121. doi: 10.1155/2015/816364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadvand H., Noori A., Dehnoo M.G., Bagheri S., Cheraghi R.A. Hypoglycemic, hypolipidemic and antiatherogenic effects of oleuropein in alloxan-induced type 1 diabetic rats. Asian Pac. J. Trop. Dis. 2014;4:442–456. [Google Scholar]
- Akhtar M.S., Ahmed M., Gulzar K., Adnan H. Hypoglycemic activity of Dodonaea viscosa leaves in normal and alloxan-induced diabetic rabbits. Diabetol. Croatica. 2011;40(3) [Google Scholar]
- Alsharidah M., Algeffari M., Abdel-Moneim A.M.H., Lutfi M.F., Alshelowi H. Effect of combined gliclazide/metformin treatment on oxidative stress, lipid profile, and hepatorenal functions in type 2 diabetic patients. Saudi Pharm. J. 2017;26:1–6. doi: 10.1016/j.jsps.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkan M.C., Hevener A.L., Greten F.R., Maeda S., Li Z.W., Long J.M., Wynshaw-Boris A. IKK-links inflammation to obesity-induced insulin resistance. Nat. Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Banerjee M., Saxen M. Interleukin-1 (IL-1) family of cytokines: Role in Type 2 Diabetes. Clinica. Chimica. Acta. 2012;413:1163–1170. doi: 10.1016/j.cca.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Birbach A., Gold P., Binder B.R., Hofer E., Martin R., Schmid J.A. Signaling molecules of the NF-κB pathway shuttle constitutively between cytoplasm and nucleus. J. Biol. Chem. 2002;277:10842–10851. doi: 10.1074/jbc.M112475200. [DOI] [PubMed] [Google Scholar]
- Cai D., Yuan M., Frantz D.F., Melendez P.A., Hansen L., Lee J., Shoelson S.E. Local and systemic insulin resistance resulting from hepatic activation of IKKand NF-B. Nat. Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capellini V.K., Baldo C.F., Celotto A.C., Batalhão M.E., Cárnio E.C., Rodrigues A.J., Evora P.R.B. Oxidative stress is not associated with vascular dysfunction in a model of alloxan-induced diabetic rats. Arq. Bras. Endocrinol. Metab. 2010;54:530–539. doi: 10.1590/s0004-27302010000600004. [DOI] [PubMed] [Google Scholar]
- Carlberg I., Mannervik B. Glutathione levels in rat brain. J. Biol. Chem. 1975;250:4480–4575. [PubMed] [Google Scholar]
- Chakraborty D., Mukherjee A., Sikdar S., Paul A., Ghosh S., Khuda-Bukhsh A.R. Gingerol isolated from ginger attenuates sodium arsenite induced oxidative stress and plays a corrective role in improving insulin signaling in mice. Toxicol. Letter. 2012;210:4–43. doi: 10.1016/j.toxlet.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Chen J., Scott S.E., Gabriel A.F., Clayton M.E. Mitochondrial reactive oxygen species and type 1 diabetes. Antioxidants Redox Signaling. 2018 doi: 10.1089/ars.2017.7346. (ja) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong K.O., Shin D.S., Bak J., Lee C., Kim K.W., Je N.K., Chung H.Y., Yoon S., Moon J.O. Hepatoprotective effects of zingerone on carbon tetrachloride and dimethylnitrosamine-induced liver injuries in rats. Arch. Pharm. Res. 2016;39:279–291. doi: 10.1007/s12272-015-0696-2. [DOI] [PubMed] [Google Scholar]
- Choi S.W., Ho C.K. Antioxidant properties of drugs used in Type 2 diabetes management: could they contribute to, confound or conceal effects of antioxidant therapy? Redox Reports. 2018;23(1):1–24. doi: 10.1080/13510002.2017.1324381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claiborne A. CRC Handbook of Methods for Oxygen Radical Research. CRC Press; Boca Raton, FL: 1985. Catalase activity; pp. 283–284. [Google Scholar]
- Conti P., Ronconi G., Kritas S.K., Caraffa A., Theoharides T.C. Activated mast cells mediate low-grade inflammation in Type 2 diabetes: IL-37 could be beneficial. Can. J. Diabetes. 2018 doi: 10.1016/j.jcjd.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Cui Y., Shi Y., Bao Y., Wang S., Hua Q., Liu Y. Zingerone attenuates diabetic nephropathy through inhibition of nicotinamide adenine dinucleotide phosphate oxidase 4. Biomed. Pharmacotherapy. 2018;99:422–430. doi: 10.1016/j.biopha.2018.01.051. [DOI] [PubMed] [Google Scholar]
