Abstract
In recent years, the decreased efficacy of existing antibiotics toward management of emergent drug-resistant strains has necessitated the search for novel antibiotics from natural products. In this regard, Bacillus sp is well known for producing variety of secondary metabolites of potential use. Therefore, we performed an investigation to isolate and identify Bacillus sp from oral cavity for production of novel antimicrobial compounds. We extracted, purified, and identified a novel bioactive compound by B. megaterium (KC246043.1). The optimal production of compound was observed on de Man Rogosa and Sharpe broth by incubating at 37 °C, and pH 7.0 for 4 days. The bioactive compound was extracted by using n-butanol (2:1 v/v), purified on TLC plates with detection at Rf 7.8 cm; further characterized and identified as a cyclic ploypeptide sharing structural similarity with bacitracin. Minimum inhibitory concentration of bioactive compound was found to be 0.25, 0.5, 1.0, 3.125 and 6.25 μg/ml against Micrococcus luteus ATCC10240, Salmonella typhi ATCC19430, Escherichia coli ATCC35218. Pseudomonas aeruginosa ATCC27853 and Staphylococcus aureus ATCC25923 respectively, with no activity against Candida albicans ATCC10231. Our findings have revealed a novel cyclic peptide compound from B. megaterium with broad spectrum antimicrobial activity against both Gram positive and Gram negative bacteria.
Keywords: Antibacterial, Bacillus megaterium, Bacitracin, Drug discovery, Peptide antibiotics
1. Introduction
Over the decades, the emergence of drug resistant strains in clinical settings has been increased tremendously due to the random and inappropriate use of antibiotics (Gordana et al., 2012). Consumption of antibiotics without prescription of doctors and under-dose use are one of the primary reasons behind this (Grigoryan, 2007). It has been reported that 95% of Staphylococcus aureus strains are resistant to penicillin and 60% to methicillin (Sakoulas and Moellering, 2008). Additionally, transfer of resistance genes among pathogenic microorganism has also boosted the spread of drug resistance. Like horizontal gene transfer through conjugative plasmids and transposons in S. aureus (Edwards et al., 2013). According to the Centers for Disease Control (CDC), one-third to one-half of all antibiotics prescribed in the U.S. are either unnecessary or inappropriate based on microorganisms’ culture and susceptibility behavior. Every year 2 million people suffer from infections and are treated with antimicrobials. Among those, at least 23,000 die from complications that result from antibiotic resistance (Centers for Disease Control and Prevention, 2015). Antibiotic resistance is an important challenge posed to the health professionals globally as it is resulting in higher medical costs, prolonged hospital stays and increased mortality. Therefore, the World Health Assembly recommends to ensure prevention and treatment of infectious diseases with safe and effective way of using medicines (World Health Organization, 2014).
According to WHO, the antimicrobials which are considered of ‘critical importance’ for human medicine include β-lactams, polymyxins, lincosamides, aminoglycosides, tetracyclines and polypeptides (World Health Organization, 2011). About 800 different peptide antibiotics which are effective against bacteria or fungi, are produced by Bacillus spp (Saxena, 2015). Of these, 66 are produced by B. subtilis, and, 23 are active metabolites of B. brevis (Li et al., 2001, Falagas and Kasiakou, 2005). Several species of the genus Bacillus biosynthesize the peptide antibiotics through a ribosomal or non-ribosomal mechanism. For example, gramicidin is produced by B. brevis (Gurnev and Nestorovich, 2014), gavaserin and saltavalin are produced by B. polymyxa (Pichard et al., 1995), whereas bacitracin and vancomycin by B. subtilis (Fang et al., 2014). B. licheniformis is reported to produce subtilin (Shobharani et al., 2015) while B. aneurinolyticus produces tyrocidines (Vosloo et al., 2013).
The Bacillus spp are spore formers and able to survive extreme environmental conditions such as high heat, low pH, dry and under adverse nutrition environments (Barbosa et al., 2005, Cutting, 2011). Additionally, they also exhibit probiotics properties including many desirable characteristics such as antagonistic or antimicrobial activities against pathogenic bacteria (Quigley, 2010), gastric and bile juice tolerance, adherence to the epithelial cells of the intestine and improvement of the intestinal microbial balance (Ouwehand et al., 2002, Ministry of Food and Drug Safety, 2015a, Ministry of Food and Drug Safety, 2015b). In particular, B. megaterium is most commonly isolated from various habitats such as soil, seawater, sediments, rice paddies, dried food, honey and milk (Lee et al., 2016). B. megaterium is highly versatile due to its adaptive behavior and is reported to produce vitamin B12, antiviral agent-oxetanocin and penicillin amidase (Salgaonkar et al., 2013).
Therefore, considering the problems of existing chemotherapeutic agents in combating drug resistance, we aimed to isolate and characterize novel antimicrobial compounds. We assume that microorganisms in the oral cavity could be adapted for antibiosis by secreting secondary metabolites against competing pathogens of varying genera. In this perspective, Bacillus spp are the most studied producers of diverse and newer antibiotics. Therefore, the extraction, and characterization of antimicrobial compounds from Bacillus spp isolated from the less explored human oral microflora, could result in discovery of newer and broad spectrum antibiotics.
2. Material and methods
2.1. Isolation of Bacillus sp
For the isolation of Bacillus spp hundred volunteers who were not on any antibiotics treatments for the previous three weeks were involved. The subjects were instructed not to drink, eat, smoke, or clean their teeth for 2 h before the sampling. 2 ml of unstimulated saliva secretions were collected in sterile eppendorf tubes. Sample were spreaded on de Man Rogosa and Sharpe (MRS) agar plates and incubated at 37 °C for 72 h under aerobic conditions according to Sarika et al., 2012. The Bacillus spp colonies with different morphologies were randomly selected, purified and maintained as frozen cultures in MRS broth with 20% glycerol at −80 °C until use.
2.2. Characterization and identification of test isolates
The identification of thirteen selected isolates was performed on the basis of morphological and biochemical characteristics according to Bergey's Manual of Systematic Bacteriology (Holt et al., 1994). These isolates were also identified and characterized on the basis of physiological and biochemical reaction tests with a colorimetric reagent card of VITEK 2 analyzer (BioMerieux, France). Furthermore, one strain BI5 was also confirmed by using an Illumina genome analyzer (Illumina MiSeq, 16 metagenomics ver 1.0.1.0). The 16 S rRNA sequencing was performed targeting the V4-V5 hypervariable region, according to Nelson et al., 2014. The 16 S rRNA sequence was compared with similar sequences of the reference organisms by NCBI Basic Local Alignment Search Tool (BLASTn) (www.ncbi.nlm.nich-gov/BLAST/). The phylogenetic tree construction and analysis was conducted using MEGA 4.0 (Tamura et al., 2007).
