Abstract
Objective
To improve quality of life (QOL) in patients at risk for post–intensive care syndrome (PICS).
Patients and Methods
We conducted a mixed-method, prospective, observational, pre-post interventional study in an adult medical and mixed medical/surgical/transplant intensive care unit (ICU) at a tertiary academic hospital. Preintervention included patients admitted from October 1 through October 31, 2016, and postintervention included patients admitted from January 15 through February 14, 2017. First, a multidisciplinary team of stakeholders identified barriers associated with decreased QOL in patients at risk for PICS. Next, interventions were designed and implemented. The effect of interventions was assessed using a mixed-method analysis. The qualitative analysis used a modified grounded theory approach. The quantitative analysis included assessment of preexisting symptoms and risk factors associated with PICS. The 36-Item Short-Form Health Status Survey (SF-36), which surveys physical and mental composite scores, was used to assess QOL.
Results
Barriers identified were lack of awareness and understanding of PICS. Interventions included educational videos, paper and online education and treatment materials, and online and in-person support groups for education and treatment. After interventions, the qualitative analysis found that patients who participated in the interventions after hospital discharge showed improved QOL, whereas education during hospitalization alone was not effective. The quantitative analysis did not find improvement in QOL, as defined by SF-36 physical or mental composite scores.
Conclusion
Interventions targeted to patients after hospitalization may offer subjective improvement in QOL for those at risk for PICS.
Abbreviations and Acronyms: ADL, activities of daily living; APACHE, Acute Physiology, Age, Chronic Health Evaluation; BMI, body mass index; ED, emergency department; ICU, intensive care unit; OT, occupational therapy; PICS, post–intensive care syndrome; PT, physical therapy; QOL, quality of life; SF-36, 36-Item Short-Form Health Status Survey
With advances in intensive care, more patients are surviving critical illness.1 In the United States there are approximately 5.7 million patients admitted to the intensive care unit (ICU) every year, with 4.85 million surviving critical illness to hospital discharge.2, 3, 4, 5 Half of these survivors of critical illness will experience at least 1 symptom of post–intensive care syndrome (PICS), described by the Society of Critical Care Medicine as “new or worsening impairment cognition, mental health, or physical function after critical illness and persisting beyond the acute care hospitalization.”6, 7
Patients with PICS experience a lower quality of life (QOL); there is, however, limited evidence on effective management.7, 8, 9, 10 Previous studies have evaluated multiple interventions, including physical and occupational therapy,11, 12, 13, 14, 15 post-ICU consultations,16, 17 outpatient PICS clinics,17, 18, 19, 20, 21 rehabilitation programs,22, 23 cognitive therapy,24 and ICU diaries.25, 26, 27 Although some of these interventions improved symptoms, they were unable to demonstrate improvement in QOL.8, 17, 28, 29, 30 The decreased QOL contributes to the increased morbidity associated with PICS, which, in turn, increases the risk of readmission and subsequent increased mortality.31, 32
Because more patients are surviving critical illness, more are at risk for PICS, yet the ability to recognize and treat PICS is limited.28 As a result, patients who survive critical illness have a decreased QOL. This study aims to (1) determine the barriers associated with decreased QOL, (2) design and implement multifaceted interventions, and (3) assess outcomes in patients who survive critical illness and are, therefore, at risk for PICS.
Patients and Methods
This study was conducted at a tertiary medical center with approximately 4500 admissions per annum in the medical and mixed medical/surgical/transplant ICUs.33 This study was reviewed by the institutional review board and determined to be nonresearch quality improvement. A mixed-method, prospective, observational, pre-post interventional study was conducted to determine barriers to design, to implement interventions, and to assess outcomes.
Barriers
A multidisciplinary team was gathered, including mental health counselors, physical therapists, critical care nurses, and physicians, to evaluate QOL in patients who survive critical illness at risk for PICS.34, 35, 36 Stakeholders were identified by posting flyers in the hospital, the local community, and the online institutional community. The flyers invited anyone who had previously experienced or cared for someone who had experienced critical illness to provide statements or participate in interviews. All stakeholders willing to participate were included. At least 20 stakeholders were included; the absolute number is unknown due to ability to remain anonymous.
A multistep qualitative analysis was performed by members of the multidisciplinary team (L.M.D., A.B.J., P.J.C., C.B., A.L.).35 First, stakeholders provided statements and participated in interviews regarding their experience with critical illness. To offer the opportunity to remain anonymous, statements and interviews were offered online using a self-selected user name.37 Stakeholders could also provide statements and interviews via email, by phone, or in person.
Statements and interviews were then reviewed and analyzed using a modified grounded theory approach.36 First, individual members of the multidisciplinary team reviewed the statements and interviews to identify established and emerging themes that were coded. Next, the coded themes were discussed with the team and agreed on, and discrepancies were resolved by consensus. Subsequently, all coded statements and interviews were again reviewed and organized into agreed on categories. Categories were summarized with a representative sample of statements selected by consensus. Using the categories identified in the qualitative analysis, a root cause analysis identified barriers associated with decreased QOL in patients at risk for PICS.
