Prevention of any disease can occur at two levels: (i) avoiding or reducing risk factors coupled with increases in protective factors (primary prevention, which is preferable when it can be practiced) and (ii) detection and intervention early in the course of disease evolution (secondary prevention). But despite substantial epidemiologic data showing that a large proportion of cancers and cancer deaths are preventable, decreases in cancer mortality rates in developed countries have lagged far behind decreases in mortality rates from heart disease,(1) another major disease amenable to prevention (for example, 18 versus 68% decrease, respectively, between 1969 and 2013 in the United States).(2) We believe that one of the main factors explaining the relatively modest reduction in cancer mortality is the limited support for cancer prevention research, which receives only 2 to 9% of global cancer research funding.(3) As a United Nations (UN) High-Level Meeting begins this week to review efforts to combat noncommunicable diseases, a key question is how to prioritize resources to realize the potential of cancer prevention.
LATE VS EARLY CANCERS
The great majority of cancer research is focused on curing late cancers that have already spread throughout the body by the time they are detected. The reasons for this heavily skewed focus are manifold. First, societies, in general, are reactive rather than proactive. Second, the final stages of treatment research (and regulatory approval) can be simpler to perform than prevention research (requiring just hundreds of patients versus tens of thousands of patients). Third, it is much more dramatic to effectively treat a patient with advanced disease than to prevent disease. Thus many patients who are effectively treated donate large sums to cancer centers; there are few thanks given for preventing cancers. Fourth, there are few financial incentives for industry to support primary prevention measures based on avoidance of risk factors. And finally, the financial incentives to develop new therapeutics are far more lucrative than those for new diagnostic tests for early detection and prevention.
Recent research has illuminated why it is so difficult to cure advanced cancers. Even the best new targeted therapies can generally only induce transient responses because hundreds to thousands of cells that are resistant to such therapies already exist within any advanced cancer.(4) These preexisting resistant cells will eventually emerge, causing relapse. On the other hand, recent research has solidified the view that many cancers are entirely preventable through changes in environment or lifestyle. A cancer that is prevented is “cured,” not simply driven into a transient remission. Moreover, primary prevention eliminates the considerable morbidity associated with surgery and adjuvant therapy.
However, not all cancers are preventable by changes in environment or lifestyle. Recent research (5, 6) has shown that mutations due to random mistakes during normal DNA replication (R) play a major role in cancer etiology, along with environment and lifestyle (collectively denoted E) and heredity (H). R can also explain the extreme variation in cancer incidence across different tissues. R, calculated from the lifetime number of cell divisions in a tissue, is correlated with the lifetime risk of cancer in that tissue, indicating a role for R, independent of E, worldwide.
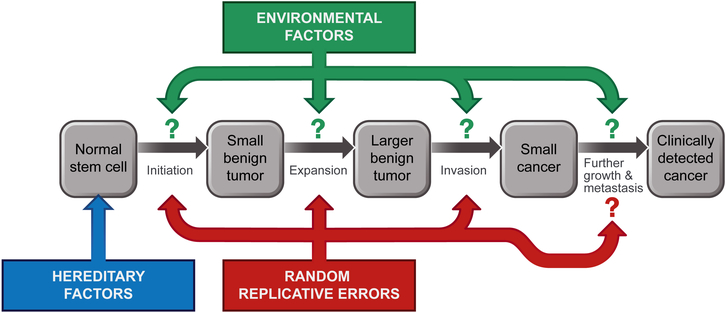
This EHR model (see the figure) highlights the connection between epidemiologic and molecular perspectives and informs cancer research and prevention strategies in two ways. First, explicit quantification of different types of mutations in cancer reinforces the importance of prevention: Cancers can still be preventable as long as one of the mutations driving toward cancer is caused by E. Second, the model highlights the heterogeneity of cancer arising in different tissues. For cancers in which most of their driver mutations are caused by E—such as lung cancers, melanomas, and cervical cancers—about 85 to 100% of incident cases could be eliminated through smoking cessation, avoidance of ultraviolet radiation exposures, and vaccination against human papillomavirus, respectively. For other cancer types that have a large proportion of R mutations—such as those of the pancreas, breast, and prostate—less than half of incident cases can be attributed to known environmental risk factors.(7, 8) Variations in cancer rates among countries and studies of migrants indicate the existence of additional environmental factors that contribute to more of these cancers than are currently known. However, mutations that occur naturally regardless of the external environment undoubtedly play a role.(6) Fortunately, many of these cancers will still be amenable to secondary prevention, with morbidity and mortality from the disease minimized.