2.3. Preparation of tester strains
Two strains of Gram positive bacteria, one each of Staphylococcus aureus ATCC25923 and Micrococcus luteus ATCC10240 and three strains of Gram negative bacteria, one each of Escherichia coli ATCC35218, Pseudomonas aeruginosa ATCC27853 and Salmonella typhi ATCC19430 were included along with one fungal strain viz. Candida albicans ATCC10231. All these strains were obtained from the ATCC Essentials of Life Science Research, USA. These stock cultures were maintained in nutrient broth with 20% glycerol at −80 °C until use. To obtain cells in the stationary growth phase, bacterial and fungal strains were subcultured twice at 37 °C and 25 °C respectively for 24 h on tryptic soy broth (TSB). Cells were harvested by centrifugation at 6000g for 2 min and washed once with a 5 mM NaCl solution. The supernatant was discarded, washed again, re-harvested and suspended in fresh TSB. A cell density of approximately 1 × 106 CFU/mL is prepared from cultures of each test strain by dilution with 0.1 M sodium phosphate buffer (pH 7.0).
2.4. Evaluation of antimicrobial activity of Bacillus extract against tester strains
Antibacterial activity of the Bacillus spp extract was evaluated using agar well diffusion method as described by Irshad et al., 2012 with some modifications. Briefly, 10 ml of MRS broth was inoculated with the Bacillus isolates and incubated at 37 °C for 3 days. 0.5 ml of the broth culture of each test strain was added to 100 ml of molten agar, which were cooled at 45 °C. Next, two layered agar plates were prepared. The base layer was sterile and clear, while the upper layer was inoculated with the test strain and left until dry. With a sterile cork borer, the wells of 8 mm were made and inoculated with a 100 μl of Bacillus extract. Gentamycin (10 μg/ml) was used as positive control. Plates were incubated at 37 °C for 24 h under aerobic conditions for bacteria. For antifungal activity determination, the agar well diffusion method as described by Ahmad et al., 2017 was used with some modifications. Briefly, 100 μl of extract was tested by inoculating the sabouraud dextrose agar with fungal tester strain (top agar) and plated over the agar base. Nystatin (0.5 mg/ml) was used as positive control. Plates were incubated at 28 °C for 72 h under aerobic conditions. The diameter of zone of inhibition was measured in millimeters (mm) and compared with the antimicrobial standards. All the experiments were done in triplicates and the data is presented as mean ± SD.
2.5. Determination of minimum inhibitory concentration of Bacillus extract against the tester strains
The minimum inhibitory concentration (MIC) of Bacillus extract was evaluated using the tube dilution method according to Phillips et al., 1991. MIC was defined as the lowest concentration of extract showing no visible turbidity of tester strains. All the experiments were done in triplicates and the data is presented as mean ± SD.
2.6. Effect of incubation period, temperature and pH on production of antimicrobial compound
Attempts were made to investigate maximum production of antimicrobial compound by Bacillus spp by optimizing the culture conditions such as the incubation period, incubation temperature and pH. The optimum incubation period and temperature for maximum antibiotic production of antimicrobial compound was determined according to the method of Chen et al., 2008. Bacillus spp isolates were cultured at temperature ranging from 25, 30, 37, 45 and 50 °C for a period ranging from 24, 48, 72, 96, 120, 144 and 168 h. Whereas, the optimum pH was determined by culturing the bacteria at various pH ranging from 5, 6, 7, 8 and 9 as adapted by Singh et al., 2014. After obtaining the optimum conditions, the antimicrobial activity of Bacillus extract was evaluated by agar well diffusion method as used by Irshad et al., 2012 and as described in the preceding section.
2.7. Extraction, purification and detection of the active compound from selected Bacillus strain
2.7.1. Extraction of the active antimicrobial compound
The active antimicrobial compound was prepared by inoculating MRS broth with culture of Bacillus sp and incubating at optimum conditions under aerobic environment. To extract the antimicrobial compound, the culture medium was centrifuged at 10,000 rpm, 4 °C for 15 min and filtered on 0.2 μm sterile nitrocellulose membrane filter (Whatman, Germany). Following the method of Azevedo et al., 1993, the filtrate was mixed with n-butanol (2:1 v/v), then vigorously shaken and allowed to separate. The supernatant was separated and concentrated under reduced pressure in a vacuum rotary pump at 4 °C (R-114, Buchi, Switzerland) until no solvent was left. Each part of the extraction process was subjected to its antimicrobial activity evaluation by the agar well diffusion method.
2.7.2. Chromatography of the antimicrobial compound
The extracted bioactive compound was analyzed by thin layer chromatography (TLC) according to the method of Batrakov et al. 2003 with slight modifications. Briefly, the samples were spotted on to 2 × 10 cm2 silica gel TLC plates (Merck, KGaA, Germany; 60 F254, 0.25 mm) using spotting tubes about 1.5–2 cm above the bottom of the plates. Subsequently, the plates were placed in a chromatography jar containing mixture of chloroform and methanol (85:15). The plates were then left in the solvent for some time, until the solvent moved across the plate from bottom to top. The plates were removed from the jar, allowed to dry, and then visualized under ultraviolet irradiations at 254 nm (CAMAG 254 nm, Switzerland) and Rf was determined.
2.7.3. Identification of the active compound
2.7.3.1. Infrared (IR) spectroscopy
The active antimicrobial compound was separated on TLC plate, and purified by lyophilization and prepared on to the solid disc (13 mm) for infrared spectroscopy (Tensor 37, Bruker, Germany, Opus 6.5 software). Following the KBr disc method, spectra were recorded in the region 4000–400 cm-1 in order to determine the functional groups and chemical bonds according to a slightly modified method from Alkotaini et al., 2013. Zinc bacitracin was used as the standard compound (Arab Center for Pharmaceuticals and Chemicals, Batch No. 230687).
2.7.3.2. High performance liquid chromatography (HPLC) analysis
The further detection of the active compound was carried out by HPLC analysis (Waters Alliance HPLC, Waters Corp, Milford, MA, USA). The single spot on TLC was collected to start the HPLC analytical method as adapted by Potts et al., 2012 with slight modifications. The active compound was run on the heated Xbridge C18 column up to 35 °C (Stainless steel, 5 μm, 4.6 mm × 150 mm). The separation pump model was e2995 and the instrument was equipped with PDA detector (model waters 2998) and Empower 2 software. The mobile phase was an isocratic system composed of 63% (methanol: acetonitrile) and 37% (buffer: water). Detector: PDA at 345 nm. Flow Rate: 1.0 ml/min. Sample volume (loop): 40 μl. The organic portion of the mobile phase was prepared by mixing methanol and acetonitrile in a ratio of 27:2. The aqueous portion of the mobile phase was prepared by mixing buffer and water in a ratio of 1:3. To this solution, EDTA was added to give a concentration of 0.1 mM. The buffer consists of monobasic phosphate and dibasic phosphate in a ratio of 4.5:1. Zinc bacitracin was used as a reference compound.
2.7.3.3. Nuclear magnetic resonance (NMR) analysis
Furthermore, to determine the chemical structure of the active compound, the NMR analysis was performed according to a slightly modified method of Muhammad and Ahmed, 2015. NMR spectra were analyzed using Bruker 300 MHz spectrometer (AVANCE III HD BRUKER, Topspin software) equipped with 5 mm of probe head for 1H analysis.