- Dugasani S., Pichika M.R., Nadarajah V.D., Balije-palli M.K., Tandra S., Korlakunta J.N. Comparative antioxidant and anti-inflammatory effects of 6-gingerol, 8-gingerol, 10-gingerol and 6-shogaol. J. Ethnopharmacol. 2010;127:515–520. doi: 10.1016/j.jep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Ferrare K., Bidel L.P., Awwad A., Poucheret P., Cazals G., Lazennec F., Azay-Milhau J., Tournier M., Lajoix A.D., Tousch D. Increase in insulin sensitivity by the association of chicoric acid and chlorogenic acid contained in a natural chicoric acid extract (NCRAE) of chicory (Cichorium intybus L.) for an antidiabetic effect. J. Ethnopharmacol. 2018 doi: 10.1016/j.jep.2017.12.035. [DOI] [PubMed] [Google Scholar]
- Ghareib S.A., El-Bassossy H.M., Elberry A.A., Azhar A., Watson M.L., Banjar Z.M., Alahdal A.M. Protective effect of zingerone on increased vascular contractility in diabetic rat aorta. Eur. J. Pharmacol. 2016;780:174–179. doi: 10.1016/j.ejphar.2016.03.046. [DOI] [PubMed] [Google Scholar]
- Gilmore T.D. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- Hany M.E., Wafaa S.A., Ahmed A.E., Mohammad I.M., Salah A., Ahmad S.A., Zainy M.B., Malcolm L.W. Zingerone alleviates the delayed ventricular repolarization and AV conduction in diabetes: effect on cardiac fibrosis and inflammation. PLOS One. 2017;10:13–71. doi: 10.1371/journal.pone.0189074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt P.D., Krauss A., Bretz L., Porsch-Ozcurumez M., Schnell-Kretschmer H., Maser E., Bretzel R.G., Zekhorn T., Klor H.U. Pancreatic exocrine function in patients with type 1 and type 2 diabetes mellitus. Acta Diabetol. 2000;37:105–110. doi: 10.1007/s005920070011. [DOI] [PubMed] [Google Scholar]
- Hemalatha K.L., Prince P.S. Preventive effects of zingerone on altered lipid peroxides and nonenzymatic antioxidants in the circulation of isoproterenol-induced myocardial infarcted rats. J. Biochem. Mol. Toxicol. 2015;29:63–69. doi: 10.1002/jbt.21668. [DOI] [PubMed] [Google Scholar]
- Hsiang C.Y., Cheng H.M., Lo H.Y., Li C.C., Chou P.C., Lee Y.C., Ho T. Ginger and zingerone ameliorate lipopolysaccharide-induced acute systemic inflammation in mice, assessed by nuclear factor-kappa B bioluminescent imaging. J. Agric. Food Chem. 2015;63:6051–6058. doi: 10.1021/acs.jafc.5b01801. [DOI] [PubMed] [Google Scholar]
- Ibrahim Y.M., Abdelatif M.A. Effects of alloxan-induced diabetes mellitus on blood metabolites and serum minerals and hormones in rabbits (Lepus cuniculus) in relation to starch supplementation and season. Adv. Biol. Compos., Biol. Properties Therapeutic Res. 2011;5(1):45–58. [Google Scholar]
- Jayachandran M., Vinayagam R., Ambati R.R., Xu B., Chung S.S.M. Guava leaf extract diminishes hyperglycemia and oxidative stress, prevents β-cell death, inhibits inflammation, and regulates NF-kB signaling pathway in STZ induced diabetic rats. BioMed. Res. Int. 2018 doi: 10.1155/2018/4601649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez A.E., Haro R.D., Terrazas L.I. Taenia crassiceps Antigens Control Experimental Type 1 Diabetes by Inducing Alternatively Activated Macrophages. Mediators Inflamm. 2017;807:29–43. doi: 10.1155/2017/8074329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jothi M.A., Parameswari C.S., Vincent S. Antidiabetic, hypolipidemic and histopathological analysis of zingerone in streptozotocin induced diabetic rats. Int. J. Pharm. Sci. Res. 2016;7(6):2385. [Google Scholar]
