Abstract
Objective
To investigate the relationship between psoriasis and interstitial pneumonia (IP).
Patients and Methods
We analyzed the clinical data of patients with psoriasis treated with biologic agents from June 1, 2008, to June 30, 2017, retrospectively. Chest computed tomography was performed in 392 patients before treatment. The clinical characteristics and radiographic findings of these patients were evaluated.
Results
Of the 392 patients with psoriasis, IP was detected in 8 patients (2%). Bilateral ground-glass and/or irregular linear (reticular) opacity in the lower lung zone was the most common chest computed tomography finding. Five of the 8 patients with IP were treated with anti–interleukin (IL) 12/IL-23 or IL-17 antibodies, leading to decreased or stable IP activity.
Conclusion
Interstitial pneumonia was detected in 2% of patients with psoriasis who needed systemic treatments. Ground-glass and/or irregular linear (reticular) opacity in the bilateral lower lobes was characteristic of IP with psoriasis. The IL-23/IL-17 axis may play important roles in the pathogenesis of IP in psoriasis.
Abbreviations and Acronyms: CT, computed tomography; IL, interleukin; IP, interstitial pneumonia; KL-6, Krebs von den Lungen-6; PASI, psoriasis area severity index; SEC, secukinumab; UST, ustekinumab
Psoriasis is a chronic inflammatory skin disease.1 Inflammation in psoriasis is not limited to the skin as surplus proinflammatory cytokines from inflamed skin affect systemic organs.1 Almost all blood flows into the pulmonary circulation, suggesting that the lung parenchyma is susceptible to systemic inflammation in psoriasis. However, no large studies demonstrating a link between psoriasis and interstitial pneumonia (IP) have been conducted.
Patients with psoriasis are routinely screened for prevalent well-known comorbidities, including cardiovascular diseases and diabetes mellitus.2, 3, 4, 5 However, lung involvement, including IP, is not intensively investigated unless respiratory symptoms are obvious. Chest computed tomography (CT) is a useful tool for finding mild IP that is not detectable on chest radiography; however, CT is not routinely performed in all patients with psoriasis. Hence, asymptomatic, mild IP associated with psoriasis may have been underdiagnosed so far.
In our hospital, chest CT is routinely performed in patients with psoriasis who need biologic agents to exclude latent and active lung tuberculosis because tuberculosis is highly prevalent in Japan. We retrospectively evaluated lung lesions in these patients. We also evaluated IP activity in relation to psoriasis activity by chest CT and serum Krebs von den Lungen-6 (KL-6) assay. The aims of this study were to determine the prevalence of IP and to reveal the clinical and radiographic characteristics of IP in patients with psoriasis.
Patients and Methods
Study Population
Five hundred twelve patients with psoriasis were treated with biologic agents at Jikei University Hospital from June 1, 2008, to June 30, 2017. Chest CT was performed in 392 patients, and the images were evaluated by 2 specialists of the Japanese Respiratory Society (H.H. and H.K.). Interstitial pneumonia was detected in 8 patients. The clinical characteristics of patients with IP and those without IP were compared. In the 8 patients with IP, the radiographic characteristics of IP were evaluated. The psoriasis area severity index (PASI) score and type of psoriasis were determined by specialists of the Japanese Dermatological Association.
This study was approved by the Ethics Committee of Jikei University (29-078 [8694]).
Statistical Analyses
Clinical indices were compared between patients with psoriasis with IP and those without IP using Welch t test, Fisher exact test, Mann-Whitney U test, and χ2 test. A 2-sided P value of less than .05 was considered statistically significant.
All statistical analysis was performed with EZR (Saitama Medical Center), which is a graphical user interface for R (The R Foundation for Statistical Computing). More precisely, it is a modified version of the R commander designed to add statistical functions frequently used in biostatistics.6
Results
Clinical Characteristics of Patients With Psoriasis With or Without IP
Lung involvement of the 392 patients with psoriasis who were treated with biologic agents was evaluated by chest CT before starting biologic therapy. Interstitial pneumonia was detected in 8 patients (2.0%). The clinical characteristics of patients with psoriasis with IP and those without IP are summarized in Table 1.
Table 1.
