Abstract
BACKGROUND:
Marital distress and depression work in tandem to escalate risks for inflammation-related disorders. Translocation of bacterial endotoxin (lipopolysaccharide, LPS) from the gut microbiota to blood circulation stimulates systemic inflammatory responses.
METHODS:
To investigate increased gut permeability (a”leakygut”) as one potential mechanistic pathway from marital distress and depression to heightened inflammation, this secondary analysis of a double-blind, randomized crossover study examined serial assessments of two endotoxin biomarkers, LPS-binding protein (LBP) and soluble CD14 (sCD14), as well as C-reactive protein ( RP), interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α) during two separate 9.5 hour visits. The 43 (N=86) healthy married couples, ages 24 to 61 (mean=38.22), discussed a marital disagreement during both visits; behavioral coding of these interactions provided data on hostile marital behaviors, a hallmark of marital distress. The Structured Diagnostic Interview for DSM-IV assessed participants’ mood disorder history.
RESULTS:
Participants with more hostile marital interactions had higher LBP than those who were less hostile. Additionally, the combination of more hostile marital interactions with a mood disorder history was associated with higher LBP/sCD14 ratios. Higher LBP and LBP/sCD14 were associated with greater CRP production; for example, only 21% of low LBP participants (lowest quartile) had average CRP across the day > 3, compared to 79% of those in the highest quartile. Higher sCD14 was associated with higher IL-6.
CONCLUSIONS:
These bacterial LPS translocation data illustrate how a distressed marriage and a mood disorder history can promote a proinflammatory milieu through increased gut permeability, thus fueling inflammation-related disorders.
Keywords: Marriage, Depression, Inflammation, Microbiome, leaky gut, intestinal permeability
1. INTRODUCTION
Unhappy marriages take a toll on mental and physical health. For example, marital stress worsened the prognosis for recurrent coronary events three-fold (Orth-Gomer et al., 2000). Among patients with congestive heart failure, marital quality was as strong a predictor of four-year survival as well as patients’ illness severity (Coyne et al., 2001). Ameta-analysis reported that the relationships between marital quality and clinical health endpoints had statistical effect sizes similar in magnitude to the health effects of diet and exercise (Robles et al., 2014).
In work from our lab, proinflammatory cytokine production increased following marital disagreements characterized by high rates of negative or punishing behaviors (e.g., hostility, sarcasm, withdrawal/disengagement), the hallmarks of marital distress (Kiecolt-Glaser et al., 2005); other studies have linked troubled marriages with chronically heightened inflammation (Shen et al., 2010; Whisman and Sbarra, 2012; Donoho et al., 2013; Kiecolt-Glaser et al., 2015c). arital discord’s notable consequences include an amplified risk for inflammation-related diseases and disorders including depression, cardiovascular disease, metabolic syndrome, diabetes, and slower wound healing (Orth-Gomer et al., 2000; Gallo et al., 2003; Kiecolt-Glaser et al., 2005; Troxel et al., 2005; Beach, 2014; Joseph et al., 2014; Whisman et al., 2014). The gut microbiota can fuel inflammation (Rogers et al., 2016), providing a potential mechanistic pathway linking marital distress to inflammation and inflammation-related diseases.
Translocation of bacterial endotoxin (lipopolysaccharide, LPS) from the gut microbiota to blood circulation – the result of a “leaky gut”-- stimulates systemic inflammatory responses (Kelly et al., 2012; Stehle et al., 2012). Hepatocytes and intestinal epithelial cells can be induced to release LBP through LPS stimulation, as well as by stimulation with inflammatory cytokines (e.g., IL-6, IL-1β, TNF-α, and IL-22) (Wan et al., 1995; Wolk et al., 2007). LPS-binding protein (LBP) and soluble CD14 (sCD14) are produced in response to bacterial translocation of endotoxin (Amar et al., 2003; Stehle et al., 2012). LBP binds LPS and presents LPS to CD14, the receptor for LPS-LBP complexes (Wright et al., 1990; Ulevitch and Tobias, 1995; Stehle et al., 2012). The endotoxin receptor sCD14 facilitates proinflammatory signaling following endotoxin exposure (Wright et al., 1990). CD14 presents LPS to Toll-like receptor (TLR)-4, a process that leads to NF-κB activation and proinflammatory cytokine production. The relative balance of LBP and sCD14 is also important; higher LBP/sCD14 ratios promote heightened inflammation (Laugerette et al., 2012; Laugerette et al., 2014).
Rodent models have shown that stress-induced changes in the gut microbiota can provoke bacterial translocation (Bailey et al., 2011; Ait-Belgnaoui et al., 2012), and that intestinal bacteria contribute to stress-induced immunopotentiation (Bailey et al., 2011; Maslanik et al., 2012). Although human data are sparse, one study demonstrated that both the prevalence and median values of serum antibodies against the LPS of six enterobacteria were greater in depressed patients than controls (Maes et al., 2008). In another study, major depressive disorder (MDD) patients had elevated expression of bacterial DNA, indicative of bacterial translocation, compared to nondepressed controls, and the magnitude was correlated with depressive symptom severity (Keri et al., 2014). These depression-related findings are relevant to the current study: unhappy marriages are a potent risk factor for depression (Beach, 2014).
The gut microbiota can impact energy balance, glucose metabolism, and obesity-related inflammation, in part through gut leakiness (Newsholme and Homem de Bittencourt, 2016). Recent work from our lab has shown that stress and a mood disorder history alter metabolic responses to high-fat meals (Kiecolt-Glaser et al., 2015b; Kiecolt-Glaser et al., 2015c; Kiecolt-Glaser et al., 2017). In a double-blind, randomized crossover study, couples ate a high-fat meal and then discussed a marital disagreement during each of two visits (Kiecolt-Glaser et al., 2015c). When combined with a mood disorder history, men and women who had more hostile marital interactions had lower post-meal energy expenditure: 128 kcal, a difference that could add ~7.7 pounds/year. Furthermore, higher levels of hostile behaviors among those who had a mood disorder history were also associated with higher post-meal insulin compared with other participants. Higher insulin levels stimulate food intake and visceral fat accumulation (Dallman, 2010), and thus would act in tandem with lower energy expenditure to promote obesity.
In this secondary analysis of the same couples, we hypothesized that higher levels of hostile behavior and a mood disorder history would be associated with higher LBP, sCD14, and a higher LBP/sCD14 ratio. We also expected that higher LBP, sCD14, and a higher LBP/sCD14 ratio would be associated with greater systemic inflammation: C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α). Our endotoxin biomarkers have relatively slow response times (Hudgins et al., 2003), and thus we did not expect them to acutely change in response to the marital conflict or the meals.
2. METHODS AND TERIALS
2.1. Design and overview
This double-blind, randomized crossover study assessed metabolic responses following high-fat meals; detailed methods have been described previously (Kiecolt-Glaser et al., 2015c). Couples completed an online screening questionnaire and an in-person screening visit. During two separate full-day visits to a hospital research unit, couples received either a high saturated fat meal or a high oleic sunflower oil meal (order randomized) after fasting for 12 hours.