- Justino A.B., Miranda N.C., Franco R.R., Martins M.M., da Silva N.M., Espindola F.S. Annona muricata Linn. leaf as a source of antioxidant compounds with in vitro antidiabetic and inhibitory potential against α-amylase, α-glucosidase, lipase, non-enzymatic glycation and lipid peroxidation. Biomed. Pharmacotherapy. 2018;100:83–92. doi: 10.1016/j.biopha.2018.01.172. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Shiratori K., Hayashi N., Fujiwara T., Horikoshi H. Effect of troglitazone on exocrine pancreas in rats with streptozotocin-induced diabetes mellitus. Pancreas. 2000;2:421–426. doi: 10.1097/00006676-200011000-00014. [DOI] [PubMed] [Google Scholar]
- Kaga A.K., Barbanera P.O., Carmo N.O., Rod L. Effect of N-acetylcysteine on dyslipidemia and carbohydrate metabolism in STZ-induced diabetic rats. Int. J. Vascular Med. 2018 doi: 10.1155/2018/6428630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A., Zaman G., Anderson R.A. Bay leaves improve glucose and lipid profile of people with Type 2 diabetes. J. Clinic. Biochem. Nutri. 2009;44:52–56. doi: 10.3164/jcbn.08-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R., Khan A.Q., Qamar W., Lateef A., Tahir M., Rehman M.U., Ali F., Sultana S. Chrysin protects against cisplatin-induced colon. toxicity via amelioration of oxidative stress and apoptosis: probable role of p38MAPK and p53. Toxicol. Appl. Pharmacol. 2012;258(3):315–329. doi: 10.1016/j.taap.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Kim M.K., Chung S.W., Kim D.H., Kim J.M., Lee E.K., Kim J.Y., Ha Y.M. Modulation of age-related NF-kappaB activation by dietary zingerone via MAPK pathway. Exp. Gerontol. 2010;45:419–426. doi: 10.1016/j.exger.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Kitabchi A.E., Umpierrez G.E., Miles J.M., Fisher J.N. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32:1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar L., Chhibber S., Harjai K. Structural alterations in Pseudomonas aeruginosa by zingerone contribute to enhanced susceptibility to antibiotics, serum and phagocytes. Life Sci. 2014;117(1):24–32. doi: 10.1016/j.lfs.2014.09.017. [DOI] [PubMed] [Google Scholar]
- Lee M.G., Choi Y.H., Lee I. Effects of diabetes mellitus induced by alloxan on the pharmacokinetics of metformin in rats: restoration of pharmacokinetic parameters to the control state by insulin treatment. J. Pharm. Pharm. Sci. 2008;11(1):88–103. doi: 10.18433/j35p4x. [DOI] [PubMed] [Google Scholar]
- Lenzen S. The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- Marklund S., Marklund G. Involvement of the superoxideanion radical in the autoxidation of pyrogallol and aconvenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Mohandas M., Marshall J.J., Duggin G.G. Differential distribution of glutathione and glutathione related enzymes in rabbit kidney. Cancer Res. 1984;44:5086–5091. [PubMed] [Google Scholar]
- Nammour F.E., Fayad N.F., Peikin S.R. Metformin-induced cholestatic hepatitis. Endocrine Practice. 2003;9(4):307–309. doi: 10.4158/EP.9.4.307. [DOI] [PubMed] [Google Scholar]
- Narayanan M., Jesudoss V.A. Hepatoprotective potential of zingerone against nonalcoholic fatty liver disease in rats fed with fructose-enriched diet. General Physiol. Biophys. 2016;35:185–194. doi: 10.4149/gpb_2015041. [DOI] [PubMed] [Google Scholar]
- Nia B.H., Khorram S., Rezazadeh H., Safaiyan A., Tarighat-Esfanjani A. The effects of natural clinoptilolite and nano-sized clinoptilolite supplementation on glucose levels and oxidative stress in rats with type 1 diabetes. Can. J. Diabetes. 2018;42:31–35. doi: 10.1016/j.jcjd.2017.01.010. [DOI] [PubMed] [Google Scholar]
