Abstract
We report the development of a Shiga toxin–producing Escherichia coli O157 gastrointestinal infection associated with hemolytic uremic syndrome in an allogenic stem cell transplant recipient with a history of gastrointestinal graft-vs-host disease receiving long-term immunosuppression.
Abbreviations and Acronyms: BMT, bone marrow transplant; GvHD, graft-vs-host disease; HUS, hemolytic uremic syndrome; LDH, lactate dehydrogenase; STEC, Shiga toxin–producing Escherichia coli; TMA, thrombotic microangiopathy
A 67-year-old woman presented to a local emergency department with abdominal pain and hematochezia that developed after she attended an agricultural state fair where she ate local products. Her medical history was notable for well-controlled type 2 diabetes and hypertension, and over 1 year previously, she had undergone matched unrelated donor allogenic peripheral blood stem cell transplant for the treatment of high-risk acute myeloid leukemia. The posttransplant course was complicated by the development of biopsy-proven grade 2 lower gastrointestinal graft-vs-host disease (GvHD) on day 39 that was successfully treated with tacrolimus and corticosteroids. At the time of current presentation, she was taking tacrolimus, 0.5 mg daily, and prednisone, 5 mg daily.
Two days before this presentation, she started experiencing loose bowel movements associated with abdominal pain that rapidly progressed to frequent episodes of bloody diarrhea, severe abdominal pain, nausea, and vomiting on the day of presentation. She was admitted to a local hospital for further management. At the time of admission, she reported no fever, chills, night sweats, or respiratory or urinary symptoms. She had eaten crepes and nutty bars at the fair 3 days before admission as well as drinking from a water fountain. She denied any sick contacts and had no relevant travel history. After admission, she underwent a diagnostic colonoscopy with random biopsies. Pathological examination revealed extensive hemorrhage within the lamina propria, extensive surface erosions, prominent crypt dropout, and foci of hyalinization within the lamina propria, suggesting severe mucosal inflammation.
She was transferred to our institution the next day for further management. On arrival, she continued to report considerable abdominal pain, nausea, and diarrhea.
Computed tomography of the abdomen and pelvis demonstrated markedly thickened and edematous colon, extending from the cecum to the splenic flexure. Findings were consistent with severe colitis (Figure 1).
Figure 1.
Computed tomography on admission. Markedly thickened and edematous colon extending from the cecum to the splenic flexure. Findings consistent with colitis, most likely infectious vs inflammatory. The arrows show the marked thickening of the colon.
Initial laboratory evaluation revealed leukocytosis, new-onset thrombocytopenia, and an elevated creatinine level. Hemoglobin on admission was within normal limits. Stool studies demonstrated positivity to Shiga toxin–producing Escherichia coli O157 serotype. At the time of transfer, she was receiving metronidazole, doxycycline, and cephalexin (initiated for a presumed diagnosis of infectious colitis), which were discontinued because of potential increased risk for transformation to hemolytic uremic syndrome (HUS). Prophylactic acyclovir and posaconazole were continued.
Supportive management with intravenous fluids was initiated. Neurological evaluation was performed every 4 hours. Hemolysis parameters were monitored with daily peripheral blood smear and haptoglobin, lactate dehydrogenase (LDH), and reticulocyte measurements. Three days after admission, mild confusion was reported overnight, and examination revealed a somnolent patient who was disoriented to time and place. The patient continued to experience diarrhea. In addition, schistocytes were identified on peripheral smear for the first time that morning (Figure 2). Laboratory values over the course of a few days continued to show deterioration with new-onset anemia, worsening thrombocytopenia, increasing creatinine levels, LDH elevation, and haptoglobin decrease. Total complement, factor B and H complement assay, and complement C3 and C4 were within normal limits, SC5b-9 complement was elevated (348 ng/mL [<251 ng/mL]), and ADAMTS13 activity was normal at 71%, consistent with the development of microangiopathy and HUS. Because of the concern for drug-induced microangiopathy, tacrolimus was withheld at the time of admission. Because of the patient’s continued decline in renal function, the multidisciplinary team decided to administer eculizumab (Soliris). Eculizumab is a monoclonal antibody against complement C5 and therefore is associated with increased risk of meningococcal infection. It is therefore recommended that patients receive meningococcal vaccination at least 2 weeks before eculizumab therapy. However, there is concern that administration of meningococcal vaccine at that time may increase complement activity and potentially worsen HUS. As a result, the decision was made to initiate azithromycin beginning 12 hours after eculizumab infusion at a dose of 500 mg intravenously daily for meningococcal prophylaxis. The decision to use azithromycin was made after considering the risks and benefits including the possibility that introducing an antibiotic could increase the risk of toxin production and worsen HUS.