Interventions and Outcomes
Based on barriers identified and in conjunction with evidence from the literature, the multidisciplinary team designed and implemented a group of interventions. Outcomes were assessed using a mixed method, as both qualitative35, 36 and quantitative28 measurements have been used previously and the optimal method of measuring QOL in patients who survive critical illness remains uncertain.38
The postintervention qualitative analysis included patients and caregivers who participated in the interventions. Statements and interviews regarding the impact of the interventions on QOL were collected, reviewed, and summarized in the same method as described previously herein.
The quantitative analysis included patients admitted to the medical and mixed medical/surgical/transplant ICUs. The preintervention phase included patients admitted from October 1 through October 31, 2016. The postintervention phase included patients admitted from January 15 through February 14, 2017. The first ICU admission during the study period was included. Patients admitted in both the preintervention and postintervention phases of study were included if a survey was completed for each phase. Patients who did not survive to discharge from the ICU were excluded. Data were collected from electronic medical records and patient survey.
The data were obtained using DataMart, an electronic database housing comprehensive data on ICU patients, and Advanced Cohort Explorer, an advanced query tool with administrative data from multiple clinical and hospital source systems.39 To account for impairments in physical, cognitive, and mental health present before hospitalization, patient characteristics on admission were extracted, including the Katz Index of Independence in Activities of Daily Living score, Hendrich II Fall Risk score, Rankin disability score, and presence of depressive symptoms. Characteristics potentially associated with PICS and QOL were extracted, including age, sex, and body mass index (calculated as the weight in kilograms divided by the height in meters squared); Acute Physiology, Age, Chronic Health Evaluation (APACHE) III40 score; Sequential Organ Failure Assessment41 score; sedation; noninvasive/invasive ventilation; ICU delirium; physical and occupational therapy; ICU and hospital length of stay; and evaluation in the emergency department (ED) or readmission to the hospital within 30 days of hospitalization.
Given the lack of a formal measurable definition of PICS,8, 42, 43 the multidisciplinary team developed a survey with the assistance of experts in survey design and study conduct from the Survey Research Center. The survey included the 36-Item Short-Form Health Status Survey (SF-36) that was previously used to assess PICS28 and QOL after critical illness.17, 28, 29, 44, 45 Questions regarding returning to home and work were added to better assess QOL after critical illness.17, 28 To determine intervention-specific measures, questions regarding participation in the interventions were added. The survey was mailed 2 months after ICU admission. To maximize survey return, phone call reminders and additional surveys were sent if the initial survey was not returned within 4 weeks. The Survey Research Center was responsible for mailing, telephoning, response tracking, instrument processing, and data file preparation.
Statistical Analyses
To compare preintervention and postintervention survey respondents, Pearson χ2 or Wilcoxon rank sum tests were used. Unadjusted and adjusted linear regressions were applied to evaluate the relationship between intervention group and SF-36 composite scores (separately, mental component and physical component). A sequential adjustment approach was used, first with a regression minimally adjusted for sex, age, body mass index, and APACHE III score at ICU admission. A second model further adjusted for invasive and noninvasive ventilation and sedation. Linear regression assumptions were assessed by evaluating plots of residuals (residual vs predicted, Q-Q plot); no violations were detected. Unadjusted and adjusted logistic regression was used to evaluate the relationship between intervention group and 30-day readmission and ED visits. The same adjusting variables were used as in the model for SF-36. A P < .05 was considered statistically significant. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc).
Results
Barriers
The root cause analysis identified lack of awareness and understanding of PICS as the main barriers in improving QOL for patients with PICS. Specifically, patients and caregivers experienced difficulty remembering and understanding what happened to them while in the ICU and what to expect after leaving the ICU (Table 1). Subsequently they felt isolated, resulting in difficulty asking for help and obtaining counseling (Table 1), ultimately contributing to decreased QOL. Health care providers did not realize what patients and caregivers could experience in the ICU (Table 1) and what life may look like after the critical illness. Multiple health care providers noted difficulty in recognizing and treating PICS, resulting in a limited ability to affect QOL for patients at risk for PICS (Table 1).
Table 1.
