Abstract
Propolis is known to possess antioxidant activity. However, there is no information on this activity in emulsions O/W. The protective effect of propolis on the oxidation and rheological properties of emulsions O/W containing wheat germ and almond oils was evaluated. Emulsions O/W were prepared with different concentration of propolis extract, almond oil and wheat germ oil. All emulsions physically stable without phase separation were stored at 37 °C for 9 weeks. Chemical composition of propolis was established by Gas chromatography coupled to mass spectrometry. Rheological characterization of different emulsions was performed evaluating consistency index and flow behavior index. The oxidation was monitored by measuring the lipid hydroperoxides and thiobarbituric acid-reactive substances (TBARS) methods. Flavonoids, phenolic acid esters, and aromatic acids were the main groups of compounds found in propolis. The results showed that popolis was good antioxidant in the concentration of 0.02 and 0.04% when lipid phase was constituted by almond oil. The rheological behavior is typical of a non-Newtonian fluid, being almond oil more adequate for having a higher stable O/W emulsion.
Keywords: Emulsion, Flavonoids, Phenolic acid esters, Almond oil, Wheat germ oil, Natural antioxidant
1. Introduction
Consumers have become more conscious and have a greater awareness of health and well-being. The demand for products with natural components instead synthetic ones has drastically risen, however, many of them still contain synthetic chemicals. Nowadays, one of the challenges of food and cosmetic industries is to formulate products not only with the adequate attributes as taste, color, fragrance, but also with functionality, safety and adequate stability, replacing some synthetic ingredients by natural ones.
Many formulated foods, pharmaceuticals and cosmetics contain a lipid phase dispersed in an aqueous medium, that is, they are oil-in-water (O/W) emulsions. The lipid phase may be constituted by vegetable oils (e.g. almond, and wheat germ oils). In cosmetic formulations, almond oil has been used in facial care, as moisturizer for excessively dry skin, sunburn and wind burn. It is used in lip balms, creams and in formulations for infants (Rabasco Alvarez and González Rodríguez, 2000). Wheat germ oil used in microemulsions, lotions and creams, has proved to be effective in treatment of sunburn and in some skin diseases such as psoriasis and eczema (Ajazuddin et al., 2013).
Although the antioxidant properties that have been attributed to almond and wheat germ oils, they may present susceptibility to oxidation, due to their triglycerides rich in polyunsaturated fatty acids (PUFAs) (Ajazuddin et al., 2013; Roncero et al., 2016).
The oil phases of the emulsion may easily oxidize due to their large surface area that facilitates not only the interactions between the lipids and the water-soluble prooxidants but also favour the availability of the oil phase to oxygen dissolved in the aqueous phase which could be produced during the emulsification process, due to the overheating of the shear. In addition, formation of free radicals is also possible to occur in the emulsification process in the case of sonication, due to acoustic cavitation (Berton-Carabin et al., 2014). The oxidation of lipids can not only disturb the emulsion stability due to the formation of the new compounds but also weaken the quality of the product. Such compounds are unpleasant odorants and therefore responsible for the decline of the product quality, with the disadvantage of forming harmful radicals for human health (Berton-Carabin et al., 2014).
Several strategies have been developed to overcome the lipid oxidation and one of them is the utilization of antioxidants (Berton-Carabin et al., 2014, Poyato et al., 2013) Butylhydroxyanisole (BHA) and butylhydroxytoluene (BHT) are two synthetic compounds generally used as antioxidant in products containing fats or oils. However, several studies have reported that these compounds may be harmful for human health (Yamaki et al., 2007), although many other studies, as reviewed by Nieva-Echevarría et al. (2015) have claimed that the toxic effects may be attributed more to BHT metabolites than to the parent compound.
Beehive is an unparalleled source of natural products, such as propolis. It is a resinous substance collected by honeybees from parts of plants, buds, and exudates, mixed with beewax and salivary enzymes. Bees use propolis to protect themselves from wind, rain, insects, microorganisms, employing it as a cement to seal cracks or open spaces in the hive, to sterilize the queen-bee posture site, and to mummify insect invaders (Marcucci, 1995). The wide application of propolis in modern medicine and the increasing demand for it, due to its health benefits and use in cosmetic and food products, have drawn growing attention to its chemical composition which is complex and varies according to the floral biodiversity of the region. Polyphenols, terpenoids including steroids, naphthalene and stilbene derivatives, fatty acids, sugars, vitamins and minerals are some examples of compounds that can be found in propolis (Barlak et al., 2011, Burdock, 1998). Propolis present a series of biological properties such as antimicrobial, anti-inflammatory, antioxidant, antidiabetic, spasmolytic, anaesthetic, anticancer, and immunomodulatory effects (Barlak et al., 2011, Burdock, 1998, Clares et al., 2011, El-Guendouz et al., 2016a, El-Guendouz et al., 2016b, Sforcin, 2016). Propolis has been used in foods and beverages, chewing gums, cosmetics, creams, skin creams, among others, not only due to their preservative properties but also as their biological attributes for maintaining or improving health (Clares et al., 2011, Duman and Ozpolat, 2015, Espinosa et al., 2015, Osés et al., 2016, Sehn et al., 2009). From the above, we can state that propolis can be a promising product that can replace the synthetic ones in the development of new GRAS (generally recognized as safe) products.
The main goal of the present work is to compare the ability of propolis (natural product) for preventing the lipid peroxidation of almond or wheat germ oils (natural products) used as lipid phase of the O/W emulsions with that of BHA (synthetic product) and at the same time evaluate its influence on some parameters of the emulsions' rheology.
2. Material and methods
2.1. Materials
BHA was purchased from Sigma Aldrich Chemie, Steinheim, Germany. Almond oil and wheat germ oil were purchased from LBCHEM Labospirit, Lda Portugal. Tween 80, Span 80, Phenonip and Xanthan gum were purchased from Guinama S.L.U Spain. Thiobarbituric acid (TCA), ferrous chloride (FeCl2) and ammonium thiocyanate (NH4SCN) were purchased from Fluka, Biochemika, Sigma-Aldrich, Steinheim, Germany. Trichloroacetic acid was purchased from VWR, Leuven, Belgium. 1-Butanol, methanol and HCl was from Fisher Scientific UK Ltd, Loughborough, UK.