25-minute baseline followed catheter insertion, and then couples ate their meals. The marital problem discussion was introduced two hours post-meal. Couples remained on the unit for ~7 hours after meal completion without further food, only water. Serum LBP, sCD14, CRP, IL-6 and TNF-α were assessed following the resting baseline, and then every 2 hours post-meal. Body composition was assessed by dual x-ray absorptiometry (DXA).
Visits occurred 1–25 weeks apart (mean=4.45, SD=4.76). Although 55% of visits occurred within 3 weeks, some were more widely spaced due to participants’ work schedules.
2.2. Participants
Using print and web-based announcements, we recruited 43 healthy couples (N=86), ages 24–61, who had been married at least 3 years. Individuals were ineligible if they or their partner had any notable chronic health problems, including gut-related disorders such as ulcerative colitis, Crohn’s disease, and celiac disease. Other exclusions included smoking, alcohol/drug abuse, diabetes, anemia, and any prescription medications except birth control pills (N=5) and levothyroxine (N=3). We prioritized recruitment of heavier sedentary individuals to maximize the likelihood of stress-related metabolic responses, and thus our inclusion criteria specified a maximum of 2 hours of vigorous activity per week for BMI < 25 and 5 hours per week for BMI > 25. Table 1 lists additional sample characteristics. The institutional review board approved this study, and each participant provided written informed consent before participation.
Table 1.
Participant Characteristics
| Men (N= 43) | Women (n=43) | Overall Sample (n=86) |
|
|---|---|---|---|
| Age, years | 39.25 (9.17) | 37.19 (7.00) | 38.22 (8.18) |
| BMI, kg/m2 | 31.96 (5.06) | 32.17 (6.58) | 32.07 (5.83) |
| Waist, cm | 106.71 (14.72) | 99.14 (13.63) | 102.93 (14.61) |
| Trunk fat, g | 19502.14 (7761.57) |
19375.02 (7382.91) |
19438.58 (7530.19) |
| Activity, hours per week | 3.52 (5.09) | 1.86 (2.00) | 2.70 (3.95) |
| Systolic blood pressure, mmHg | 127.12 (12.18) | 111.67 (12.30) | 119.40 (14.44) |
| Diastolic blood pressure, mmHg | 76.00 (7.21) | 67.72 (8.22) | 71.85 (8.74) |
| Years married | 11.49 (6.64) | ||
| Race | |||
| White | 35 (81%) | 35 (81%) | 70 (81%) |
| Black | 8 (19%) | 8 (19%) | 16 (19%) |
| Education | |||
| Graduate degree | 17 (40%) | 20 (47%) | 37 (43%) |
| College graduate | 13 (30%) | 8 (19%) | 21 (24%) |
| Partial college | 6 (14%) | 10 (23%) | 16 (19%) |
| High school graduate | 5 (12%) | 5 (12%) | 10 (12%) |
| ≤ 11 years high school | 2 (5%) | 0 (0%) | 2 (2%) |
| Hostile behavior score* | 22.7 (29.1) | ||
| Couples’ Satisfaction Index** | 124.2 (31.6) |
2.3. Standardized pre-study meals
On the day before each study visit, couples received three standardized meals to reduce the variability associated with recent food intake, as previously described (Kiecolt-Glaser et al., 2015c). Participants’ last meals, eaten no later than 7:30 PM the night before admission, were light and low in fat.
2.4. Research meals
Both research meals during the visit included 930 kcals, with 60 grams fat, 59 grams carbohydrate, and 36 grams protein (percent of total kcals = 60, 25, 15, respectively). However, to address the parent study’s metabolic questions (Kiecolt-Glaser et al., 2015c), the high saturated fat meal contained 16.84 g palmitic and 13.5 g oleic, compared to 8.64 g palmitic and 31.21 g oleic for the high oleic sunflower oil meal. Compliance was good: participants consumed 91.18 + 8.62% of these meals.
2.5. Interview data
The mood disorder modules of the Structured Clinical Interview for DSM-IV, nonpatient version (SCID-NP) provided data on lifetime prevalence. Interviews were administered by trained clinical psychology graduate students or staff. Consensus meetings reviewed the recorded interviews to obtain diagnoses. SCID-NP data showed that 16 people met criteria for a past mood disorder (MDD=13, and 1 each for depression NOS, bipolar, and dysthymia). Average time since diagnosis was 7.95 years (SD=6.27). Two currently met criteria (1 MDD, 1 dysthymia).
2.6. Questionnaires
The 32-item Couples Satisfaction Index (CSI) assessed marital satisfaction (Funk and Rogge, 2007). Developed using item response theory, the CSI discriminates well between satisfied and dissatisfied couples with greater precision than the most commonly used marital scales (Funk and Rogge, 2007). The Pittsburgh Sleep Quality Index (PSQI) evaluated sleep quality and disturbances over a one-month interval (Buysse et al., 1989). The CHAMPS assessed the weekly frequency and duration of various physical activities (Harada et al., 2001; Stewart et al., 2001).The Center for Epidemiological Studies Depression (CES-D) Scale assessed depressive symptoms in the past week (Radloff, 1977).
2.7. Marital problem discussion
Hostile behavior, a hallmark of marital distress, has predicted couples’ physiological changes more reliably than self-reports (Kiecolt-Glaser and Newton, 2001). To obtain behavioral data, the experimenter first conducted a 10–20 minute interview to identify the most conflictual topics (Kiecolt-Glaser and Newton, 2001; Kiecolt-Glaser et al., 2005), based on each spouse’s Relationship Problem Inventory ratings (Knox, 1971). Couples were then asked to discuss and try to resolve one or more marital issues that the interviewer judged to be the most conflict-producing, e.g., money, communication, or in-laws. The research team remained out of sight while videotaping the subsequent 20-minute discussion.
Marital interaction tapes were coded using the Rapid Marital Interaction Coding System (RMICS) which discriminates well between distressed and nondistressed couples (Heyman, 2004). Distressed marriages are characterized by negative affect, conflictual communication, and poor listening skills (Kiecolt-Glaser and Newton, 2001; Kiecolt-Glaser et al., 2005; Robles et al., 2014). Accordingly, the composite index summed each of the four following RMICS codes, which we refer to collectively as hostility: psychological abuse (e.g., disgust, contempt, belligerence, as well as nonverbal behaviors like glowering), distress-maintaining attributions (e.g., “You’re only being nice so I’ll have sex with you tonight” or “You were being mean on purpose”), hostility (e.g., criticism, hostile voice tone, or rolling the eyes dramatically) and withdrawal (behaviors that suggest pulling back from the interaction or not listening).