Representative Sample of Statements From the Preintervention and Postintervention Qualitative Analysisab
| Category | Representative statements |
|---|---|
| Preintervention phase | |
| Patients and caregivers explained their experience of being in the ICU | “All I remember is darkness. Nothingness. Couldn’t walk or talk because of the tubes in my throat. No one told me what happened so I was very confused as to where I was and why.” “I was instantly convinced that I was being held prisoner.” “When I was allowed to come out of sedation I couldn’t lift my head, arms, or legs. I was terrified.” “Later on, when I asked my husband, who is my caregiver, to explain to me what had occurred, I was shocked. So much had occurred that I didn’t even realize.” “In all honesty, I do not even remember being in ICU.” “For [my husband] I believe it is more difficult. He had to stand there, helpless, and watch me as death became a real possibility. While I was so sick that I did not fully comprehend.” |
| Patients and caregivers explained their experience since being in the ICU | “I can walk and talk again and I’m just now trying to make sense of it all. Still confused. Still cry all the time.” “I too am very emotional and will cry out of nowhere. I feel like no one understands and people do not even ask.” “My husband had a very brief stay in the ICU after a major health scare and I can tell you, even as a healthy young man, the stay traumatized him and affected him psychologically long after he recovered physically—it is something he will never forget.” “My husband gave me a hard time about looking rather grim and sour. I guess I did. My son mentioned that I wasn’t smiling anymore. I think they expected me to be my happy, driven self sooner than it happened.” “The depression monster and its friend the anxiety monster are constant companions for me. My brain won’t work for me anymore.” |
| Health care providers' responses after hearing the patient and caregivers’ experience | “I had no idea.” “We focus so much on saving lives, we often forget about what happens in recovery outside of the ICU.” |
| Patients, caregivers, and health care providers on PICS | “I was not familiar with name of the condition [PICS]. However, the condition was there all the time.” “No one mentioned PICS to me.” “No one plans for a stay in the intensive care, but those that find themselves there have to deal with how life-changing the experience can be.” |
| Patients on factors contributing to decreased quality of life with PICS | “I don’t have anyone to share with who would understand my thoughts and feelings.” “My wife just wants me to stop talking about it but I am consumed with it.” “It is so stressful for the patient and family but also the staff caring for patients with life-threatening or severe illness or conditions.” “I feel like people that do not go through [it] do not get it” “My family and loved ones are reluctant to discuss the events because, I believe, it is too painful for them.” “I need help, and I know it. But getting help is almost impossible…. I don’t even know who to ask anymore.” |
| Postintervention phase | |
| Patients and caregivers on impact of interventions on quality of life | “I have learned a couple of things since I have been participating in these discussions: I am not alone in my struggles; it helps to talk (or write) about it; there is support for me; and I have found that I can support others and that feels good!” “I’m so glad that Mayo Clinic Connect has created a space to talk about the stresses and consequences of being seriously ill.” “It’s reassuring to hear that the chaotic experience has faded some over so many years.” “I just needed someone to talk to and care tonight. Thanks for your caring on this site.” “I am so glad I found this forum/thread! I thought, until now, that I was the only one who suffered from this! And it’s such a relief to give it a name, instead of thinking that I am going 'crazy'!” “Your post was exactly what I needed to hear.” “Thank you for normalizing what I am experiencing. It really does help to know I’m not alone in this.” “I was relieved to find this support group and to learn that this is a diagnosable condition known as PICS.” |
ICU = intensive care unit; PICS = post–intensive care syndrome.
Continuous data are presented as median (25th-75th) percentiles unless specified otherwise (ADL total score reported as mean (standard deviation))
Interventions
To overcome the barriers identified, the multidisciplinary team determined that the interventions should be targeted to improve PICS education and treatment resources. Based on the analysis, along with a review of the literature, the multidisciplinary team determined that interventions should be easily accessed, individualized,38 involve caregivers,28 provide more information about recovery,36, 46, 47 and include more reassurance from experts.28, 36, 46 In addition, to optimize the sustainability of the interventions they should not be resource intensive and should be widely available, according to the multidisciplinary team.17, 27, 28
For that reason, interventions used multiple integrated formats for easy access and individualization, including (1) educational videos, (2) paper and online education and treatment materials, and (3) online and in-person support groups for education and treatment. Information provided in the interventions was redundant in each intervention, and interventions were linked to one another. Within each intervention, sections individualized information for patients, caregivers and health care providers and also separated symptoms of cognitive, physical, and/or mental impairments. To provide information about recovery and include reassurance from experts, the support groups included participation from long-term survivors of critical illness and providers with experience in PICS. All the interventions were publicly available and used preestablished platforms to aid in sustainability and availability and to limit resource utilization.
The 3 educational videos were created to improve education. These included information for patients and caregivers, management options for patients and caregivers, and information for health care providers. Patients and caregivers watched videos before dismissal from the ICU that they could reaccess online for later viewing. Health care providers were shown the information for the health care providers’ video as part of monthly continued medical education.
The paper material included information on patients’ dismissal summary, and online material used the preexisting platform, an institutional free and open-access webpage.37 This webpage was regularly updated by members of this team and experts in PICS.
The online support group was created using the preexisting Mayo Clinic Connect platform, a free and open-access webpage with discussion groups and individual messaging.48 The site is continually monitored by Mayo Clinic Connect personnel, and when medical questions are posed or resources are requested, the monitor refers members of the multidisciplinary team to participate in the online discussion. An in-person support group was created with health care providers with PICS experience and a mental health counselor as part of the Thrive initiative supported by the Society of Critical Care Medicine. The support group met monthly at a local coffee shop that was selected for ease of access for patients who may have physical and cognitive impairments.
Outcomes
The qualitative analysis demonstrated that patients and caregivers participating in the interventions after hospitalization demonstrated increased understanding of life after critical illness and PICS. In addition, patients and caregivers participating in the support groups noted a sense of community, with decreased feelings of being left alone to cope with their ailment (PICS) (Table 1).