2.2. Propolis extract
Extracts were obtained as reported by El-Guendouz et al. (2016b). Briefly, one gram of propolis from Morocco (region of Fez-Boulmane) was chopped into small pieces and extracted by maceration using 30 mL of 70% ethanol and maintained for 1 week at 37 °C under agitation (200 rpm). The resulting solution was filtered under vacuum. A clear solution, without further purification, was used to prepare the emulsions.
2.3. GC–MS analysis of propolis extract
The analysis was performed with a Hewlett–Packard gas chromatograph 5890 series II Plus linked to a Hewlett–Packard 5972 mass spectrometer (Hewlett-Packard, Wilmington, DE, USA) system equipped with a 30 m long, 0.25 mm i.d., and 0.5 µm film thickness HP5-MS capillary column. The work conditions were the same as previously reported (El-Guendouz et al., 2016b). Semi-quantification was carried out by internal normalization with the area of each compound. The addition of individual areas of the compounds corresponds to 100% area. Compound identification was performed using commercial libraries and comparison of mass spectra and retention times of reference compounds.
2.4. Preparation and storage of O/W emulsion
Oil-in-water (o/w) emulsions were prepared using Tween 80 and Span 80 as surfactants. The oil phases were constituted by the vegetable oils, wheat germ and almond. Phenonip (antimicrobial) and xanthan gum (stabilizer) (Krstonošić et al., 2015) were also used. Two antioxidants, propolis and butylated hydroxyanisole BHA (positive control), were tested with three different concentrations 0.01% (w/w), 0.02% (w/w) and 0.04% (w/w) each. Briefly, O/W emulsions were prepared by mixing almond oil and wheat germ with different concentration each (A: 100% of wheat germ oil/0% almond oil; B: 75% of wheat germ oil/25% almond oil; C: 50% of wheat germ oil/50% almond oil; D: 25% of wheat germ oil/75% almond oil and E: 0% of wheat germ oil/100% almond oil). For each formulation (A, B, C, D and E) Tween 80 (1.1%, w/w), Span 80 (0.9%, w/w), Phenonip (0.8%, w/w), xanthan gum (0.9%, w/w), and propolis extract or BHA were added. Afterwards distilled water was added to 100% (w/w) and all the component were mixed with an ultra turrax homogenizer (IKA T18D Ultra Turrax, 50/60 HZ, 500 W, Germany) working at 16,000 rpm for 5 min. All emulsions physically stable were transferred to round glass amber bottles and stored in an incubator at 37 °C for 9 weeks. The assays were prepared in triplicate. A similar procedure was repeated to prepare a negative control (emulsions without antioxidant). The concentrations of each ingredient used in this work to prepare the O/W emulsions were chosen after different assays (data not shown), since they produced the most stable emulsions.
The chemical characteristics of almond and wheat germen oils are described in Table S1, according to the specifications of the trade marks.
2.5. Peroxide content determination in cosmetic emulsions
For the determination of primary oxidation products, lipid hydroperoxides were measured as describe by (Malinowska et al., 2014) with slight modification. One hundred (100 µL) of emulsion were mixed with 9.5 mL of methanol/butanol (2:1, v/v) by vortexing (10 s), then 0.1 mL of 30% ammonium thiocyanate and 0.1 mL of 0.02 M ferrous chloride in 3.5% hydrochloric acid were added. The absorbance of the solution was measured at 510 nm, 3 min after addition of the ferrous chloride against the control sample. Hydroperoxide content was expressed as absorbance (A510). All emulsions were tested at weeks 0, 2, 4, 6 and 9.
2.6. Determination of lipid oxidation products (thiobarbituric acid-reactive substances)
Thiobarbituric acid-reactive substances (TBARS) were determined on the emulsions according to some authors (Hebishy et al., 2013) with minor modifications. TBARS reagent was prepared by mixing 15% (w/v) trichloroacetic acid, 0.375% (w/v), 2-thiobarbituric acid in 1.76 mL of 12 M hydrochloric acid, then distilled water was added to 100 mL (w/v). Then one mL of emulsion sample and two mL the TBARS reagent were well vortexed for 30 s, placed in a boiling water bath for 15 min and then cooled down in room temperature for 30 min. 1-Butanol (2 mL) was added and mixture was centrifuged at 5000 rpm, for 10 min. The colorful supernatant was collected and the absorbance was measured at 532 nm.
2.7. Rheological studies
Rheological properties of the different samples were determined, using a Brookfield programmable rotational viscometer LVDV-II+Pro (Brookfield Engineering Laboratories Inc) equipped with the software Rheocalc 32 (version 2.4.). The experimental conditions were adjusted in preliminary tests and the studies were performed at 25 ± 1 °C and spindle LV-3C speeds ranging from 20 to 100 rpm, with an up-down rate ramp. The viscosity (η) was determined at shear rates between 2 and 21 s−1. Each shear rate was imposed during 1 min to stabilized the viscosity. The assays were performed under the same conditions and identical container and were repeated at least two times and the experimental data fitted to the Power Law model (Eq. (1)) to establish the type of non-Newtonian emulsion behavior.
| (1) |
where τ is the shear stress, is the shear rate, K is the consistency index, and n is index of fluid behavior.
The viscosity was related with the equation of Power-law model by the Eq. (2).
| (2) |
From this model it can be inferred that if n > 1, the fluid is showing shear thickening behavior; if n < 1, it shows shear thinning behavior; if n = 1, it is exhibiting Newtonian behavior.
To eliminate the hypothesis of xanthan gum conditioned the rheological behavior of the emulsions, we proceed to the measure of viscosity in the same conditions of the assay. The viscosity of xanthan gum solution 0.9% (w/w) was 1240 ± 53 mPa·s, determined at a shear rate of 6.3 s−1 and present a shear thinning behavior.