Marital behavior, as measured by the composite hostile behavior scores, was highly correlated across visits (Spearman r = 0.77, p<0.0001) and within couples (Spearman r = 0.81, p<0.0001), and thus the couple’s hostile behavior sum was averaged across visits for use as a predictor in our analyses. Interrater agreement for the RMICS hostility composite (Holley and Guilford, 1964; Xu and Lorber, 2014) was high (Holley and Gilford’s G index = 0.88). This hostility composite score shared a moderate, negative association with couples’ self-reported marital satisfaction (Spearman r = −0.33, p<0.05).
2.8. Assays
Serum IL-6, TNF-α, CRP, and LBP were multiplexed and measured using an electrochemilluminescence method with Meso Scale Discovery kits, while sCD14 levels were measured using a Quantikine ELISA kit from R&D Systems. Each couple’s stored samples from both visits were assayed for each marker in one run, thus using the same controls for all time points. Sensitivity for IL-6 and TNF-α was 0.3 pg/ml, CRP was 0.7 ng/mL, LBP was 0.038 ng/mL, and sCD14 was 125 pg/mL The intra-assay coefficient of variation for IL-6 was 3.42%, and the inter-assay coefficient of variation was 8.425%; corresponding values for TNF-α were 2.59% and 8.14%, 6.28% and 7.36% for CRP, 2.74% and 8.33% for LBP, and 5.5% and 6.3% for sCD14.
2.9. Statistical Methods
To summarize the repeated measurements of endotoxin and inflammatory markers, area under the curve with respect to ground (AUCG) was calculated from baseline (pre-meal) to the last time point (7.5 hours post-meal) for serum TNF-α, IL6, CRP, LBP, sCD14, and LBP/sCD14 ratio (Pruessner et al., 2003). We used AUCG as a summary measure since it captures the overall intensity of exposure, and we did not expect acute meal- or conflict-related changes in the endotoxin markers due to their relatively slow response times (Hudgins et al., 2003). Figure 1 shows trajectories of all outcomes across the day; complete summary statistics are provided in eTable 1. All three endotoxin markers showed statistically significant, though small, changes, but LBP and the LBP/sCD14 ratio actually decreased across the day, and there were no meal-related differences in these slopes (ps>0.12). These endotoxin markers also showed remarkable stability from one visit to the next (LBP, Pearson r = 0.86, p<0.0001; sCD14, Pearson r = 0.85, p<0.0001). Previous analyses (Kiecolt-Glaser et al., 2015b; Kiecolt-Glaser et al., 2015c) showed an increase in IL-6 post-meal, but no change in TNF-α, and no meal-related differences; similar analyses showed no change in CRP post-meal and no meal effects. AUCG for both LBP and sCD14 was rescaled by dividing values by 1000 to avoid extremely small regression coefficients. Several of the AUCG variables had skewed distributions, thus all regression models were fit using generalized estimating equations (GEE) to relax parametric assumptions. In these models, we used an independent working correlation matrix and robust standard errors to account for the clustering of spouses and multiple visits per subject. Results from these models are presented as the average across visits, since the majority of outcomes did not have significant visit effects.
Figure 1:
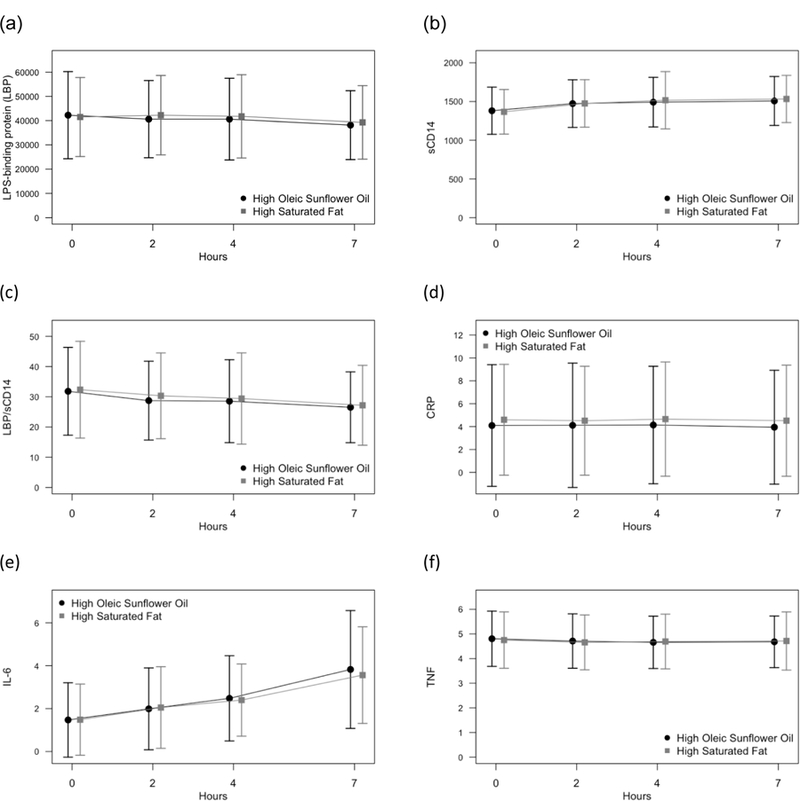
Mean (a) LBP, (b) sCD14, (c) LBP/sCD14 ratio, (d) CRP, (e) IL-6, and (f) TNF across the day, separately by meal type. Error bars show +/− 1 SD.
We tested for an interaction between mood disorder history and hostile behaviors in models predicting endotoxins, because this interaction was significant in previous analyses of other post-meal outcomes (Kiecolt-Glaser et al., 2015c). Non-significant interactions (p>0.1) were removed in constructing the final models. In secondary analyses, we substituted depressive symptoms (CES-D) for mood disorder history and marital satisfaction (CSI) for hostile behavior to explore the patterns’ stability.
All models controlled for age, race (white vs. African-American), trunk fat, gender, sleep (PSQI), and physical activity (average calories expended per week from the CHAMPS) to guard against potential confounding (all measured at the first visit). Models for outcomes measured at both visits (endotoxins and inflammatory markers) also controlled for visit number (first or second) and meal type to account for the study design, though these effects were not of primary interest. All analyses were conducted in SAS version 9.4 (Cary, NC).
In the analyses there were five subjects who only contributed data for one visit: of these, two partners did not have a second visit, and three subjects’ modestly elevated temperature indicated acute illness at one visit. Data from these five visits were excluded from all models, but data from these subjects’ other visits were included. Additionally, there were seven subjects with one visit excluded from the CRP analyses because they were missing the last measurement of CRP and thus AUCG could not be calculated. Data from other outcomes for these subjects’ visits were available and thus they were included in the other models.