Fig 1. Connecting epidemiologic and genetic perspectives on cancer.
Solid tumors of adults evolve through a stepwise accumulation of three or more genetic alterations, but these are not the only determinants of cancer incidence. Hereditary factors can predispose individuals to cancer, generally via an initiating mutation to all stem cells. Random, replicative errors every time a cell divides can contribute to cancers at the indicated phases of tumorigenesis. Environmental factors can play a role during all phases.
CONNECTIONS AND MECHANISMS
More research is needed to strengthen the connection between epidemiology and molecular biology. It will be important to identify mechanisms through which diet, exercise, and other lifestyle factors that are unambiguously associated with cancer lead to the disease.(9) Can such factors be associated with genetic or epigenetic signatures that are often found in cancers, tying sequencing and epidemiology together? At present, most molecular signatures identified in genome-wide sequencing studies of cancer cannot be attributed to any environmental factor.
A specific example might put these challenges in perspective. Obesity is appreciated to be a major risk factor for cancer. Although the mechanisms through which obesity increases cancer risk remain to be fully understood, several explanations can be offered.(10) First, obesity may increase the number of mutations by increasing the concentration of endogenous mutagenic substances, such as reactive oxygen species. Second, obesity could increase the number of mutations in a tissue by increasing cell divisions in that tissue through hormones, growth factors, metabolites, or other chemicals produced by adipocytes and other tissues (for example, estrogens, insulin, adipokines, and prostaglandins). Because cell division is intrinsically mutagenic, each time a cell divides, three to five new mutations occur. Moreover, increased proliferation itself, in a tumor that already harbors mutations, could determine whether a tumor becomes clinically evident and thus enumerated in epidemiologic studies. Third, obesity may promote cancer by influencing gene expression through epigenetic mechanisms that can be reversed by weight loss. Finally, adipose tissue is a source of mesenchymal stem cells, which may increase the pool of cells at risk through their recruitment to support tumor growth and progression. These potential pathways warrant further molecular and epidemiologic research. By incorporating the role played by R, the HER model highlights the potential for new chemopreventive strategies, including those that reduce epigenetic changes or reduce errors made during cellular DNA replication.(6)
Regardless of the precise mechanisms, substantial epidemiologic data show that at least 35% of all cancer deaths in the world could be avoided by modifying known risk factors,(8) that is, through primary prevention. Preventability estimates will hopefully increase with further research, because additional preventable causes are likely to be discovered. For example, early-life exposures may play a critical role in cancer initiation, but epidemiologic studies have mostly focused on midlife risk factors.(11) Moreover, the potential of chemopreventive strategies is generally not considered in the preventability estimate, partly because of uncertainty in the risk-benefit profile. For example, there is convincing evidence that regular use of aspirin can prevent colorectal cancer,(12) and that tamoxifen and raloxifene lower the risk of estrogen receptor-positive breast cancer.(13)
CHANGING RESEARCH PRIORITIES
The World Health Organization Global Action Plan for the Prevention and Control of Noncommunicable Diseases calls for a 25% reduction in cancer mortality rates by 2025, and the UN Sustainable Development Goals program calls for a 33% reduction by 2030. Achieving these goals requires not only more research but also strong government commitments to sustainable investments in primary cancer prevention strategies through legislative, regulatory, financial, and educational approaches. As highlighted by the U.S. National Cancer Institute’s Cancer Moonshot Blue Ribbon Panel, there is an urgent need for more behavioral and policy research on overcoming barriers to adoption of effective evidence-based interventions.