- Ogurtsova K., da Rocha Fernandes J.D., Huang Y., Linnenkamp U., Guariguata L., Cho N.H., Cavan D., Shaw J.E., Makaroff L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- Omodanis E., Aboua Y.G., Oguntibeju O.O. Assessment of the antihyperglycaemic, antiinflammatory and antioxidant activities of the methanol extract of Moringa Oleifera in diabetes-induced nephrotoxic male wistar rats. Molecules. 2017;22:439. doi: 10.3390/molecules22040439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panahi Y., Khalili N., Sahebi E., Namazi S., Karimian M.S., Majeed M., Sahebkar A. Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: a randomized controlled trial. Inflammopharmacology. 2017;25:25–31. doi: 10.1007/s10787-016-0301-4. [DOI] [PubMed] [Google Scholar]
- Patel S., Santani D. Role of NF-KB in the pathogenesis of diabetes and its associated complications. Pharmacol. Rep. 2009;61:595–603. doi: 10.1016/s1734-1140(09)70111-2. [DOI] [PubMed] [Google Scholar]
- Perry R.J., Peng L., Cline G.W., Wang Y., Song J.D., Zhang D., Zhang X.M., Nozaki Y., Dufour S., Petersen K.F., Shulman G.I. Mechanisms by which a very-low-calorie diet reverses hyperglycemia in a rat model of type 2 diabetes. Cell Metabol. 2018;27(210–217):e3. doi: 10.1016/j.cmet.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen K.E., Lykkesfeldt J., Raun K., Rakipovski G. Brief Communication: plasma lipid oxidation predicts atherosclerotic status better than cholesterol in diabetic apolipoprotein E deficient mice. Experiment. Biol. Med. 2017;242:88–91. doi: 10.1177/1535370216650520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince P.S.M., Kamalakkannan N., Menon V.P. Antidiabetic and antihyperlipidaemic effect of alcoholic Syzigium cumini seeds in alloxan induced diabetic albino rats. J. Ethnopharmacol. 2004;91(2–3):209–213. doi: 10.1016/j.jep.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Qaisar N., Lin S., Ryan G., Yang C., Oikemus S.R., Brodsky M.H., Bortell R., Mordes J.P., Wang J.P. A critical role for the type I interferon receptor in virus-induced autoimmune diabetes in rats. Diabetes. 2017;66(1):145–157. doi: 10.2337/db16-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan I., Narayanan N., Rabindran R., Jayasree P.R., Kumar M. Zingerone protects against stannous chloride-induced and hydrogen peroxide-induced oxidative DNA damage in vitro. Biol. Trace Elem. Res. 2013;155:455–459. doi: 10.1007/s12011-013-9801-x. [DOI] [PubMed] [Google Scholar]
- Rao B.N., Rao B.S. Antagonistic effects of Zingerone, a phenolic alkanone against radiation-induced cytotoxicity, genotoxicity, apoptosis and oxidative stress in Chinese hamster lung fibroblast cells growing in vitro. Mutagenesis. 2010;25:577–587. doi: 10.1093/mutage/geq043. [DOI] [PubMed] [Google Scholar]
- Rao B.N., Rao B.S., Aithal B.K., Kumar M.S. Radiomodifying and anticlastogenic effect of Zingerone on Swiss albino mice exposed to whole body gamma radiation. Mutat. Res./Genetic Toxicol. Environ. Mutagen. 2009;677(1):33–41. doi: 10.1016/j.mrgentox.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Reddy K.R. Talapotaka pushpa: a promising herbal remedy for diabetes mellitus. Asian J. Pharm. 2018;11:04. [Google Scholar]
- Rehman M.U., Rashid S.M., Rasool S., Shakeel S., Ahmad B., Ahmad S.B., Madkhali H., Ganaie M.A., Majid S., Bhat S.A. Zingerone (4-(4-hydroxy-3-methylphenyl)butan-2-one) ameliorates renal function via controlling oxidative burst and inflammation in experimental diabetic nephropathy. Arch Physiol. Biochem. 2018;14:1–9. doi: 10.1080/13813455.2018.1448422. [DOI] [PubMed] [Google Scholar]
- Rehman M.U., Tahir M., Khan A.Q., Khan R., Lateef A., Oday-O-Hamiza Qamar W, Ali F., Sultana S. Chrysin suppresses renal carcinogenesis via amelioration of hyperproliferation, oxidative stress and inflammation: plausible role of NF-κB. Toxicol Lett. 2013;4;216(2–3):146–158. doi: 10.1016/j.toxlet.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Rifai N., Warnick G.R. Elsevier Saunders; St. Louis, MO, USA: 2006. Lipids, lipoproteins, apolipoproteins, and other cardiovascular risk factors; pp. 903–981. [Google Scholar]