3. RESULTS
3.1. Marital discord, past mood disorders, and endotoxin biomarkers
As shown in Figure 2a, there was a strong, significant association between hostile behavior and LBP UCG (p=0.0005), such that participants with more hostile marital interactions had higher LBP AUCG (eTables 2–6 provide details for analyses). Each one unit increase in hostile behavior frequency was associated with a 1.3 unit increase in LBP AUCG (95% CI: 0.55 to 2.0). As a result, a participant with higher hostile behavior (75th percentile) had 7.2% higher LBP AUCG than a participant with lower hostile behavior (25th percentile). The effect of mood disorder history was not significant (p=0.13) but trended towards higher LBP AUCG among participants with a mood disorder history. There was not a significant association between sCD14 AUCG and either mood disorder history (p=0.59) or hostile behavior (p=0.22), but the trend was for more hostile behaviors to be associated with lower sCD14. There was a significant interaction of hostile behavior and mood disorder history in predicting the LBP/sCD14 ratio (p=0.01, Figure 2b). The relationship between hostile behaviors and LBP/sCD14 ratio was stronger among participants with a mood disorder history (slope = 2.5, 95% CI: 1.6 to 3.5, p<0.0001) compared to participants without a history of mood disorder (slope = 1.1, 95% CI: 0.38 to 1.8, p=0.003).
Figure 2:
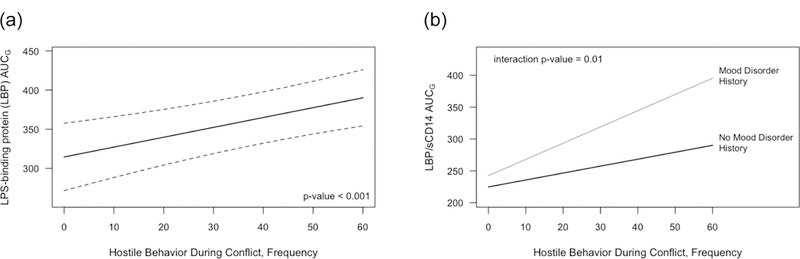
Estimated (a) LBP AUCG and (b) LBP/sCD4 ratio AUCG as a function of couples’ hostile behavior, averaged across visits. Results are from GEE models controlling for age, sex, race, trunk fat, sleep, daily activity level, meal type, and visit order. Dotted lines are a 95% confidence interval.
In secondary analyses, self-reported marital satisfaction using the couple’s average CSI scores was used in place of hostile behavior frequency in the models, and patterns of results were similar. Poorer marital satisfaction (lower CSI) trended towards being associated with higher LBP AUCG (p=0.055), and there was a significant interaction between marital satisfaction and mood disorder history in predicting the LBP/sCD14 ratio (p<0.0001). Poorer marital satisfaction (lower CSI) was associated with higher LBP/sCD14 ratio among participants with a mood disorder history (slope = −2.3; 95% CI: −2.8 to −1.8, p<0.0001) but was not associated with LBP/sCD14 for participants without a history of mood disorder (slope = 0.060; 95% CI: −0.39 to 0.51, p=0.79). Unlike the models using hostile behaviors, there was a significant interaction between marital satisfaction and mood disorder history in predicting sCD14 (p=0.001); poorer marital satisfaction (lower CSI) was significantly associated with lower sCD14 for participants with a history of a mood disorder (slope = 0.030; 95% CI: 0.009 to 0.051, p=0.006) but not for participants without a mood disorder history (slope = −0.011; 95% CI: −0.027 to 0.005, p=0.17).
3.2. Endotoxin biomarkers and inflammation
Both LBP AUCG (p=0.007) and LBP/sCD14 ratio AUCG (p=0.02) were significantly associated with CRP AUCG (Figure 3). The estimated CRP AUCG for a participant with high LBP AUCG (75th percentile) was 80% higher than for a participant with low LBP (25th percentile),corresponding to an average estimated CRP level across the day of 6.21 ng/mL for a participant with high LBP AUCG compared to 3.46 ng/mL for a participant with low LBP AUCG. This effect was also seen when looking at the unadjusted AUC values; among values in the lowest quartile of LBP AUCG, only 21% had an average CRP > 3 ng/mL across the day, while 79% of those in the highest quartile of LBP AUCG had an average CRP > 3ng/mL across the day. The effect for LBP/sCD14 ratio AUCG was similar though smaller in magnitude. The estimated CRP AUCG for a participant with high LBP/sCD14 AUCG (75th percentile) was 50% higher than for a participant with low LBP/sCD14 AUCG (25th percentile), corresponding to average estimated RP across the day of 5.91 ng/mL compared to 3.94 ng/mL. However, sCD14 AUCG was not associated with CRP AUCG (p=0.12), though the trend was for higher sCD14 to be associated with higher CRP AUCG.
Figure 3:
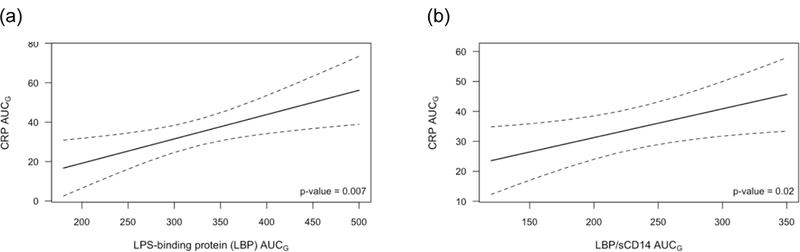
Estimated CRP AUCG as a function of (a) LBP AUCG and (b) LBP/sCD14 AUCG, averaged across visits. Results are from GEE models controlling for age, sex, race, sleep, daily activity level, trunk fat, meal type, and visit order. Dotted lines are a 95% confidence interval.
There was a nonsignificant trend towards higher LBP AUCG associated with higher IL-6 AUCG (p=0.12), and higher sCD14 AUCG was associated with higher IL-6 AUCG (p=0.03). Compared to low sCD14 participants (25th percentile), those in the 75th percentile had 15% higher IL-6 AUCG (Figure 4). The LBP/sCD14 ratio was not significantly associated with IL-6 AUCG (p=0.35). None of the endotoxin markers (LBP, sCD14, LBP/sCD14 ratio) were significantly associated with TNF-α AUCG (ps>0.4).
Figure 4:
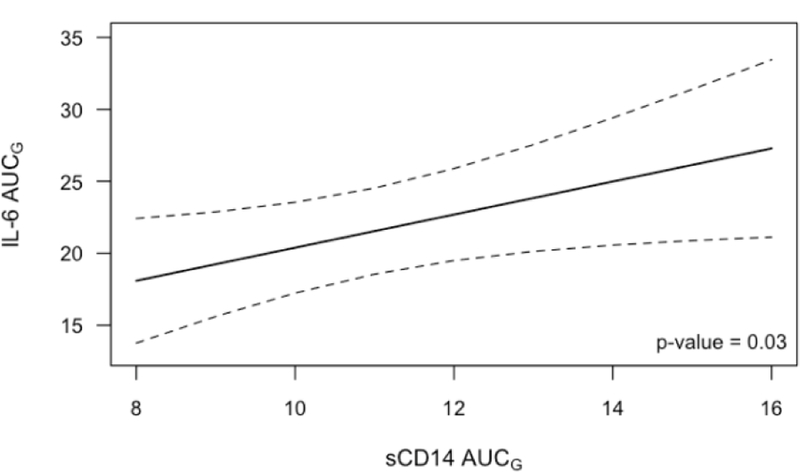
Estimated IL-6 AUCG as a function of sCD14 AUCG, averaged across visits. Results are from GEE models controlling for age, sex, race, sleep, daily activity level, trunk fat, meal type, and visit order. Dotted lines are a 95% confidence interval.