2.8. Statistical analysis
TBARS Data were analyzed by two-way ANOVA using GraphPad Prism version 5.03 for Windows (GraphPad Software, La Jolla California, USA). Statistical significance was set as p < 0.05; when the analysis was statistically significant, the Duncan post hoc test was done. Rheological analyses were performed on duplicated samples. Statistical analysis was conducted with a SigmaPlot 12 software (Systat Software, version 12 for Windows), implementing the one-way ANOVA method. Significant differences between means (at the level of P < 0.05) were determined by Student–Newman–Keuls test.
3. Results and discussion
3.1. Chemical composition of propolis extract
Table 1 depicts the chemical composition of the hydro-alcoholic extract of propolis, and Fig. S1 shows the chromatographic profile. Flavonoids (51.9%), phenolic acid esters (22.4%), sugars and sugar derivatives (9.2%) and aromatic acids (4.3%) were the main groups of compounds found in this extract. Flavonoids, the major group of phenol components, were mainly represented by galangin (8.7%), pinocembrin chalcone (6.6%), pinocembrin (6.6%), pinobanksin acetate (5.4%) and chrysin (5.4%), all of them with concentrations higher than 5%. The phenolic acid ester were the second abundant group in the propolis component and some of them were present also at a concentrations higher than 5%: pentenyl caffeate isomers (7.1% and 5.2%) (Table 1). The phenolic composition of propolis extract is somehow similar to that of poplar origin (Bankova et al., 2002).
Table 1.
Chemical composition of hydro-alcoholic extract of Moroccan propolis.
| Aromatic acids | % | Phenolic acid esters | % | Flavonoids | % | Sugars and sugar derivatives | % |
|---|---|---|---|---|---|---|---|
| E-caffeic acid | 2.2 | Pentenyl caffeate (isomere) | 7.1 | Galangin | 8.7 | Disaccharides | 1.1 |
| Dimethoxycinnamic acid | 0.7 | Pentenyl caffeate (isomere) | 5.2 | Pinocembrin chalcone | 6.6 | Glycerol | 0.6 |
| Z-Caffeic acid | 0.6 | Pentenyl caffeate (isomere) | 3.6 | Pinocembrin | 6.6 | Fructofuranose | 2.0 |
| Ferulic acid | 0.5 | CAPE | 2.2 | Pinobanksin acetate | 5.4 | Glucopyranose | 1.8 |
| p-Coumaric acid | 0.3 | Benzyl caffeate | 1.3 | Chrysin | 5.4 | Sugar | 0.9 |
| Pentyl caffeate | 1.3 | Pinobanksin | 4.8 | Sugar | 0.7 | ||
| Pentenyl caffeate (isomere) | 0.7 | Pinobanksin chalcone | 2.7 | Sugar | 0.7 | ||
| Pentenyl caffeate | 0.4 | Tectochrysin | 1.8 | Sugar | 0.5 | ||
| Pentenyl p-coumarate (isomere) | 0.3 | Pinobanksin acetate chalcone | 1.6 | Sugar | 0.5 | ||
| Pentenyl p-coumarate (isomere) | 0.2 | Kaempferol | 1.4 | Sugar | 0.2 | ||
| Pentenyl p-coumarate | 0.1 | Kaempferol methyl ether (isomere) | 1.4 | Sugar | 0.2 | ||
| Quercetin methyl ether (isomere) | 1.1 | ||||||
| Pinostrobin chalcone | 0.9 | ||||||
| Quercetin | 0.7 | ||||||
| Quercetin methyl ether. | 0.7 | ||||||
| Kaempferol methyl ether | 0.7 | ||||||
| Alpinone chalcone | 0.7 | ||||||
| Quercetin | 0.7 | ||||||
| Total | 4.3 | Total | 22.4 | Total | 51.9 | Total | 9.2 |
Standard deviation does not succeed 6% for any of the constituents.
3.2. Protective effect of propolis extract on oxidative stability of W/O emulsion measured through peroxide content
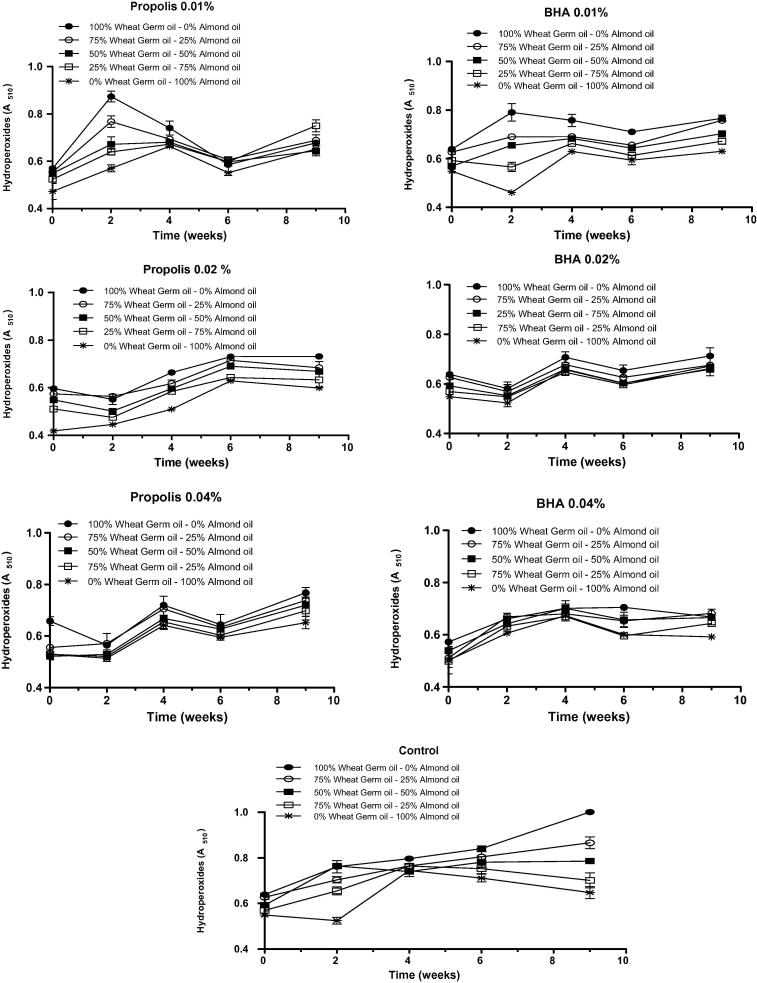
Fig. 1 showed that hydroperoxides increased as time increased to nine weeks for all emulsion comparing with the control (Blank). Propolis extracts and BHA at higher concentrations (0.02% and 0.04%) present the best protecting effect on the oxidation at the formulation D and E (Fig. 1).