Characteristics of Patients With Psoriasis With or Without IP
| Characteristic | With IP (n=8) | Without IP (n=384) | P value |
|---|---|---|---|
| Age (y) | 72±5.98 | 53.5±15.3 | <.001 |
| Sex: male (%) | 6 (75) | 271 (70.6) | >.99 |
| Disease duration (y) | 11±8.49 | 13.9±10.4 | .21 |
| Ex-smoker (%) | 5 (62.5) | 221 (57.9) Unknown: n=2 |
>.99 |
| BI | 832±455 | 551±478 | .13 |
| Family history of psoriasis | 0 (0) | 21 (5.5) Unknown: n=2 |
>.99 |
| PASI score | 12.1±9.93 | 12.2±8.48 | .76 |
| KL-6 (U/mL) | 504±149 | 261±172 | .003 |
| Type of psoriasis | .003 | ||
| PsV | 4 (50%) | 290 (75.52%) | |
| PsA | 1 (12.5%) | 81 (21.09%) | |
| GPP | 2 (25 %) | 9 (2.34%) | |
| PsE | 1 (12.5%) | 1 (0.26%) | |
| PsG | 0 (0%) | 1 (0.26%) | |
| PPP | 0 (0%) | 2 (0.52%) |
BI = Brinkman index; GPP = generalized pustular psoriasis; IP = interstitial pneumonia; KL-6 = Krebs von den Lungen-6; PASI = psoriasis area severity index; PPP = palmoplantar pustulosis; PsA = psoriatic arthritis; PsE = erythrodermic psoriasis; PsG = guttate psoriasis; PsV = psoriasis vulgaris.
Patients with psoriasis with IP were significantly older than those without IP (P<.01). The serum sialylated carbohydrate antigen KL-6 levels in patients with psoriasis with IP were higher than those in patients without IP (P=.003). Generalized pustular psoriasis, the most severe type of psoriasis, was frequent in patients with psoriasis with IP. Sex, smoking status, family history of psoriasis, PASI scores, and underlying diseases were not different between the 2 groups. It is highly likely given the small sample size in the psoriasis with IP group that there is not sufficient statistical power to detect meaningful differences between the psoriasis with IP group and the psoriasis without IP group. The coexistence of any other connective tissue diseases that might cause IP was excluded by clinical evaluation and laboratory tests including autoantibodies.
Biologic agents were used in 392 patients (infliximab in 88 cases, adalimumab in 129 cases, ustekinumab [UST] in 141 cases, secukinumab [SEC] in 33 cases, and brodalumab in 1 case).
Detailed characteristics of the 8 patients with psoriasis with IP are presented in Table 2. Of the eight patients, 6 were men and 2 were women (age range, 61-81 years; median age, 73.5 years). Psoriasis disease duration was 1 to 26 years, with a median of 11 years. Five patients were former smokers, and the other 3 were nonsmokers. One patient had respiratory symptoms, whereas the others had no symptoms. Four patients had a PASI score higher than 10 (average, 12.1; range, 0.6-20.4), and KL-6 levels were elevated in 4 patients (3 of the 4 patients with a PASI score of >10) (average, 504; range, 307-770). Of the 8 patients, 5 patients were treated with UST, 2 were treated with adalimumab, and 1 was treated with infliximab.
Table 2.
Clinical Characteristics of Patients With Psoriasis With Interstitial Pneumonia
| Case | Age (y) | Sex | Disease duration (y) | Smoking status | PASI score | KL-6 (U/mL) | Type of psoriasis | Treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | 61 | M | 3 | Ex | 6.2 | 352 | GPP | ADA |
| 2 | 67 | M | 2 | Ex | 0.6 | 507 | PsA | ADA |
| 3 | 68 | M | 1 | Ex | 32 | 530 | PsE | IFX |
| 4 | 75 | M | 26 | Never | 10.4 | 467 | PsV | UST |
| 5 | 75 | M | 16 | Never | 2.7 | 407 | PsV | UST |
| 6 | 72 | M | 15 | Ex | 18 | 770 | PsV | UST/SEC |
| 7 | 77 | F | 18 | Ex | 6.6 | 307 | GPP | UST |
| 8 | 81 | F | 7 | Never | 20.4 | 688 | PsV | UST |
ADA = adalimumab; GPP = generalized pustular psoriasis; F = female; IFX = infliximab; KL-6 = Krebs von den Lungen-6; M = male; PASI = psoriasis area severity index; PsA = psoriatic arthritis; PsE = erythrodermic psoriasis; PsV = psoriasis vulgaris; SEC = secukinumab; UST = ustekinumab.