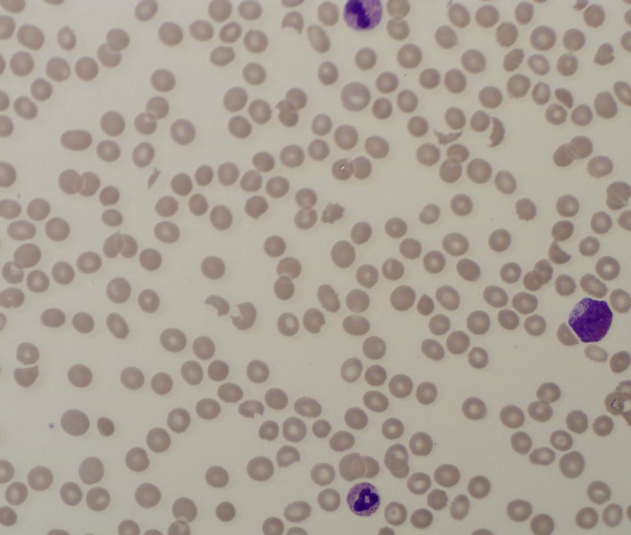
Figure 2.
Peripheral smear at at the time of eculizumab administration.
During the 3 days following the eculizumab infusion, the patient’s neurological status continued to worsen, with reports of visual hallucinations and agitation. Microangiopathic parameters continued to worsen, with hemoglobin decreasing to 7.8 g/dL, platelet count decreasing to 12 × 109/L, LDH increasing to 804 U/L, and creatinine increasing to 2.6 mg/dL (Table). Interestingly, the patient’s hematochezia improved and her diarrhea resolved.
Table.
Blood Markers Before and After Eculizumab
| Blood marker | 3 mo before admission | Admission | Day before eculizumab administration (day 0) | Morning before second eculizumab dose (day 13) | 1 mo after first dose | 12 mo after eculizumab |
|---|---|---|---|---|---|---|
| Plasma creatinine (mg/dL) | 1.1 | 1.29 | 2.09 | 3.5 | 5.2 | 3.3 |
| Platelet count (×103/μL) | 152 | 77 | 26 | 23 | 92 | 210 |
| Lactate dehydrogenase (U/L) | 239 | 410 | 596 | 574 | 478 | 196 |
| Hemoglobin (g/dL) | 12.69 | 13.89 | 9.39 | 8.2 | 7.7 | 12.7 |
| Absolute reticulocytes (×109/L) | 83.69 | 85.80 | 203 | |||
| Haptoglobin (mg/dL) | <14 | <14 | <14 | <14 | ||
| Peripheral blood smear | No schistocytes | Slight schistocytes and helmet cells | Moderate polychromasia, moderate schistocytes, and helmet cells |
Six days after the eculizumab infusion, the patient’s neurological status improved. She was alert and oriented on examination. She continued to have borderline renal function. Diuretics were administered because of evidence of hypervolemia.
A second dose of eculizumab was administered 7 days after the initial dose. Creatinine continued to rise, and there was ongoing evidence of hemolysis, requiring a total of 5 units of packed red blood cell transfusions over a period of 15 days (transfusion threshold of <7 g/dL). Platelets remained at less than 20 × 109/L, with subsequent increase to 78 × 109/L 15 days after the second infusion. No platelet transfusion was required.
The patient's serum creatinine level peaked at 5.5 mg/dL at 20 days after admission. She experienced volume overload and hyperkalemia, managed only with diuretics. Dialysis was never required during the disease course; interestingly, persistent polychromasia, moderate schistocytes, and helmet cells remained present (Figure 3).
Figure 3.
Peripheral blood smear 14 days after.