3.3. Covariate Effects
The effects of controlling variables varied slightly depending on which other predictors were included (e.g., hostile behaviors versus marital satisfaction; LBP versus sCD14) (see eTables 2–8). In general, older age was associated with higher LBP/sCD14 AUCG, and higher trunk fat was associated with higher LBP AUCG, RP AUCG, and TNF AUCG, with a trend towards higher IL-6 AUCG. Men had significantly lower sCD14 AUCG, CRP AUCG, and IL-6 AUCG than women, and white participants had higher sCD14 AUCG, a lower LBP/sCD14 ratio, lower IL-6 AUCG, and higher TNF AUCG than African-American participants. Higher physical activity (higher average calories expended per week) was associated with lower IL-6 AUCG. Neither sleep nor meal type were significant predictors in any model.
3.4. Ancillary Analyses
To investigate the associations with current affective symptoms, mood disorder history was replaced by current depressive symptoms in all models. However, neither the main effect of CES-D nor the interactions between CES-D and hostile behavior or self-reported marital satisfaction were significant predictors of the endotoxin markers (LBP, sCD14, LBP/sCD14 ratio; ps>0.1).
4. DISCUSSION
Consistent with our hypotheses, participants with more hostile marital interactions had higher LBP. Additionally, partners who had more hostile marital interactions had higher LBP/sCD14 ratios, with the strongest effects among those who had a prior mood disorder. Neither hostile behavior nor mood disorder history was related to sCD14 alone, but instead predicted the relative balance of LBP and sCD14. Likewise, higher LBP and LBP/sCD14 ratios, but not sCD14, were associated with greater CRP production. Indeed, compared to participants with low LBP (25th percentile), participants with high LBP (75th percentile) had 79% higher CRP across the day. Similarly, relative to participants with lower LBP/sCD14 ratios (25th percentile), participants with higher LBP/sCD14 ratios (75th percentile) had 45% higher CRP over the study visit. Higher sCD14 was associated with higher IL-6. Mirroring our findings, LBP was associated with CRP but not IL-6 in two other studies (Stehle et al., 2012; Romani et al., 2013), while sCD14 was significantly related to IL-6 but not CRP (Stehle et al., 2012).
CRP has clinically relevant prognostic significance, discriminating among people with low, moderate and high risk of future heart attack and stroke (<1, 1 – 3, and > 3 mg/L, respectively) (Ridker, 2003). Thus it is noteworthy that only 21 % of low LBP participants (lowest quartile) had average CRP values > 3, compared to 79% of those in the highest quartile. The higher CRP values in this sample reflect this study’s inclusion criteria that prioritized overweight and sedentary couples.
LBP has been described as a surrogate marker of microbial translocation (Stehle et al., 2012). Thus, higher levels of LBP reflect greater amounts of endotoxin from commensal microbes translocating from their niche on the body to the blood, where they stimulate LBP production by the liver. Every surface of the body can be colonized by commensal bacteria, but the vast majority of microbes reside in the gut. Many of these are Gram-negative microbes with LPS in their cell wall. Thus, high levels of LBP often reflect translocation of Gram-negative bacteria from the gut to the interior of the body. It should be noted, however, that in addition to LPS, cytokines (such as IL-6, IL-1β, TNF-α, and IL-22) can increase LBP production in hepatocytes and colonic epithelial cells (Wan et al., 1995; Wolk et al., 2007). In stressor-exposed animals, cytokine increases have been associated with bacterial translocation and influenced by the gut microbiota (Bailey et al., 2011; Maslanik et al., 2012; Lafuse et al., 2017), supporting the rationale for bacterial translocation from a leaky gut as a new mechanistic pathway among marital distress, a history of depression, and inflammation-related disorders.
In studies with MDD patients, the gut microbiota’s composition has differed from that of healthy controls (Kelly et al., 2015; Kelly et al., 2016; Rogers et al., 2016). After a disturbance, the gut microbiota community typically returns to the pre-disturbance composition. However, severe insults may produce long-term—perhaps permanent—changes in the microbiota’s composition, leading to problematic alterations in the commensal gut microbiota that regulate local and systemic inflammation and immunity, as well as maintenance of gut barrier function.
In fact, the differences we found between individuals with a mood disorder history compared to those without may reflect persistent psychological and physiological vulnerabilities, in a way that past-week depressive symptoms did not capture. Indeed, impairments in marital relationships can persist for years after an acute depressive episode (Bothwell and Weissman, 1977; Levkovitz et al., 2003). People with a history of depression experience more major and minor stressors than those without a similar history, and past depression can boost emotional reactivity to daily stressors (Hammen, 1991; Gunthert et al., 2007; Husky et al., 2009). Repeated intermittent stressors also characterize distressed marriages (Story and Bradbury, 2004). For example, in a study that collected daily reports of stress and marital functioning, daily stress among hostile couples was driven in part by the prior day’s marital conflict (Timmons et al., 2017). In turn, daily stress spilled over to increase the next day’s marital conflict, fueling a vicious cycle.
Greater and more frequent emotional reactivity to stressors has implications for bacterial translocation; two weeks of daily 15-minute stress exposures produced persistent increases in gut permeability in mice that were still evident two weeks after the last session (Rodrigues et al., 2017). Thus, it is possible that the associations in our data related to distressed marriages and past mood disorders may reflect longer-term microbiota alterations. leaky gut may serve to maintain low-grade inflammation that could be exacerbated by stress, thereby enhancing risk for recurrent mood disorder episodes.
Just as hostile behaviors remained similar from one visit to the next, endotoxin biomarkers were very highly correlated across visits, suggesting more chronic exposure. Indeed, steady, lasting associations seem to exist between marital distress and intestinal permeability in a way that may chronically fuel inflammation. In addition to their stability across visits, the endotoxin biomarkers showed little change within each of the study visits (Figure 1), consistent with evidence that they are relatively slow-moving. For example, in one study LBP finally peaked 12 hours after an endotoxin injection (Hudgins et al., 2003), a far more substantial inflammatory stimulus than a marital discussion or a high-fat meal.
The bidirectional microbiota-gut-brain communication involves multiple depression- and stress-responsive pathways including the sympathetic nervous system (SNS), the hypothalamic-pituitary-adrenal (HPA) axis, the vagus nerve, and the immune system. For example, SNS-stimulated catecholamine production can elevate both pathogenic and commensal bacterial levels 10,000-fold while simultaneously enhancing pathogenic virulence (Freestone et al., 2002). In addition, The HPA axis activation that accompanies stress and depression can disrupt the functioning of the intestinal barrier by promoting corticotropin-releasing hormone (CRH) release, which prompts ACTH and cortisol production (Vanuytsel et al., 2014).