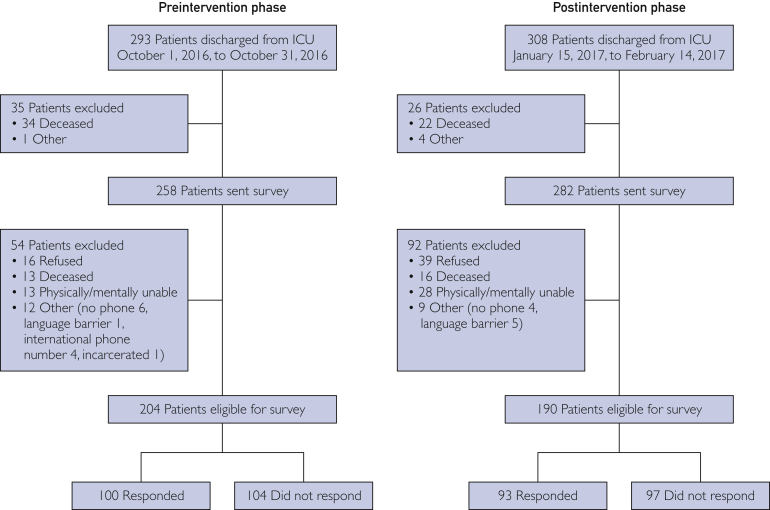
The quantitative analysis included 293 and 308 patients discharged from the ICU in the preintervention and postintervention periods, respectively. Thirty-five patients were excluded from the preintervention group and 26 from the postintervention group before the follow-up survey because of death, age younger than 18 years, or an inability to receive mail. Of 258 surveys in the preintervention group and 282 in the postintervention group, 100 surveys (38.8%) were returned from the preintervention group and 93 (33.0%) from the postintervention group (Figure). Survey responders tended to be older and have lower Rankin disability scores, fall risk scores, activities of daily living scores, depressive symptoms, and ICU delirium (Supplemental Table, available online at http://www.mayoclinicproceedings.org).
Figure.
Flow diagram of included and excluded patients in the preintervention and postintervention phases. ICU = intensive care unit.
Baseline characteristics between respondents from the preintervention and postintervention groups were generally similar, although the fall risk score was higher in the preintervention group (Table 2). Patients’ responses to participating in interventions were identical (Table 3). The QOL as measured by the physical and mental composite scores were not different between the 2 groups (Table 3). After adjustment for patient characteristics, there was no evidence of a difference in average physical composite score (difference = −1.23; 95% CI = −4.60 to 2.13; P=.47) or mental composite score (difference = −0.16; 95% CI = −3.49 to 3.17; P=.92) (Table 4). There were also no differences in discharge location, readmission rates, or rate of return to work or home (Table 3).
Table 2.
| Characteristic | Preintervention phase (n=100) | Postintervention phase (n=93) | P value |
|---|---|---|---|
| Female sex (No. [%])c | 44 (44.4) | 50 (53.8) | .20 |
| Age (y) | 64.4 (58.2-77.8) | 62.5 (54.4-72.1) | .09 |
| BMI | 28.6 (24.2-33.0) | 28.9 (25.6-34.6) | .20 |
| ICU length of stay (d) | 1.4 (0.8-2.0) | 1.2 (0.8-2.1) | .71 |
| Hospital length of stay (d) | 5.8 (3.5-11.1) | 5.4 (2.8-9.2) | .49 |
| APACHE III score | 34.0 (27.0-47.0) | 39.0 (29.0-49.0) | .21 |
| Sequential Organ Failure Assessment score | 4.0 (2.0-6.0) | 4.0 (2.0-6.0) | .84 |
| Invasive ventilator use (No. [%])c | 19 (19.2) | 21 (22.6) | .56 |
| Duration of invasive ventilator use (d) | 0.9 (0.3-1.6) | 0.9 (0.3-1.3) | .80 |
| Noninvasive ventilator use (No. [%])c | 19 (19.2) | 17 (18.3) | .87 |
| Duration of noninvasive ventilator use (d) | 0.3 (0.2-1.0) | 0.3 (0.2-0.8) | .72 |
| Exposure to sedation (No. [%]) | |||
| Any | 77 (77.0) | 62 (66.7) | .11 |
| Opioid | 68 (68.0) | 55 (59.1) | .20 |
| Benzodiazepine | 55 (55.0) | 53 (57.0) | .78 |
| Propofol | 42 (42.0) | 43 (46.2) | .55 |
| Ketamine | 32 (32.0) | 28 (30.1) | .78 |
| Dexmedetomidine | 10 (10.0) | 11 (11.8) | .68 |
| Rankin disability score (No. [%])c | .46 | ||
| No significant | 62 (62.6) | 50 (54.3) | |
| Slight | 24 (24.2) | 25 (27.2) | |
| Moderate | 9 (9.1) | 12 (13.0) | |
| Moderately severe | 4 (4.0) | 3 (3.3) | |
| Severe | 0 | 2 (2.2) | |
| Depressive symptoms (No. [%]) | 3 (3.0) | 5 (5.4) | .42 |
| Fall risk score | 3.0 (1.0-5.0) | 2.0 (1.0-4.0) | .01 |
| ADL total score, mean ± SD | 0.5±1.2 | 0.3±0.9 | .53 |
| ICU delirium (No. [%])c | 19 (19.2) | 16 (17.2) | .72 |
| Therapy during ICU admission (No. [%]) | |||
| Physical therapy | 37 (37.0) | 26 (28.0) | .18 |
| Occupational therapy | 14 (14.0) | 16 (17.2) | .54 |
| Therapy during hospital admission (No. [%]) | |||
| Physical therapy | 51 (51.0) | 37 (39.8) | .12 |
| Occupational therapy | 18 (18.0) | 23 (24.7) | .25 |
ADL = activities of daily living; APACHE = Acute Physiology, Age, Chronic Health Evaluation; BMI = body mass index; ICU = intensive care unit.