Radiographic Findings in Patients With Psoriasis With IP
Chest CT scans of the patients with psoriasis with IP are shown in Figure 1, Figure 2, Figure 3, Figure 4. A summary of the radiographic findings is presented in Table 3.
Figure 1.
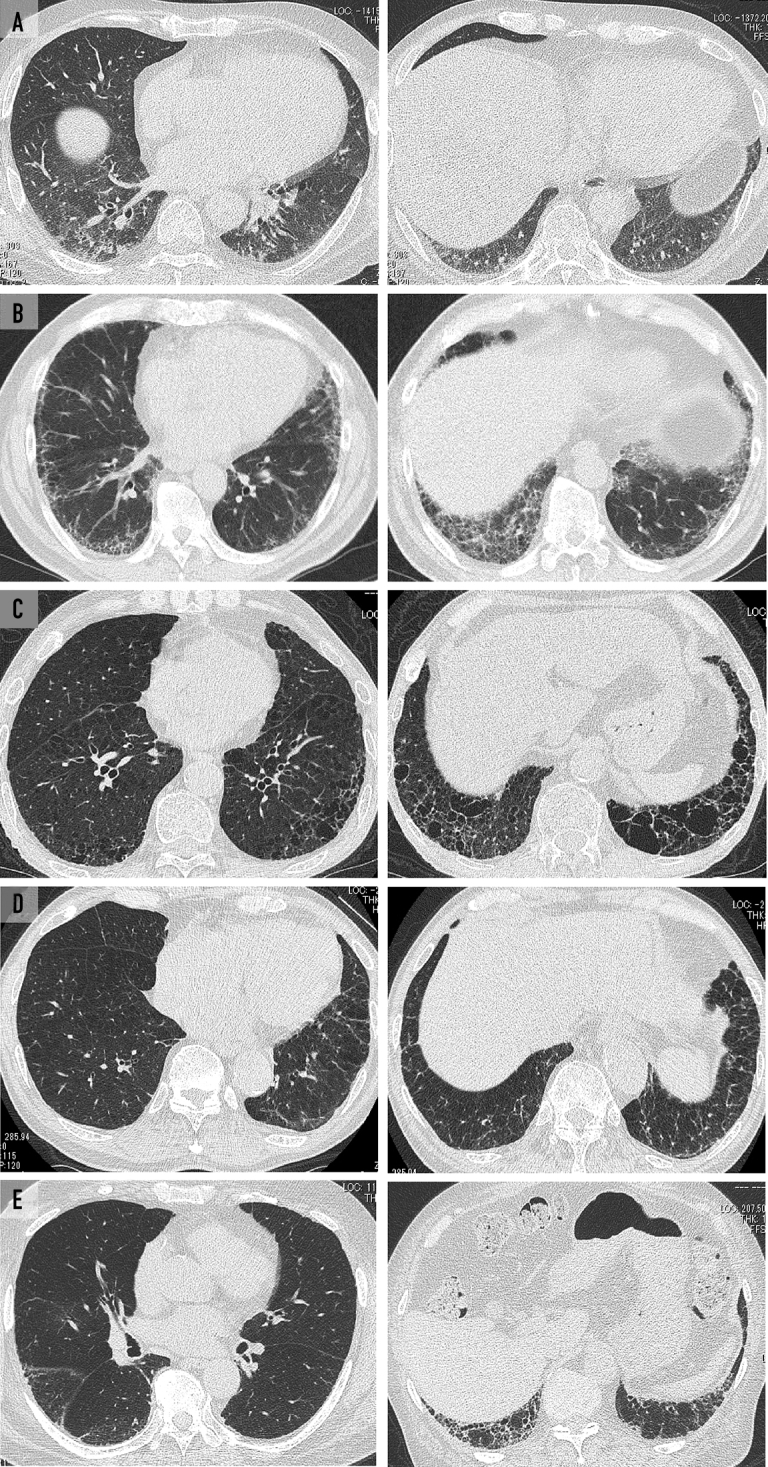
A, Ground-glass and irregular linear (reticular) opacity was distributed along the bronchial vascular bundle in the lower lobes on chest computed tomography (CT) of case 1 (61-year-old man). Chest CT in case 2 (67-year-old man) (B) and case 5 (75-year-old man) (E) showed subpleural ground-glass and irregular linear (reticular) opacity distributed in the lower lobes. Subpleural linear opacity was shown on CT in case 3 (68-year-old man) (C) and case 4 (75-year-old man) (D).