Concomitantly, advances in genetics and other scientific areas promise new vistas in secondary prevention. Many patients have early, potentially curable cancers, or advanced precursor lesions, that can be detected through mutations or other tumorspecific markers present in their circulation or other bodily fluids and removed early to prevent further progression.(14) Large-scale, prospective studies to determine the ability of such biomarkers to save lives will be required to gain confidence in such tests. But one can imagine a day when heathy individuals are routinely tested for these biomarkers to detect early cancers, along with lipid concentrations to detect early cardiac disease, at periodic visits to their physicians. Similar advances in physics (for example, in imaging) could provide orthogonal tests to detect early cancers. Interventions based on improvements in minimally invasive surgical procedures, accomplished through endoscopic means, are equally important initiatives.
Of course, primary and secondary prevention strategies should be balanced and prioritized on the basis of country-specific cancer demographics, risk factor profile, and resource availability. As in developed countries, primary prevention is preferable in developing countries, but secondary prevention with simple, cost-effective tests—such as Pap smears followed by surgical excisions for prevention of cervical cancer—is feasible in the developing world. Moreover, in developing countries, the costs of expensive new drugs that treat advanced disease are not feasible.(15) Optimally, prevention efforts will also confer co-benefits for other chronic diseases with shared risk factors (for example, type 2 diabetes, depression, and dementia).
We believe that both epidemiologic and molecular research support a congruent public olicy recommendation for consideration by the UN: A much greater emphasis on cancer prevention research is needed to avoid, detect, and treat early cancers and premalignant lesions. With such further research, we envision that cancer death rates could be reduced by 70% around the world, even without the development of any new therapies.(7) Such a reduction, similar to that for heart disease over the past six decades, will only come about if research priorities are changed.
Acknowledgments:
This work is supported by the National Institutes of Health (K99 CA215314 and R00 CA215314 to M.S.; UM1 CA167552 and UM1 CA176726 to W.C.W.; and P30 CA006973 to C.T.), the American Cancer Society (MRSG-17–220-01 – NEC to M.S.), The Lustgarten Foundation for Cancer Research (B.V.), The Virginia and D.K. Lud-wig Fund for Cancer Research (B.V.), The Marcus Foundation (B.V. and C.T.), The John Templeton Foundation (C.T.), and The Maryland Cigarette Restitution Fund (C.T.).
Footnotes
Competing interests: PapGene, Inc. has li-censed technologies from Johns Hopkins University, and B.V, and C.T. receive equity or royalties from these licenses. The terms of these arrangements are being managed by Johns Hopkins University in accordance with its conflict of interest policies. B.V. is founder of Personal Genome Diagnostics, Inc. and PapGene, Inc. and is on the Scien-tific Advisory Board of Sysmex-Inostics.
References
- 1.Mortality GBD, C. Causes of Death, Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1459–1544 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma J, Ward EM, Siegel RL, Jemal A, Temporal Trends in Mortality in the United States, 1969–2013. JAMA 314, 1731–1739 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Eckhouse S, Lewison G, Sullivan R, Trends in the global funding and activity of cancer research. Mol Oncol 2, 20–32 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz LA Jr. et al. , The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 486, 537–540 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomasetti C, Vogelstein B, Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 347, 78–81 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomasetti C, Li L, Vogelstein B, Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science 355, 1330–1334 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Islami F et al. , Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA. Cancer J. Clin, (2017). [DOI] [PubMed] [Google Scholar]
- 8.Danaei G et al. , Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet 366, 1784–1793 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Golemis EA et al. , Molecular mechanisms of the preventable causes of cancer in the United States. Genes Dev 32, 868–902 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy N, Jenab M, Gunter MJ, Adiposity and gastrointestinal cancers: epidemiology, mechanisms and future directions. Nat Rev Gastroenterol Hepatol, (2018). [DOI] [PubMed] [Google Scholar]
- 11.Clarke MA, Joshu CE, Early Life Exposures and Adult Cancer Risk. Epidemiol Rev 39, 11–27 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bibbins-Domingo K, U. S. P. S. T. Force, Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 164, 836–845 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Cuzick J et al. , Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet 381, 1827–1834 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siravegna G, Marsoni S, Siena S, Bardelli A, Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 14, 531–548 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Kantarjian H, Steensma D, Rius Sanjuan J, Elshaug A, Light D, High cancer drug prices in the United States: reasons and proposed solutions. J Oncol Pract 10, e208–211 (2014). [DOI] [PubMed] [Google Scholar]