Interventions implemented included (1) educational videos, (2) paper and online education and treatment materials, and (3) online and in-person support groups for education and treatment. Continuous data are presented as medians (25th-75th percentiles) unless specified otherwise.
Data was not available in the data base used.
Table 3.
| Measure | Preintervention phase (n=100) | Postintervention phase (n=93) | P value |
|---|---|---|---|
| Process measures | |||
| Aware of PICS (No. [%]) | 13 (13.0) | 18 (19.4) | .23 |
| Participated in a support group for post-ICU patients (No. [%]) | 3 (3.0) | 2 (2.1) | .71 |
| Participated in PT or OT after hospitalization (No. [%]) | 38 (38.0) | 26 (28.0) | .14 |
| Outcome measuresc | |||
| Readmitted to hospital within 28 d (No. [%]) | 27 (28.4) | 19 (21.6) | .29 |
| Returned to the emergency department within 28 days, No. ([%]) | 30 (32.6) | 22 (25.3) | .28 |
| Back to work (No. [%]) | .83 | ||
| Yes (full-time) | 10 (1.1) | 13 (14.6) | |
| Yes (part-time) | 8 (8.1) | 7 (7.9) | |
| No (unable) | 14 (14.1) | 12 (13.5) | |
| No (not working) | 67 (67.7) | 57 (64.0) | |
| Returned home (No. [%]) | 87 (88.8) | 83 (91.2) | .28 |
| Aware of PICS (No. [%]) | 13 (13.4) | 18 (19.8) | .24 |
| SF-36 physical composite score | 31.1 (24.4-41.3) | 31.1 (23.6-39.5) | .84 |
| SF-36 mental composite score | 51.6 (39.2-57.6) | 5.2 (4.0-56.0) | .60 |
ICU = intensive care unit; OT = occupational therapy; PICS = post–intensive care syndrome; PT = physical therapy; SF-36 = 36-Item Short-Form Health Status Survey.
Interventions implemented included (1) educational videos, (2) paper and online education and treatment materials, and (3) online and in-person support groups for education and treatment. Continuous data are presented as medians (25th-75th percentiles).
Data was not available in the data base used.
Table 4.
| Outcome | Unadjusted |
Adjusted model 1c |
Adjusted model 2d |
|||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | P value | Estimate (95% CI) | P value | Estimate (95% CI) | P value | |
| SF-36 physical composite score | −0.30 (−3.66 to 3.06) | .86 | −0.28 (−3.69 to 3.13) | .87 | −1.23 (−4.60 to 2.13) | .47 |
| SF-36 mental composite score | −0.25 (−3.61 to 3.11) | .88 | −0.09 (−3.40 to 3.21) | .96 | −0.16 (−3.49 to 3.17) | .92 |
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
|---|---|---|---|---|---|---|
| 30-d readmission to hospital | 1.05 (0.52 to 2.11) | .89 | 1.08 (0.53 to 2.21) | .84 | 1.05 (0.51 to 2.19) | .89 |
| 30-d emergency department evaluation visit | 1.28 (0.58 to 2.80) | .54 | 1.24 (0.55 to 2.80) | .60 | 1.20 (0.53 to 2.74) | .66 |
SF-36 = 36-Item Short-Form Health Status Survey.
Regression outcomes comparing the preintervention and postintervention groups. Interventions implemented included (1) educational videos, (2) paper and online education and treatment materials, and (3) online and in-person support groups for education and treatment.
Model 1 adjusted for sex, age, body mass index (BMI), and Acute Physiology, Age, Chronic Health Evaluation (APACHE) III score.
Model 2 adjusted for sex, age, BMI, APACHE III score, invasive ventilator use, noninvasive ventilator use, and sedation.
Overall, the 30-day readmission rate was 20.8%, and 15.6% of patients had an ED visit within 30 days of discharge. After adjusting for potential confounders, there was no evidence of an association between intervention group and 30-day readmission rate (odds ratio = 1.05; 95% CI = 0.51 to 2.19; P=.89) or 30-day ED evaluation rate (odds ratio = 1.20; 95% CI = 0.53 to 2.74; P=.66).
Discussion
We found that lack of awareness and understanding of PICS were the main barriers to improving QOL for patients at risk for PICS. To overcome these barriers, interventions focused on improving PICS education and treatment resources. With this, we showed a subjective improvement in QOL. We could not identify an objective improvement in QOL. The inability to quantify improved QOL was likely in part due to the inability to target patients at increased risk for PICS and the early implementation of interventions while patients were still critically ill.