Figure 2.
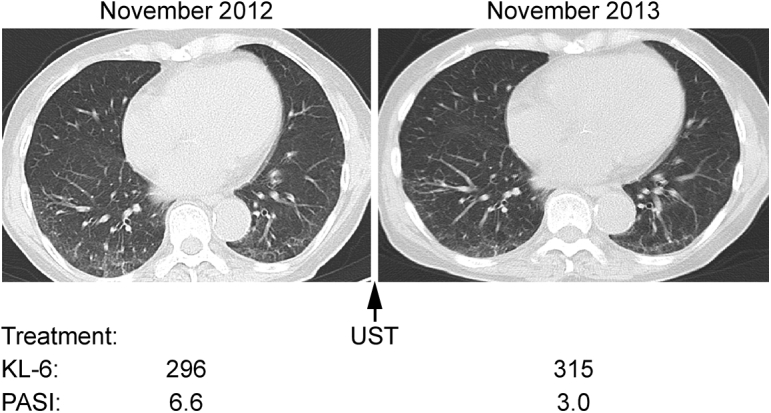
The relationship between IP activity and psoriasis activity in case 6, a 72-year-old man.7 Interstitial pneumonia activity was evaluated by chest CT and serum KL-6, and psoriasis activity was assessed by PASI scores. Chest CT showed subpleural ground-glass and irregular linear (reticular) opacity distributed in the lower lobes. Interstitial pneumonia activities fluctuated in accordance with psoriasis activity. Used with permission of Eur J Dermatol.7 CT = computed tomography; IP = interstitial pneumonia; KL-6 = Krebs von den Lungen-6; PASI = psoriasis area severity index; UST = ustekinumab.
Figure 3.
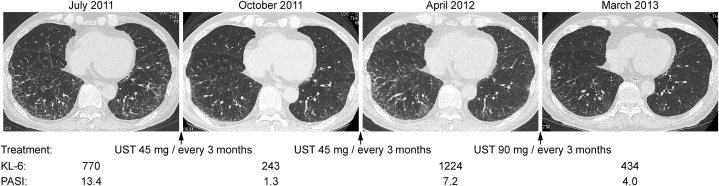
The chest CT, KL-6 levels, and PASI score of case 7 (77-year-old man) are shown. Ground-glass opacities along the bronchial vascular bundle were ameliorated by treatment with UST with decreased KL-6 levels and PASI scores. CT = computed tomography; KL-6 = Krebs von den Lungen-6; PASI = psoriasis area severity index; UST = ustekinumab.
Figure 4.
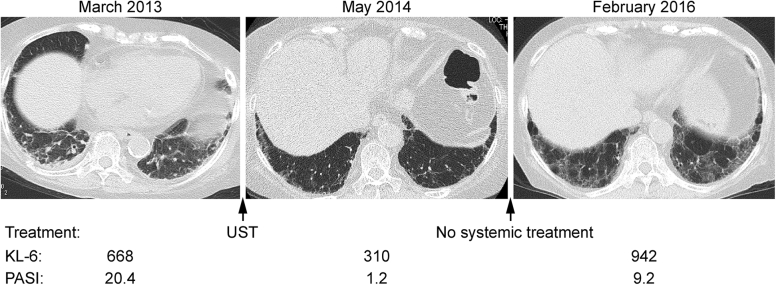
The chest CT, KL-6 levels, and PASI score of case 8 (81-year-old man) are shown. Subpleural ground-glass and irregular linear (reticular) opacity distributed in the lower lobes improved with UST treatment temporarily. Discontinuation of the treatment worsened both IP and psoriasis. CT = computed tomography; IP = interstitial pneumonia; KL-6 = Krebs von den Lungen-6; PASI = psoriasis area severity index; UST = ustekinumab.