Fig. 1.
Effect of adding propolis extract and BHA at different concentrations (0.01%; 0.02% and 0.04%) on hydroperoxides (ABS510) of O/W emulsions made with germ wheat oil and almond oil under storage conditions of 9 weeks at 37 °C. Values of mean (n = 6) ± standard deviation.
The results depicted in Table S2 indicated that a significant (p < 0.05) increase of absorbance at λ = 510 nm, which means an evolution of primary oxidation products, was observed in all emulsions in comparison to the control before and after nine weeks of storage at 37 °C. It can also be seen from Table S2, in the presence of propolis, or BHA that the higher concentration of almond oil is the best preventing lipid peroxidation (D: 75% almond oil and 25% wheat germ, E: 100% almond oil). Regarding the different concentration of the propolis and BHA (0.01%; 0.02% and 0.04%), propolis revealed to have a similar effect as BHA in preventing oxidation.
Neither BHA nor propolis extract were so good for preventing lipid peroxidation of wheat germ as they were with almond oil. This may be explained by the predominance of polyunsaturated fatty acids constituting the triacylglycerols of wheat germ. Each additional double bond introduced into a fatty acid, at least doubles its rate of oxidation (Berton-Carabin et al., 2014). It might be concluded that the combination between propolis or BHA and almond oil has a protective effect against oxidation of O/W emulsions.
3.3. Protective effect of propolis extract on oxidative stability of W/O emulsion measured through TBARS method
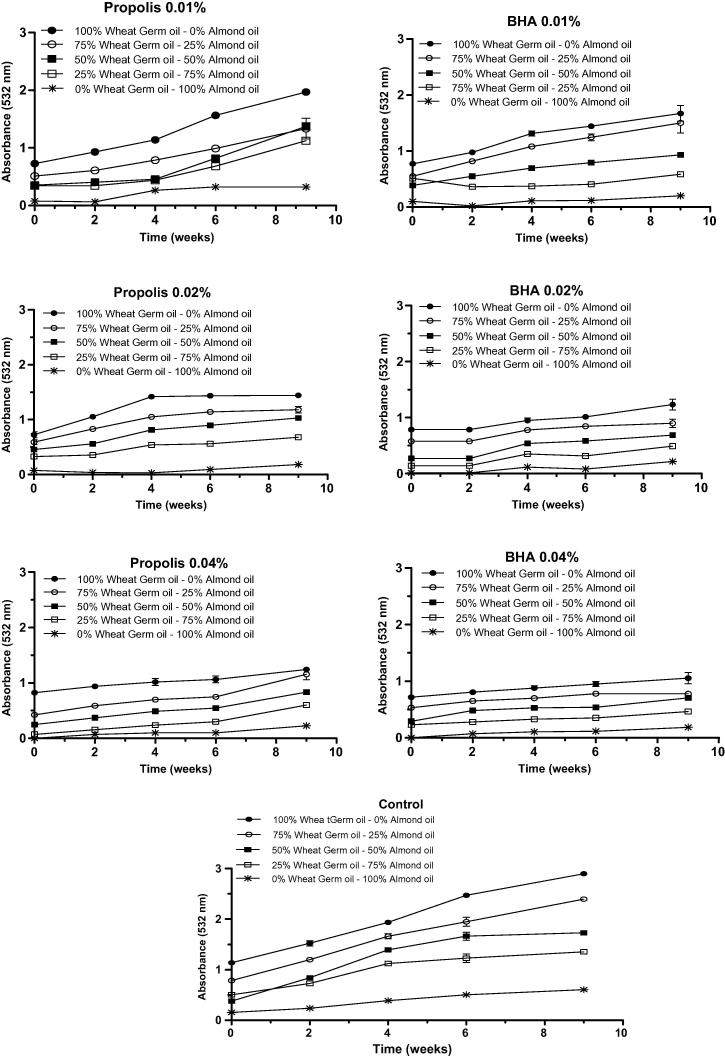
The reaction of reactive substances (degradation products of lipidic peroxidation, including malondialdehyde) with thiobarbituric acid, originates a pink pigment that is directly proportional to the amount of those reactive substances produced, which absorbance can be measured at λ = 532 nm. The increase of absorbance reveals, therefore, accumulation of reactive substances, including malondialdehyde, with thiobarbituric acid, which means oxidation. Fig. 2 depicts the evolution of absorbance at λ = 532 nm of emulsions O/W with various proportions of almond and wheat germ oils in the presence of three concentrations of propolis extract or BHA at three concentrations (0.01, 0.02 and 0.04%), over time (9 weeks). A blank was performed without BHA or propolis extract. In all cases, the absorbance rose over time, being clearly evident that in the absence of any antioxidant (BHA or propolis extract), the accumulation of malondialdehyde after 9 weeks was higher when compared to the samples in which an antioxidant was added. regardless of the antioxidant used, the results also showed that they gradually lose their ability for preventing lipid peroxidation as the wheat germ concentration increases in emulsions (Fig. 2). The Figure also shows that the raise of antioxidant ability is dependent on the concentration of BHA or propolis extract added to emulsions.