She was discharged from the hospital 23 days after admission with close outpatient follow-up. She continued to experience borderline renal function and required monitoring of her hemoglobin with periodic red blood cell transfusions. Two weeks after hospitalization, she received immunizations with the Menomune meningococcal polysaccharide vaccine, BEXSERO meningococcal group B vaccine, pneumococcal vaccine series, and Haemophilus influenzae vaccination.
Seven months after her initial presentation to the emergency department with hematochezia, the patient’s renal function remained stable, no signs of GvHD were identified, her energy level improved significantly, and dialysis has not been required.
Discussion
This case highlights a rare but important differential diagnosis for the development of microangiopathic hemolysis in an allogeneic transplant recipient—Shiga toxin–producing Escherichia coli (STEC)–associated HUS. It also presents the current challenges in the management of STEC-HUS, the role of antibiotics and complement-directed therapy. Our patient presented with bloody diarrhea accompanied by abdominal cramps. Symptoms progressed to encephalopathy, microangiopathy, and renal failure. After a multidisciplinary discussion, a decision was made to use eculizumab. Encephalopathy improved over a period of 2 weeks, hemolysis ceased after 4 months, and kidney function stabilized without any requirement for dialysis.1
Shiga toxin–producing Escherichia coli–associated HUS is defined as the triad of hemolytic anemia with erythrocyte fragmentation, thrombocytopenia, and acute kidney injury that occurs after a prodromal infection by a Shiga toxin–producing strain of bacteria.2 This results from a direct binding of the potent Shiga cytotoxin to cell membrane glycolipid Gb3. The incidence of STEC-HUS ranges from 6 in 100,000 children younger than 5 years to 2 in 100,000 in the overall population including adults. Escherichia coli O157:H7 remains the most common bacterial strain associated with STEC-HUS.2, 3 Common vehicles of transmission include ground beef, unpasteurized milk, and municipal or swimming water. Shiga toxin–associated HUS is a main cause of acute renal failure in young children; in contrast, in adults, the HUS with prodromal diarrhea, indicating an infectious cause, is a rare event.1, 4 The reported mortality is up to 5%, as demonstrated in the wide German outbreak.5
Although the association between bone marrow transplant (BMT) and thrombotic microangiopathy (TMA) is a well-documented and potentially lethal complication, the outcomes of STEC-HUS after allogeneic transplant are unknown. The overall incidence of postallogeneic transplant TMA is reported to be between 0.5% and 63.6%.6 Since its first description in 1978,6 numerous cases of transplant-associated TMA have been reported. A number of pathogenic factors have been implicated in post-BMT TMA, including immunosuppressive agents such as calcineurin inhibitors, muromonab-CD3,7 total body irradiation, and acute GvHD as well as systemic viral infections, including cytomegalovirus, parvovirus, adenovirus, and influenza A virus.
In early May 2011, northern Germany was the principal site of a massive HUS epidemic caused by a single clone of a strain of enterohemorrhagic E coli classified as O104:H4. This represents the largest reported case series in the literature. The outbreak involved 298 adults in 23 hospitals, and more than 50% of patients required dialysis. The reported mortality rate was 4%.1 More than 20% of patients received eculizumab treatment. Although there was no considerable improvement in outcomes in patients receiving eculizumab, a small group of patients that received antimicrobials and eculizumab had improved outcomes (lower rates of seizures and improved mortality). Notably, the use of plasma exchange did not improve outcomes either.
Another outbreak of STEC-HUS was reported in Bordeaux, France, in June 2011.8 This involved 24 cases, of which 9 developed HUS. Although the report is limited by the small sample size, patients who received early treatment with eculizumab (day 0-4) had rapid improvement in symptoms.8 The association between the initiation of eculizumab therapy and platelet count recovery in the French outbreak may also be a coincidental finding. On the basis of the reported experience in the German outbreak, patients treated with best supportive care had worsening of disease activity 6 to 8 days after diarrhea, followed by improvement. Therefore, the observed improvement after eculizumab might be the natural course, and no concrete conclusions can be drawn on eculizumab-specific efficacy for STEC-HUS.8
On the basis of these reports and after a multidisciplinary discussion that involved hematology, nephrology, and infectious disease services, we elected to treat our patient with eculizumab on day 3 of her presentation.