Table 3.
Radiographic Characteristics of IP in Patients With Psoriasis
| Case | Abnormal chest radiography findings | Distribution | GGO | Reticular opacity | Emphysema | IP activity during psoriasis treatment | Respiratory failure due to IP |
|---|---|---|---|---|---|---|---|
| 1 | Yes | Bilateral lower lobe | Yes | Yes | No | Stable | No |
| 2 | Yes | Bilateral lower lobe | Yes | Yes | No | Stable | Yes |
| 3 | Yes | Bilateral lower lobe | No | Yes | Yes | Stable | No |
| 4 | No | Bilateral lower lobe | No | Yes | No | Stable | No |
| 5 | No | Bilateral lower lobe | Yes | Yes | No | Stable | No |
| 6 | No | Bilateral lower lobe | Yes | Yes | Yes | Improved | No |
| 7 | No | Bilateral lower lobe | Yes | Yes | No | Improved | No |
| 8 | Yes | Bilateral lower lobe | Yes | Yes | No | Improved | No |
GGO = ground-glass opacity; IP = interstitial pneumonia.
Lung lesions could be detected by chest radiography in 4 patients. Bilateral ground-glass and/or irregular linear (reticular) opacity was the most common CT finding in these patients. These opacities predominantly involved the lower lung zone in both lungs.
Correlation Between IP Activity and Psoriasis Activity
Interstitial pneumonia activity was evaluated by chest radiography, CT, and serum KL-6 periodically in patients with psoriasis with IP. Psoriasis activity was evaluated by PASI scores.
Five of the 8 patients were treated with anti–interleukin (IL) 12/IL-23p40 antibodies; UST or anti–IL-17 antibodies; SEC or anti–IL-17 receptor antibodies; or brodalumab, and disease activity decreased in 3 patients (cases 6, 7, and 8) and was stable in 2 patients.
Details on the clinical course of cases 6, 7, and 8 are as follows. In case 6, UST induction treatment improved not only skin lesions but also IP lesions.7 After the recurrence of skin lesions during UST maintenance treatment, IP became worse. An increased dose of UST ameliorated both skin and lung lesions (Figure 2). The IP became worse again with further relapse of psoriasis, which was improved by treatment with SEC (data not shown). In case 7, lung lesions (ground-glass and irregular linear [reticular] opacity) on chest CT regressed with UST treatment, which was effective for psoriasis skin lesions (Figure 3). In case 8, both lung and skin lesions regressed with UST treatment. Both lesions were exacerbated after the discontinuation of UST (Figure 4).
In these 3 patients, IP activity was aligned with psoriasis activity. Intriguingly, patients who were treated with biologic agents other than an IL-23/IL-17 axis blocker showed no clear association between IP activity (assessed by CT and KL-6) and psoriasis activity (PASI score).
Discussion
In this study, we demonstrated that IP was detected in patients with psoriasis who needed biologic agents (8 of 392 [2%]). Bilateral ground-glass and irregular linear (reticular) opacity in the lower lobes was the most common chest CT finding in these patients. Most of the IPs were mild without respiratory symptoms, and half of the IPs were not detectable by chest radiography. The activities of IP were aligned with the activities of psoriasis in 3 of 5 patients treated with anti–IL-12/IL-23 or IL-17 antibodies, suggesting an intimate link between psoriasis and IP.
Increasing evidence suggests that psoriasis has systemic effects and is associated with several systemic comorbidities, including cardiometabolic diseases.1, 3, 4, 5 Although the high prevalence of chronic obstructive pulmonary disease8, 9 and pneumonia10 in psoriasis has been demonstrated by population-based studies, the association between psoriasis and IP remains to be determined. Indeed, there are only a few case reports demonstrating the simultaneous existence of psoriasis and IP,11, 12, 13, 14, 15 including case 6 in this report.7 No epidemiologic studies searching for comorbidities in psoriasis have revealed a high prevalence of IP in psoriasis, indicating that clinically relevant IP might be rare in patients with psoriasis. However, in this study, we showed that IP was involved in 2% of patients with psoriasis who needed biologic agents. Considering that the prevalence of idiopathic pulmonary fibrosis, one of the most common idiopathic forms of IP, is 0.01% in the general population in Japan,16 the prevalence of IP in psoriasis in this study seems high.