Fig. 2.
Effect of adding propolis extract and BHA at different concentrations (0.01%; 0.02% and 0.04%) on TBARS (ABS532) of O/W emulsions made with germ wheat oil and almond oil under storage conditions of 9 weeks at 37 °C. Values of mean (n = 6) ± standard deviation.
In Table S3 it is possible to conclude that propolis extracts at higher concentrations (0.02 and 0.04%) work better as antioxidants than BHA, when the lipid phase was only constituted by almond oil (E), or at 0.04% when the oil phase was constituted by 75% almond oil and 25% wheat germ (0.04%). For all of the other samples, BHA revealed to be better than propolis extract as antioxidant.
The antioxidant capacity of propolis has been studied and in a review study made by Miguel et al. (2014), the authors reported that the ability for preventing lipid peroxidation measured through the method of thiobarbituric acid, as done in the present work, was dependent on the flavonoids, caffeic acid and its derivatives caffeic acid phenethyl ester and 1,1-dimethylallylcaffeate. In short, either flavonoids or phenolic acids and their esters need to have two ortho-hydroxyl groups in the aromatic ring to give high antioxidant ability to the compound (Miguel, 2013). The intermediate o-hydroxyl phenoxyl radical formed during the reaction of oxidation is stable owing to the unpaired electrons that can easily delocalize across the molecule (Gregoris and Stevanato, 2010, Wu et al., 2007). In the present work, the chemical composition of propolis extract showed relative high amounts of caffeic acid derivatives with two ortho-hydroxyl groups, albeit also possessing a great percentage of flavonoids with two meta-hydroxyl groups (pinocembrin, pinobanksin, chrysin and their derivatives). In another study, Espinosa et al. (2015) found that sinapic acid and rutin hydrate were the most efficient compounds, extracted from red propolis, to delay lipid oxidation of a functional emulsion, among 11 natural phenolic compounds (vanillic acid, caffeic acid, trans-cinnamic acid, 2,4-dihydroxycinnamic acid, p-coumaric acid, quercetin, trans-ferulic acid, trans,trans-farnesol, rutin, gallic acid or sinapic acid).
3.4. Rheological properties of W/O emulsion
For stabilizing an emulsion O/W, two categories of molecules can be used: those that adsorb at the oil-water interface and reduce interfacial tension (surfactants), and those that provide long-term emulsion stability by, for instance, modifying the viscosity of aqueous phase, that is, they are thickening agents (stabilizers) (Krstonošić et al., 2015). In order to improve the stability of emulsions O/W, in some cases, surfactants or emulsifiers and stabilizers are used combined, due to the fact that they can act synergistically (Desplanques et al. (2012). Xanthan gum has been used a stabilizer of emulsions not only to its high thickening attributes but also to its relative high solubility in cold water (Desplanques et al. (2012). In the present work, the percentages of surfactants (Tween 80 and Span 80) and stabilizer (xanthan gum) were constant in all emulsions O/W.
Rheological characterization of the emulsions formulations, using different percentage of germ wheat oil and almond oil, with propolis extract and BHA as oxidants at different concentrations were performed. The rheological characteristics of the emulsion samples were measured applying a shear rate from 2.10 to 21.00 s−1, with a 2.10 increment.
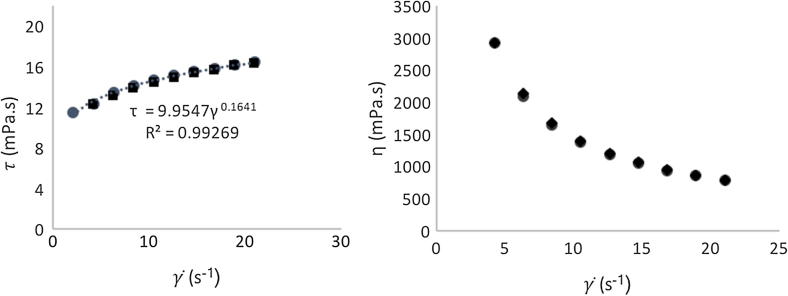
The thixotropic behavior was determined using the increment of the shear rate, as ascending scanning and decreasing the values of shear rate, as a descending scanning. It was found that for the same shear rate value, identical values of shear stress were obtained, which indicated that the emulsions do not present thixotropic behavior, as can be seen by Fig. 3a, that shows the data for the fit to the power law model. The consistency index (K) and the flow behavior index (n) were obtained by fitting the viscosity vs shear rate data with the power-low equation. The flow curves, shear stress versus shear rate, show that when shear rate increases, the values of viscosity decrease (Fig. 3b), i.e. a shear thinning behavior (Fig. 3a). The xanthan gum polymer has been characterized rheologically and exhibits, like the emulsion, a shear thinning behavior.
Fig. 3.
Flow behavior curve and Power law model (….….) fitting (a) and relation between apparent viscosity and the shear rate (b), at 25 ± 1 °C with an up-down rate ramp.