Currently there is no formal way to predict PICS, limiting the ability to specifically identify patients at increased risk for PICS. Previous studies have overcome these limitations by including all patients, as we did in the quantitative analysis.11, 25, 35, 49 However, by including all patients we included those at lower risk for PICS. This limited our ability to determine whether there was a difference in QOL specifically in patients at higher risk for PICS. As an example, in the quantitative analysis, the median ICU stay was less than 2 days and the duration of mechanical ventilation was less than 1 day, which may be associated with a lower risk of PICS than a longer ICU stay or a longer duration of mechanical ventilation. This is in contrast to the qualitative analysis that included patients who self-selected to participate, where most also endorsed symptoms of PICS. The qualitative analysis demonstrated an improvement in QOL, perhaps due to a higher proportion of patients with PICS and, therefore, a higher-risk group. Therefore, the inability to quantify the impact of PICS-specific interventions may, in part, be due to the inability to specifically target patients at higher risk for PICS.
PICS encompasses a spectrum of symptoms, with a range of severity, over an inconstant amount of time to recovery. The impact of this variability was demonstrated when comparing the cognitive impairment in patients who participated in interventions while still in the ICU, who were early into their recovery from critical illness, with the patients who participated later in recovery from critical illness. The quantitative analysis intervention was initiated while patients were in the ICU, where most patients could not recall participating in the interventions or PICS ever being mentioned. Although the patients in the qualitative analysis were also unable to recall many events that occurred in the ICU, they were able to recall participating in the PICS-specific interventions. Note that most patients in the qualitative analysis were weeks, months, and even years into recovery from critical illness. Suggesting that it was not the cognitive impairment experienced in the ICU that limited the interventions but rather the cognitive impairment at the time of intervention participation that limited the patient’s ability to understand and retain information. This, in turn, limited the ability of participation in the intervention to affect QOL. Therefore, interventions in the ICU, when patients are still early in recovery from critical illness, may have limited ability to affect QOL in patients with PICS. Perhaps interventions focused later in recovery from critical illness will have the most impact, as the support group members in this study noted.11, 12, 13, 14, 15, 25, 26, 27
We were unable to track which patients participated in each intervention; therefore, we were unable to determine the effect of individual interventions. Furthermore, patients outside the health system who participated in the interventions and qualitative analysis were unable to be included in the quantitative analysis, again limiting the ability to determine the quantitative impact of the interventions.
We used the SF-36, which does not directly assess cognitive impairment, and cognitive impairment may interfere with the ability of the SF-36 to measure QOL.43 Also note that the nonresponders had an increased rate of delirium, perhaps suggesting higher risk of cognitive impairment. Likewise, critical illness may affect the ability to assess QOL as measured by the SF-36.50 In addition, this study was performed in a health care system that already had preexisting online platforms that we were able to use for this group of interventions, limiting the ability for other systems to implement similar interventions without using additional resources.
Last, although previous studies have reported a low return of results of 67.1%, the present return of surveys was notably lower at 35.7%.17, 29 Part of this discrepancy may be due to study design because we did not directly contact patients while in the hospital to discuss consent. This may have limited the ability to exclude patients not participating, noting that previous studies have consented patients while still hospitalized and have found declined participation in up to 30%, whereas we found that only 10.2% declined.51 In addition, the present surveys were mailed and required return by mail, not given as part of in-person follow-up, as in previous studies with higher response rates.17, 25, 29
Conclusion
Patients who survive critical illness continue to be at risk for PICS and an associated decreased QOL. While the intervention was able to provide PICS education and treatment, the early implementation of the intervention limited the ability to specifically target patients at higher risk for PICS. More research is needed to best target interventions and determine the appropriate timing of interventions as the population of patients surviving critical illness continues to grow.
Footnotes
Dr Daniels is now with the Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, Department of Medicine, Emory University School of Medicine, Atlanta, GA.
Grant Support: Survey management and statistical analysis was supported in part by the Critical Care Independent Multidisciplinary Program at Mayo Clinic in Rochester and by a grant from Thrive Peer Support Collaborative of the Society of Critical Care Medicine (A.B.J.).
Potential Competing Interests: The authors report no competing interests.
Supplemental Online Material
Supplemental material can be found online at http://mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
References
- 1.Kaukonen K.M., Bailey M., Suzuki S., et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311(13):1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 2.Wunsch H., Angus D.C., Harrison D.A., et al. Variation in critical care services across North America and Western Europe. Crit Care Med. 2008;36(10):2787–2793. doi: 10.1097/CCM.0b013e318186aec8. e1-e9. [DOI] [PubMed] [Google Scholar]
- 3.Vincent J.L., Marshall J.C., Namendys-Silva S.A., et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2(5):380–386. doi: 10.1016/S2213-2600(14)70061-X. [DOI] [PubMed] [Google Scholar]
- 4.Adhikari N.K., Fowler R.A., Bhagwanjee S., et al. Critical care and the global burden of critical illness in adults. Lancet. 2010;376(9749):1339–1346. doi: 10.1016/S0140-6736(10)60446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Critical care statistics: critical care patients. http://www.sccm.org/Communications/Critical-Care-Statistics Society of Critical Care Medicine website.