3. Results and discussion
A large number of different microbial species have been identified from human microbiome (Dewhirst et al., 2010), but lesser investigations have been carried out to exploit these rich flora for the production of novel antimicrobial compounds. Therefore, we attempted to extract and characterize a novel bioactive antimicrobial compounds from bacterial isolates of oral microflora.
3.1. Isolation and characterization of Bacillus isolates
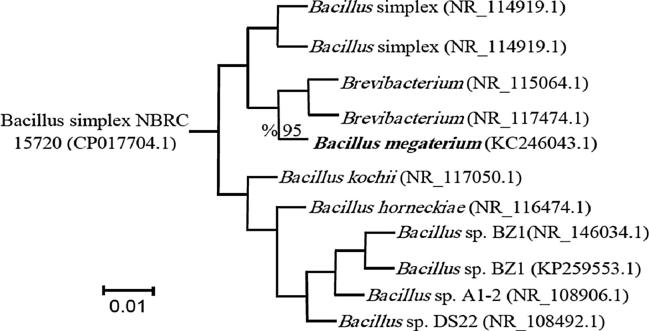
Bacillus isolates were identified on the basis of morphological, biochemical characteristics, VITEK 2 analyzer (BioMerieux, France) giving 92% probability and conformed by using an Illumina genome analyzer. Morphologically the colony of B. megaterium strain was yellowish-creamy in color, 3 mm in diameter, no pigmentation, and round to irregular shapes with entire to undulate margins on TSA. B. megaterium is a Gram positive bacteria with motile rod-shape cells. It is catalase positive and grows aerobically at 4–45 °C, with optimum growth at pH 7 and temperature 30 °C. B. megaterium was primarily identified using the API 50 BCL system, VITEK 2 analyzer as shown in Table 1. Furthermore, according to 16S rRNA sequence based phylogenetic analysis with the sum of branch length 0.01, the isolate was identified to be B. megaterium (Accession number: KC246043.1) and showed 95% similarity to reference sequences from NCBI as shown in Fig. 1.
Table 1.
Physiochemical test of the strain B. megaterium as determined using the API 50 BCL system by using VITEK 2 analyzer.
| Biochemical test | Results | Biochemical test | Results |
|---|---|---|---|
| Beta–Xylosidase | + | Tetrazolium red | − |
| Beta–Galactosidase | − | Leucine arylamidase | − |
| Ala–Phe–Pro arylamidase | + | Alanine arylamidase | − |
| Ellman | + | Glycogen | − |
| D–Mannose | − | Maltotriose | − |
| Beta–Mannosidase | + | Palatinose | − |
| Inulin | − | α – Glucosidase | + |
| Oleandomycin resistance | − | Putrescine assimilation | − |
| L–Lysine arylamidase | − | Polmixin–B resistance | − |
| L–Pyrrolydonyl arylamidase | − | Phenylalanine arylamidase | + |
| Cyclodextrin | + | Tyrosine arylamidase | + |
| Methyl D – Xyloside | − | Myo inositol | + |
| D–Melezitose | − | Glycine arylamidase | − |
| Phosphorl choline | − | L – Rhamnose | − |
| D–Glucose | − | D – Tagatose | − |
| Esculine hydrolysis | + | NaCl 6.5% | + |
| L– Asartate arylamidase | − | L – Proline arylamidase | − |
| Methyl α–D Glucopyranoside acidification | − | β – N – Acetyl Glucosaminidase | − |
| D–Galactose | − | A–Galactosidase | + |
| A–Mannosidase | − | D – Mannitol | + |
| N–Acetyl D–Glucosamine | − | β – Glucosidase | + |
| Pyruvate | − | D – Trehalose | − |
| D–Ribose | − | Kanamycin resistance | − |
+, positive reaction; −, negative reaction.
Fig. 1.
Phylogenetic tree for 16S rRNA sequence of the strain B. megaterium using the neighbor-joining method.
3.2. Bacillus extract and its antimicrobial activity
The active metabolites produced (at optimized conditions of incubation at 37 °C and pH 7.0 for 4 days) by thirteen different isolates could show varied antibacterial activities as shown in Table 2. Out of these isolates, BI-5 exhibited highest antibacterial activity against the tester strains including both Gram positive (S. aureus ATCC25923 and M. luteus ATCC10240) and Gram negative bacteria (E. coli ATCC35218, P. aeruginosa ATCC27853 and S. typhi ATCC19430) with the zone of inhibition ranging from 9 to 22 mm. Whereas, no antimicrobial activity was recorded against C. albicans ATCC10231. Strain BI-5 was characterized and identified as Bacillus megaterium.
Table 2.
Antibacterial activity of Bacillus isolates against the tester strains as determined by spot lawn method.
| Bacterial strains | Diameter of zone of inhibition (mm) |
|||||
|---|---|---|---|---|---|---|
| S. aureus ATCC 25,923 | M. luteus ATCC 10,240 | E. coli ATCC 35,218 | P. aeruginosa ATCC 27,853 | S. typhi ATCC 19,430 | C. albicans ATCC 10,231 | |
| BI-1 | 8.33 ± 0.76 | 8.45 ± 0.81 | 8.21 ± 0.78 | 8.33 ± 0.76 | 8.54 ± 0.76 | 8.33 ± 0.45 |
| BI-2 | 8.33 ± 0.76 | 8.67 ± 0.93 | 8.33 ± 0.76 | 8.33 ± 0.76 | 8.33 ± 0.76 | 8.47 ± 0.91 |
| BI-3 | 11.33 ± 0.92 | 14.89 ± 1.75 | 9.78 ± 0.98 | 8.91 ± 1.03 | 8.66 ± 0.55 | 8.33 ± 0.76 |
| BI-4 | 8.78 ± 0.67 | 8.33 ± 0.76 | 8.51 ± 0.55 | 8.33 ± 0.76 | 8.67 ± 0.33 | 8.33 ± 0.71 |
| BI-5 | 18.33 ± 1.55 | 22.69 ± 1.86 | 20.67 ± 1.14 | 9.67 ± 1.10 | 16.89 ± 1.64 | 8.92 ± 0.77 |
| BI-6 | 8.22 ± 0.72 | 8.11 ± 0.76 | 8.67 ± 0.93 | 8.33 ± 0.76 | 8.99 ± 0.99 | 8.11 ± 0.76 |
| BI-7 | 8.42 ± 0.76 | 8.99 ± 0.76 | 8.79 ± 0.91 | 8.33 ± 0.76 | 8.39 ± 0.89 | 8.33 ± 0.76 |
| Bl-8 | 8.33 ± 0.45 | 8.25 ± 0.78 | 8.44 ± 0.66 | 8.21 ± 0.76 | 8.93 ± 0.76 | 8.44 ± 0.71 |
| BI-9 | 8.21 ± 0.33 | 8.53 ± 0.93 | 8.13 ± 0.95 | 8.45 ± 1.03 | 8.33 ± 0.76 | 8.91 ± 1.03 |
| BI-10 | 10.28 ± 0.98 | 13.76 ± 0.84 | 10.33 ± 0.99 | 8.54 ± 0.76 | 9.98 ± 1.18 | 8.67 ± 0.93 |
| BI-11 | 8.23 ± 0.46 | 8.33 ± 0.76 | 8.71 ± 0.81 | 8.93 ± 0.84 | 8.33 ± 0.76 | 8.47 ± 0.91 |
| BI-12 | 8.66 ± 0.55 | 8.53 ± 0.76 | 8.21 ± 0.78 | 8.33 ± 0.76 | 8.67 ± 0.94 | 8.31 ± 0.76 |
| BI-13 | 8.33 ± 0.76 | 8.63 ± 0.76 | 8.67 ± 0.93 | 8.43 ± 0.56 | 8.11 ± 0.76 | 8.35 ± 0.76 |
Zone of inhibition ≤ 9 = absence of antimicrobial activity.