The use of antimicrobial therapy is generally controversial in patients with STEC-HUS. Several studies showed that it did not affect outcomes.8 Furthermore, some studies reported that antibiotic treatment triggered the development of HUS.7 On that basis, antibiotics were discontinued at the time of admission in our patient and the initiation of prophylactic azithromycin was delayed until 12 hours after eculizumab use.
It is well established that patients undergoing allogeneic transplant have a substantially increased risk of bacterial, fungal, and viral infections due to continued immunosuppression and due to the development of GvHD in most patients.9, 10 However, it is unclear whether there is an increased susceptibility to STEC. In 2012, Eriguchi et al11 described that in mice, damage to Paneth cells by GvHD resulted in dramatically reduced expression of α-defensins in the small intestines and perturbed the normal intestinal environment. The diversity of the intestinal microflora was lost with overwhelming expansion of specific bacteria, such as E coli, which are normally a very small proportion of the intestinal microbial communities. This study confirms and further extends a recent study showing the intestinal flora change, with an increase in gram-negative Enterobacteriaceae family members including E coli after allogeneic BMT in mice.11
In summary, we report a case of STEC-HUS as a differential diagnosis for the development of microangiopathy in an allogeneic transplant recipient and discuss important treatment decisions in an immunocompromised host, including the controversial role of eculizumab and antimicrobial therapy.
Footnotes
Grant Support: The study was supported by a grant (N.L.) from Omeros.
Potential Competing Interests: Dr Leung has board membership with Takeda and BTG and is a consultant for Prothena. The rest of the authors report no competing interests.
References
- 1.Frank C., Werber D., Cramer J.P., et al. HUS Investigation Team Epidemic profile of Shiga-toxin–producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med. 2011;365(19):1771–1780. doi: 10.1056/NEJMoa1106483. [DOI] [PubMed] [Google Scholar]
- 2.Trachtman H., Austin C., Lewinski M., Stahl R.A. Renal and neurological involvement in typical Shiga toxin-associated HUS. Nat Rev Nephrol. 2012;8(11):658–669. doi: 10.1038/nrneph.2012.196. [DOI] [PubMed] [Google Scholar]
- 3.Tarr P.I., Gordon C.A., Chandler W.L. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365(9464):1073–1086. doi: 10.1016/S0140-6736(05)71144-2. [DOI] [PubMed] [Google Scholar]
- 4.Boyer O., Niaudet P. Hemolytic uremic syndrome: new developments in pathogenesis and treatment. Int J Nephrol. 2011;2011:908407. doi: 10.4061/2011/908407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tahden M., Manitz J., Baumgardt K., Fell G., Kneib T., Hegasy G. Epidemiological and ecological characterization of the EHEC O104:H4 outbreak in Hamburg, Germany, 2011. PloS One. 2016;11(10) doi: 10.1371/journal.pone.0164508. e0164508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obut F., Kasinath V., Abdi R. Post-bone marrow transplant thrombotic microangiopathy. Bone Marrow Transplant. 2016;51(7):891–897. doi: 10.1038/bmt.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong C.S., Jelacic S., Habeeb R.L., Watkins S.L., Tarr P.I. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med. 2000;342(26):1930–1936. doi: 10.1056/NEJM200006293422601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delmas Y., Vendrely B., Clouzeau B., et al. Outbreak of Escherichia coli O104:H4 haemolytic uraemic syndrome in France: outcome with eculizumab. Nephrol Dial Transplant. 2014;29(3):565–572. doi: 10.1093/ndt/gft470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serody J. Bacterial sepsis and GI tract GVHD: more commensal than you think. Blood. 2012;120(1):6–7. doi: 10.1182/blood-2012-05-427435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poutsiaka D.D., Munson D., Price L.L., Chan G.W., Snydman D.R. Blood stream infection (BSI) and acute GVHD after hematopoietic SCT (HSCT) are associated. Bone Marrow Transplant. 2011;46(2):300–307. doi: 10.1038/bmt.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eriguchi Y., Takashima S., Oka H., et al. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of α-defensins [published correction appears in Blood. 2014;124(7):1201] Blood. 2012;120(1):223–231. doi: 10.1182/blood-2011-12-401166. [DOI] [PubMed] [Google Scholar]