The high prevalence of IP in patients with psoriasis in this study is partly due to the high detection rate of asymptomatic and subclinical IP by chest CT. In previous studies, chest CT would not be performed in most patients because IP has not been recognized as a comorbidity in psoriasis. Subclinical IP associated with psoriasis might be underdiagnosed in those studies. Intriguingly, Scherak et al17 reported that lymphocytes in the bronchoalveolar lavage fluid of asymptomatic seronegative patients with arthritis (8 patients with psoriasis of a total of 13 patients were included) increased.17 The lung might be affected insidiously in psoriasis.
Another reason for the high prevalence of IP in this study was that the study population included patients with severe psoriasis who needed biologic agents. In particular, generalized pustular psoriasis, the most severe type of psoriasis,18 was more frequent in patients with psoriasis with IP than in those without IP. The severity of psoriasis is associated with the high prevalence of comorbidities of psoriasis.19 A large surplus of proinflammatory cytokines from inflamed skin might have affected the lungs in the patients with severe psoriasis in this study.
In this study, we investigated the radiographic characteristics of IP associated with psoriasis. Most of the IPs associated with psoriasis were mild and affected only a small part of the lung; hence, half of the IPs were not detectable by chest radiography. Bilateral ground-glass and/or irregular linear (reticular) opacity in the lower lung zone on CT scans was a characteristic radiographic finding.
Increasing evidence suggests that the IL-23/IL-17 axis plays central roles in the pathogenesis of psoriasis,1, 20 and clinically available anti–IL-23 or IL-17 antibodies improve psoriasis skin lesions.21, 22, 23 In this study, we showed that the disease activities of IP were aligned with the activities of psoriasis in 3 of 5 patients treated with anti–IL-12/IL-23 or IL-17 antibodies. Inhibition of the IL-23/IL-17 axis ameliorated not only psoriasis skin lesions but also IP, indicating that this signaling pathway is involved in the development of both psoriasis and IP. In addition to the 3 patients in this report, Miyachi et al13 recently reported a case showing the improvement in IP during psoriasis by IL-23/IL-17 inhibition. Inhibition of this pathway with biologic agents may be effective for IP associated with psoriasis, although biologic agents also potentially induce IP.24, 25 Intriguingly, inhibition of the IL-23/IL-17 axis also ameliorated inflammation-induced lung fibrosis in mouse models through several mechanisms.26, 27, 28 The IL-23/IL-7 axis is also up-regulated in many autoimmune diseases, including rheumatoid arthritis and inflammatory bowel diseases,29 although the pathogenetic roles of the IL-23/IL-17 axis remain unknown. Rheumatoid arthritis and inflammatory bowel diseases frequently affect the lungs,30, 31 suggesting that the IL-23/IL-17 axis may play some roles in the development of lung involvement in these diseases. The precise mechanisms of the intimate relationship between IP and autoimmune diseases, including psoriasis, need further studies.
This study has several limitations. First, this study was retrospectively designed and performed in a single center. In addition, the sample size was small. Larger studies are necessary to confirm our results. Second, psoriasis affects 2% to 3% of the general population in Europe, whereas it affects 0.34% in Japan.32 The genetic background may differ between patients in Japan and those in other countries. Hence, our conclusions might not be applicable to patients in other countries. Finally, our study population consisted of patients with severe psoriasis who needed the biologic agents described. The prevalence of IP in psoriasis will be lower in patients with mild or topical psoriasis than in patients with severe psoriasis in this study.