- 6.Needham D.M., Davidson J., Cohen H., et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40(2):502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 7.Maley J.H., Brewster I., Mayoral I., et al. Resilience in survivors of critical illness in the context of the survivors' experience and recovery. Ann Am Thorac Soc. 2016;13(8):1351–1360. doi: 10.1513/AnnalsATS.201511-782OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffiths J., Hatch R.A., Bishop J., et al. An exploration of social and economic outcome and associated health-related quality of life after critical illness in general intensive care unit survivors: a 12-month follow-up study. Crit Care. 2013;17(3):R100. doi: 10.1186/cc12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oeyen S.G., Vandijck D.M., Benoit D.D., et al. Quality of life after intensive care: a systematic review of the literature. Crit Care Med. 2010;38(12):2386–2400. doi: 10.1097/CCM.0b013e3181f3dec5. [DOI] [PubMed] [Google Scholar]
- 10.Marra A., Pandharipande P.P., Girard T.D., et al. Co-occurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Crit Care Med. 2018;46(9):1393–1401. doi: 10.1097/CCM.0000000000003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schweickert W.D., Pohlman M.C., Pohlman A.S., et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris P.E., Goad A., Thompson C., et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–2243. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 13.Mehta S., Burry L., Cook D., et al. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: a randomized controlled trial. JAMA. 2012;308(19):1985–1992. doi: 10.1001/jama.2012.13872. [DOI] [PubMed] [Google Scholar]
- 14.Kayambu G., Boots R., Paratz J. Early physical rehabilitation in intensive care patients with sepsis syndromes: a pilot randomised controlled trial. Intensive Care Med. 2015;41(5):865–874. doi: 10.1007/s00134-015-3763-8. [DOI] [PubMed] [Google Scholar]
- 15.Tipping C.J., Harrold M., Holland A., et al. The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med. 2017;43(2):171–183. doi: 10.1007/s00134-016-4612-0. [DOI] [PubMed] [Google Scholar]
- 16.Tan T., Brett S.J., Stokes T., et al. Rehabilitation after critical illness: summary of NICE guidance. BMJ. 2009;338:b822. doi: 10.1136/bmj.b822. [DOI] [PubMed] [Google Scholar]
- 17.Cuthbertson B.H., Rattray J., Campbell M.K., et al. The PRaCTICaL study of nurse led, intensive care follow-up programmes for improving long term outcomes from critical illness: a pragmatic randomised controlled trial. BMJ. 2009;339:b3723. doi: 10.1136/bmj.b3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones C., Griffiths R.D. Patient and caregiver counselling after the intensive care unit: what are the needs and how should they be met? Curr Opin Crit Care. 2007;13(5):503–507. doi: 10.1097/MCC.0b013e3282efb83e. [DOI] [PubMed] [Google Scholar]
- 19.Modrykamien A.M. The ICU follow-up clinic: a new paradigm for intensivists. Respir Care. 2012;57(5):764–772. doi: 10.4187/respcare.01461. [DOI] [PubMed] [Google Scholar]
- 20.Crocker C. A multidisciplinary follow-up clinic after patients' discharge from ITU. Br J Nurs. 2003;12(15):910–914. doi: 10.12968/bjon.2003.12.15.11420. [DOI] [PubMed] [Google Scholar]
- 21.Peskett M., Gibb P. Developing and setting up a patient and relatives intensive care support group. Nurs Crit Care. 2009;14(1):4–10. doi: 10.1111/j.1478-5153.2008.00302.x. [DOI] [PubMed] [Google Scholar]
- 22.Griffiths J.A., Barber V.S., Cuthbertson B.H., et al. A national survey of intensive care follow-up clinics. Anaesthesia. 2006;61(10):950–955. doi: 10.1111/j.1365-2044.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- 23.Egerod I., Risom S.S., Thomsen T., et al. ICU-recovery in Scandinavia: a comparative study of intensive care follow-up in Denmark, Norway and Sweden. Intensive Crit Care Nurs. 2013;29(2):103–111. doi: 10.1016/j.iccn.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Brummel N.E., Girard T.D., Ely E.W., et al. Feasibility and safety of early combined cognitive and physical therapy for critically ill medical and surgical patients: the Activity and Cognitive Therapy in ICU (ACT-ICU) trial. Intensive Care Med. 2014;40(3):370–379. doi: 10.1007/s00134-013-3136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones C., Backman C., Capuzzo M., et al. Intensive care diaries reduce new onset post traumatic stress disorder following critical illness: a randomised, controlled trial. Crit Care. 2010;14(5):R168. doi: 10.1186/cc9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garrouste-Orgeas M., Coquet I., Perier A., et al. Impact of an intensive care unit diary on psychological distress in patients and relatives*. Crit Care Med. 2012;40(7):2033–2040. doi: 10.1097/CCM.0b013e31824e1b43. [DOI] [PubMed] [Google Scholar]
- 27.Mehlhorn J., Freytag A., Schmidt K., et al. Rehabilitation interventions for postintensive care syndrome: a systematic review. Crit Care Med. 2014;42(5):1263–1271. doi: 10.1097/CCM.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 28.Jensen J.F., Thomsen T., Overgaard D., et al. Impact of follow-up consultations for ICU survivors on post-ICU syndrome: a systematic review and meta-analysis. Intensive Care Med. 2015;41(5):763–775. doi: 10.1007/s00134-015-3689-1. [DOI] [PubMed] [Google Scholar]