Zone of inhibition > 9 = presence of antimicrobial activity.
In the present study, n-butanol was used to extract bioactive metabolites from B. megaterium. The purified extract displayed lowest MICs of 0.25, 0.5 and 1 μg/ml against M. luteus ATCC10240, S. typhi ATCC19430 and E. coli ATCC35218, respectively. Whereas highest MICs of 3.125 and 6.25 μg/ml were observed against P. aeruginosa ATCC27853 and S. aureus ATCC25923, respectively (Table 3). A study conducted by Lisboa et al., 2006 has revealed a bacteriocin-like substance produced by Bacillus amyloliquefaciens, which inhibited pathogenic and food-spoilage bacteria (L. monocytogenes, Bacillus cereus, Serratia marcescens, and Pasteurella haemolytica). Ebrahimipour et al., 2014 isolated and purified an antimicrobial compound upon extracting the culture of Bacillus atrophaeus by methanol and acetone. Al-Saraireh et al., 2015 extracted the supernatant of Bacillus isolates from soil and reported its antibacterial activity against Gram positive bacteria, namely B. subtilis, M. luteus and S. aureus. Ramachandran et al., 2014 isolated an antimicrobial peptide B. subtilis RLID 12.1 that showed a broad-spectrum antimicrobial activity against Streptococcus pyogenes. Lee et al., 2016 isolated Bacillus sp LM7 from the korean traditional fermented soybean food, which produced an antimicrobial lipopeptides inhibiting the growth of Gram positive bacteria (L. monocytogenes and B. cereus), but it could not inhibit lactic acid bacteria (Lactobacillus plantarum and Lactococcus lactis).
Table 3.
Minimum inhibitory concentration of purified active antimicrobial compound produced by the B. megaterium against the tester strains.
| Bacterial strains | Minimum inhibitory concentration (µg/ml) |
|---|---|
| S aureus ATCC 25,923 | 6.25 ± 0.0 |
| Micrococcus luteus ATCC 10,240 | 0.25 ± 0.3 |
| P. aeruginosa ATCC 27,853 | 3.125 ± 0.0 |
| Escherichia coli ATCC 35,218 | 1 ± 0.0 |
| S. typhi ATCC 19,430 | 0.5 ± 0.3 |
| Candida albicans ATCC 10,231 | ND |
Values are mean with ± SD of three replications; ND: Not determined.
3.3. Purification and characterization of the active compound from B. Megaterium
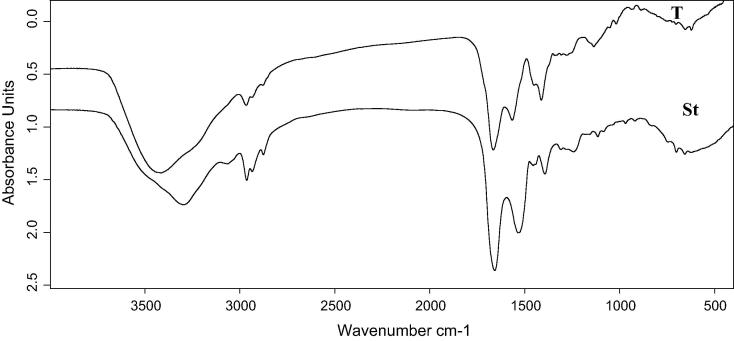
As shown in Fig. 2, the active compound appeared as a yellowish solid on the TLC plate with Rf 7.8 cm. The separation spot was scratched and prepared for identification processes. The Fourier transform-infrared (FT-IR) spectrum of the purified active antimicrobial compound is shown in Fig. 3. A 1665 cm–1 band was recorded due to the stretching mode of the CO-N bond (amide I band in helical protein). Also, 3414 and 1565 cm–1 bands were recorded as a result of the deformation mode of the N—H bond combined with C—N stretching mode (amide II band). Both of these observations indicated the presence of a peptide component in the active compound. Whereas, 2966, 2878, 1412 and 1400 cm−1 bands were the result of a typical CH stretching vibration in an aliphatic chain in the hydrophobic amino acids.
Fig. 2.

Separation of the crude antimicrobial components obtained from the B. megaterium supernatant on TLC plate.
Fig. 3.
FTIR spectrum of bacitracin standard and purified active antimicrobial components produced by the strain B. megaterium.
Compared with previous studies, Lin et al., 1994 observed strong bands indicating the presence of a peptide component. A 3300 cm−1 band resulting from the N—H stretching mode, 1655 cm−1 band resulting from the stretching mode of the CO—N bond and 1535 cm−1 band resulting from the deformation mode of the N—H bond combined with the C—N stretching mode. Moreover, the bands at 2960–2860 and 1470–1370 cm−1 resulting from the C—H stretching mode suggested the presence of an aliphatic chain related to the predominance of hydrophobic amino acids such as L-isoleucine, L-leucine or it contains a fatty acid in their structure. In a study conducted by Bechard et al., 1998, the FT-IR spectrum of a purified bacitracin showed characteristic absorption valleys at 1540, 1650 and 3300 cm−1 indicating that antibiotic contains peptide bonds. A lactone ring is suggested by the absorption at 1740 cm−1 and valleys that result from C—H stretching (2950, 2850, 1460 and 1400 cm−1) indicated the presence of an aliphatic chain. Additionally, the studies from Maquelin et al., 2002 had shown the spectrum of typical proteins and peptide absorption bands corresponding to N—H stretching (amide A) at 3200 cm−1. The peaks at 1506, 1645 cm−1 (Gauzian amide bond) and band at 3371 cm−1 (Hydrogen bonded OH group) indicated the presence of peptide bonds. The spectral analysis indicated typical absorption peaks corresponding to N—H stretching of proteins and peptide bonds. Romero et al., 2007 performed the FT-IR analysis on active extracts from Bacillus subtilis strains. Their study showed bands in the range of 1630–1680 cm–1 resulting from the stretching mode of the CO—N bond (amide I band) and at 1570–1515 cm–1 resulting from the deformation mode of the N—H bond combined with C—N stretching mode (amide II band). Both of these have indicated the presence of a peptide component and also bands at 2855–2960 cm–1 resulting from typical CH stretching vibration in the alkyl chain. Benitez et al., 2010 recorded a peak at 1645 cm−1 associated with spectrum between 3500 and 3200 cm−1 indicating the amide functional group. Ajesh et al., 2013 observed the absorption valley at 2936 cm−1 resulting from CH stretching indicating the existence of an aliphatic chain. The peak at 1415 cm−1 arises from the amide II band which results from the deformation of N—H bond combined with the C—N stretching molecule. Muhammad et al., 2016 isolated an antibacterial polypeptide from the B. brevis MH9 and described its structural details by FT-IR spectrum showing absorption peaks at the regions of 794 cm-1 (C C), 1257 cm-1 (C—O), 1700 cm-1 (N—H), 1940 cm-1 (O—N—O), 2350 cm-1 (—C N), 2810 cm-1 ( C—H), 3430 cm-1 (—N—C—H), 3490 cm-1 (H—O—H) and in the region of 3620 cm-1 (—OH).