- Rohilla A., Ali S. Alloxan induced diabetes: mechanisms and effects. Int. J. Res. Pharm. Biomed. Sci. 2012;3:819–823. [Google Scholar]
- Sathishsekar D., Subramanian S. Beneficial effects of Momordica charantia seeds in the treatment of STZ-induced diabetes in experimental rats. Biol. Pharm. Bull. 2005;28:978–983. doi: 10.1248/bpb.28.978. [DOI] [PubMed] [Google Scholar]
- Shimobayashi M., Albert V., Woelnerhanssen B., Frei I.C., Weissenberger D., Meyer-Gerspach A.C., Clement N., Moes S., Colombi M., Meier J.A., Swierczynska M.M., Jenö P., Beglinger C., Peterli R., Hall M.N. Insulin resistance causes inflammation in adipose tissue. J. Clin. Invest. 2018;128(4) doi: 10.1172/JCI96139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawanshi N.P., Bhutey A.K., Nagdeote A.N., Jadhav A.A., Manoorkar G.S. Study of lipid peroxide and lipid profile in diabetes mellitus. Ind. J. Clin. Biochem. 2006;21:126–130. doi: 10.1007/BF02913080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001;50:536–546. [PubMed] [Google Scholar]
- Takizawa M., Sato M., Kusuoku H., Sakasai M. Google Patents; 2012. Lipolysis stimulator. [Google Scholar]
- Thounaojam M.C., Powell F.L., Patel S., Gutsaeva D.R., Tawfik A., Smith S.B., Nussbaum J., Block N.L., Martin P.M., Schally A.V., Bartoli M. Protective effects of agonists of growth hormone-releasing hormone (GHRH) in early experimental diabetic retinopathy. Procee. Nat. Acad. Sci. 2017;114(50):13248–13253. doi: 10.1073/pnas.1718592114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma, S., Nibha Sagar, N., Vats, P., Shukla, K.N., Abbas, M., Banerjee, M., 2018. Antioxidant enzyme levels as markers for type 2 diabetes mellitus. 2, 685–690.
- Vinothkumar R., Sudha M., Nalini N. Chemopreventive effect of zingerone against colon carcinogenesis induced by 1,2-dimethylhydrazine in rats. Eur. J. Can. Prev. 2014;23:361–371. doi: 10.1097/CEJ.0b013e32836473ac. [DOI] [PubMed] [Google Scholar]
- Welsh N., Cnop M., Kharroubi I., Bugliani M., Lupi R., Marchetti P., Eizirik D.L. Is there a role for locally produced interleukin-1 in the deleterious effects of high glucose or the type 2 diabetes milieuto human pancreatic islets? Diabetes. 2005;54:3238–3244. doi: 10.2337/diabetes.54.11.3238. [DOI] [PubMed] [Google Scholar]
- Whiting D.R., Guariguta L., Weil C., Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2017;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Wright J.R., Colby H.D., Miles P.R. Cytosolic factorswhich affect microsomal lipid peroxidation in lung and liver. Arch. Biochem. Biophys. 1981;206:296–304. doi: 10.1016/0003-9861(81)90095-3. [DOI] [PubMed] [Google Scholar]
- Xie X., Sun S., Zhong W., Soromou L.W., Zhou X. Zingerone attenuates lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol. 2014;19:103–109. doi: 10.1016/j.intimp.2013.12.028. [DOI] [PubMed] [Google Scholar]
- Xiong H., Zhang S., Zhao Z., Zhao P., Chen L., Mei Z. Antidiabetic activities of entagenic acid in type 2 diabetic db/db mice and L6 myotubes via AMPK/GLUT4 pathway. J. Ethnopharmacol. 2018;211:366–374. doi: 10.1016/j.jep.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Yigit M., Sogut O., Tataroglu O., Yamanoglu A., Yigit E., Güler E.M., Ozer O.F., Kocyigit A. Oxidative/antioxidative status, lymphocyte DNA damage, and urotensin-2 receptor level in patients with migraine attacks. Neuropsychiatr. Dis. Treat. 2018;14:367–374. doi: 10.2147/NDT.S156710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Thakur K., Hu F., Zhang J.G., Wei Z.J. Cross talk between 10-gingerol and its anti-cancerous potential: a recent update. Food Funct. 2017;8:2635–2649. doi: 10.1039/c7fo00844a. [DOI] [PubMed] [Google Scholar]