Conclusion
Mild asymptomatic IP was detected in 2% of patients with psoriasis who needed biologic agents. Ground-glass and/or irregular linear (reticular) opacity in the bilateral lower lobes was characteristic of IP with psoriasis. Individuals with severe psoriasis should be counseled and screened for IP by chest CT and serum KL-6. The intimate relationship between psoriasis and IP indicated that common pathways were involved in the development of both psoriasis and IP. The IL-23/IL-17 axis may play important roles in the pathogenesis of both psoriasis and IP although a direct role for this pathway in the IP observed in the patients with psoriasis remains to be elucidated. Inhibition of this pathway may be effective treatment for IP associated with psoriasis. Additional therapy for IP with immunosuppressants can be avoided in some cases.
Acknowledgments
Drs Kawamoto and Hara contributed equally to this work.
Footnotes
Potential Competing Interests: H. Nakagawa is a consultant and/or has received research grants and/or speaker honoraria from Kyowa Hakko Kirin, AbbVie, Mitsubishi Tanabe, Janssen, Eli Lilly, Leo Pharma, Maruho, and MSD.
A. Asahina is a consultant and/or has received speaker honoraria from Kyowa Hakko Kirin, AbbVie, Mitsubishi Tanabe, Taiho, Torii, Janssen, Eli Lilly, Leo Pharma, and Maruho.
K. Kuwano is a consultant and has received consulting fees and research grants from ONO Pharmaceutical Co., LTD; he also has received research grants from Astellas Pharma Inc., KYORIN Pharmaceutical Co., LTD, and Nippon Boehringer Ingelheim Co., LTD. The rest of the authors report no competing interests.
References
- 1.Greb J.E., Goldminz A.M., Elder J.T., et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082. doi: 10.1038/nrdp.2016.82. [DOI] [PubMed] [Google Scholar]
- 2.Furue K., Ito T., Tsuji G., Kadono T., Nakahara T., Furue M. Autoimmunity and autoimmune co-morbidities in psoriasis. Immunology. 2018;154(1):21–27. doi: 10.1111/imm.12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito T., Takahashi H., Kawada A., Iizuka H., Nakagawa H., Japanese Society for Psoriasis Research Epidemiological survey from 2009 to 2012 of psoriatic patients in Japanese Society for Psoriasis Research. J Dermatol. 2018;45(3):293–301. doi: 10.1111/1346-8138.14105. [DOI] [PubMed] [Google Scholar]
- 4.Ryan C., Kirby B. Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol Clin. 2015;33(1):41–55. doi: 10.1016/j.det.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Takeshita J., Grewal S., Langan S.M., et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76(3):377–390. doi: 10.1016/j.jaad.2016.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hara H., Kuwano K., Kawamoto H., et al. Psoriasis-associated interstitial pneumonia. Eur J Dermatol. 2018;28(3):395–396. doi: 10.1684/ejd.2018.3264. [DOI] [PubMed] [Google Scholar]
- 8.Ungprasert P., Srivali N., Thongprayoon C. Association between psoriasis and chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Dermatolog Treat. 2016;27(4):316–321. doi: 10.3109/09546634.2015.1107180. [DOI] [PubMed] [Google Scholar]
- 9.Balci D.D., Celik E., Genc S., Çelik M.M., Inan M.U. Impaired pulmonary function in patients with psoriasis. Dermatology. 2016;232(6):664–667. doi: 10.1159/000456032. [DOI] [PubMed] [Google Scholar]
- 10.Kao L.T., Lee C.Z., Liu S.P., Tsai M.C., Lin H.C. Psoriasis and the risk of pneumonia: a population-based study. PLoS One. 2014;9(12):e116077. doi: 10.1371/journal.pone.0116077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta R., Espiritu J. Azathioprine for the rare case of nonspecific interstitial pneumonitis in a patient with psoriasis. Ann Am Thorac Soc. 2015;12(8):1248–1251. doi: 10.1513/AnnalsATS.201505-274LE. [DOI] [PubMed] [Google Scholar]
- 12.Messina M., Scichilone N., Guddo F., Bellia V. Rapidly progressive organising pneumonia associated with cytomegalovirus infection in a patient with psoriasis. Monaldi Arch Chest Dis. 2007;67(3):165–168. doi: 10.4081/monaldi.2007.489. [DOI] [PubMed] [Google Scholar]