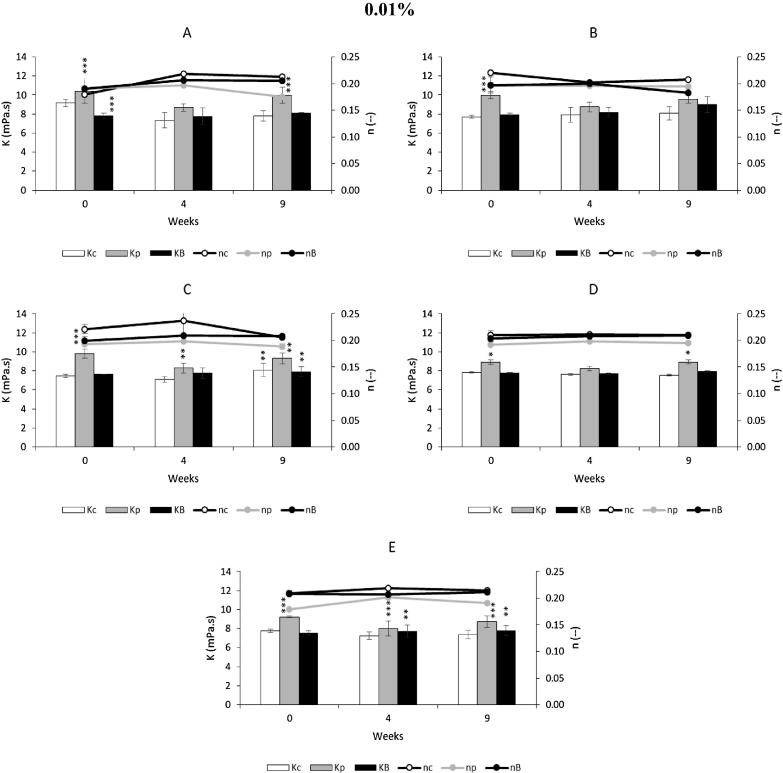
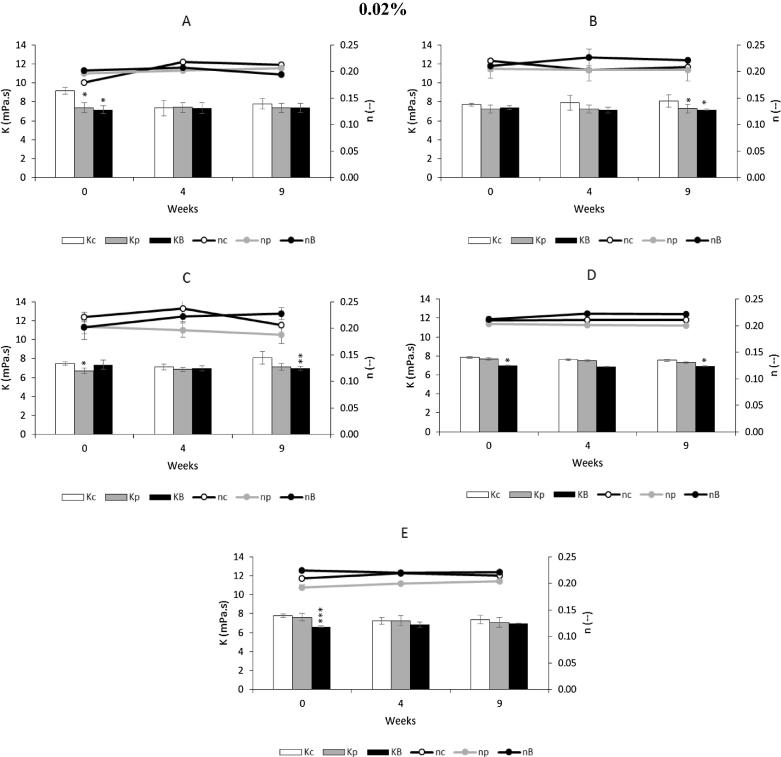
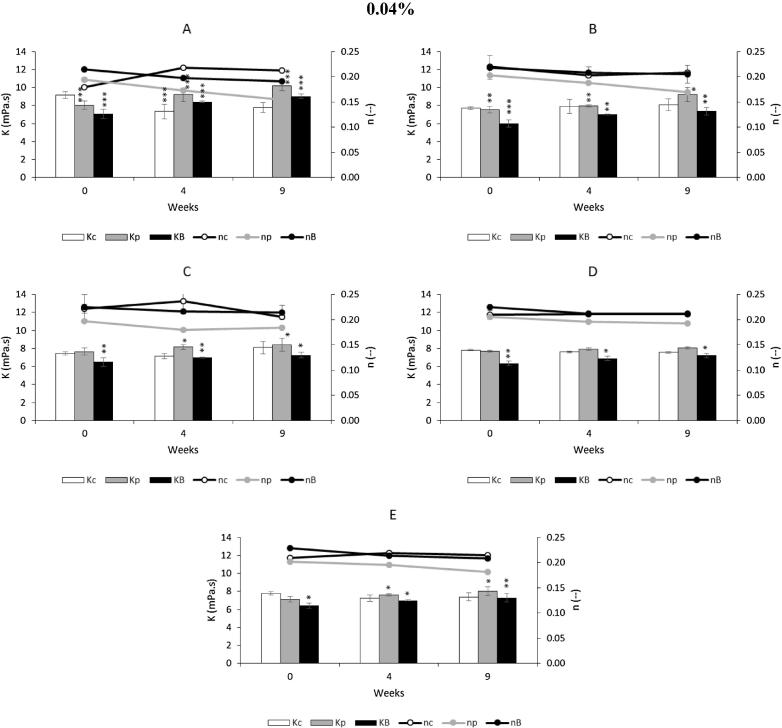
Fig. 4, Fig. 5, Fig. 6, present the values of consistency index (K) and the fluid behavior index (n) for a control and for the various emulsions, with the antioxidants propolis extract and BHA, at different concentrations. All emulsions clearly show a fluid behavior index less than 1, more specifically n < 0.25, typical of shear thinning, which confirms the aforementioned results.
Fig. 4.
Effect of adding propolis extract (0.01%) and BHA on rheological characterization, with determination of consistency index (Kc, Kp, KB) and fluid behavior index (nc, np, nB), under storage conditions (37 °C for 9 weeks). Bars mean standard deviation (n = 4). Level of significance for the test ANOVA one-way: * P < 0.05; **P < 0.01; ***P < 0.001. A contains 100% of Germ wheat oil and 0% almond oil. B contains 75% of Germ wheat oil and 25% almond oil. C contains 50% of Germ wheat oil and 50% almond oil. D contains 25% of Germ wheat oil and 75% almond oil. E contains 0% of Germ wheat oil and 100% almond oil. Symbols: c, control; p, propolis; B, BHA.
Fig. 5.