In a recent study of the FT-IR spectrum of bacitracin by Li et al., 2017, the comparison of bacitracin-palladium nanoparticles to bacitracin, showed absorption peak at 3400 cm-1 derived from the stretching vibrations of —OH and/or —NH groups. Where, the absorption peaks at 2930 and 1540 cm-1 are attributed to the stretching vibrations of C—H and C C, respectively. The absorption peak at 1660 cm-1 is assigned to the C—C stretching of aromatic, whereas, the absorption peaks at 1100 and 702 cm-1 are associated with the C—O stretching vibrations and C—S bonds, respectively.
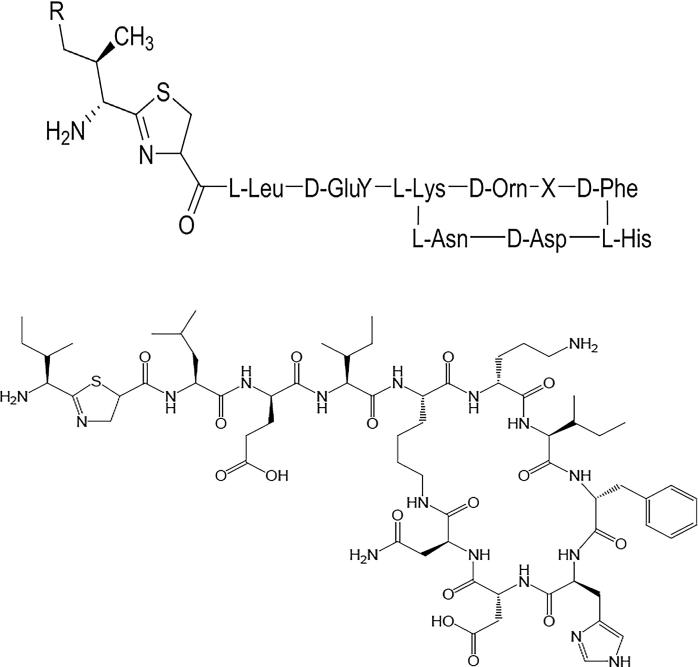
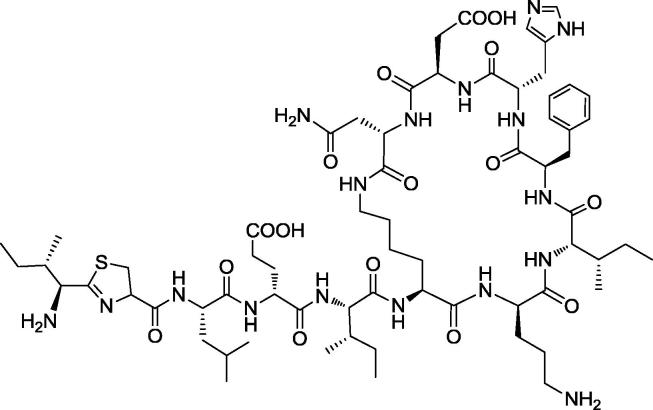
Therefore, the comparison of our FTIR analysis with literature has indicated that the pure antimicrobial compound isolated from BI-5 (B. megaterium) is a kind of cyclic ploypeptide and shares structural similarity with bacitracin. Bacitracin (C66H103N17O16S) is a polypeptide antibiotic produced as a mixture of closely related compounds by B. subtilis or B. licheniformis, and widely used as a medicine active against Gram positive bacteria (Kim and Jeon, 2016). Bacitracin consists of multi-amino acids in chain viz. l-cysteine, d-glutamic acid, l-histidine, d-phenylalanine, l-lysine, l-isoleucine, l-leucine, d-ornithine and l-aspartic acid as shown in Fig. 4 (Sarri et al., 2006).
Fig. 4.
Bacitracin peptide sequencing and chemical structure.
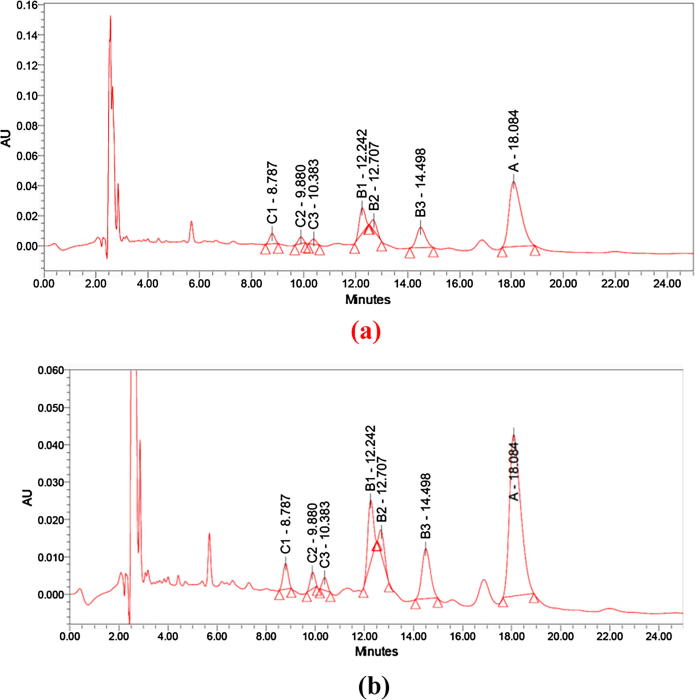
This is to be suggested here that microbial flora of human body show mutualism and antagonism among microbial population for survival. It is quite possible that the B. megaterium strain in oral microbiota is producing this kind of peptide antibiotics to antagonize other Gram positive and Gram negative bacterial strains for its survival. This strategy could be exploited to isolate newer antimicrobial compounds from natural human microflora. Further to ensure chemical nature of this compound, we performed HPLC analysis and confirmed the similarity between the purified active antimicrobial and bacitracin standard as shown in Fig. 5(a) and (b). The purified active antimicrobial compound was detected as bacitracins B1, B2, B3 and A corresponding to peaks which appeared at the retention time 12.242, 12.707, 14.498 and 18.084 min, respectively. Compared with previous studies, Potts et al., 2012 detected the standard bacitracin B1, B2, B3 and A at the retention time 12.1, 12.7, 14.9 and 17.9 min, respectively.
Fig. 5.
HPLC analysis of (a) bacitracin standard (b) purified active antimicrobial components produced by the strain B megaterium.