- 29.Douglas S.L., Daly B.J., Kelley C.G., et al. Chronically critically ill patients: health-related quality of life and resource use after a disease management intervention. Am J Crit Care. 2007;16(5):447–457. [PMC free article] [PubMed] [Google Scholar]
- 30.Norman B.C., Jackson J.C., Graves J.A., et al. Employment outcomes after critical illness: an analysis of the bringing to light the risk factors and incidence of neuropsychological dysfunction in ICU survivors cohort. Crit Care Med. 2016;44(11):2003–2009. doi: 10.1097/CCM.0000000000001849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prescott H.C., Langa K.M., Iwashyna T.J. Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA. 2015;313(10):1055–1057. doi: 10.1001/jama.2015.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brinkman S., de Jonge E., Abu-Hanna A., et al. Mortality after hospital discharge in ICU patients. Crit Care Med. 2013;41(5):1229–1236. doi: 10.1097/CCM.0b013e31827ca4e1. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen J., Graff C., Kanters J.K., et al. Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs. 2011;25(6):473–490. doi: 10.2165/11587800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.George M.L., Rowlands D., Price M., Maxey J. McGraw-Hill; New York, NY: 2005. The Lean Six Sigma Pocket Toolbook: A Quick Reference Guide to nearly 100 Tools for Improving Quality, Speed, and Complexity. [Google Scholar]
- 35.Garrouste-Orgeas M., Perier A., Mouricou P., et al. Writing in and reading ICU diaries: qualitative study of families' experience in the ICU. PLoS One. 2014;9(10):e110146. doi: 10.1371/journal.pone.0110146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prinjha S., Field K., Rowan K. What patients think about ICU follow-up services: a qualitative study. Crit Care. 2009;13(2):R46. doi: 10.1186/cc7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayo Clinic Connect Post intensive care syndrome (PICS) https://connect.mayoclinic.org/page/pics/# Mayo Clinic website.
- 38.Aitken L.M., Marshall A.P. Monitoring and optimising outcomes of survivors of critical illness. Intensive Crit Care Nurs. 2015;31(1):1–9. doi: 10.1016/j.iccn.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Herasevich V., Pickering B.W., Dong Y., et al. Informatics infrastructure for syndrome surveillance, decision support, reporting, and modeling of critical illness. Mayo Clin Proc. 2010;85(3):247–254. doi: 10.4065/mcp.2009.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knaus W.A., Wagner D.P., Draper E.A., et al. The APACHE III prognostic system: risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 41.Vincent J.L., Moreno R., Takala J., et al. on behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 42.Jackson J.C., Pandharipande P.P., Girard T.D., et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369–379. doi: 10.1016/S2213-2600(14)70051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pandharipande P.P., Girard T.D., Ely E.W. Long-term cognitive impairment after critical illness. N Engl J Med. 2014;370(2):185–186. doi: 10.1056/NEJMc1313886. [DOI] [PubMed] [Google Scholar]
- 44.Wieske L., Dettling-Ihnenfeldt D.S., Verhamme C., et al. Impact of ICU-acquired weakness on post-ICU physical functioning: a follow-up study. Crit Care. 2015;19:196. doi: 10.1186/s13054-015-0937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holmes A., Hodgins G., Adey S., et al. Trial of interpersonal counselling after major physical trauma. Aust N Z J Psychiatry. 2007;41(11):926–933. doi: 10.1080/00048670701634945. [DOI] [PubMed] [Google Scholar]
- 46.Pattison N., Dolan S. Exploring patients' experiences of a nurse-led follow-up service after critical care. Nurs Times. 2009;105(19):16–19. [PubMed] [Google Scholar]
- 47.Glimelius Petersson C., Bergbom I., Brodersen K., et al. Patients' participation in and evaluation of a follow-up program following intensive care. Acta Anaesthesiol Scand. 2011;55(7):827–834. doi: 10.1111/j.1399-6576.2011.02474.x. [DOI] [PubMed] [Google Scholar]
- 48.Mayo Clinic Connect Post-intensive care syndrome (PICS) - let's talk. https://connect.mayoclinic.org/discussion/post-intensive-care-syndrome-pics-lets-talk/?pg=2#post-256257 Mayo Clinic website.
- 49.Balas M.C., Vasilevskis E.E., Olsen K.M., et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Crit Care Med. 2014;42(5):1024–1036. doi: 10.1097/CCM.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ely E.W., Inouye S.K., Bernard G.R., et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 51.Knowles R.E., Tarrier N. Evaluation of the effect of prospective patient diaries on emotional well-being in intensive care unit survivors: a randomized controlled trial. Crit Care Med. 2009;37(1):184–191. doi: 10.1097/CCM.0b013e31819287f7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.