- 13.Miyachi H., Nakamura Y., Nakamura Y., Matsue H. Improvement of the initial stage of interstitial lung disease during psoriasis treatment with secukinumab. J Dermatol. 2017;44(12):e328–e329. doi: 10.1111/1346-8138.14026. [DOI] [PubMed] [Google Scholar]
- 14.Penizzotto M., Retegui M., Arrien Zucco M.F. Organizing pneumonia associated with psoriasis [in Spanish] Arch Bronconeumol. 2010;46(4):210–211. doi: 10.1016/j.arbres.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Webber N.K., Elston C.M., O’Toole E.A. Generalized pustular psoriasis and cryptogenic organizing pneumonia. Br J Dermatol. 2008;158(4):853–854. doi: 10.1111/j.1365-2133.2007.08421.x. [DOI] [PubMed] [Google Scholar]
- 16.Natsuizaka M., Chiba H., Kuronuma K., et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med. 2014;190(7):773–779. doi: 10.1164/rccm.201403-0566OC. [DOI] [PubMed] [Google Scholar]
- 17.Scherak O., Kolarz G., Popp W., Wottawa A., Ritschka L., Braun O. Lung involvement in rheumatoid factor-negative arthritis. Scand J Rheumatol. 1993;22(5):225–228. doi: 10.3109/03009749309095127. [DOI] [PubMed] [Google Scholar]
- 18.Choon S.E., Lai N.M., Mohammad N.A., Nanu N.M., Tey K.E., Chew S.F. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53(6):676–684. doi: 10.1111/ijd.12070. [DOI] [PubMed] [Google Scholar]
- 19.Yeung H., Takeshita J., Mehta N.N., et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149(10):1173–1179. doi: 10.1001/jamadermatol.2013.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogawa E., Sato Y., Minagawa A., Okuyama R. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45(3):264–272. doi: 10.1111/1346-8138.14139. [DOI] [PubMed] [Google Scholar]
- 21.Krueger G.G., Langley R.G., Leonardi C., et al. CNTO 1275 Psoriasis Study Group A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356(6):580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 22.Langley R.G., Elewski B.E., Lebwohl M., et al. ERASURE Study Group. FIXTURE Study Group Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 23.Rich P., Sigurgeirsson B., Thaci D., et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168(2):402–411. doi: 10.1111/bjd.12112. [DOI] [PubMed] [Google Scholar]
- 24.Kajihara I., Yamada-Kanazawa S., Maeda-Otsuka S., Jinnin M., Akaike K., Ihn H. Secukinumab-induced interstitial pneumonia in a patient with psoriasis vulgaris. J Dermatol. 2017;44(12):e322–e323. doi: 10.1111/1346-8138.13986. [DOI] [PubMed] [Google Scholar]
- 25.Kikuchi S., Umezawa Y., Hayashi M., et al. Interstitial pneumonia in two patients with psoriasis during ustekinumab treatment. J Dermatol. 2016;43(6):712–713. doi: 10.1111/1346-8138.13250. [DOI] [PubMed] [Google Scholar]
- 26.Gasse P., Riteau N., Vacher R., et al. IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis. PLoS One. 2011;6(8):e23185. doi: 10.1371/journal.pone.0023185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mi S., Li Z., Yang H.Z., et al. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-beta1-dependent and -independent mechanisms. J Immunol. 2011;187(6):3003–3014. doi: 10.4049/jimmunol.1004081. [DOI] [PubMed] [Google Scholar]
- 28.Wilson M.S., Madala S.K., Ramalingam T.R., et al. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 2010;207(3):535–552. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwakura Y., Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116(5):1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Songur N., Songur Y., Tuzun M., et al. Pulmonary function tests and high-resolution CT in the detection of pulmonary involvement in inflammatory bowel disease. J Clin Gastroenterol. 2003;37(4):292–298. doi: 10.1097/00004836-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Hyldgaard C., Hilberg O., Pedersen A.B., et al. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis. 2017;76(10):1700–1706. doi: 10.1136/annrheumdis-2017-211138. [DOI] [PubMed] [Google Scholar]
- 32.Kubota K., Kamijima Y., Sato T., et al. Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open. 2015;5(1):e006450. doi: 10.1136/bmjopen-2014-006450. [DOI] [PMC free article] [PubMed] [Google Scholar]