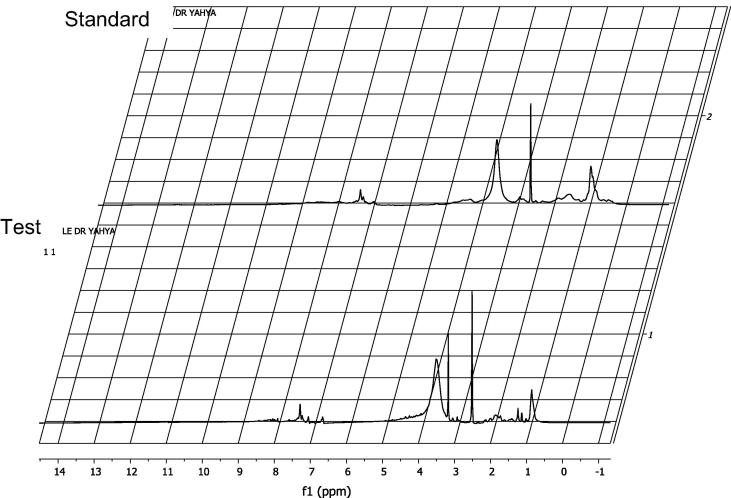
Furthermore, in our findings, the 1H NMR spectrum of active antimicrobial components showed the signals of aliphatic groups. A —CH3 resonance was observed at 1.11 ppm, while —C—CH2—C— (sp3 hydrogen) were recorded at 1.37 ppm. The resonance of alkyl amines (RNH2) was observed at 3.99 ppm. In 1H NMR spectrum, the superimposition of the aliphatic protons of the active antimicrobial components with the aliphatic protons of the standard bacitracin zinc was observed. Three new signals appeared as one doublet at 1.13 ppm, J = 6.3, one singlet at 1.24 ppm, and another singlet at 3.17 ppm. This may be due to the residual of the ethanol and n-butanol used in the purification process. The superimposition of the aromatic and de-shielded protons of the active antimicrobial components with the corresponding protons of the standard bacitracin zinc was also recorded. Two additional signals appeared as dd at 6.67 ppm and doublet at 7.05 ppm. Two protons confirmed of the active antimicrobial components with the corresponding protons of the standard bacitracin zinc at 7.1–9.5 ppm as shown in Fig. 6. The predicted structure of the active antimicrobial components based on 1H NMR data is shown in Fig. 7. Similar to our findings, Muhammad and Ahmed, 2015 also found the active antimicrobial compound (Aeritracin), which showed with 1H NMR spectrum, an aliphatic group signals –CH3 resonance being observed at 1.195 ppm, while —C—CH2—C— (sp3 hydrogen) recorded at 1.571 ppm. The resonance of alkyl amines (RNH2) was observed at 3.996 ppm. Epperson and Ming, 2000 observed the resonance of alkyl amines (RNH2) at 3.996 ppm and detected the isotropically shifted 1H NMR spectral features of the high-potent bacitracin analogues, including bacitracins A1, B1, and B2, which were virtually identical.
Fig. 6.
1H NMR spectrum of the purified active antimicrobial components (lower) and the standard bacitracin zinc (upper).
Fig. 7.
Predicted structure of active antibacterial compound isolated from the strain B. megaterium based on 1H NMR data.
Bacitracin is an effective antimicrobial agent against Gram positive bacteria and used intramuscularly and topically. The intramuscular application is limited to infants and topically as an ointment (Sarri et al., 2006, Economou et al., 2013). The drug is used as ophthalmic solution and as a food supplement in veterinary medicine to prevent breeding animals from infections (Eichenwald, 1983). Bacitracin is only effective against Gram positive bacteria, whereas, the antimicrobial compound isolated in this study is not only effective against Gram positive bacteria but is also equally effective against Gram negative bacteria. This has indicated the multiple modes of action by this compound and, therefore, could be a potential drug candidate against the drug-resistant pathogens. We suggest that antibacterial compound revealed in our investigation could be a novel and potential agent exhibiting broad spectrum activity and its mechanism of action could be similar to bacitracin and polymixin B. Some other antimicrobial proteins or peptides are reported from Bacillus spp such as lichenin produced by B. licheniformis; megacin by B. megaterium; coagulin by B. coagulans; polyfermenticin by B. polyfermenticus; cerein by B. cereus; thuricins, tochicin, kurstakin, entomocin and bacthuricin by B. thuringiensis (Abdulhameed et al., 2017). Ma et al., (2016) isolated B. megaterium from the intestines of marine fish producing 40 variants of lipopeptides such as iturin, fengycin A and B esperin (isoforms of surfactins). However, our study has revealed the production of a broad spectrum lipopeptide from the strain B. megaterium, which is quite effective to combat Gram positive and Gram negative bacterial pathogens. Since Bacillus sp could also be used as probiotics, it further strengthens the exploitation of the strain B. megaterium as a replacement of antimicrobial compound, as it could maintain the host’s normal microflora in the form of probiotics.
4. Conclusion
Currently, it is the need of hour to isolate newer and natural bioactive compounds to combat failure of existing chemotherapeutic agents in treating infectious diseases due to the increased drug resistance. Bacillus spp from environmental samples have been well known to produce a variety of antimicrobial compounds. Our findings have highlighted that even human microflora could be a good source for isolating natural and newer bioactive compounds from microorganisms. We isolated and characterized a bacitracin like peptide compound from B. megaterium, which has exhibited potential broad spectrum antimicrobial activity. Therefore, we suggest exploration of such compounds from human microflora isolates to obtain antimicrobial compounds that can meet the demand of searching for novel chemotherapeutic agents.
5. Ethical disclosures
The authors announce that no experiments were performed on animals and no data were collected from patient in this research. The authors have obtained informed consent from volunteer and the written approval of the Teaching Dental Hospital, Umm Al-Qura University, Makkah, Saudi Arabia to perform this study.
Acknowledgements
The authors would like to thank Institution of Scientific Research and Revival of Islamic Heritage at Umm Al-Qura University (project # 43509007) for the financial support. The authors are grateful to Teaching Dental Hospital staff for their valuable contribution in the work.