Heightened SNS and HPA responses have also been observed in laboratory studies with distressed couples. For example, more hostile couples produced larger and more persistent catecholamine, ACTH, and cortisol responses during and following marital problem discussions compared to their less hostile counterparts (Kiecolt-Glaser and Newton, 2001), as well as greater elevations in these hormones throughout the remainder of the day and night (Kiecolt-Glaser et al., 1996).
The vagus nerve provides neural communication between peripheral gut microbes and centrally mediated behavioral processes (Kelly et al., 2015). Lower vagal activation has been associated with poorer marital satisfaction in both cross-sectional and longitudinal studies (Smith et al., 2011; Donoho et al., 2015), and a meta-analysis showed that depression also lowers vagal activation (Kemp et al., 2010). Importantly, although antidepressant treatment reduces depressive symptoms, it does not change HRV (Kemp et al., 2010). Persistent HRV reductions could drive long-term maladaptive communication between gut microbes and behavior.
The immune system helps to maintain intestinal homeostasis, and immune dysregulation can provoke gut dysbiosis (microbial imbalance), thus promoting bacterial translocation (Bailey et al., 2011; Ait-Belgnaoui et al., 2012). Both hostile marital interactions and depression can dysregulate cellular and inflammatory immune responses (Kiecolt-Glaser and Newton, 2001; Kiecolt-Glaser et al., 2005; Robles et al., 2014; Kiecolt-Glaser et al., 2015a). Marital behavior is stable across time, as reflected in the high correlation in hostile behaviors between the two study visits, also shown in other work (Kiecolt-Glaser and Newton, 2001). Accordingly, persistent marital distress and past depression could spur gut dysbiosis and bacterial translocation through multiple routes, including heightened SNS and HPA activation in tandem with lower vagal activation and immune dysregulation.
Heightened endotoxin exposure has important health implications. Atherosclerosis, cardiovascular disease, and diabetes have all been associated with chronic bacterial endotoxin exposure (Stoll et al., 2004; Pussinen et al., 2011; Kelly et al., 2012), and each has been linked to marital discord and depression (Orth-Gomer et al., 2000; Gallo et al., 2003; Jones et al., 2003; Troxel et al., 2005; Joseph et al., 2014; Whisman et al., 2014). Furthermore, aging is accompanied by increased gut bacteria translocation as well as less diversity in the gut microbiota’s bacterial composition, and both of these changes promote persistent low-grade inflammation (Stehle et al., 2012). Higher levels of LBP have been associated with poorer physical function and higher levels of inflammation even among healthy older adults independent of age, gender, BMI, aerobic fitness, and inflammatory biomarkers (Stehle et al., 2012).
Our sample’s average age was 38, a feature of the study that probably underestimates the associations among middle-aged and older populations. Marital distress and depression likely interact with age-related increases in gut bacterial translocation to heighten age-associated risks. This study did not include gut microbiota assessments, another limitation. Additionally, although convergent data from two studies suggests that a mood disorder history can alter metabolic responses to high-fat meals (Kiecolt-Glaser et al., 2015b; Kiecolt-Glaser et al., 2015c), the relatively small number of participants with a past mood disorder suggest that those results should be interpreted cautiously.
Both marital discord and depression have notable physiological repercussions, as documented in the poorer clinical outcomes for conditions ranging from cardiovascular disease to metabolic syndrome and diabetes (Orth-Gomer et al., 2000; Gallo et al., 2003; Jones et al., 2003; Troxel et al., 2005; Joseph et al., 2014; Whisman et al., 2014). This study illustrates novel pathways through which a troubled marriage and a mood disorder history could contribute to each of these high risk conditions. Accordingly, treatments that address marital distress and/or depression could also benefit both mental and physical health.
Our data demonstrate how a distressed marriage and a depression history can promote a proinflammatory milieu through increased gut permeability, with broad health implications. Indeed, this study shows how the gut microbiota can fuel a range of stress-associated pathologies.
Supplementary Material
Table 2.
Endotoxins and Inflammatory markers at baseline and summarized across the day (area under the curve; AUC)
| Outcome | N* | Mean (SD) | Median (IQR) | |
|---|---|---|---|---|
| LBP | Baseline | 154 | 41876 (17105) | 39700 (30100–50100) |
| (82 subjects) | AUC | 154 | 334717 (128817) | 327165 (241018–397420) |
| sCD14 | Baseline | 157 | 1374 (296) | 1350 (1190–1560) |
| (83 subjects) | AUC | 157 | 12113 (2441) | 11873 (10582–13445) |
| LBP/sCD14 | Baseline | 154 | 32 (15) | 31 (21–39) |
| (82 subjects) | AUC | 154 | 239 (110) | 224 (162–305) |
| CRP | Baseline | 160 | 4.3 (5.1) | 2.6 (0.92–5.9) |
| (86 subjects) | AUC | 160 | 35 (41) | 20 (8.0–49) |
| IL-6 | Baseline | 159 | 1.5 (1.7) | 1.0 (0.69–1.5) |
| (86 subjects) | AUC | 159 | 20 (13) | 17 (11–25) |
| TNF-a | Baseline | 160 | 4.8 (1.1) | 4.7 (3.9–5.5) |
| (86 subjects) | AUC | 160 | 38 (8.4) | 38 (33–44) |
Highlights.
- At two visits, couples discussed marital disagreements and gave blood samples.
- Hostile behaviors and depression predicted greater bacterial LPS translocation.
- In turn, greater bacterial translocation was related to higher inflammation.
- Bacterial translocation explained hostility and depression’s links to CRP.
Acknowledgements
The study was supported in part by NIH grants R21 CA154054, K05 CA172296, R01 AG057032, T32 DE014320, K99 AG056667, and UL1TR001070. The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. We are grateful to Michael Di Gregorio, M.A., for his role as a key organizer and experimenter, and Susan Glaser, hD, who conducted the interviews to identify the best topics for couples’ conflict discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors had no potential conflicts of interest.