Effect of adding propolis extract (0.02%) and BHA on rheological characterization, with determination of consistency index (Kc, Kp, KB) and fluid behavior index (nc, np, nB), under storage conditions (37 °C for 9 weeks). Bars mean standard deviation (n = 4). Level of significance for the test ANOVA one-way: * P < 0.05; **P < 0.01; ***P < 0.001. A contains 100% of Germ wheat oil and 0% almond oil. B contains 75% of Germ wheat oil and 25% almond oil. C contains 50% of Germ wheat oil and 50% almond oil. D contains 25% of Germ wheat oil and 75% almond oil. E contains 0% of Germ wheat oil and 100% almond oil. Symbols: c, control; p, propolis; B, BHA.
Fig. 6.
Effect of adding propolis extract (0.04%) and BHA on rheological characterization, with determination of consistency index (Kc, Kp, KB) and fluid behavior index (nc, np, nB), under storage conditions (37 °C for 9 weeks). Bars mean standard deviation (n = 4). Level of significance for the test ANOVA one-way: * P < 0.05; **P < 0.01; ***P < 0.001. A contains 100% of Germ wheat oil and 0% almond oil. B contains 75% of Germ wheat oil and 25% almond oil. C contains 50% of Germ wheat oil and 50% almond oil. D contains 25% of Germ wheat oil and 75% almond oil. E contains 0% of Germ wheat oil and 100% almond oil. Symbols: c, control; p, propolis; B, BHA.
In Fig. 4 the consistency index (K) for 0.01% emulsions with propolis extracts present a value slightly higher than the control and BHA emulsions, at same concentration. This index is a measure of the system consistency and is related with the viscosity. In this case, almost all of the viscosity values of emulsions with propolis extract are higher than those obtained with other samples, at the same concentration of 0.01% (Table S4). For the emulsions with propolis extracts with 100% of germ wheat oil, the initial viscosity was 2312 ± 11 mPa·s, against 2020 ± 32 and 1760 ± 00 mPa·s, of control and BHA, respectively. After 9 weeks, propolis extract had an viscosity of 2184 ± 79 and control and BHA, 1834 ± 59 and 1936 ± 34 mPa·s, respectively.
Also, the flow behavior index (n) was tracked over time for the different concentrations of propolis extracts. The flow behavior index presented a more pronounced shear thinning behavior, as observed in the curves of Figs. 4(A, C, E), 5(C) and 6, the n values decrease more sharply during the 9 weeks of storage. The n values of BHA had a similar behavior to the control, for the different concentrations and formulations.
Depending on the composition of oils percent, wheat germ and almond oil, there is change in the viscosity tendency to increase or decrease, as the antioxidant added. Thus, in the case of propolis extract 0.01%, while the percentage of wheat germ oil decreases from 100% to 50% (A to C), the initial viscosity of the samples decreases, stabilizing the value when the percentage of almond oil prevails (D and E) (Table S4). The same happened with the storage period, where the values of viscosity of 0 weeks and 9 weeks decreased until the percentage of almond oil was 75% or higher, as can be seen in Table S4. The decrease of viscosity, observed in 0.01% propolis extract emulsion and control, during the storage period may be occurred due to two possible reasons: a diffusion of water molecules from internal to the external aqueous phase or bursting of multiple globules due to osmotic pressure (Tirnaksiz and Kalsin, 2005). The same was observed by Mahmood and Akhtar, (2013) in studies with multiple emulsions encapsulated with 5% green tea extract.
The different emulsions with 0.04% of antioxidant, propolis extract or BHA, showed an increase of viscosity with the storage period (9 weeks). In this case, this may be due to storage conditions where the temperature was 37 °C, enhancing the evaporation of the aqueous phase of the emulsion. Also, the antioxidants are prepared as a hydro-alcoholic solution, evaporation can be more severe and cause increased viscosity and accentuating the shear thinning character, visible in Fig. 6, the curves representing the propolis extract behavior index.
The characterization of the behavior of the culture under the storage conditions may allow evaluating the maintenance of the emulsion rheological properties and these results indicate that almond oil could have a stabilizing effect of the emulsion by minimizing flow variations with time aspect reflected in the measurement of viscosity after storage at 37 °C. The addition of antioxidant at a concentration of 0.01% seems also a beneficial effect in the emulsion.
Acknowledgments
Acknowledgements
The authors wish to acknowledge the financial support provided by the Portuguese National Funding Agency for Science, Research and Technology (Fundação para a Ciência e Tecnologia – FCT; Portugal), under the projects UID/BIA/04325/2013–MEDTBIO and UID/MAR/00350/2013.
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jsps.2018.05.017.