Conflict of interest
Authors declare no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Saraireh H., Al-Zereini W.A., Tarawneh K.A. Antimicrobial activity of secondary metabolites from a soil Bacillus sp. 7B1 isolated from south Al-Karak, Jordan. Jord. J. Biol. Sci. 2015;8:127–132. [Google Scholar]
- Abdulhameed S., Pradeep N.S., Sugathan S. In: Antimicrobials from Microbes. Francis D., editor. Springer Nature, Singapore Pte. Ltd; 2017. Bioresources and bioprocess in biotechnology; pp. 291–326. [Google Scholar]
- Ahmad N., Amir M.K., Ayaz S., Ahmad Jan A., Ashraf J.S., Zuhra F. Antimicrobial profile of the selected medicinal plants. Int. J. Chem. Lif. Sci. 2017;1(2):1039–1041. [Google Scholar]
- Ajesh K., Sudarslal S., Arunan C., Sreejith K. Kannurin, a novel lipopeptidefrom Bacillus cereus strain AK1: isolation, structural evaluation and antifungal activities. J. Appl. Microbiol. 2013;115(6):1287–1296. doi: 10.1111/jam.12324. [DOI] [PubMed] [Google Scholar]
- Alkotaini B., Anuar N., Kadhum A.A., Sani A.A. Detection of secreted antimicrobial peptides isolated from cell-free culture supernatant of Paenibacillus alvei AN5. J. Ind. Microbiol. Biotechnol. 2013;40(6):571–579. doi: 10.1007/s10295-013-1259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo E.C., Rios E.M., Fukushima K., Campos-Takaki G.M. Bacitracin production by a new strain of Bacillus subtilis extraction, purification and characterization. Appl. Biochem. Biotechnol. 1993;42(1):1–7. doi: 10.1007/BF02788897. [DOI] [PubMed] [Google Scholar]
- Barbosa T.M., Serra C.R., La Ragione R.M., Woodward M.J., Henriques A.O. Screening for bacillus isolates in the broiler gastrointestinal tract. Appl. Environ. Microbiol. 2005;71(2):968–978. doi: 10.1128/AEM.71.2.968-978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batrakov S.G., Rodionova T.A., Esipov S.E., Polyakov N.B., Sheichenko V.I., Shekhovtsova N.V., Lukin S.M., Panikov N.S., Nikolaev Y.A. A novel lipopeptide, an inhibitor of bacterial adhesion, from the thermophilic and halotolerant subsurface Bacillus licheniformis strain 603. Biochim. Biophys. Acta. 2003;1634(3):107–115. doi: 10.1016/j.bbalip.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Bechard J., Eastwell K., Sholberg P., Mazza G., Skura B. Isolation and partial chemical characterization of an antimicrobial peptide produced by a strain of Bacillus subtilis. J. Agric. Food Chem. 1998;46(12):5355–5361. [Google Scholar]
- Benitez L.B., Velho R.V., Lisboa M.P., Medina L.F.D.C., Brandelli A. Isolation and characterization of antifungal peptides produced by Bacillus amyloliquefaciens LBM5006. J. Microbiol. 2010;48(6):791–797. doi: 10.1007/s12275-010-0164-0. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2015. Antibiotics/antimicrobial resistance. http://www.cdc.gov/drugresistance/ (accessed 20.09.15).
- Chen H., Wang L., Su C.X., Gong G.H., Wang P., Yu Z.L. Isolation and characterization of lipopeptide antibiotics produced by Bacillus subtilis. Lett. Appl. Microbiol. 2008;47(3):180–186. doi: 10.1111/j.1472-765X.2008.02412.x. [DOI] [PubMed] [Google Scholar]
- Cutting S.M. Bacillus probiotics. Food Microbiol. 2011;28(2):214–220. doi: 10.1016/j.fm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Dewhirst F.E., Chen T., Izard J., Paster B.J., Tanner A.C.R., Yu W., Lakshmanan A., Wade W.G. The human oral microbiome. J. Bacteriol. 2010;192(19):5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimipour G.H., Khosravibabadi Z., Sadeghi H., Aliahmadi A. Isolation, partial purification and characterization of an antimicrobial compound, produced by Bacillus atrophaeus. Jundisha. J. Microbiol. 2014;7:e11802. doi: 10.5812/jjm.11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economou N.J., Cocklin S., Loll P.J. High-resolution crystal structure reveals molecular details of target recognition by bacitracin. Proc. Natl. Acad. Sci. U.S.A. 2013;110(35):14207–14212. doi: 10.1073/pnas.1308268110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J.S., Betts L., Frazier M.L., Pollet R.M., Kwong S.M., Walton W.G., Ballentine W.K., Huang J.J., Habibi S., Del Campo M. Molecular basis of antibiotic multiresistance transfer in Staphylococcus aureus. Proc. Natl. Acad. Sci. 2013;110(8):2804–2809. doi: 10.1073/pnas.1219701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenwald H.F. Antibiotic drug therapy in the newborn. Pediatr. Pharmacol. 1983;3:181–187. [PubMed] [Google Scholar]
- Epperson J.D., Ming L.J. Proton NMR atudies of Co(II) complexes of the peptide antibiotic bacitracin and analogues: insight into structure-activity relationship. Biochemistry. 2000;39(14):4037–4045. doi: 10.1021/bi991997p. [DOI] [PubMed] [Google Scholar]
- Falagas M.E., Kasiakou S.K. Colistin: the revival of polymyxins for the management of multidrug-resistant Gram-negative bacterial infections. Clin. Infect. Dis. 2005;40(9):1333–1341. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- Fang C., Stiegeler E., Cook G.M., Mascher T., Gebhard S. Bacillus subtilis as a platform for molecular characterisation of regulatory mechanisms of Enterococcus faecalis resistance against cell wall antibiotics. PLos One. 2014;9(3):e93169. doi: 10.1371/journal.pone.0093169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordana M., Andric B., Terzic D., Lopicic M., Dupanovic B. Antibiotic susceptibility of Salmonella sp: a comparison of two surveys with a five years interval. J. IMAB Ann. Proc. (Scientific Papers) 2012;18(1):216–219. [Google Scholar]
- Grigoryan, L., 2007. Self-medication with Antibiotics in Europe and its Determinants. Dissertation, Groningen University.
- Gurnev P.A., Nestorovich E.M. Channel-forming bacterial toxins in biosensing and macromolecule delivery. Toxins (Basel) 2014;6(8):2483–2540. doi: 10.3390/toxins6082483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J.G., Krieg N.R., Sneath P.H.A., Staley J.T., William S.T. Williams and Walkins; Baltimore: 1994. Bergey's Manual of Determinative Bacteriology. [Google Scholar]
- Irshad S., Mahmood M., Parveen F. In-vitro antibacterial activities of three medicinal plants using agar well diffusion method. Res. J. Biol. 2012;2(1):1–8. [Google Scholar]
- Kim J.C., Jeon B. Novel adjuvant strategy to potentiate bacitracin against MDR MRSA. J. Antimicrob. Chemother. 2016;71(5):1260–1263. doi: 10.1093/jac/dkv463. [DOI] [PubMed] [Google Scholar]
- Lee M.H., Lee J., Nam Y.D., Lee J.S., Seo M.J., Yi S.H. Characterization of antimicrobial lipopeptides produced by Bacillus sp. LM7 isolated from chungkookjang, a Korean traditional fermented soybean food. Int. J. Food Microbiol. 2016;221:12–18. doi: 10.1016/j.ijfoodmicro.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Li J., Turnidge J., Milne R., Nation R.L., Coulthard K. In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob. Agents Chemother. 2001;45(3):781–785. doi: 10.1128/AAC.45.3.781-785.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang Z., Li X., Yin T., Bian K., Gao D. Facile synthesis of bacitracin-templated palladium nanoparticles with superior electrocatalytic activity. J. Power Sources. 2017;341:183–191. [Google Scholar]
- Lin S.C., Minton M.A., Sharma M.M., Georgiou G. Structural and immunological characterization of a biosurfactant produced by Bacillus licheniformis JF-2. Appl. Environ. Microbiol. 1994;60(1):31–38. doi: 10.1128/aem.60.1.31-38.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisboa M.P., Bonatto D., Bizani D., Henriques J.A., Brandelli A. Characterization of a bacteriocin-like substance produced by Bacillus amyloliquefaciens isolated from the Brazilian Atlantic forest. Int. Microbiol. 2006;9(2):111–118. [PubMed] [Google Scholar]
- Ma Y., Kong Q., Qin C., Chen Y., Chen Y., Lv R., Zhou G. Identification of lipopeptides in Bacillus megaterium by two–step ultrafiltration and LC–ESI–MS/MS. AMB. Expr. 2016;6(1):79–93. doi: 10.1186/s13568-016-0252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquelin K., Kirschner C., Choo-Smith L.P., van den Braak N., Endtz H.P., Naumann D., Puppels G.J. Identification of medically relevant microorganisms by vibrational spectroscopy. J. Microbiol. Methods. 2002;51(3):255–271. doi: 10.1016/s0167-7012(02)00127-6. [DOI] [PubMed] [Google Scholar]
- Ministry of Food and Drug Safety, 2015a. http://www.foodnara.go.kr/hfoodi/ (accessed 21.04.15).