References
- Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L, Theodorou V, 2012. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 37, 1885–1895. [DOI] [PubMed] [Google Scholar]
- Amar J, Ruidavets JB, Bal Dit Sollier C, Bongard V, Boccalon H, Chamontin B, Drouet L, Ferrieres J, 2003. Soluble CD14 and aortic stiffness in a population-based study. J. Hypertens 21, 1869–1877. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M, 2011. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain. Behav. Immun 25, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach SRH, 2014. The couple and family discord model of depression: Updates and future directions, in: Agnew CR, South SC (Eds.), Interpersonal relationships and health: Social and clinical psychological mechanisms Oxford University Press, New York, NY, US, pp. 133–155. [Google Scholar]
- Bothwell S, Weissman MM, 1977. Social impairments four years after an acute depressive episode. Am. J. Orthopsychiatry 47, 231–237. [DOI] [PubMed] [Google Scholar]
- Buysse J, Reynolds CF, Monk TH, Berman SR, Kupfer DJ, 1989. Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Coyne JC, Rohrbaugh MJ, Shoham V, Sonnega JS, Nicklas JM, Cranford JA, 2001. Prognostic importance of marital quality for survival of congestive heart failure. Am. J. Cardiol 88, 526–529. [DOI] [PubMed] [Google Scholar]
- Dallman MF, 2010. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab 21, 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoho CJ, Crimmins EM, Seeman TE, 2013. Marital quality, gender, and markers of inflammation in the MIDUS cohort. Journal of Marriage and Family 75, 127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoho CJ, Seeman TE, Sloan RP, Crimmins EM, 2015. Marital status, marital quality, and heart rate variability in the MIDUS cohort. J. Fam. Psychol 29, 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freestone PP, Williams PH, Haigh RD, Maggs AF, Neal CP, Lyte M, 2002. Growth stimulation of intestinal commensal escherichia coli by catecholamines: A possible contributory factor in trauma-induced sepsis. Shock 18, 465–467. [DOI] [PubMed] [Google Scholar]
- Funk JL, Rogge RD, 2007. Testing the ruler with item response theory: Increasing precision of measurement for relationship satisfaction with the Couples Satisfaction Index. J. Fam. Psychol 21, 572–583. [DOI] [PubMed] [Google Scholar]
- Gallo LC, Troxel WM, Kuller LH, Sutton-Tyrrell K, Edmundowicz D, Matthews K., 2003. Marital status, marital quality, and atherosclerotic burden in postmenopausal women. Psychosom. Med 65, 952–962. [DOI] [PubMed] [Google Scholar]
- Gunthert K, Cohen L, Butler A, Beck J, 2007. Depression and next-day spillover of negative mood and depressive cognitions following interpersonal stress. Cognit. Ther. Res 31, 521–532. [Google Scholar]
- Hammen C, 1991. Generation of stress in the course of unipolar depression. J. Abnorm. Psychol 100, 555–561. [DOI] [PubMed] [Google Scholar]
- Harada ND, Chiu V, Stewart AL, 2001. An evaluation of three self-report physical activity instruments for older adults. Med. Sci. Sports Exerc 33, 962–970. [DOI] [PubMed] [Google Scholar]
- Heyman R., 2004. Rapid marital interaction coding system (RMICS), in: Kerig PK, Baucom DH (Eds.), Couple observational coding systems Lawrence Erlbaum Associates, Nahwah, New Jersey, pp. 67–94. [Google Scholar]
- Holley JW, Guilford JP, 1964. A note on the g-index of agreement. Educational and Psychological Measurement 24, 749–753. [Google Scholar]
- Hudgins LC, Parker TS, Levine DM, Gordon BR, Saal SD, Jiang XC, Seidman CE, Tremaroli JD, Lai J, Rubin AL, 2003. A single intravenous dose of endotoxin rapidly alters serum lipoproteins and lipid transfer proteins in normal volunteers. J. Lipid Res 44, 1489–1498. [DOI] [PubMed] [Google Scholar]
- Husky M, Mazure C, Maciejewski P, Swendsen J, 2009. Past depression and gender interact to influence emotional reactivity to daily life stress. Cognit. Ther. Res 33, 264–271. [Google Scholar]
- Jones DJ, Bromberger JT, Sutton-Tyrrell K, Matthews KA, 2003. Lifetime history of depression and carotid atherosclerosis in middle-aged women. Arch. Gen. Psychiatry 60, 153–160. [DOI] [PubMed] [Google Scholar]
- Joseph NT, Kamarck TW, Muldoon MF, Manuck SB, 2014. Daily marital interaction quality and carotid artery intima-medial thickness in healthy middle-aged adults. Psychosom. Med 76, 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CJ, Colgan SP, Frank DN, 2012. Of microbes and meals: The health consequences of dietary endotoxemia. Nutr. Clin. Pract 27, 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JR, Borre Y, C OB, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald, Ross P, Stanton C, Clarke G, Cryan JF, Dinan TG, 2016. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res 82, 109–118. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Kennedy J, Cryan JF, Dinan TG, Clarke G, Hyland NP, 2015. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front ell Neurosci 9, 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp H, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM, 2010. Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biol. Psychiatry 67, 1067–1074. [DOI] [PubMed] [Google Scholar]
- Keri S, Szabo C, Kelemen O, 2014. Expression of toll-like receptors in peripheral blood mononuclear cells and response to cognitive-behavioral therapy in major depressive disorder. Brain Behavior and Immunity 40, 235–243. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Derry HM, Fagundes CP, 2015a. Inflammation: Depression fans the flames and feasts on the heat. Am. J. Psychiatry 172, 1075–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Fagundes CP, Andridge R, Peng J, Malarkey WB, Habash D, Belury MA, 2017. Depression, daily stressors and inflammatory responses to high-fat meals: When stress overrides healthier food choices. Mol. Psychiatry 22, 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Habash DL, Fagundes CP, Andridge R, Peng J, Malarkey WB, Belury MA, 2015b. Daily stressors, past depression, and metabolic responses to high-fat meals: A novel path to obesity. Biol. Psychiatry 77, 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Jaremka L, Andridge R, Peng J, Habash D, Fagundes CP, Glaser R, Malarkey WB, Belury MA, 2015c. Marital discord, past depression, and metabolic responses to high-fat meals: Interpersonal pathways to obesity. Psychoneuroendocrinology 52, 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell J., Malarkey WB, Lemeshow S, Dickinson SL, Glaser R, 2005. Hostile marital interactions, proinflammatory cytokine production, and wound Healing. Arch. Gen. Psychiatry 62, 1377–1384. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton T, Cacioppo JT, MacCallum RC, Glaser R, Malarkey WB, 1996. Marital conflict and endocrine function: Are men really more physiologically affected than women? J. Consult. Clin. sychol 64, 324–332. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL, 2001. Marriage and health: His and hers. Psychol. Bull 127, 472–503. [DOI] [PubMed] [Google Scholar]
- Knox D, 1971. Marriage happiness Research Press, Champaign, IL. [Google Scholar]