Appendix A. Supplementary material
References
- Ajazuddin, Alexander A., Khichariya A., Gupta S., Patel R.J., Giri T.K., Tripathi D.K. Recent expansions in an emergent novel drug delivery technology: Emulgel. J. Control. Release. 2013;171:122–132. doi: 10.1016/j.jconrel.2013.06.030. [DOI] [PubMed] [Google Scholar]
- Bankova V., Popova M., Bogdanov S., Sabatini A.G. Chemical composition of European propolis: expected and unexpected results. Zeitschrift fur Naturforsch. – Sect. C J. Biosci. 2002;57:530–533. doi: 10.1515/znc-2002-5-622. [DOI] [PubMed] [Google Scholar]
- Barlak Y., Değer O., Colak M., Karataylı S.C., Bozdayı A.M., Yücesan F. Effect of Turkish propolis extracts on proteome of prostate cancer cell line. Proteome Sci. 2011;9:74. doi: 10.1186/1477-5956-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton-Carabin C.C., Ropers M.H., Genot C. Lipid oxidation in oil-in-water emulsions: involvement of the interfacial layer. Compr. Rev. Food Sci. Food Saf. 2014;13:945–977. [Google Scholar]
- Burdock, G.A., 1998. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 10.1016/S0278-6915(97)00145-2. [DOI] [PubMed]
- Clares, P., Galvez, V.G., M.A.R., 2011. Elaboration, characterization, and stability study of a sunscreen emulsion for use as a towelette application in pediatric photoprotection. J. Cosmet. Sci. 62, 371–382. [PubMed]
- Desplanques S., Renou F., Grisel M., Malhiac C. Impact of chemical composition of xanthan and accacia gums on the emulsification and stability of oil-in-water emulsions. Food Hydrocol. 2012;27:401–410. [Google Scholar]
- Duman M., Ozpolat E. Effects of water extract of propolis on fresh shibuta (Barbus grypus) fillets during chilled storage. Food Chem. 2015;189:80–85. doi: 10.1016/j.foodchem.2014.08.091. [DOI] [PubMed] [Google Scholar]
- El-Guendouz S., Aazza S., Lyoussi B., Antunes M.D., Faleiro M.L., Miguel M.G. Anti-acetylcholinesterase, antidiabetic, anti-inflammatory, antityrosinase and antixanthine oxidase activities of Moroccan propolis. Int. J. Food Sci. Technol. 2016;51:1762–1773. [Google Scholar]
- El-Guendouz S., Aazza S., Lyoussi B., Bankova V., Lourenço J.P., Rosa Costa A.M., Mariano J.F., Miguel M.G., Faleiro M.L. Impact of biohybrid magnetite nanoparticles and moroccan propolis on adherence of methicillin resistant strains of Staphylococcus aureus. Molecules. 2016;21 doi: 10.3390/molecules21091208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa R.R., Inchingolo R., Alencar S.M., Rodriguez-Estrada M.T., Castro I.A. Antioxidant activity of phenolic compounds added to a functional emulsion containing omega-3 fatty acids and plant sterol esters. Food Chem. 2015;182:95–104. doi: 10.1016/j.foodchem.2015.02.130. [DOI] [PubMed] [Google Scholar]
- Gregoris E., Stevanato R. Correlations between polyphenolic composition and antioxidant activity of Venetian propolis. Food Chem. Toxicol. 2010;48:76–82. doi: 10.1016/j.fct.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Hebishy E., Buffa M.M., Guamis B., Trujillo A.J. Stability of sub-micron oil-in-water emulsions produced by ultra high pressure homogenization and sodium caseinate as emulsifier. Chem. Eng. Trans. 2013:1813–1818. [Google Scholar]
- Krstonošić V., Dokić L., Nikolić I., Milanović M. Influence of xanthan gum on oil-in-water emulsion characteristics stabilized by OSA starch. Food Hydrocoll. 2015;45:9–17. [Google Scholar]
- Mahmood, T., Akhtar, N., 2013. Stability of a cosmetic multiple emulsion loaded with green tea extract. Scientific World Journal Article ID 153695. 10.1155/2013/153695. [DOI] [PMC free article] [PubMed]
- Malinowska P., Gliszczynska-swiglo A., Szymusiak H. Protective effect of commercial acerola, willow, and rose extracts against oxidation of cosmetic emulsions containing wheat germ oil. Eur. J. Lipid Sci. Technol. 2014;116:1553–1562. [Google Scholar]
- Marcucci M.C. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26:83–99. [Google Scholar]
- Miguel M.G. Chemical and biological properties of propolis from the western countries of the Mediterranean basin and Portugal. Int. J. Pharm. Pharm. Sci. 2013;5:403–409. [Google Scholar]
- Nieva-Echevarría B., Manzanos M.J., Goicoechea E., Guillén M.D. 2,6-Di-tert-butyl-hydroxytoluene and its metabolites in foods. Compr. Rev. Food Sci. Food Saf. 2015;14:67–80. doi: 10.1111/1541-4337.12121. [DOI] [PubMed] [Google Scholar]
- Osés S.M., Pascual-Maté A., Fernandez-Muiño M.A., López-Diaz T.M., Sancho M.T. Bioactive properties of honey with propolis. Food Chem. 2016;196:1215–1223. doi: 10.1016/j.foodchem.2015.10.050. [DOI] [PubMed] [Google Scholar]
- Poyato C., Navarro-Blasco I., Calvo M.I., Cavero R.Y., Astiasarán I., Ansorena D. Oxidative stability of O/W and W/O/W emulsions: effect of lipid composition and antioxidant polarity. Food Res. Int. 2013;51:132–140. [Google Scholar]
- Rabasco Alvarez A.M., González Rodríguez M.L. Lipids in pharmaceutical and cosmetic preparations. Grasas y Aceites. 2000;51:74–96. [Google Scholar]
- Roncero J.M., Álvarez-Ortí M., Pardo-Giménez A., Gómez R., Rabadán A., Pardo J.E. Virgin almond oil: extraction methods and composition. Grasas y Aceites. 2016;67 [Google Scholar]
- Sehn E., Hernandes L., Franco S.L., Gonçalves C.C.M., Baesso M.L. Dynamics of reepithelialisation and penetration rate of a bee propolis formulation during cutaneous wounds healing. Anal. Chim. Acta. 2009;635:115–120. doi: 10.1016/j.aca.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Sforcin, J.M., 2016. Biological properties and therapeutic applications of propolis. Phyther. Res. 10.1002/ptr.5605. [DOI] [PubMed]
- Tirnaksiz F., Kalsin O. A topical w/o/w multiple emulsions prepared with Tetronic 908 as a hydrophilic surfactant: formulation, characterization and release study. J. Pharm. Pharmaceut. Sci. 2005;8:299–315. [PubMed] [Google Scholar]
- Wu W.-M., Lu L., Long Y., Wang T., Liu L., Chen Q., Wang R. Free radical scavenging and antioxidative activities of caffeic acid phenethyl ester (CAPE) and its related compounds in solution and membranes: a structure-activity insight. Food Chem. 2007;105:107–115. [Google Scholar]
- Yamaki K., Taneda S., Yanagisawa R., Inoue K., Takano H., Yoshino S. Enhancement of allergic responses in vivo and in vitro by butylated hydroxytoluene. Toxicol. Appl. Pharmacol. 2007;223:164–172. doi: 10.1016/j.taap.2007.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.