- Ministry of Food and Drug Safety, 2015b. Korean Food Standards Codex. http://www.foodnara.go.kr/residue/RS/jsp/menu02_01_01.jsp (accessed 27.11.15).
- Muhammad S.A., Ahmed S. Production and characterization of a new antibacterial peptide obtained from Aeribacillus pallidus SAT4. Biotechnol. Rep. (Amst) 2015;21(8):72–80. doi: 10.1016/j.btre.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad S.A., Ali A., Naz A., Hassan A., Riaz N., Saeed-ul-Hassan S., Andleeb S., Barh D. A new broad-spectrum peptide antibiotic produced by Bacillus brevis strain MH9 isolated from Margalla Hills of Islamabad., Pakistan. Int. J. Pept. Res. Ther. 2016;22(2):271–279. [Google Scholar]
- Nelson M.C., Morrison H.G., Benjamino J., Grim S.L., Graf J. Analysis, optimization and verification of Illumina-generated 16S rRNA gene amplicon surveys. Plos One. 2014;9(4):e94249. doi: 10.1371/journal.pone.0094249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwehand A.C., Salminen S., Isolauri E. Probiotics: An overview of beneficial effects. Antonie. Van. Leeuwenhoek. 2002;82(1–4):279–289. [PubMed] [Google Scholar]
- Phillips I., Andrews J.M., Bridson E., Cooke E.M., Holt H.A., Spencer R.C., Wise R., Bint A.J., Brown D.F.J., Greenwood D., King A., Williams R.J. A guide to sensitivity testing, report of the working party on antibiotic sensitivity testing of the British society for antimicrobial chemotherapy. J. Antimicrob. Chemother. 1991;27:1–50. [PubMed] [Google Scholar]
- Pichard B., Larue J.P., Thouvenot D. Gavaserin and saltavalin, new peptide antibiotics produced by Bacillus polymyxa. FEMS. Microbiol. Lett. 1995;133(3):215–218. doi: 10.1111/j.1574-6968.1995.tb07887.x. [DOI] [PubMed] [Google Scholar]
- Potts A.R., Psurek T., Jones C., Parris L., Wise A. Validation of a quantitative HPLC method for bacitracin and bacitracin zinc using EDTA as a mobile-phase modifier. J. Pharm. Biomed. Anal. 2012;70:619–623. doi: 10.1016/j.jpba.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Quigley E.M. Prebiotics and probiotics; modifying and mining the microbiota. Pharmacol. Res. 2010;61(3):213–218. doi: 10.1016/j.phrs.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Ramachandran R., Chalasani A.J., Lal R., Roy U. A broad-spectrum antimicrobial activity of Bacillus subtilis RLID 12.1. Scient. World J. 2014:1–10. doi: 10.1155/2014/968487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero D., de Vicente A., Rakotoaly R.H., Dufour S.E., Veening J.W., Arrebola E., Cazorla F.M., Kuipers O.P., Paquot M., Pérez-García A. The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca. Mol. Plant Microbe Interact. 2007;20(4):430–440. doi: 10.1094/MPMI-20-4-0430. [DOI] [PubMed] [Google Scholar]
- Sakoulas G., Moellering R. Increasing antibiotic resistance among methicillin resistant Staphylococcus aureus strains. J. Clin. Infect. Dis. 2008;46(11):360–367. doi: 10.1086/533592. [DOI] [PubMed] [Google Scholar]
- Salgaonkar B.B., Mani K., Braganca J.M. Characterization of polyhydroxyalkanoates accumulated by a moderately halophilic salt pan isolate Bacillus megaterium strain H16. J. Appl. Microbiol. 2013;114:1347–1356. doi: 10.1111/jam.12135. [DOI] [PubMed] [Google Scholar]
- Sarika A.R., Lipton A.P., Aishwarya M.S., Dhivya R.S. Isolation of a bacteriocin-producing lactococcus lactis and application of its bacteriocin to manage spoilage bacteria in high-value marine fish under different storage temperatures. Appl. Biochem. Biotechnol. 2012;167(5):1280–1289. doi: 10.1007/s12010-012-9701-0. [DOI] [PubMed] [Google Scholar]
- Sarri A.K., Megoulas N.C., Koupparis M.A. Development of a novel liquid chromatography evaporative light scattering detection method for bacitracin and applications to quality control of pharmaceuticals. Anal. Chim. Acta. 2006;573–574:250–257. doi: 10.1016/j.aca.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Saxena S. In: Applied Microbiology. Saxena S., editor. Springer; New Delhi, India: 2015. Microbes in production of fine chemicals (Antibiotics, drugs, vitamins, and amino acids) pp. 83–120. [Google Scholar]
- Shobharani P., Padmaja R.J., Halami P.M. Diversity in the antibacterial potential of probiotic cultures Bacillus licheniformis MCC2514 and Bacillus licheniformis MCC2512. Res. Microbiol. 2015;166(6):546–554. doi: 10.1016/j.resmic.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Singh L.S., Sharma H., Talukdar N.C. Production of potent antimicrobial agent by actinomycete, Streptomyces sannanensis strain SU118 isolated from phoomdi in Loktak Lake of Manipur, India. BMC Microbiol. 2014;14:278–291. doi: 10.1186/s12866-014-0278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol.Evol. 2007;24(8):1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Vosloo J.A., Stander M.A., Leussa A.N., Spathelf B.M., Rautenbach M. Manipulation of the tyrothricin production profile of Bacillus aneurinolyticus. Microbiology. 2013;159(Pt 10):2200–2211. doi: 10.1099/mic.0.068734-0. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2011. Critically important antimicrobials for human medicine (third revision). http://www.who.int/foodsafety/publications/antimicrobials-third/en/ (accessed 15.04.15).
- World Health Organization . Surveillance of Antimicrobial Drug Resistance in Disease-Specific Programs. WHO Library; Geneva: 2014. Antimicrobial resistance: global report on surveillance; pp. 1–256. [Google Scholar]