- Lafuse WP, Gearinger R, Fisher S, Nealer C, Mackos AR, Bailey MT, 2017. Exposure to a social stressor induces translocation of commensal lactobacilli to the spleen and priming of the innate immune system. J. Immunol 198, 2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugerette F, Alligier M, Bastard JP, Drai J, Chanseaume E, Lambert-Porcheron S, Laville M, Morio B, Vidal H, Michalski MC, 2014. Overfeeding increases postprandial endotoxemia in men: Inflammatory outcome may depend on LPS transporters LBP and sCD14. Molecular Nutrition & Food Research 58, 1513–1518. [DOI] [PubMed] [Google Scholar]
- Laugerette F, Furet JP, Debard C, Daira P, Loizon E, Geloen A, Soulage CO, Simonet C, Lefils-Lacourtablaise J, Bernoud-Hubac N, Bodennec J, Peretti N, Vidal H, Michalski MC, 2012. Oil composition of high-fat diet affects metabolic inflammation differently in connection with endotoxin receptors in mice. American Journal of Physiology-Endocrinology and Metabolism 302, E374–E386. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Lamy D, Ternochiano P, Treves I, Fennig S, 2003. Perception of dyadic relationship and emotional states in patients with affective disorder. J. Affect. Disord 75, 19–28. [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis JC, 2008. The gut-brain barrier in major depression: Intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuroendocrinol. Lett 29, 117–124. [PubMed] [Google Scholar]
- Maslanik T, Tannura K, Mahaffey L, Loughridge AB, Benninson L, Ursell L, Greenwood BN, Knight R, Fleshner M, 2012. Commensal bacteria and mamps are necessary for stress-induced increases in il-1β and il-18 but not IL-6, il-10 or mcp-1. PLOS ONE 7, e50636. [DOI] [PMC free article] [PubMed]
- Newsholme P, Homem de Bittencourt PI Jr., 2016. Gut associated bacteria are critical to metabolism, inflammation and health. Curr Opin Clin Nutr Metab Care 19, 245–249. [DOI] [PubMed] [Google Scholar]
- Orth-Gomer K, Wamala SP, Horsten M, Schenck-Gustafsson K, Schneiderman N, Mittleman MA, 2000. Marital stress worsens prognosis in women with coronary heart disease - the Stockholm female coronary risk study. JAMA 284, 3008–3014. [DOI] [PubMed] [Google Scholar]
- Pruessner J., Kirschbaum C, Meinlschmid G, Hellhammer DH, 2003. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinolgy 28, 916–931. [DOI] [PubMed] [Google Scholar]
- Pussinen PJ, Havulinna AS, Lehto M, Sundvall J, Salomaa V, 2011. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care 34, 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS, 1977. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement 1, 385–401. [Google Scholar]
- Ridker PM, 2003. C-reactive protein - a simple test to help predict risk of heart attack and stroke. Circulation 108, E81–E85. [DOI] [PubMed] [Google Scholar]
- Robles TF, Slatcher RB, Trombello JM, McGinn MM, 2014. Marital quality and health: A meta-analytic review. Psychol. Bull 140, 140–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues MED, Bekhbat M, Houser MC, Chang JJ, Walker DI, Jones DP, do Nascimento C, Barnum CJ, Tansey MG, 2017. Chronic psychological stress and high-fat high-fructose diet disrupt metabolic and inflammatory gene networks in the brain, liver, and gut and promote behavioral deficits in mice. Brain Behavior and Immunity 59, 158–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S, 2016. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 21, 738–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani J, Caixas A, Escote X, Carrascosa JM, Ribera M, Rigla M, Vendrell J, Luelmo J., 2013. Lipopolysaccharide-binding protein is increased in patients with psoriasis with metabolic syndrome, and correlates with C-reactive protein. Clin. Exp. Dermatol 38, 81–84. [DOI] [PubMed] [Google Scholar]
- Shen BJ, Farrell KA, Penedo FJ, Schneiderman N, Orth-Gomer K, 2010. Waist circumference moderates the association between marital stress and C-reactive protein in middle-aged healthy women. Ann. Behav. Med 40, 258–264. [DOI] [PubMed] [Google Scholar]
- Smith TW, Cribbet MR, Nealey-Moore JB, Uchino BN, Williams PG, Mackenzie J, Thayer JF, 2011. Matters of the variable heart: Respiratory sinus arrhythmia response to marital interaction and associations with marital quality. J. Pers. Soc. Psychol 100, 103–119. [DOI] [PubMed] [Google Scholar]
- Stehle JR Jr., Leng X, Kitzman W, Nicklas BJ, Kritchevsky SB, High KP, 2012. Lipopolysaccharide-binding protein, a surrogate marker of microbial translocation, is associated with physical function in healthy older adults. J. Gerontol. A. Biol. Sci. Med. Sci 67, 1212–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL, 2001. Champs physical activity questionnaire for older adults: Outcomes for interventions. Med. Sci. Sports Exerc 33, 1126–1141. [DOI] [PubMed] [Google Scholar]
- Stoll LL, Denning GM, Weintraub NL, 2004. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler. Thromb. Vasc. Biol 24, 2227–2236. [DOI] [PubMed] [Google Scholar]
- Story LB, Bradbury TN, 2004. Understanding marriage and stress: Essential questions and challenges. Clin. Psychol. Rev 23, 1139–1162. [DOI] [PubMed] [Google Scholar]
- Timmons AC, Arbel R, Margolin G, 2017. Daily patterns of stress and conflict in couples: Associations with marital aggression and family-of-origin aggression. Journal of Family Psychology : JFP : Journal of the Division of Family Psychology of the American Psychological Ass 31, 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxel WM, Matthews KA, Gallo LC, Kuller LH, 2005. Marital quality and occurrence of the metabolic syndrome in women. Arch. Intern. Med 165, 1022–1027. [DOI] [PubMed] [Google Scholar]
- Ulevitch RJ, Tobias PS, 1995. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol 13, 437–457. [DOI] [PubMed] [Google Scholar]
- Vanuytsel T, van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E, Salim Rasoel S, Tomicronth J, Holvoet L, Farre R, Van Oudenhove L, Boeckxstaens G, Verbeke K, Tack J, 2014. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 63, 1293–1299. [DOI] [PubMed] [Google Scholar]
- Wan Y, Freeswick D, Khemlani LS, Kispert PH, Wang SC, Su GL, Billiar TR, 1995. Role of lipopolysaccharide (LPS), interleukin-1, interleukin-6, tumor necrosis factor, and dexamethasone in regulation of LPS-binding protein expression in normal hepatocytes and hepatocytes from LPS-treated rats. Infect. Immun 63, 2435–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisman MA, Li A, Sbarra DA, Raison CL, 2014. Marital quality and diabetes: Results from the Health and Retirement Study. Health Psychol 33, 832–840. [DOI] [PubMed] [Google Scholar]
- Whisman MA, Sbarra DA, 2012. Marital adjustment and interleukin-6 (IL-6). J. Fam. Psychol 26, 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk K, Witte E, Hoffmann U, Doecke W-D, Endesfelder S, Asadullah K, Sterry W, Volk H-D, Wittig BM, Sabat R, 2007. Il-22 induces lipopolysaccharide-binding protein in hepatocytes: A potential systemic role of il-22 incrohn’s disease. The Journal of Immunology 178, 5973. [DOI] [PubMed] [Google Scholar]
- Wright SD, Ramos RA, Tobias PS, Ulevitch J, Mathison JC, 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249, 1431–1433. [DOI] [PubMed] [Google Scholar]
- Xu S, Lorber MF, 2014. Interrater agreement statistics with skewed data: Evaluation of alternatives to Cohen’s kappa. J. Consult. Clin. Psychol 82, 1219–1927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


